Abstract
Obesity is a risk factor for cardiovascular disease and is associated with increased plasma levels of the adipose-derived hormone leptin. Vascular smooth muscle cells (VSMC) express leptin receptors (LepR); however, their physiological role is unclear. We hypothesized that leptin, at levels to mimic morbid obesity, impairs vascular relaxation. To test this, we used control and VSM-LepR knockout mice (VSM-LepR KO) created with a tamoxifen-inducible specific Cre recombinase to delete the LepR gene in VSMC. Control (10–12 wk old) and VSM-LepR KO (10–12 wk old) mice were fed a diet containing tamoxifen (50 mg/kg) for 6 wk, after which vascular reactivity was studied in isolated carotid arteries using an organ chamber bath. Vessels were incubated with leptin (100 ng/ml) or vehicle (0.1 mM Tris·HCl) for 30 min. Leptin treatment resulted in significant impairment of vessel relaxation to the endothelial-specific agonist acetylcholine (ACh). When these experiments were repeated in the presence of the superoxide scavenger tempol, relaxation responses to ACh were restored. VSM-LepR deletion resulted in a significant attenuation of leptin-mediated impaired ACh-induced relaxation. These data show that leptin directly impairs vascular relaxation via a VSM-LepR-mediated mechanism, suggesting a potential pathogenic role for leptin to increase cardiovascular risk during obesity.
Keywords: endothelial function, obesity, acetylcholine, hypertension, Cre recombinase
obesity is a significant risk factor for the development of cardiovascular disease, as it often appears clinically with other risk factors such as diabetes, hypertension, and hyperlipidemia, giving rise to the metabolic syndrome. Another common cardiovascular risk factor associated with obesity is impaired vascular relaxation. Studies in animal models of obesity have documented that obesity is associated with impaired vascular relaxation (13, 24, 29, 39, 42). Similar observations have also been made in human patient populations (5, 58). Although impaired vascular relaxation has been widely documented in obesity, the underlying cause responsible for this phenotype has yet to be established.
One potential candidate for mediating the altered vascular relaxation in obesity is leptin. Leptin is a hormone produced from white adipocytes that acts on its receptors in the hypothalamus to control food intake and energy expenditure. Mutations in leptin and its receptor cause genetic obesity in rodents and humans (30, 40, 46, 62, 64). Although leptin receptors are highly expressed in the brain and nervous system, they are also present in the periphery including the vasculature. Leptin receptors are expressed in both endothelial and vascular smooth muscle (VSM) cells (54). Interestingly, in obesity, plasma leptin levels are very high, suggesting a central resistance to the effects of leptin on appetite and energy expenditure; however, the peripheral actions are believed to remain intact (15, 35).
Leptin has been reported to have several conflicting effects on the vasculature. Leptin has been found to both promote and inhibit VSM cell growth and proliferation (8, 22, 32, 44, 51, 56, 63). Studies in both animal models as well as human patient populations have linked leptin to the development and progression of atherosclerosis (7, 36, 50, 57). Leptin also has confounding effects on vascular resistance. It can promote the release of the potent vasoconstrictor, endothelin, and increase the expression of its receptors in both endothelial and VSM cells (12, 23, 48). Leptin also increases the expression of renin-angiotensin system components (angiotensinogen, angiotensin receptors), and it is well known that renin-angiotensin activation has profound vascular effects (63). However, leptin also increases the levels and activity of both endothelial and neuronal nitric oxide (NO) synthase (NOS) to increase NO levels, which is a major vasodilator (4, 31, 51, 53, 59). Leptin signals via the long form of the leptin receptor through activation of signal transducer and activator of transcription 3, Shp2, and phosphatidylinositol 3-kinase (PI3K)/Akt pathways and acutely increases NO levels through activation of the PI3K/Akt pathway (20, 59). However, prolonged exposure to leptin has been reported to attenuate NO production and phosphorylation of NOS3 through upregulation of suppressor of cytokine signaling-3 expression (6).
Although these diverse actions of leptin in the vasculature are established in the literature, the specific effect of leptin in VSM cells has yet to be elucidated. We utilized a Cre-loxP-based approach to effectively knock out leptin receptors from VSM cells to determine the effect of VSM cell leptin receptors on vascular reactivity. We utilized a mouse model in which exon 1 of the leptin receptor gene was flanked by loxP sites to create a model with loss of all leptin receptor splice variants in VSM cells in vivo (14, 27). We bred these floxed leptin receptor mice with a tamoxifen-inducible Cre recombinase expressed specifically in VSM cells to test the hypothesis that VSM cell leptin receptors mediate the alterations in vascular relaxation in response to high leptin levels typically observed in obesity.
