Abstract
Published research supports a role for central glucagon-like peptide 1 (GLP-1) signaling in suppressing food intake in rodent species. However, it is unclear whether GLP-1 neurons track food intake and contribute to satiety, and/or whether GLP-1 signaling contributes to stress-induced hypophagia. To examine whether GLP-1 neurons track intake volume, rats were trained to consume liquid diet (LD) for 1 h daily until baseline intake stabilized. On test day, schedule-fed rats consumed unrestricted or limited volumes of LD or unrestricted volumes of diluted (calorically matched to LD) or undiluted Ensure. Rats were perfused after the test meal, and brains processed for immunolocalization of cFos and GLP-1. The large majority of GLP-1 neurons expressed cFos in rats that consumed satiating volumes, regardless of diet type, with GLP-1 activation proportional to intake volume. Since GLP-1 signaling may limit intake only when such large proportions of GLP-1 neurons are activated, a second experiment examined the effect of central GLP-1 receptor (R) antagonism on 2 h intake in schedule-fed rats. Compared with baseline, intracerebroventricular vehicle (saline) suppressed Ensure intake by ∼11%. Conversely, intracerebroventricular injection of vehicle containing GLP-1R antagonist increased intake by ∼14% compared with baseline, partly due to larger second meals. We conclude that GLP-1 neural activation effectively tracks liquid diet intake, that intracerebroventricular injection suppresses intake, and that central GLP-1 signaling contributes to this hypophagic effect. GLP-1 signaling also may contribute to satiety after large volumes have been consumed, but this potential role is difficult to separate from a role in the hypophagic response to intracerebroventricular injection.
Keywords: cFos; Exendin-3 (9–39), Ex9; meal patterns
food intake is regulated by a complex, neurochemically diverse neural network distributed within the brain stem, hypothalamus, and limbic forebrain. Food intake is the product of meal number (determined by signals that initiate feeding bouts) and meal size (determined by signals that terminate feeding bouts) (32). When rats in a controlled laboratory environment have unlimited access to standard chow, meal size is largely determined by feeding-generated satiety signals arising from the gastrointestinal (GI) tract that are delivered by vagal sensory inputs to the caudal nucleus of the solitary tract (cNST) (4, 12, 14–18). The cNST relays satiety signals to a variety of central regions, including brain-stem pattern generator and premotor circuits that control the motoric components of feeding (i.e., licking, chewing, and swallowing) (31, 34). While it is clear that the cNST is critically involved in receiving and processing signals that modulate ingestive consummatory behaviors, details regarding the neurochemical identity and circuit connectivity of cNST neurons that limit meal size are just beginning to emerge.
Recent studies support the hypothesis that glucagon-like peptide-1 (GLP-1)-immunopositive cNST neurons participate in meal termination. In rats, GLP-1 neurons are activated to express the immediate-early gene product cFos in response to experimental treatments that reduce meal size, including restraint stress (7), intraperitoneal injection of cholecystokinin (24, 27, 38), or gastric distension (8, 36). A recent study in our laboratory explored whether food intake itself activates cFos expression by GLP-1 neurons (21). Rats in that study were food deprived for 24 h for the first time and then allowed to refeed to satiety on palatable liquid Ensure. Although rats consumed ∼5% of their body weight within 30 min, intake of these large satiating volumes activated cFos expression in only ∼30% of GLP-1 neurons (21), which is only slightly more than the 20–25% of GLP-1 neurons expressing cFos under baseline conditions in ad libitum-fed controls (24, 25). Since the proportion of GLP-1 neurons expressing cFos was significantly and positively correlated with intake volume in our earlier report (21), we posited that GLP-1 neuronal activation might “track” the amount of food consumed, such that rats consuming even larger amounts would display proportionately higher levels of GLP-1 neural activation. To examine this, the first experiment in the present study quantified GLP-1 neural activation in rats that were trained over 5 days to consume significantly larger volumes of liquid diet within a 1-h period. Consistent with our hypothesis, intake of larger volumes by meal-entrained rats activated larger proportions of GLP-1 neurons (i.e., up to 90%; see results).
Although GLP-1 neurons appear to track gastric distension or some other feeding-related sensory signal in rats (Ref. 21 and present report), experimental support for a role of central GLP-1R signaling in satiation has been equivocal. Pharmacological antagonism of GLP-1Rs by intracerebroventricular infusion of Exendin-3 (9–39) (Ex9) has been reported to increase cumulative intake by rats in some feeding paradigms (3, 10, 17, 35, 39), but not in others (3, 17, 35, 39). For example, Ex9 administered into the 4th ventricle increased chow intake in rats compared with intake by vehicle-injected controls, but only after rats had first consumed a ∼3% body weight preload of Ensure (17). Intake by noninjected rats was not reported in that study, making it unclear whether GLP-1 receptor (R) antagonism increased baseline intake and/or countered a potentially hypophagic effect of intracerebroventricular injection. Similarly, another research group reported convincing evidence that intracerebroventricular Ex9 dose-dependently increased cumulative dark-onset food intake in ad libitum-fed rats compared with intake by intracerebroventricular vehicle-injected controls (10), but comparable dark-onset food intake by rats under noninjected baseline conditions was not reported. Results from that study differ from others in which intracerebroventricular Ex9 did not increase chow intake in ad libitum-fed rats compared with intake by control rats treated with intracerebroventricular vehicle (3, 13, 20, 25, 43). One study reported an effect of Ex9 to increase meal size in rats (9), suggesting a role for GLP-1 signaling in satiety, but that study also did not report comparable intake data from noninjected rats, and did not report whether increased meal size resulted in increased cumulative intake. In other studies, rats that were food-deprived for the first time subsequently consumed the same amount of chow regardless of whether they were pretreated intracerebroventricularly with Ex9 or vehicle before refeeding (35, 39), although deprivation-induced intake by noninjected controls was not reported.
It is possible that central GLP-1R signaling limits food intake only under unusual conditions in which many GLP-1 neurons are activated, e.g., when rats consume very large amounts of food and/or after hypophagic stress (23, 25, 26). Indeed, we recently reported no significant effect of intracerebroventricular Ex9 on 1 h dark-onset chow intake in ad libitum-fed control rats compared with 1 h intake under noninjected baseline conditions; interestingly, however, intracerebroventricular Ex9 blocked the hypophagic effect of acute restraint stress, which robustly activated GLP-1 neurons (25). In that study, intracerebroventricular injection of either saline vehicle or Ex9 by itself activated cFos expression by ∼60% of GLP-1 neurons, but neither injection significantly altered dark-onset chow intake compared with intake under noninjected baseline conditions, which averaged less than 2% body weight (25). Since food intake was relatively modest and was assessed for only 1 h in that study, the second experiment in the present study used intracerebroventricularly cannulated rats trained to consume large, stable volumes of Ensure during a daily 2-h period to determine whether cumulative intake and/or meal patterns are altered after intracerebroventricular injection of NaCl vehicle (SAL) or Ex9.
METHODS
Experiments were conducted in accordance with the National Institutes of Health (NIH) “Guide for the Care and Use of Laboratory Animals” and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) were individually housed in hanging wire cages in a temperature-controlled room (lights on from 0700 to 1900), with ad libitum access to food (Purina Rat chow #5001) and water, unless otherwise noted. Rats were acclimated to this environment for at least 3 days prior to experimental manipulations.
Experiment 1: Feeding-Induced GLP-1 Neuronal Activation in Schedule-fed Rats
Meal entrainment.
Ad libitum-fed rats (n = 29, 230–300 g body wt at time of final testing) were pre-exposed overnight to a ball-tipped drinking spout attached to a graduated cylinder containing 10 ml of Ensure (milk chocolate flavor, 1.06 kcal/g; 14% protein, 64% carbohydrate, 22% fat by kcal; 1 g/ml; Abbott Nutrition, Columbus, OH) in addition to chow and water. This pre-exposure was performed to reduce neophobic responses to Ensure during a subsequent deprivation-induced feeding test in a subset of rats, described below. Every rat consumed all of the available Ensure during overnight pre-exposure. While only a subset of rats was assigned to an Ensure feeding group on the final test day (see below), all rats were pre-exposed similarly to standardize their treatment histories.