METHODS
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. Experiments were performed on male and female mice between 10 and 15 wk of age. Breeding pairs of floxed leptin receptor mice (LepRflox) mice were obtained from Dr. Jeffrey M. Friedman, Rockefeller University, and offspring were maintained on a C57BL/6 background as previously described (21). These mice contain loxP sites, which flank exon 1 of the leptin receptor gene (14). Because this exon contains the signal sequence, Cre-mediated deletion of this exon results in the loss of all leptin receptor splice variants (14). VSM-specific inducible Cre mice (SM22-MerCreMer) were as originally described (27). All mice were fed a standard diet containing 0.29% NaCl and provided water ad libitum. Tamoxifen was administered via a specially prepared diet, which was identical in composition to the standard diet but contained tamoxifen at a concentration of 50 mg/kg (Harlan Laboratories, Madison, WI). Mice were fed the tamoxifen diet for a period of 6 wk, after which time they were switched back to the standard diet. Experiments were then performed 2 wk after switching back to the standard diet.
Blood pressure.
Blood pressure was measured in mice by radiotelemetry. Mouse radiotelemetry transmitters (TA11PA-C10; Data Sciences International, Minneapolis, MN) were implanted into the carotid artery of mice as previously detailed (10). Mice were allowed 10 days to fully recover from surgery. After this time, blood pressures were measured for 10 s every 15 min for 7 days. Mice were then euthanized and tissues collected. Blood pressure data were analyzed using Dataquest ART version 3.1 software (Data Sciences International). Mean arterial blood pressure (MAP) was separated into daytime and nighttime values, and then two readings were averaged to get a daily MAP. MAP over each of the 7 days of recording was then averaged to get an individual measurement for each mouse as well as reported daytime and nighttime averages.
Vascular ring preparation.
Mouse carotid arteries were removed and prepared for vessel reactivity studies as previously published (52). Carotid arteries were incubated in Krebs buffer (in mmol/l: pH 7.4, 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 11.0 glucose) saturated with 95% O2-5% CO2. Resting tension was adjusted stepwise to reach a final tension of 0.25 g. For each animal, at least two vessel segments were studied with the averaged response equal to an n of 1. Concentration-dependent relaxation (10−8-10−4 mol/l) to acetylcholine (ACh) and sodium nitroprusside (SNP) were assessed in vessel segments precontracted with the thromboxane A2 mimetic U46619 (0.4 μg/ml). A subset of vessels was incubated with leptin (100 ng/ml) or vehicle (Tris·HCl 0.1 mM) for 30 min before and during the concentration response curve to ACh. These studies were conducted in the presence or absence of Tempol (1 mM) to test the role of reactive oxygen species.
PCR of leptin receptor deletion product.
PCR was performed on genomic DNA isolated from the aorta, brain vessels, carotid artery, coronary arteries, kidney arteries, and mesenteric arteries using the Direct PCR kit (Viagen Biotech, Los Angeles, CA) according to manufacturer's guidelines. Vessels from the brain, heart, and kidney were prepared by passing the tissue through a 75-μm sieve and collecting the vascular tissue retained. Individual mesenteric arteries were cleared of all surrounding adipose tissue and collected by microdissection. PCR for the leptin deletion product was performed using the following primers: LepRprimer1-5′-GTCACCTAGGTTAATGTATTC-3′ and LepRprimer3-5′-GCAATTCATATCAAAACGCC-3′. PCR was performed using the following protocol: 95°C for 30 s, 50°C for 30 s, and 72°C for 30 s for 35 cycles. PCR products were visualized on 2% agarose gels.
Real-time PCR.
Real-time PCR was performed on RNA isolated from aortas of wild-type and KO mice. RNA was isolated from aortas using Nucleo Spin RNA kit (Macherey-Nagel, Durew, Germany) according to manufacturer's guidelines. cDNA was derived from 500 ng of the purified total RNA using the iScript RT kit (Bio-Rad, Hercules, CA). Real-time PCR was performed on 1 μl of the cDNA using iTaq University SYBR-Green Kit (Bio-Rad). PCR was performed using the following protocol: 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s for 40 cycles. The difference in the threshold cycle (Ct) for the target gene of each sample was compared with the Ct for 18S rRNA to yield the delta Ct. The results are expressed as the fold difference for each target gene compared with the level in wild-type mice. The PCR primers used for real-time PCR were as follows: LEPR+, 5′CTATGTGGTTTTGTTACACTGG; LEPR, 5′AGGTGAGAGAAAGGAGTCATC; Nox1 forward+, 5′AATGCCCAGGATCGAGGT; Nox1 reverse+, 5′GATGGAAGCAAAGGGAGTGA; Nox2 forward+, 5′CCCTTTGGTACAGCCAGTGAAGAT; Nox2 reverse, CAATCCCGGCTCCCACTAACATCA; Nox4 forward, 5′GGATCACAGAAGGTCCCTAGCAG; Nox4 reverse, 5′GCGGCTACATGCACACCTGAGAA; SOD1 forward, 5′CGGCTTCTCGTCTTGCTCTC; SOD1 reverse, 5′CGAAGTGGATGGTTCCCTGC; SOD2 forward, 5′TTAACGCGCAGATCATGCA; SOD2 reverse, 5′GGTGGCGTTGAGATTGTTCA; SOD3 forward, 5′CAGAACGGCAATGCAGGTCG; SOD3 reverse, 5′CTGAGTGTGGCTTAAGTGGTCTTG; 18S+ 5′TTAGTCCCTGCCCTTTGTACACA; 18S- 5′-GATCCGAGGGCCTCACTAAAC.
Western blot.
Total abundance of NOS3 and phosphorylation of NOS3 at Ser 1177 were determined by Western blot. The thoracic aorta was homogenized in 250 μl of lysis buffer, centrifuged at 10,000 g for 10 min at 4°C, and the homogenate was then mixed with loading buffer and heated to 85°C for 5 min. Rat kidney inner medulla was used as a positive control. Homogenized sample (22.5 μl) was separated by electrophoresis (200 V, 40 min, 4–15% TGX Stain-Free Precast gel, Bio-Rad) and transferred onto nitrocellulose membranes (Bio-Rad Trans-Blot Turbo, High MW program). The membrane was then blocked for 1 h and cut at the 75-kDa marker. The top half was incubated with the rabbit polyclonal phosphorylated NOS3-Ser1177 antibody (Cell Signaling Technology, Beverly, MA) overnight at 4°C, and the lower half was incubated with β-actin (Sigma, St. Louis, MO). Membranes were then incubated with the secondary antibodies (rabbit 1:1,000 for p-NOS3 and mouse 1:30,000 for actin, Bio-Rad) for 1 h at room temperature and then developed with enhanced chemiluminescent reagents (Thermo Scientific, Rockford, IL). The top half of the membrane was stripped (ReBlot Plus Mild; Millipore, Billerica, MA) and incubated overnight with a primary antibody against NOS3 (1:250; BD Transduction, San Jose, CA) before incubation with the mouse secondary antibody (mouse, 1:1,000). Bands were then quantified using the VersaDoc Imaging System and Image Lab 3.0 Software (Bio-Rad). NOS3 phosphorylation was calculated as the integrated optical density (IOD) of the phospho-NOS3 band to the IOD of the NOS3 band, and NOS3 abundance was calculated as the ratio of the IOD of NOS3 to the IOD of β-actin.
Statistics.
All data are presented as means ± SE. Differences between treatment groups were determined using an unpaired t-test or a one-way ANOVA with a post hoc test (Dunnett's). P < 0.05 was considered to be significant. All analyzes were performed with SigmaStat (Systat Software, Richmond, CA).
RESULTS
Tamoxifen-mediated deletion of leptin receptors in vascular smooth muscle cells.
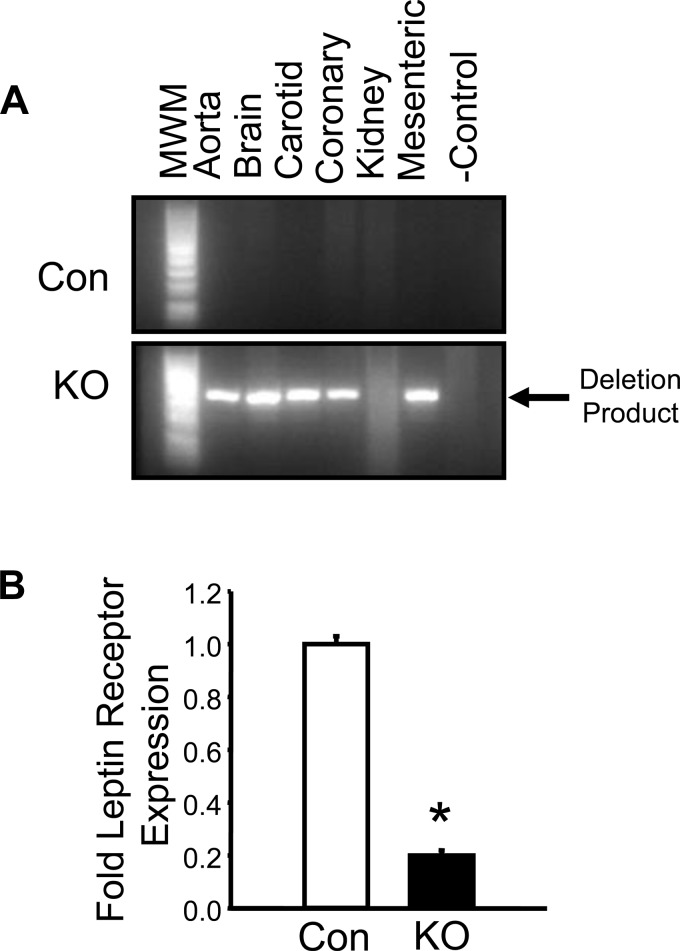
Detection of the deleted leptin receptor was performed using a specific PCR-based assay, which specifically amplifies the deletion product of the leptin receptor gene. Mice were administered tamoxifen for 6 wk in their chow. After a 2-wk recovery period, mice were euthanized, and blood vessels from different vascular beds were collected. PCR of several different vascular segments was then performed from control flox and flox mice expressing the inducible VSM cell-specific Cre recombinase (Fig. 1A). The 400-bp PCR deletion product was only observed in vascular segments from the tamoxifen-treated KO mice and not from control (Con) mice (Fig. 1A). Levels of leptin receptor mRNA were next determined from aorta by real-time PCR. Leptin receptor mRNA levels were decreased by 80% in the aorta of KO compared with wild-type mice (Fig. 1B).