At least 48 h later, all rats were fasted overnight (∼22 h, beginning at ∼1600) to initiate a 5-day meal entrainment (scheduled feeding) protocol. Fasting was discontinued after this initial night. On the first and subsequent days, rats received access to an excess volume of a standard, calorically dilute liquid diet (LD; 0.5 kcal/ml; 20% protein, 70% carbohydrate, 10% fat by kcal; Research Diet, New Brunswick, NJ) presented in their home cage for 1 h each afternoon (beginning at 1430), with the amount consumed recorded on each of the 5 acclimation days (see results). This calorically dilute LD was used for meal entrainment to encourage larger intake volumes during scheduled 1-h feeding periods and also to determine whether even larger meals would be consumed on the final test day by rats given access to the more palatable Ensure at the same or a higher caloric density (see Final treatment groups, below). Water was removed during daily 1-h LD access. On the first and subsequent days, 1.5–2 h after the end of LD access (i.e., at 1700–1730), rats were given 12–13 g of chow (3.0 kcal/g) to consume overnight. Each rat consumed the entirety of its chow ration by the next morning on each of the 5 acclimation days. Rats maintained or increased their body weight by up to 5 g per day during the 5-day acclimation period. One-hour LD intake and body weights were recorded daily.
Final treatment groups.
On day 6, meal-entrained (schedule-fed) rats were divided into five experimental groups to examine cFos activation in rats consuming test meals of different volumes and caloric densities. One group received their usual 1-h access to an unrestricted volume of LD (LD rats, n = 6). A second group received 1-h access to an unrestricted volume of Ensure, which was diluted by 50% with water to match the caloric density of LD (0.5 kcal/ml, diEN rats, n = 5). A third group received 1-h access to an unrestricted volume of undiluted Ensure (1.06 kcal/ml, EN rats, n = 5). A fourth group received 1-h access to a restricted volume of LD (RES-LD rats; n = 7), equivalent to 60% of the average LD volume consumed by all rats on the previous day (i.e., on day 5 of the feeding schedule). The fifth group of rats was not fed during the final 1-h period (NF rats, n = 6).
Perfusion fixation.
Thirty to 45 min after the end of the final 1-h feeding period, rats were deeply anesthetized with pentobarbital sodium (Fatal Plus; 100 mg/kg body wt ip; Butler Schein, Columbus, OH) and then transcardially perfused with 50–100 ml saline followed by 100 ml of 2% paraformaldehyde (PF; Sigma, St. Louis, MO) containing 1.5–2.0% acrolein (Polysciences, Warrington, PA), followed by 100 ml of 2% PF. After perfusion, clamps were placed at the distal esophagus and proximal duodenum, stomachs were excised, and gastric contents removed and weighed. Brains were postfixed overnight in situ in 2% PF at 4°C, then removed from the skull, blocked, cryoprotected in 20% sucrose, frozen, and sectioned at 35 μm using a sliding microtome. Sections were collected serially in six sets such that each contained a complete rostrocaudal series of sections spaced by 210 μm. Sections were stored at −20°C in cryopreservant solution (37) to await immunohistochemical processing.
Immunohistochemistry.
Tissue sections were removed from cryopreservant, rinsed in 0.1 M phosphate buffer (pH 7.2), and pretreated in 0.5% sodium borohydride followed by 0.5% H2O2. For dual cFos and GLP-1 immunoperoxidase labeling, one set of pretreated sections from each rat was incubated overnight at room temperature or for 48–72 h at 4°C in primary rabbit antiserum raised against cFos protein (1:20,000, no. PC38; EMD Chemicals, San Diego, CA). After being rinsed, sections were incubated in biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature, rinsed, then incubated in avidin-biotin complex (Vectastain Elite reagents, Vector Labs, Burlingame, CA) for 1.5 h. Diaminobenzidine (DAB, Sigma) intensified with nickel sulfate was catalyzed by H2O2 to produce a blue-black nuclear cFos reaction product.
After cFos immunolabeling, sections were incubated overnight in rabbit anti-GLP-1 (1:10,000, no. T-4363; Peninsula, San Carlos, CA) at room temperature. After being rinsed, sections were incubated in biotinylated donkey anti-rabbit IgG and then avidin-biotin complex, as described above. After being rinsed, tissue underwent an H2O2-catalyzed reaction in a solution of DAB in 0.1 M Tris buffer to produce a brown cytoplasmic GLP-1 peroxidase reaction product.
Quantification of feeding-induced GLP-1 neuronal activation.
In each rat, the number of GLP-1 neurons within the caudal NST (cNST) and medullary reticular formation (MRF) was quantified in sections (spaced by 210 μm) from the upper cervical spinal cord through the midlevel of the area postrema using a ×20 objective on a light microscope. GLP-1 neurons were classified as cFos-positive if their nucleus contained visible cFos immunolabeling, regardless of intensity. GLP-1 neuronal activation was quantified as the proportion (%) of GLP-1-positive cNST or MRF neurons that also were cFos-positive.
Data analysis.
Analyzed data included the amount of LD, diEN, or EN consumed by each rat (expressed as volume, and also as weight, with the latter converted to % body wt), the postmortem weight of gastric contents, the percentage of the consumed amount and the total amount of diet (expressed as kcal) that emptied from the stomach before gastric contents were collected postperfusion, and the proportions of identified GLP-1 neurons activated to express cFos within the cNST and the MRF. Data were combined by final feeding group on day 6 and are presented in graphs and tables as group means ± SE. Statistically significant effects of feeding group on experimental outcomes were identified using ANOVA, with feeding group as the independent variable, followed by post hoc Tukey t-tests (corrected for multiple comparisons) to detect differences between individual feeding groups. For all comparisons, an alpha level of 0.05 was used as a marker of statistical significance. Correlational analyses also were performed to examine relationships between feeding-related measures and cFos activation of GLP-1 neurons.
Experiment 2: Ensure Intake After GLP-1R Blockade in Schedule-Fed Rats
To determine whether and how cumulative intake and/or meal patterns are altered after intracerebroventricular injection of SAL vs. Ex9, a separate group of rats was fitted with intracerebroventricular cannulas and then trained to consume large, stable volumes of Ensure during a daily 2-h period. The longer 2-h scheduled feeding period used in this experiment permitted analysis of potentially delayed treatment effects occurring after a significant amount had already been consumed.
Cannulation procedures.
Experimentally naïve, individually housed male rats (n = 24, 287–308 g body wt at time of surgery) were anesthetized with isoflurane (1.5–2% in oxygen) and placed into a stereotaxic frame. Rats were fitted with a unilateral chronic indwelling 21-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) positioned above the lateral ventricle, 1.5 mm lateral and 0.90 mm caudal to bregma, with the tip protruding 2.7 mm below the surface of the skull. Cannulas were fixed to the skull with anchor screws and dental acrylic and fitted with removable obturators that sat flush with the tip of the guide cannula. Correct cannula placement was verified 5–6 days later, after presurgery body weights were attained. For this purpose, water-replete rats were injected intracerebroventricularly with 2 μl of sterile 0.15 M SAL containing 5 ng of angiotensin II (ANG II, Sigma). All rats included in this report began drinking water within 30 s and drank at least 6 ml in 30 min, evidence for accurate cannula placement (19).
Meal-entrainment protocol using lickometer-equipped feeding boxes.
Beginning 2–7 days after the ANG II screening test, cannulated rats (now 292–344 g body wt) were acclimated to a feeding schedule that was maintained for 7 days, as follows: from 1600 to 1800 each day, rats were individually placed into clear Plexiglas feeding boxes (12 × 10-inch floor, 8-inch height) with stainless steel rod floors (Med Associates, Georgia, VT) in a room adjacent to the housing room. Feeding boxes contained a contact lickometer connected to a metal ball-spout and graduated cylinder that supplied unrestricted amounts of Ensure (0.93 kcal/ml). The volume of Ensure within the graduated cylinder was recorded at 0 and again at 120 min after placing the rat in the box. Lickometers were connected to a computer with software (Med PC) set to record the cumulative number of licks made by each rat every 60 s over the 2-h feeding period. Each rat was placed in the same feeding box equipped with the same ball-spout each day. After each 2-h feeding period, rats were immediately returned to their home cages and given an excess amount of preweighed chow that they could consume ad libitum overnight. Each morning (between 0900 and 1000), remaining chow and spillage were collected and weighed to determine intake, and rat body weights were recorded. During daily collection of body weight data, rats were gently restrained by hand and cannula obturators manipulated to acclimate rats to handling procedures that would accompany intracerebroventricular injection on day 7.