Fig. 1.
Molecular characterization of the vascular smooth muscle (VSM) leptin receptor (LepR) knockout (KO) mice. A: PCR validation of LepR KO in vessels isolated from various tissues in control (Con) and KO mice. B: expression of LepR mRNA levels in the aorta of Con and KO mice via real-time PCR. MWM, molecular weight marker. *P < 0.05 vs. Con, n = 4/group.
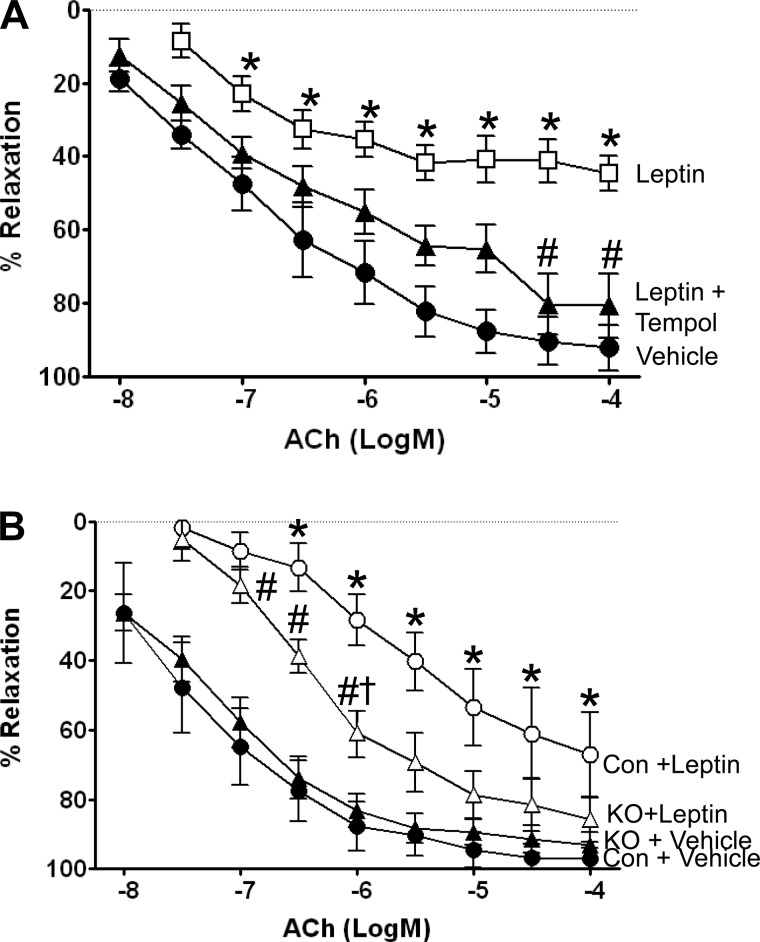
Acute incubation with leptin inhibits ACh but not SNP-mediated relaxation, and it is restored with the superoxide scavenger tempol.
Acute incubation of isolated carotid arteries from control mice with a concentration of leptin that mimics levels achieved in morbid obesity caused significantly impaired vessel relaxation to the endothelial-specific agonist ACh (Fig. 2A) but not to smooth muscle-mediated relaxation caused by SNP (data not shown). When these experiments were repeated in the presence of the superoxide scavenger tempol, relaxation responses to ACh were restored, suggesting that leptin-mediated oxidative stress contributed to the impairment.
Fig. 2.
A: acute incubation with leptin inhibits acetylcholine (ACh)-mediated relaxation and is restored with the superoxide scavenger tempol (1 mM). Leptin was added to the bath for acute exposure. P < 0.05 vs. vehicle-treated mice. #P < 0.05 vs. leptin-treated mice, n = 6/group. B: leptin impairment of ACh-mediated relaxation of the carotid artery is blunted in VSM LepR KO mice. Leptin was added to the bath for acute exposure, and the ACh response was paired from the same artery. *P < 0.05 vs. Con + vehicle and KO + vehicle. #P < 0.05 vs. Con + vehicle and KO + vehicle. †P < 0.05 vs. Con + Leptin, n = 6/group.
Leptin-mediated impairment of ACh relaxation is blunted in VSM LepR KO mice.
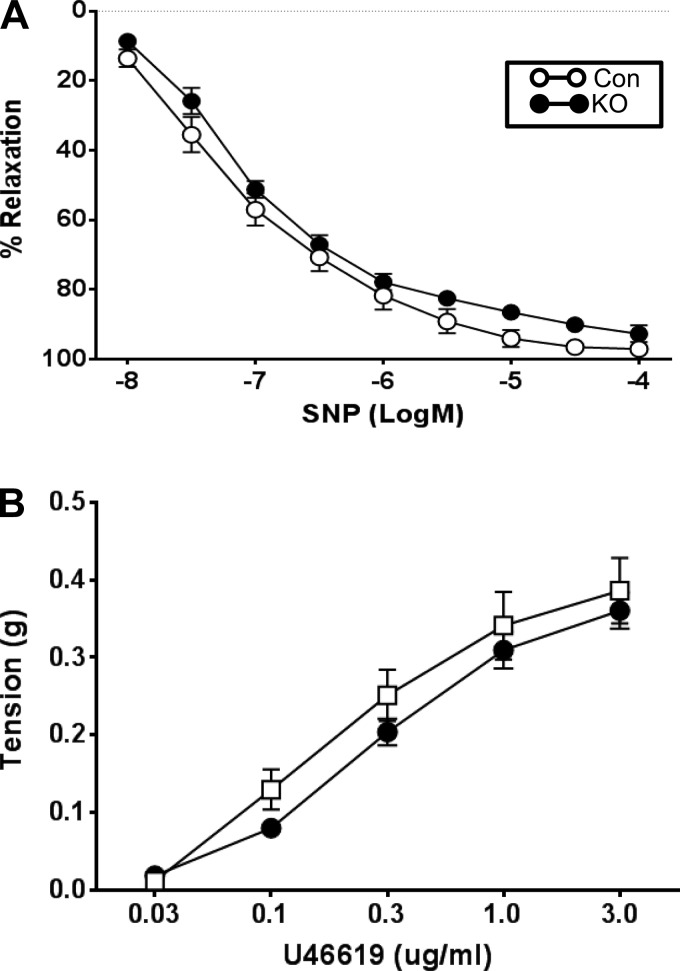
To specifically test the role of VSM LepR in mediating vascular responses to leptin, we utilized VSM LepR KO mice and littermate controls (Con). Conditional KO of the LepR did not change ACh-mediated relaxation in the carotid artery (Fig. 2B). Consistent with our data above, carotid arteries from control littermates incubated with leptin exhibited significant impairment to ACh-mediated relaxation. However, this impairment in response to acute incubation with leptin was significantly attenuated in carotid arteries from VSM LepR KO mice. No differences in the concentration response curves to SNP (Fig. 3A) or U46619 (Fig. 3B) were observed in carotid arteries from control and KO mice.
Fig. 3.
Responses of the carotid artery of VSM LepR KO and Con mice to sodium nitroprusside (SNP) (A) and the thromboxane receptor agonist, U46619 (B); n = 6/group.
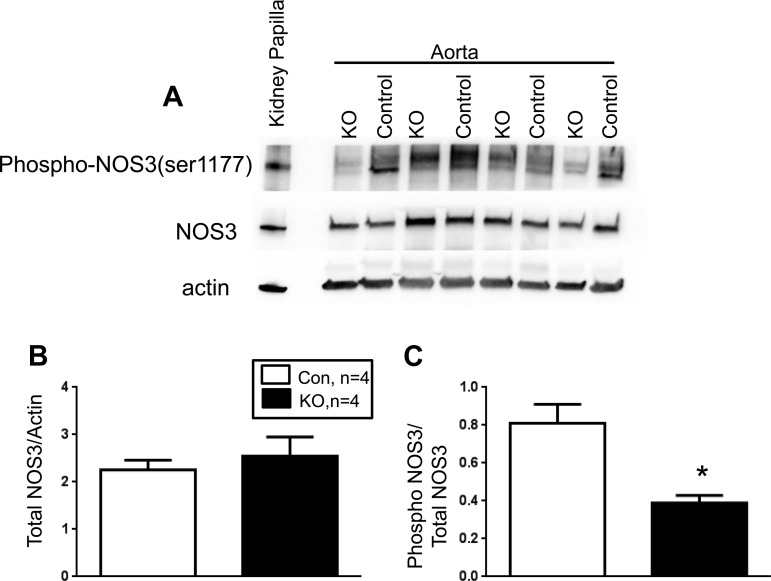
VSM deletion of leptin receptor decreases vascular phospho-NOS3 without affecting total NOS3 levels.
The levels of both total NOS3 and phospho Ser 1177 NOS3 were determined by Western blot using aortic lysates from control and VSM LepR KO mice. Total NOS3 levels did not differ in the aorta between control and VSM LepR KO mice (Fig. 4, A and B). However, the levels of phospho Ser 1177 NOS3 were significantly diminished in the aorta VSM LepR KO compared with control mice (Fig. 4, A and C).
Fig. 4.
Western blot analysis of total and Ser 1177 phosphorylated NADPH oxidase 3 (NOS3) from the aorta of VSM LepR KO and Con mice. A: representative Western blot. B: ratio of total NOS3 to actin. C: ratio of phospho NOS3 to total NOS3. *P < 0.05 vs. Con.