Intracerebroventricular injections and ensure intake.
On day 7 of the feeding schedule, rats were randomly assigned to receive an intracerebroventricular infusion of either 3 μl of sterile 0.15 M NaCl vehicle (SAL, n = 12) or vehicle containing 100 μg Ex9 (Ex9, n = 12), a dose previously reported in adult rats to increase food intake after intracerebroventricular infusion compared with intake after intracerebroventricular vehicle (20, 35). Infusates were administered at ∼1530 using a Hamilton syringe attached to polyethylene tubing attached to a 26-gauge injector tip (Plastics One) that extended 2.5 mm beyond the tip of the guide cannula, to enter the ventricular space. After intracerebroventricular infusion, rats were returned to their home cages for 15–30 min and then were placed into the lickometer chambers from ∼1600 to 1800 for 2 h Ensure intake as usual.
After completing the experiment, to confirm proper intracerebroventricular cannula placement and patency, we deeply anesthetized rats with Fatal Plus, infused intracerebroventricularly with ∼5 μl of black India ink, and then transcardially perfused them with 100–150 ml of 4% PF. All data included in this report were obtained from rats with visible ink at the base of the hypothalamus and within the cerebral aqueduct, confirming proper intracerebroventricular cannula placement and patency.
Data Analysis.
CUMULATIVE INTAKE VOLUME.
The total volume of Ensure consumed by each rat (converted to grams and then expressed as % body weight) during the 2-h feeding period was analyzed and compared at baseline (day 6) and after intracerebroventricular injection (day 7). Cumulative Ensure intake data are presented both as absolute intake values on baseline and intracerebroventricular injection days, and as injection day intake compared with each animal's own baseline intake (% change from baseline). To account for potential pre-existing (prior to intracerebroventricular injection) between-group differences in cumulative Ensure intake, we treated baseline intake as a covariate when analyzing an effect of intracerebroventricular injection group on injection-day intake via ANCOVA. Within-subject effects of intracerebroventricular injection on intake volume (% change from baseline) were averaged within each injection group and then compared between the two groups using a t-test. For all comparisons, an alpha level of 0.05 was used as a marker of statistical significance.
MEAL STRUCTURE.
Lick data were used to evaluate meal structure within the 2 h feeding period on baseline day 6 and after intracerebroventricular injection on day 7. Based on published criteria for rats consuming a liquid diet (2, 29, 38), a meal was defined as at least three licks, while a pause of at least 5 min between licks defined the end of the previous meal. A previous study demonstrated that varying the pause criterion from 2 to 60 min does not significantly change the number of meals detected under such experimental conditions (29), and preliminary analysis of our own data confirmed this. Analyzed feeding measures included meal number, meal size (number of licks), meal duration (minutes), and intermeal interval (IMI, minutes). Statistical comparison of intracerebroventricular injection group effects on each feeding measure was performed using ANCOVA, with baseline values for the same measures as covariate. Independent samples t-tests were used to reveal an effect of intracerebroventricular injection group on within-subjects (% change from baseline) measures. For all comparisons, an alpha level of 0.05 was used as a marker of statistical significance.
RESULTS
Experiment 1: Feeding Acclimation and Final Test Meal Measures in Meal-Entrained Rats
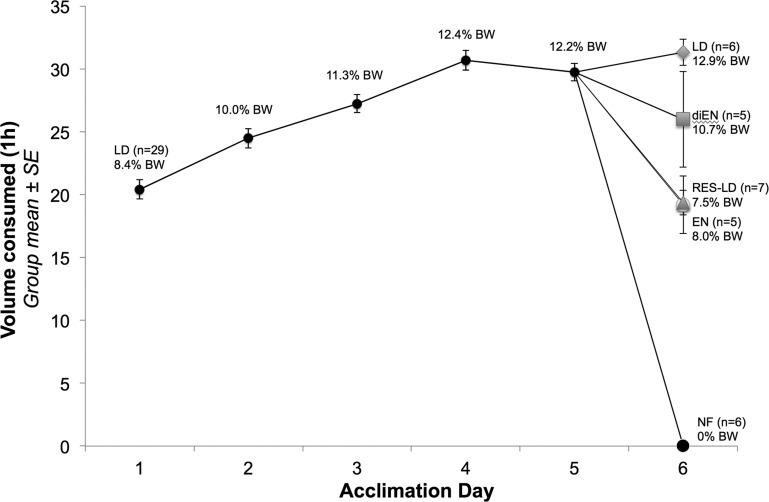
Daily 1-h LD intakes for all rats during the 5-day scheduled feeding acclimation period are presented in Fig. 1 and Table 1 as volume consumed (ml) and as % body wt. LD intake increased progressively between days 1 and 4 (Fig. 1), whereas intake on day 5 was not significantly different from intake on day 4 [paired-samples t-test: t(28) = 1.27, P > 0.05]. On the final intake test and perfusion day (day 6), rats assigned to the LD group consumed ∼12.9% of their body weight (∼31 ml, Table 1) within 60 min, similar to LD intake of all rats on acclimation days 4 and 5 (Fig. 1). Conversely, rats assigned to the diEN, EN, or RES-LD groups consumed smaller volumes (i.e., ∼10.7, 8, and 7.5% of their body weight, respectively; Fig. 1; see y-axis and also Table 1 for mls consumed). No rats were still consuming food when bottles were removed at the end of the 1 h feeding period, including RES-LD rats, each of which consumed its entire ration. At the time of perfusion (30 min after the end of the 1-h feeding period), postmortem gastric content assessment indicated that more calories had emptied from the stomach into the intestines in LD and diEN rats (∼9.8 and 9.3 kcal, respectively; Table 1) compared with calories emptied in EN and RES-LD rats (∼6.8 and 7.2 kcal, respectively; Table 1).
Fig. 1.
Average daily 1-h LD intake (volume on y-axis, % body wt indicated at each data point) by all rats (n = 29) during 5 days of acclimation to scheduled feeding (meal entrainment) and by rats in each subgroup on the final testing day (day 6). There was no significant difference in 1-h intake on acclimation days 4 and 5 (paired-samples t-test, P > 0.05), evidence that intakes had stabilized. On day 6, rats were randomly assigned to 1 of 5 feeding groups (n = 5–7 per group, as indicated), and the average amount of diet consumed by rats within each group is indicated (by different symbols). LD, standard liquid diet; EN, Ensure; diEN, diluted Ensure; RES-LD, restricted LD; NF, not fed; BW, body weight.
Table 1.
Final test meal-related statistics from schedule-fed rats in experiment 1
| Feeding Condition | Premeal Body Wt, g | Meal Size, ml | Meal Size, % body wt | Postmortem Weight of Gastric Contents, g | Intestinal Nutrient Exposure, kcal |
|---|---|---|---|---|---|
| LD (n = 6) | 242.47 ± 2.08a | 31.33 ± 1.05a | 12.92 ± 0.47a | 11.80 ± 0.38a,b | 9.77 ± 0.63a |
| diEN (n = 5) | 243.30 ± 8.76a | 26.00 ± 3.81a | 10.69 ± 0.67a | 7.32 ± 1.43a,c | 9.34 ± 0.22a |
| EN (n = 5) | 241.40 ± 2.91a | 19.20 ± 2.29b | 7.96 ± 0.94b | 12.41 ± 1.90b | 6.80 ± 0.77b |
| RES-LD (n = 7) | 258.57 ± 1.94b | 19.36 ± 0.97b | 7.49 ± 0.38b | 5.01 ± 0.78c | 7.17 ± 0.19b |
| NF (n = 6) | 244.00 ± 4.73a | 0c | 0c | — | — |
Values represent group means ± SE.