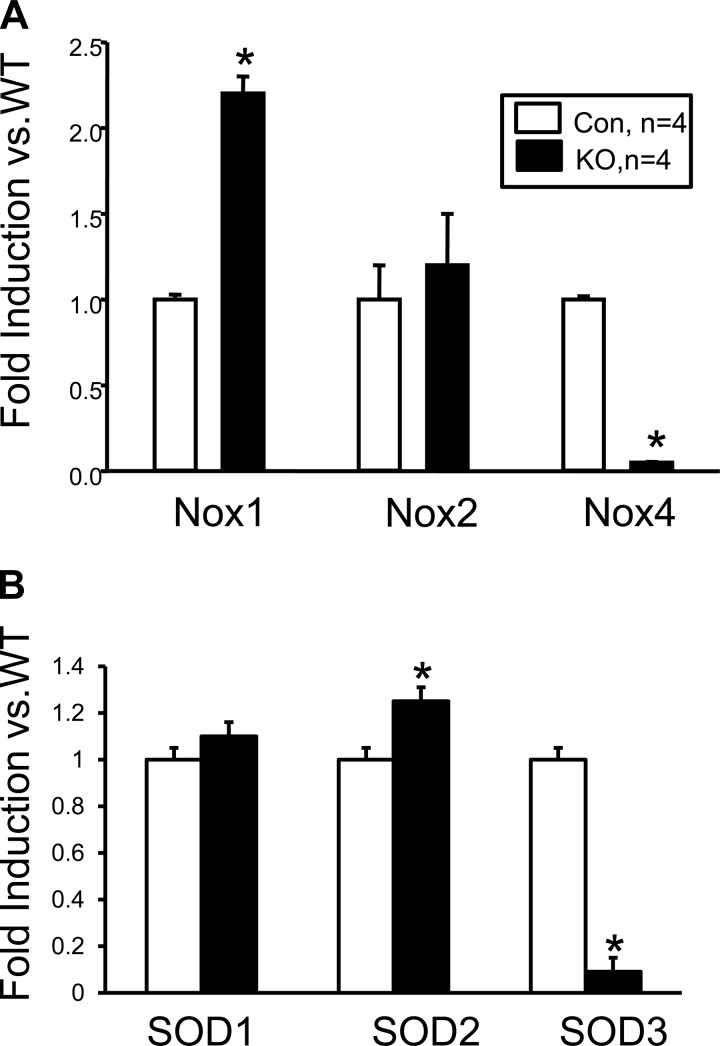
VSM deletion of leptin receptor decreases vascular NOX4 and SOD3 levels.
The effect of VSM deletion of the leptin receptor on enzymes involved in regulating the production of vascular superoxide was determined by real-time PCR using RNA derived from the aorta of control and VSM LepR KO mice. NADPH oxidases are a family of proteins responsible for the generation of superoxide in the vasculature. NOX4, which is the major isoform of NADPH oxidase present in the vasculature, was decreased by 90% in the aortas of VSM LepR KO mice compared with control mice (Fig. 5A). This significant decrease in vascular NOX4 expression was associated with a twofold induction of NOX1 expression in the aorta of VSM LepR KO compared with control mice (Fig. 5A). VSM LepR KO mice also exhibit a 90% decrease superoxide dismutase (SOD) 3 expression and a 20% increase in SOD2 expression in the aorta compared with control mice (Fig. 5B).
Fig. 5.
Expression levels of NOX isoforms (A) and superoxide dismutase (SOD) isoforms (B) from the aorta of VSM LepR KO and control mice. WT, wild-type. *P < 0.05 vs. Con.
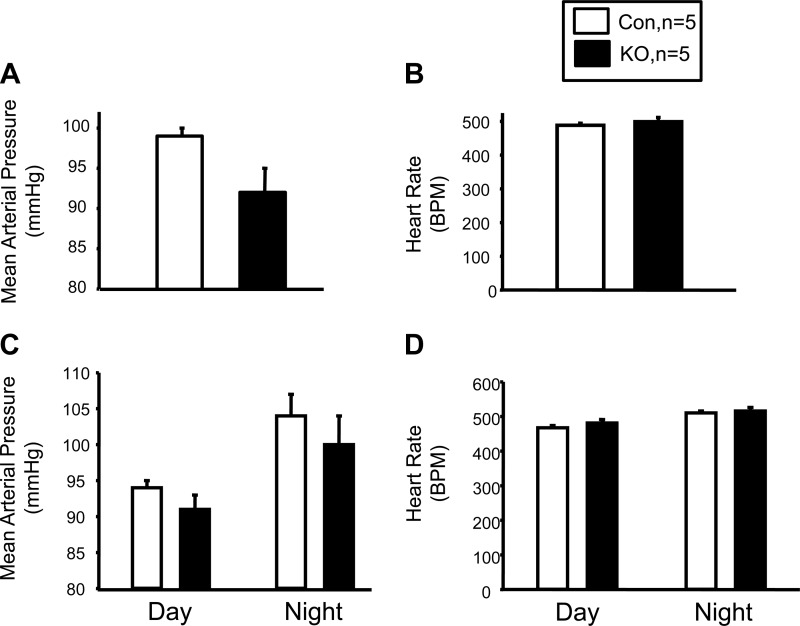
VSM deletion of leptin receptor has no effect on blood pressure or heart rate.
To determine the effect of loss of VSM leptin receptors on basal blood pressure, we measured conscious 24-h blood pressure in tamoxifen-treated control and KO mice for 7 days by radiotelemetry. KO of VSM leptin receptors did not have any effect on 24-h MAP or heart rate (Fig. 6, A and B). Additionally, no effect of VSM leptin receptor KO was observed on either daytime or nighttime MAP (Fig. 6, C and D). In addition, no differences in body weight, heart weight, heart weight-to-body weight ratio, or heart weight-to-body length ratio were found between control and KO mice (Table 1).