LD, liquid diet; EN, Ensure; diEN, diluted Ensure; RES-LD, restricted liquid diet; NF, nonfed. Within each column, values with different superscript letters (a, b, c) are significantly different (P < 0.05) between feeding condition groups (ANOVA with Tukey HSD post hoc tests).
Feeding-Induced Activation of GLP-1 Neurons in Meal-Entrained Rats
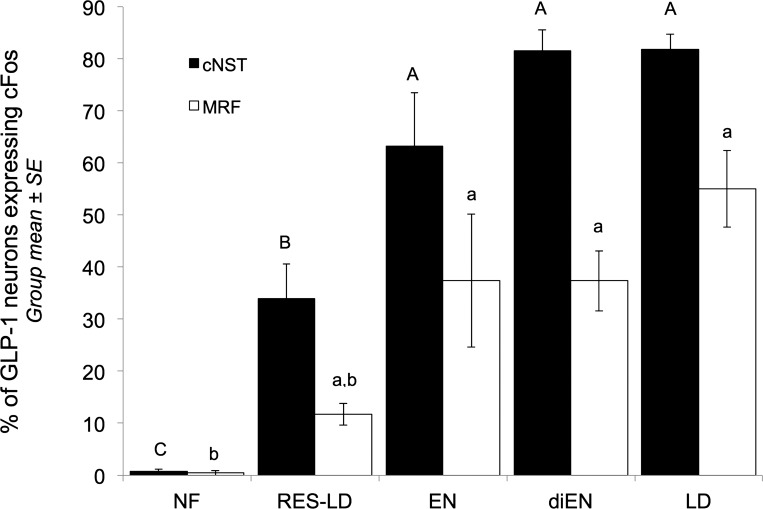
As expected, there was no main effect of feeding condition on the number of GLP-1-positive neurons counted within either the cNST [66.69 ± 5.08 neurons per rat; between-groups comparison F(4, 24) = 1.82, P > 0.05] or the MRF [54.41 ± 3.95 neurons per rat; between-groups comparison F(4, 24) = 0.95, P > 0.05]. Also as expected, very few GLP-1-positive neurons in either medullary region were cFos-positive in NF rats, whereas cFos expression by GLP-1 neurons in both regions was increased in rats within all four fed groups (Fig. 2). GLP-1 neural activation within the cNST in a representative rat from the LD group is shown in Fig. 3.
Fig. 2.
Proportion (%) of glucagon-like peptide (GLP)-1-immunopositive neurons within the caudal nucleus of the solitary tract (cNST, filled bars) and medullary reticular formation (MRF, open bars) activated to express cFos in rats consuming final test meals of different sizes or types. Within each regional subgroup of GLP-1 neurons, bars with different letters (A, B, C for the cNST; a, b for the MRF) are significantly different between feeding condition groups (P < 0.05). LD, standard liquid diet; EN, Ensure; diEN, diluted Ensure; RES-LD, restricted LD; NF, not fed.
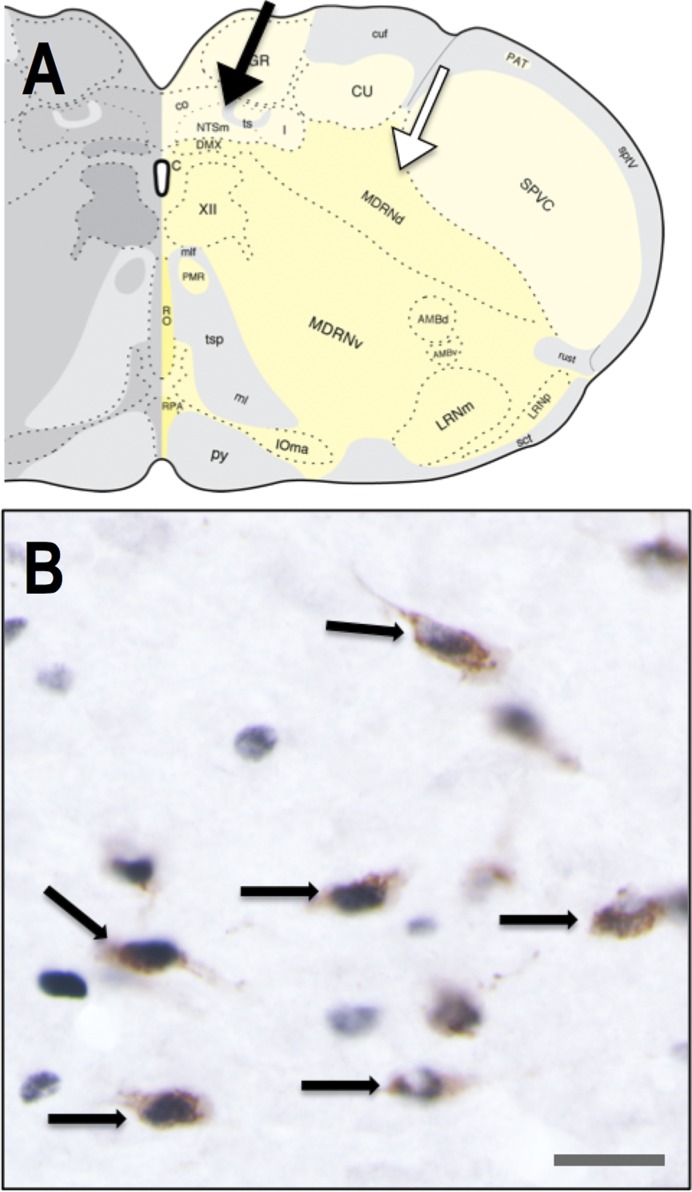
Fig. 3.
GLP-1-positive (brown cytoplasmic labeling, B) neurons within the cNST (region indicated by black arrow in A) are activated to express cFos (black nuclear labeling, B) in a representative rat that consumed 14% of its body weight in LD. The white arrow in A indicates the MRF, where GLP-1-positive neurons also reside. Black arrows in B point out some of the double-labeled neurons in which nuclear cFos immunolabeling is colocalized with cytoplasmic GLP-1. Scale bar in B = 25 μm. Brain stem schematic in A, 14.3 mm caudal to bregma (adapted from Ref. 33, available by CC BY-NC 4.0, https://creativecommons.org/licenses/by-nc/4.0/).
Within the cNST, ANOVA revealed a significant main effect of feeding condition on the proportion of GLP-1 neurons expressing cFos [F(4, 24) = 37.62, P < 0.001]. The proportion of cNST GLP-1 neurons activated to express cFos in RES-LD rats was significantly higher than the proportion activated in NF rats, but significantly lower than the proportions activated in rats with unrestricted access to Ensure (EN), diluted (di)EN, or LD (Fig. 2). In this regard, it is noteworthy that significantly fewer cNST GLP-1 neurons were activated to express cFos in RES-LD rats compared with EN rats, despite nearly identical intake volumes in these two groups (ml and % body wt, Fig. 1 and Table 1). However, gastric volumes assessed postmortem were more than twice as large in EN rats compared with RES-LD rats (Table 1), evidence for more prolonged gastric distension in the EN group. Among rats that consumed unrestricted (i.e., satiating) amounts of EN, diEN, or LD, there were no between-groups differences in cFos activation of cNST GLP-1 neurons (P > 0.05 for each between-group comparison, Fig. 2).
ANOVA also revealed a significant main effect of feeding condition on the proportion of GLP-1 neurons expressing cFos within the MRF [F(4, 24) = 11.98, P < 0.001]. However, the difference in MRF GLP-1 activation between NF and RES-LD groups was not significant (P = 0.64). Interestingly, significantly smaller proportions of MRF GLP-1 neurons were activated in all four fed groups compared with activation of GLP-1 neurons within the cNST (P < 0.05 for each within-feeding group comparison, Fig. 2). Among rats that consumed satiating amounts of EN, diEN, or LD, there were no between-groups differences in activation of MRF GLP-1 neurons (P > 0.05 for each between-group comparison, Fig. 2).
Correlational Analyses of GLP-1 Neuronal Activation vs. Amount Consumed
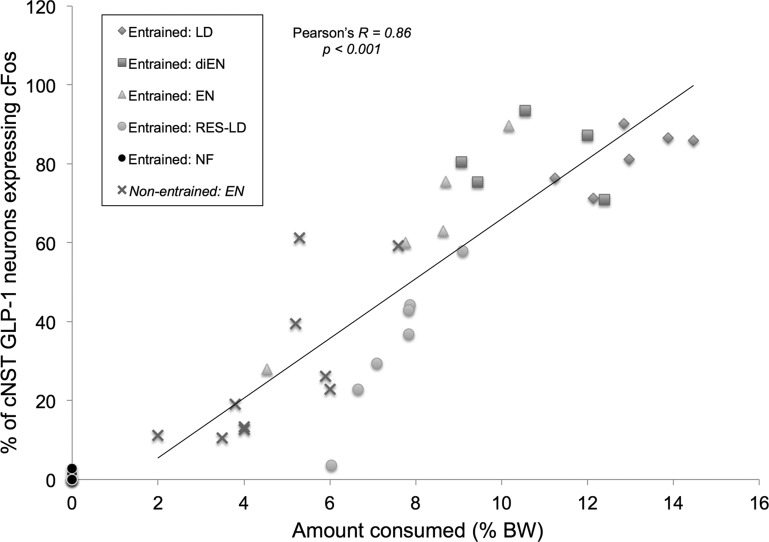
Figure 4 plots the proportion (%) of cNST GLP-1 neurons activated to express cFos in individual rats from each feeding condition group as a function of the amount of test diet consumed by each rat (expressed as % body wt) in experiment 1. For purposes of comparison, food intake (as % body wt) and GLP-1 cFos activation data from our recently published study (21) using nonentrained rats consuming unrestricted amounts of Ensure also are plotted (X symbols in Fig. 4). In that earlier study, experimentally naïve rats fasted overnight and then refed Ensure for the first time consumed, on average, 4.7% body wt and displayed cFos activation of ∼27.5% of cNST GLP-1 neurons (21). Across both studies, activation of cNST GLP-1 neurons was positively (Pearson's R = 0.86) and significantly (P < 0.001) correlated with amount consumed (as % body wt) across all five experimental fed groups (note that Entrained: NF group data are plotted in Fig. 4 but are not included in the correlation). Similarly, when only Entrained rats in the present study are considered, activation of cNST GLP-1 neurons was positively (Pearson's R = 0.82) and significantly (P < 0.001) correlated with amount consumed. Activation of cFos expression by cNST GLP-1 neurons in the present study also was positively and significantly correlated with the calculated value of calories emptied from the stomach (i.e., reaching the intestines; Table 1) by the time of perfusion, although this correlation (Pearson's R = 0.66, P = 0.001) was somewhat weaker than the correlation between GLP-1 neural activation and amount consumed.
Fig. 4.
Relationship between amount consumed and cNST GLP-1 neuronal activation in meal-entrained rats consuming different liquid diets for 1 h. Each symbol represents data from 1 rat. Data points (Xs) from our previously published report using food deprived, nonentrained rats (23) are added for comparison (“Nonentrained: EN”). Data points from Entrained: NF rats are plotted for comparison, but the indicated correlation value is derived only from the 5 fed groups.
Experiment 2: Ensure Intake and Meal Patterns in Schedule-Fed Rats after Intracerebroventricular SAL vs. Ex9
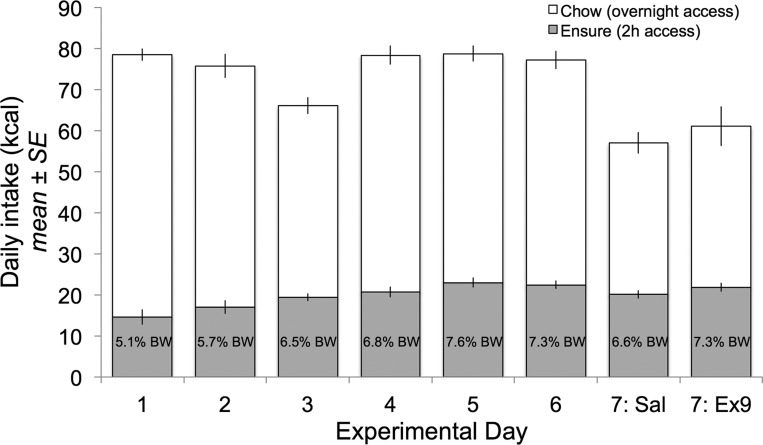
Daily 2-h Ensure intake (as kcal and as % body wt) increased progressively during the first few days of the 6-day acclimation period and was stable on days 5 and 6 (Fig. 5). During this period, overnight chow intake (kcal) decreased from day 1 to day 3, then rebounded, and remained stable from days 4 to 6, resulting in overall stable total daily caloric intake from day 4 to day 6 (Fig. 5). Across all rats (n = 24), cumulative daily 2 h Ensure intake was highly consistent for the 4 days prior to intracerebroventricular injection [acclimation days 3–6, intraclass correlation coefficient (1,1) = 0.46, P < 0.01]. Average body weight increased across the acclimation period (313 g ± 4.2 on day 1 and 323 g ± 3.1 on day 6; n = 24) and was similar for rats in each intracerebroventricular injection group both on the final day of acclimation [day 6; t(22) = −0.07, P = 0.95] and on the subsequent intracerebroventricular injection day [day 7; t(22) = 0.23, P = 0.82]. Intake data collected on acclimation day 6 were used as baseline intake values.
Fig. 5.
Daily food intake (expressed as kcal) during the scheduled feeding acclimation period for rats with intracerebroventricular cannulas (experiment 2). Data from all 24 rats are represented for each day (except for 1 rat with missing data on days 1 and 2, n = 23 on those days). Values within the gray bars indicate Ensure intake expressed as % body weight on that day. Open bars depict post-Ensure overnight chow intake.
Absolute intake.
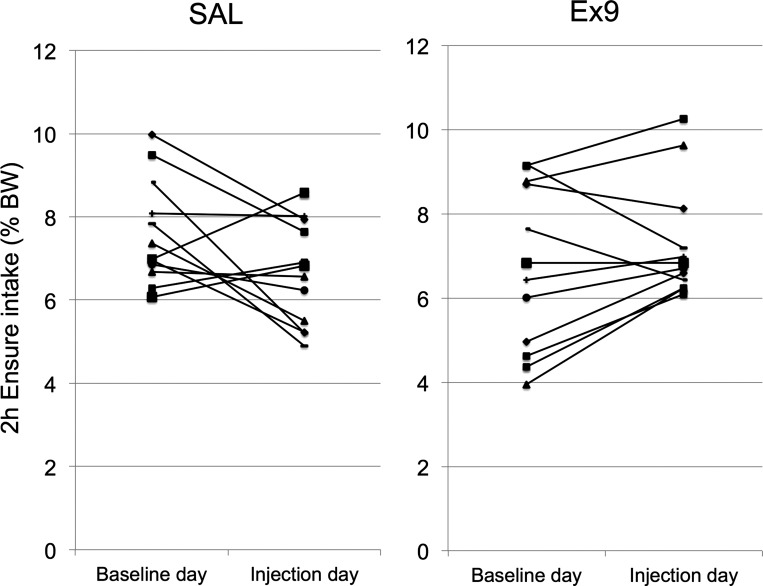
Cumulative 2-h Ensure intake on baseline day 6 was similar between groups destined to receive intracerebroventricular injection of SAL (n = 12, 7.6% body wt ± 0.36) vs. Ex9 (n = 12, 6.72% body wt ± 0.57) the following day [F(1,23) = 1.8, P = 0.195]. Repeated-measures ANOVA (with Ensure intake on baseline vs. intracerebroventricular injection day as the within-subjects repeated measure) revealed a significant interaction between testing day and intracerebroventricular injection group on 2 h intake [F(1,22) = 6.8, P = 0.016], but no main effect of testing day [F(1,22) = 0.54, P = 0.5; data not shown]. Cumulative 2-h Ensure intake was slightly higher in rats injected intracerebroventricular with Ex9 (7.3% body wt) compared with rats injected intracerebroventricular with SAL (6.6% body wt, Fig. 5), but this between-groups difference was not statistically significant based on ANOVA [F(1,23) = 1.5, P = 0.238] (as opposed to ANCOVA, see below). Cumulative intake by SAL rats on baseline day was not significantly correlated with intake by the same rats after intracerebroventricular SAL (R = 0.186, P = 0.56), suggesting an inconsistent effect of intracerebroventricular SAL (Fig. 6). Compared with baseline day intake, intake after intracerebroventricular SAL was strongly increased in one of the 12 rats, was moderately increased in two rats, was stable or moderately reduced in three rats, and was strongly reduced in six rats (Fig. 6). Conversely, within the Ex9 group, cumulative intake on intracerebroventricular injection day was strongly and significantly correlated with intake on baseline day (R = 0.76, P < 0.01; Fig. 6), suggesting that the effect of intracerebroventricular Ex9 on intake was more consistent than the effect of intracerebroventricular SAL. Compared with baseline day intake, intake after intracerebroventricular Ex9 was stable or was increased in eight of the 12 rats but was reduced in three rats.