Fig. 6.
blood pressure and heart rate in VSM LepR KO and Con mice. A: 24-h mean arterial pressure. B: 24-h heart rate. C: circadian blood pressure. D: circadian heart rate; n = 5.
Table 1.
Body weight, heart weight, and indices of cardiac hypertrophy in wild-type and VSM LepR KO mice
| Parameter | Wild-type | VSM LepR KO | P Value |
|---|---|---|---|
| Body weight, g | 28.2 + 1.2 | 27.6 + 1.2 | 0.69 |
| Heart Weight, mg | 113 + 7.2 | 115 + 7.8 | 0.85 |
| HW:BW, mg:g | 4 + 0.2 | 4.2 + 0.2 | 0.6 |
| HW:BL, mg:cm | 11.6 + 0.6 | 11.8 + 0.8 | 0.83 |
All values are given as means + SE; n = 5. VSM, vascular smooth muscle; LepR, leptin receptor; KO, knockout; HW, heart weight; BW, body weight; BL, body length.
DISCUSSION
Obesity is associated with an increased incidence of cardiovascular diseases. As it often appears with other metabolic abnormalities such as diabetes, hyperlipidemia, hypercholesterolemia, and inflammation, it is difficult to specifically determine the importance of obesity alone in the development of cardiovascular disease such as hypertension and heart disease. One of the potential links between obesity and the development of hypertension is the hormone leptin, which is released primarily from white adipose tissue and is elevated in obesity. Elevated leptin levels as observed in obesity can directly cause hypertension through a central mechanism resulting in increased sympathetic outflow to peripheral organs like the kidney (11, 41, 49, 55). High plasma levels may also promote hypertension and cardiovascular disease through impairment of vascular relaxation. In our study, we observed a significant effect of acute high leptin levels similar to those observed in obese individuals to attenuate ACh-mediated vascular relaxation. This response was likely due in part to leptin-mediated increases in VSM cell oxidative stress, as treatment with the antioxidant compound tempol significantly attenuated the effect of high leptin levels on ACh-mediated vascular relaxation. The effect of leptin to attenuate ACh-mediated relaxation was also a VSM cell-dependent effect, as deletion of leptin receptors also prevented this response. The alterations in the ability of leptin to attenuate ACh-mediated relaxation in VSM LepR KO mice were not due to alterations in vascular reactivity in these mice, as they exhibited normal responses to sodium nitroprusside and U46619. Previous studies have reported that leptin can cause increases in the levels of oxidative stress in endothelial cells and cardiomyocytes (9, 17). The mechanism by which leptin increases vascular oxidative stress is not known but may be mediated via stimulation of NAD(P)H oxidases (18). NOX4 and NOX1 are thought to be the major isoforms of NAD(P)H oxidase in the vasculature responsible for superoxide production (3, 33). VSM LepR KO mice exhibited a >90% decrease in vascular expression of NOX4 compared with control mice and an increase in vascular NOX1 expression. It is possible that the leptin receptor acting either directly or through increases in angiotensin II or endothelin is responsible for maintaining NOX4 expression levels in the vasculature to increase NAD(P)H-mediated superoxide production (43, 60, 63). It is not known whether the observed increase in vascular NOX1 levels in the VSM LepR KO mice is a compensatory response to loss of vascular NOX4; however, it is clear that the enhanced NOX1 expression is not sufficient to mediate the effect of leptin to attenuate ACh-mediated relaxation in the VSM LepR KO mice. SODs are a family of proteins that are responsible for protecting the vasculature from reactive oxygen species (ROS), such as superoxide (37). We observed significant decreases in the expression of the extracellular isoform of SOD, SOD3, in the vasculature of VSM LepR KO compared with control mice. SOD3 expression is believed to be regulated by factors such as angiotensin II and endothelin-1, which may be attenuated in VSM-lacking leptin receptors (45, 47). Recent studies have demonstrated that, although VSM-specific deletion of SOD3 is associated with enhanced superoxide production and attenuation of bioavailable NO, it does not have a significant effect on blood pressure either under basal conditions or in response to angiotensin II infusion (34). It is possible that the decrease in SOD3 expression in the vasculature of VSM LepR KO mice is reflective of the NOX4-mediated decrease in vascular superoxide production. However, additional studies are required to fully elucidate this relationship.
Our findings of attenuation of vascular relaxation by high leptin levels, which are mediated by increased oxidative stress, are in agreement with a recent study that demonstrated that acute leptin treatment impairs vascular relaxation in second-order branches of mesenteric arteries (61). This study demonstrated that the effect of leptin to impair vascular relaxation was also mediated by a central mechanism, as celiac ganglionectomy blocked the effect of leptin on endothelial function (61). These results, when combined with the results of the present study, suggest that leptin may impair vascular relaxation by two mechanisms, a centrally mediated mechanism and a direct leptin receptor-mediated effect on VSM cells. It is possible that each of these mechanisms plays a different role in the regulation of vascular function in smaller resistance arteries vs. larger arterioles with the direct effect of leptin being more pronounced in the larger arteries and the central sympathetic mechanism regulating smaller resistance arterioles. It should be noted that VSM cell leptin receptors in our model were knocked out in both large arterioles such as the carotid artery and aorta as well as small mesenteric resistance arteries (Fig. 1A). However, we did not address the role of leptin in mediating vascular function in small mesenteric arterioles in the present study.