Fig. 6.
Two-hour Ensure intake (expressed as % body wt) from each rat within the NaCl vehicle (SAL)-assigned group (left, n = 12) and the Exendin-3(9–39) (Ex9)-assigned group (right, n = 12) is depicted on baseline day (day 6) and on intracerebroventricular injection day (day 7).
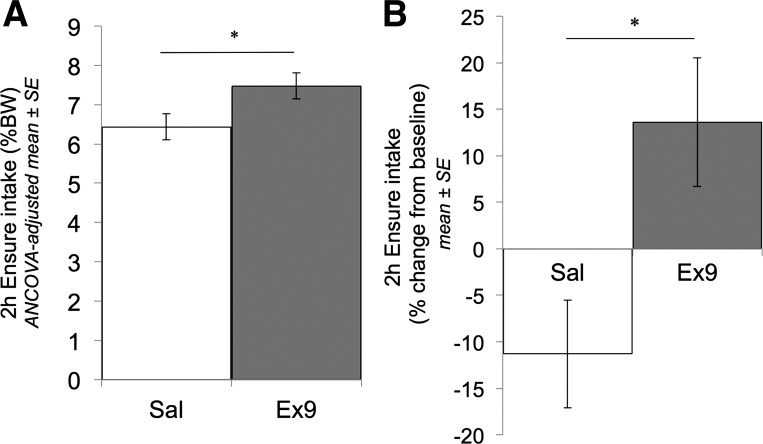
After we controlled for baseline-day Ensure intake as a covariate, a modest but significant difference in cumulative 2-h Ensure intake after intracerebroventricular SAL vs. Ex9 injection was revealed [ANCOVA, F(1,21) = 4.64, P < 0.05; Fig. 7A]. Furthermore, when 2-h Ensure intake after intracerebroventricular injection was expressed as a within-subjects change from baseline intake, intake was reduced by 11.3% ± 5.8 after SAL injection, whereas Ex9 injection increased intake by 13.6% ± 6.9 (Fig. 7B). This differential effect of intracerebroventricular treatment was statistically significant [t(22) = −2.75, P = 0.01]. Paired-samples t-tests (within subjects) revealed that the intracerebroventricular SAL-induced suppression of intake below baseline just met the alpha level criterion for significance (P = 0.054), whereas the Ex9-induced increase in intake above baseline did not (P = 0.163).
Fig. 7.
Cumulative 2-h Ensure intake in ad libitum-fed, meal-entrained rats (experiment 2). A: the between-groups comparison of cumulative intake (as % body wt) by intracerebroventricular SAL- vs. Ex9-injected rats (day 7), with group means adjusted for baseline intake on the previous noninjection day (day 6) (ANCOVA, *P < 0.05). In B, cumulative 2 h intake on the intracerebroventricular injection day is normalized to each rat's own baseline intake and then averaged within each intracerebroventricular injection group (t-test, *P < 0.05).
After intracerebroventricular injection on day 7, overnight chow intake was significantly reduced by 24.5% ± 6.85 below baseline in the SAL group (paired t-test, P < 0.05), and by 16.85% ± 10.13 below baseline in the Ex9 group (paired t-test, P < 0.05) (Fig. 5). The magnitude of these change-from-baseline values were not significantly different between the two intracerebroventricular injection groups (t-test, P > 0.05).
Meal structure.
Meal structure was assessed based on lickometer data collected during 2-h Ensure intake on baseline and intracerebroventricular injection days. Meal number, size, duration, and intermeal interval (IMI) values for individual rats were averaged by intracerebroventricular injection group and are reported in Table 2 as absolute values and also as % change (within-subjects) on intracerebroventricular injection day compared with baseline day.
Table 2.
Meal pattern data in schedule-fed rats on baseline and intracerebroventricular injection days (experiment 2)
| Group | Treatment Day | Meals, n | Meal 1 Size, licks | Meal 1 Duration, min | Meal 2 Size, licks | Meal 2 Duration, min | IMI, min |
|---|---|---|---|---|---|---|---|
| SAL | baseline | 2.7 ± 0.3 | 5,364.6 ± 542.3 | 18.3 ± 1.2 | 2,557.6 ± 424.8 | 8.6 ± 1.3 | 44.6 ± 5.9 |
| icv SAL | 2.3 ± 0.2 | 5,825.3 ± 400.6 | 18.3 ± 1.2 | 1,534.6 ± 439.0 | 5.8 ± 1.3 | 54.5 ± 6.9 | |
| % change | 2.19% ± 7.6 | 18.6% ± 8.7 | 3.0% ± 7.8 | −19.3% ± 16.0 | −28.1% ± 12.0 | 2.2% ± 11.4 | |
| Ex9 | baseline | 2.8 ± 0.3 | 5,041.3 ± 406.3 | 16.9 ± 1.0 | 2,009.8 ± 401.3 | 6.6 ± 1.1 | 55.8 ± 9.1 |
| i.c.v. Ex9 | 2.3 ± 0.2 | 6,190.8 ± 400.6 | 17.0 ± 1.2 | 2,641.5 ± 439.0 | 7.0 ± 1.3 | 63.0 ± 6.9 | |
| % change | 5.5 ± 9.0 | 11.7 ± 11.1 | −5.6 ± 4.8 | 63.6 ± 39.6 | 18.3 ± 18.5 | 20.5 ± 23.5 | |
| SAL vs. Ex9 (icv day, ANCOVA-adjusted values | P = 0.996 | P = 0.527 | P = 0.470 | P = 0.094 | P = 0.544 | P = 0.401 | |
| SAL vs. Ex9 (% change from baseline) | P = 0.785 | P = 0.629 | P = 0.358 | P = 0.067 | P = 0.049* | P = 0.493 |
Within each intracerebroventricular (icv) injection group, baseline values for each meal-related parameter are absolute values (group means ± SE), and icv values for each parameter are adjusted for ANCOVA by controlling for baseline day values as a covariate. The % change values reflect the % change from baseline day (calculated for each animal and then averaged within icv injection group) for a given meal-related parameter.
IMI, intermeal interveal.
Significantly greater in Ex9- vs. NaCl vehicle (SAL)-treated rats (t-test, P < 0.05).
ABSOLUTE VALUES.
Rats typically consumed two meals separated by an IMI of ∼45–60 min during the 2-h feeding period, regardless of treatment day or intracerebroventricular injection group (Table 2). All rats (n = 24) consumed at least two meals on baseline day. However, on intracerebroventricular injection day, two rats (n = 1 per injection group) consumed only one meal. Therefore, in Table 2, n = 11/group for meal 2 size, meal 2 duration, and IMI. Since only a few rats consumed more than two meals on either baseline or intracerebroventricular injection days, statistical comparisons of meal size and duration were conducted only on the first and second meals (Table 2). When meal-related parameter values on intracerebroventricular injection day were adjusted using baseline day values for each parameter as a covariate, ANCOVA revealed no significant effect of intracerebroventricular injection group on any meal-related parameter (Table 2). There also was no effect of intracerebroventricular injection group on any meal-related parameter on injection day when nonadjusted t-comparisons were performed (t-tests, P > 0.05 for all comparisons; data not shown).
WITHIN-SUBJECTS EFFECTS OF INTRACEREBROVENTRICULAR TREATMENT (% CHANGE, INTRACEREBROVENTRICULAR INJECTION VS. BASELINE).
As shown in Table 2, the duration of the second meal after intracerebroventricular SAL injection was reduced by ∼28% compared with within-subjects baseline, whereas the duration of the second meal after intracerebroventricular Ex9 injection was increased by ∼18% compared with baseline. The differential effect of intracerebroventricular treatment on second meal duration was significantly different (Table 2). The size (in licks) of the second meal in SAL rats was reduced by ∼19% after intracerebroventricular injection compared with baseline, whereas the size of the second meal in Ex9 rats was increased by ∼64% compared with baseline (Table 2); the differential effect of intracerebroventricular injection on meal 2 size trended toward but did not achieve statistical significance (Table 2, P = 0.067). For all other meal parameters analyzed, the % change from baseline after intracerebroventricular SAL vs. Ex9 did not differ between groups (Table 2).
DISCUSSION
The present study was designed to investigate feeding-induced recruitment of hindbrain GLP-1 neurons in rats entrained to consume unusually large volumes of liquid diet and also to explore a role for central GLP-1R signaling in controlling intake volume (meal size). Our cFos results indicate that GLP-1 neurons are progressively recruited in rats consuming progressively larger volumes of liquid diet. Consistent with previous reports (3, 9, 17, 20, 43), rats injected intracerebroventricularly with Ex9 consumed slightly but significantly more Ensure during 2 h access compared with rats injected intracerebroventricularly with SAL. Furthermore, when the effect of intracerebroventricular injection on 2 h intake within subjects was expressed as change from baseline intake (% change), the effect of intracerebroventricular SAL to reduce intake was significantly different from the effect of intracerebroventricular Ex9 to increase intake. When cumulative intake by rats after intracerebroventricular Ex9 was compared with intake by the same rats under noninjected baseline conditions (within-subjects comparison), however, the hyperphagic effect of Ex9 failed to reach significance, whereas intracerebroventricular SAL had a nearly significant hypophagic effect. We interpret these results as evidence that intracerebroventricular Ex9 increases Ensure intake primarily by counteracting the hypophagic effect of intracerebroventricular injection. Additional insight into the differential effects of intracerebroventricular treatment were gained from meal pattern analysis, which revealed that increased cumulative intake after Ex9 vs. SAL was due to longer second meals consumed during the latter portion of the 2-h feeding period. Thus, in addition to attenuating the hypophagic effects of intracerebroventricular injection, blockade of central GLP-1R signaling may also contribute to limiting food intake after a substantial amount of food has already been consumed, a proposal that is consistent with a previous report (17). However, this conclusion remains tentative, since intracerebroventricular SAL treatment reduced the duration of the second meal by nearly 30% compared with nontreated baseline conditions. Our results highlight the importance of considering both cumulative intake and meal pattern data collected under both nonmanipulated control and experimental conditions, particularly when assessing the role of stress-sensitive neuropeptide systems such as GLP-1 (23) in controlling food intake and other motivated behaviors. In this regard, a report documenting significant hyperphagic effects of chronic GLP-1 peptide knockdown or pharmacological antagonism of central GLP-1Rs in rats (6) needs confirmation after evaluating how these manipulations alter food intake compared with intake by untreated control rats under baseline conditions, rather than only compared with intake by surgically manipulated and intracerebroventricular-injected control rats. The latter controls are essential, but insufficient.
Feeding-Induced Activation of GLP-1 Neurons: cNST
In experiment 1, 60–80% of GLP-1 neurons in the cNST were activated to express cFos after rats ate to satiety by consuming 7–13% of their body weight in liquid diet during the allotted 1-h feeding period (Fig. 2). This level of GLP-1 activation was two to three times higher than in our previous report (21), in which rats were not trained to consume large meals. After an overnight fast, rats in the earlier study consumed only ∼5% of their body weight, and only ∼30% of GLP-1 neurons were activated (21). In the present study, there were no differences in GLP-1 activation between rats consuming the entrained LD vs. the more novel Ensure, regardless of dilution, suggesting that neither caloric density, novelty, nor flavor/palatability contributed significantly to feeding-induced activation of GLP-1 neurons. In a previous study, Vrang et al. (36) reported that ∼30–40% of GLP-2-positive neurons (which also are GLP-1-positive) were activated to express cFos in rats after inflation of a gastric balloon with 9 ml of saline, similar to the volume of Ensure voluntarily consumed by nonentrained rats in our previous study (21). Thus, GLP-1 neuronal activation likely is due to stimulation of vagal mechanoreceptors that are sensitive to gastric distension. Indeed, correlational analyses in the present study indicated that cNST GLP-1 neural activation was best predicted by the volume ingested, although we cannot rule out a possible additional contribution of intestinal nutrient exposure.
Despite eating more voluminous meals and emptying more calories into the intestines, RES-LD rats in the present study consumed ∼19 ml (∼7.5% body wt) but displayed similar proportions of cNST GLP-1 neuronal activation (∼35%) as nonentrained, satiated rats in our previous study that consumed only ∼9 ml (∼5% body wt) (21). Considered together, these results suggest that the ability of a given amount of feeding-induced gastric distension to activate cNST GLP-1 neurons varies based on the animal's experience consuming large meals. Specifically, meal entrainment (scheduled feeding) may shift the intake-response curve to the right, such that greater amounts of food must be consumed by meal-entrained rats to recruit GLP-1 neurons that are recruited after smaller amounts of food are consumed by nonentrained rats. For a given volume of liquid diet intake and gastric load, the lower levels of GLP-1 activation observed in meal-entrained RES-LD rats (present study) vs. the higher levels of GLP-1 neurons activated in nonentrained rats in our previous study (see Fig. 4 in Ref. 21) may reflect physiological adjustments that permit larger meals to be consumed after scheduled feeding/entrainment (40–42).
Feeding-Induced Activation of GLP-1 Neurons: MRF
Interestingly, although approximately half of all identified GLP-1 neurons in the caudal brain stem reside in the MRF, GLP-1 neurons within the cNST were more sensitive to feeding-induced activation, consistent with a previous report (11). These GLP-1 regional differences in cFos activation may be due to the fact that the cNST receives direct vagal sensory input, including input arising from the GI tract, whereas sensory inputs to the MRF are indirect. The neural pathway(s) through which MRF GLP-1 neurons are recruited in rats after food intake is unknown. However, given that MRF neurons are recruited in an intake volume-dependent manner, similar to the progressive recruitment of cNST GLP-1 neurons (Figs. 2, 4), the recruitment of MRF GLP-1 neurons could reflect relayed input from the cNST (1, 5).
GLP-1R Blockade in Meal-Entrained Rats
Our cFos data provide convincing evidence that GLP-1 neuronal activation closely reflects the amount consumed, in a manner that is modified by feeding experience (i.e., meal entrainment). Importantly, however, rats must consume at least 5–6% of their body weight within a 1-h period (experimentally feasible only after food deprivation or under scheduled feeding conditions) before GLP-1 neural activation increases above baseline activation levels measured in rats with ad libitum food access (Refs. 24, 25, and present study). Therefore, insofar as cFos is an indicator of GLP-1 neural activation, our results indicate that GLP-1 neurons are relatively insensitive to sensory feedback signals generated by food intake, except when rats consume unusually large amounts during a relatively short period of time. It follows that central blockade of GLP-1R signaling should have little impact on food intake when endogenous GLP-1 signaling is relatively low, whereas an effect may be revealed when increased GLP-1 signaling accompanies the consumption of very large meals. Indeed, several studies have reported that intracerebroventricular Ex9 does not increase chow intake in ad libitum-fed rats compared with intake by control rats treated with intracerebroventricular vehicle (3, 13, 20, 25, 43).
The new Ex9 experiment in the present study used ad libitum-fed rats that were trained to consume large volumes of palatable Ensure within a 2-h period. According to our hypothesis, this is the type of feeding scenario in which endogenous GLP-1 signaling may contribute to the termination of intake. If so, then central administration of Ex9 should block such signaling, promoting more excessive intake. Cumulative 2 h intake in rats after intracerebroventricular Ex9 was compared both to baseline intake by the same rats in the absence of injection (i.e., within subjects) and also to intake by a different group of rats after intracerebroventricular SAL treatment (i.e., between subjects). Ex9 significantly increased 2 h cumulative intake compared with intake by different rats treated with intracerebroventricular SAL, but not compared with baseline intake by the same Ex9-treated rats. Meal structure analyses revealed that Ex9 increased the duration of the second (but not the first) meal compared with rats treated with intracerebroventricular SAL, but not compared with baseline values in the same Ex9-treated rats. The delayed effect of intracerebroventricular SAL injection to reduce meal size and duration may reflect a delayed hypophagic response to intracerebroventricular injection that was not apparent during the first meal. The differential effect of Ex9 vs. SAL treatment on second meal duration was accompanied by a strong trend toward larger second meal size after Ex9. Interestingly, rats in both intracerebroventricular injection groups consumed significantly less chow overnight following injection compared with chow intake during the previous three nights (Fig. 5), supporting the view that intracerebroventricular injection promotes stress-induced hypophagia that persists for many hours. The similar magnitude of this long-lasting hypophagic effect in both SAL- and Ex9-injected rats could indicate that Ex9 is effective in counteracting the hypophagic effect of intracerebroventricular injection for only a few hours.
The current analysis of cumulative intake and meal pattern data in rats under both noninjected control conditions and after experimental treatment provides novel insights into the potential role of endogenous GLP-1 signaling in regulating food intake. To our knowledge, only one previous study has reported the effect of central Ex9 treatment on meal structure, with no reported effects on cumulative intake (9). In that study, ad libitum-fed rats were trained to consume sweetened condensed milk or sucrose solution for 2 h each day and then received either vehicle or Ex9 (in counterbalanced order) infused unilaterally into the nucleus accumbens (NAcc) before test meals. Compared with intake after vehicle infusion, Ex9 increased meal size and duration and altered licking microstructure in a manner consistent with increased palatability, suggesting a site-specific role for GLP-1 signaling within the NAcc to reduce food palatability (9). However, meal structure data under noninjected baseline conditions were not reported, leaving open the possibility that intra-NAcc Ex9 also exerts at least some of its effects by attenuating or counteracting the effect of experimental treatment (i.e., central infusion).
The effect of Ex9 (vs. SAL) to increase food intake/meal duration in rats consuming 6–7% of their body weight (experiment 2) is consistent with a substantial recruitment (40–50%) of GLP-1 neurons in rats that consumed similar volumes of liquid diet (experiment 1), and also with recent evidence that intracerebroventricular injection alone activates ∼60% of GLP-1 neurons (25). Thus, a behavioral effect of blocking endogenous GLP-1 signaling may be revealed only when significant proportions of GLP-1 neurons have been activated. However, in considering the potential role of hindbrain GLP-1 neurons in the control of food intake, it is important to remember that GLP-1 is not their sole signaling molecule. In rats, GLP-1 neurons are glutamatergic (44) and likely express a variety of additional neuropeptides, including inhibin-β, enkephalin, and/or somatostatin (30). Thus, the relatively subtle effect of pharmacological GLP-1R antagonism to increase food intake under certain experimental conditions does not preclude a potentially more important and/or more physiological role of GLP-1 neurons to control food intake that involves signaling by one or more coexpressed transmitter molecules.
Perspectives and Significance
Results from this study indicate that the large majority of GLP-1 neurons are activated to express cFos in rats that consume large satiating volumes of liquid diet within a 1-h period, with GLP-1 activation proportional to intake volume. Since central GLP-1R signaling may play a role in limiting food intake only when such large proportions of GLP-1 neurons are activated, we examined whether central GLP-1R antagonism would increase intake in schedule-fed rats. However, the intracerebroventricular injection procedure itself (necessary to deliver Ex9 antagonist) tended to decrease intake, an effect that was counteracted by Ex9. Thus, the previously reported hyperphagic effect of intracerebroventricular Ex9 is likely attributable, at least in part, to drug-induced attenuation of the hypophagic effects of intracerebroventricular vehicle injection. Central administration of Ex9 also attenuates the hypophagic effects of other stressful treatments, including systemically administered lithium chloride (26) or endotoxin (13, 22), centrally administered oxytocin (28), and acute restraint stress (25). Although previous studies have reported that rats consume more food after intracerebroventricular Ex9 than after intracerebroventricular vehicle, these studies did not report baseline food intake under noninjected control conditions (3, 9, 17, 20, 43). To our knowledge, no published reports investigating the effect of central vehicle or Ex9 (or other drugs) on food intake have included a relevant direct comparison of intake by the same rats under nonmanipulated baseline conditions. Thus, results from our study revealing rats' hypophagic response to intracerebroventricular saline are novel and important. Our findings should encourage investigators to optimize experimental designs by including intake measurements collected under nonmanipulated conditions along with measurements collected after drug or vehicle administration. The current findings provide additional insight into the role of endogenous GLP-1 signaling in controlling food intake, i.e., by reducing food intake near the end of the feeding period when meal-entrained rats have already consumed large amounts of food, and also under experimental conditions that promote stress-induced hypophagia.
GRANTS
This research was funded by NIH Grants MH-059911 and DK-100685 (L. Rinaman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.D.K. and L.R. conception and design of research; A.D.K. performed experiments; A.D.K. and L.R. analyzed data; A.D.K. and L.R. interpreted results of experiments; A.D.K. and L.R. prepared figures; A.D.K. drafted manuscript; A.D.K. and L.R. edited and revised manuscript; A.D.K. and L.R. approved final version of manuscript.
REFERENCES
- 1.Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Res 693: 51–63, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Aja S, Schwartz GJ, Kuhar MJ, Moran TH. Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am J Physiol Regul Integr Comp Physiol 280: R1613–R1619, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, Tome D, Ballet N, Chaumontet C. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav 104: 675–683, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Davis JD, Campbell CS. Peripheral control of meal size in the rat. Effect of sham feeding on meal size and drinking rate. J Comp Physiol Psychol 83: 379–387, 1973. [DOI] [PubMed] [Google Scholar]
- 9.Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab 304: E1314–E1320, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaykema RP, Daniels TE, Shapiro NJ, Thacker GC, Park SM, Goehler LE. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res 1294: 61–79, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill HJ, Carmody JS, Amanda Sadacca L, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol 287: R1190–R1193, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 33, Suppl 1: S11–S15, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 201: 267–269, 1978. [DOI] [PubMed] [Google Scholar]
- 16.Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 254: R853–R856, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves alpha1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes 60: 2701–2709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res 86: 399–418, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav 136: 47–54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langhans W, Balkowski G, Savoldelli D. Differential feeding responses to bacterial lipopolysaccharide and muramyl dipeptide. Am J Physiol Regul Integr Comp Physiol 261: R659–R664, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Front Neurosci 6: 199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav 121: 35–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci 35: 10701–10714, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol Regul Integr Comp Physiol 277: R1537–R1540, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol 277: R582–R590, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 283: R99–R106, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Rushing PA, Houpt TA, Henderson RP, Gibbs J. High lick rate is maintained throughout spontaneous liquid meals in freely feeding rats. Physiol Behav 62: 1185–1188, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Sawchenko PE, Arias C, Bittencourt JC. Inhibin beta, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 291: 269–280, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition 16: 814–820, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev 20: 41–46, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Swanson L. Structure of the Rat Brain. Amsterdam: Elsevier, 2004. [Google Scholar]
- 34.Travers JB, Rinaman L. Identification of lingual motor control circuits using two strains of pseudorabies virus. Neuroscience 115: 1139–1151, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285: R470–R478, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159, 1986. [DOI] [PubMed] [Google Scholar]
- 38.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol Regul Integr Comp Physiol 246: R776–R787, 1984. [DOI] [PubMed] [Google Scholar]
- 39.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods SC. The eating paradox: how we tolerate food. Psychol Rev 98: 488–505, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Woods SC. The house economist and the eating paradox. Appetite 38: 161–165, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res 110: 175–182, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 36: 1522–1528, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct 220: 3011–3022, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]