Leptin has been previously described to promote vascular relaxation by enhancement of NO production from endothelial cells (2, 25, 31). The ability of leptin to increase NO production is thought to be an important counterbalance to its central effects to raise blood pressure (1, 19, 28). Acute leptin treatment (hours) has been demonstrated to increase NOS3 phosphorylation through a PI3K-Akt mediated pathway (59). Enhancement of NOS3 phosphorylation at Ser 1177 results in increased activity and is one mechanism by which the activity of the protein is regulated (16, 38). VSM cell deletion of leptin receptors was associated with decreased levels of phospho Ser 1177 NOS3. Because NOS3 is expressed in endothelial cells of the vasculature, it is unclear how VSM-specific deletion of leptin receptors could influence the regulation of endothelial NOS3 activity. One possibility is that decreased leptin-mediated VSM superoxide production increases the bioavailability of NO, which results in a downregulation of NOS3 phosphorylation in endothelial cells. Although several studies have demonstrated that leptin can promote vascular relaxation via enhancement of NO production, other studies have demonstrated that high doses of leptin promotes vascular dysfunction via uncoupling of NOS3 and increased peroxynitrite formation (26). Our results would suggest that the VSM leptin receptor plays an important role in leptin-induced vascular dysfunction through a mechanism dependent on superoxide generation.
We did not find any effect of VSM cell deletion of leptin receptors on basal blood pressure or heart rate in the present study. Thus it is apparent that VSM cell leptin receptors do not contribute to the regulation of blood pressure under basal conditions. However, we did not determine the effect of loss of VSM cell leptin receptors on leptin-induced hypertension, as it was not the focus of the present study. It is possible that loss of VSM cell leptin receptors could attenuate leptin-induced hypertension by reducing leptin-mediated oxidative stress in the vasculature and enhancing leptin-mediated NO production from endothelial cells. It would also be interesting to determine the effect of chronic leptin treatment on relaxation in the VSM cell leptin receptor KO mice. Both of these questions need to be addressed in future experiments.
Perspectives and Significance
We developed a novel model of VSM cell-specific KO of leptin receptors to assess the impact of high circulating levels of leptin on vascular function. We found that high leptin levels attenuate ACh-mediated vasorelaxation, and this effect was reversed by pretreatment of vascular segments with the antioxidant compound tempol. We further demonstrate that VSM cell-specific loss of leptin receptors attenuated the effect of leptin to inhibit ACh-mediated vascular relaxation. VSM deletion of the leptin receptor was associated with decreased aortic expression of NOX4 and SOD3 and phosphorylation of NOS3 at Ser 1177. VSM cell-specific KO of leptin receptors had no effect on body weight, basal blood pressure, heart rate, and cardiac hypertrophy. This model could be beneficial for determining the role of VSM cell leptin receptors in other vascular pathologies, such as atherosclerosis, and in understanding the role of leptin receptors in vascular stiffness.
GRANTS
This work was supported by grants from the National Heart, Lung and Blood Institute (PO1HL-051971) and the National Institute of General Medical Sciences (P20GM-104357).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.J.R. and D.E.S. conception and design of research; M.J.R., T.T.C., J.M.S., K.M.P., M.W.H., and D.E.S. performed experiments; M.J.R., T.T.C., J.M.S., K.M.P., M.W.H., and D.E.S. analyzed data; M.J.R., J.M.S., M.W.H., and D.E.S. interpreted results of experiments; M.J.R., T.T.C., J.M.S., and D.E.S. prepared figures; M.J.R., T.T.C., J.M.S., K.M.P., M.W.H., and D.E.S. edited and revised manuscript; M.J.R., T.T.C., J.M.S., K.M.P., M.W.H., and D.E.S. approved final version of manuscript; D.E.S. drafted manuscript.
REFERENCES
- 1.Beltowski J, Jochem J, Wojcicka G, Zwirska-Korczala K. Influence of intravenously administered leptin on nitric oxide production, renal hemodynamics and renal function in the rat. Regul Pept 120: 59–67, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Beltowski J, Wojcicka G, Borkowska E. Human leptin stimulates systemic nitric oxide production in the rat. Obes Res 10: 939–946, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol 30: 849–854, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Benkhoff S, Loot AE, Pierson I, Sturza A, Kohlstedt K, Fleming I, Shimokawa H, Grisk O, Brandes RP, Schroder K. Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arterioscler Thromb Vasc Biol 32: 1605–1612, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee R, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatr 10: 8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanquicett C, Graves A, Kleinhenz DJ, Hart CM. Attenuation of signaling and nitric oxide production following prolonged leptin exposure in human aortic endothelial cells. J Investig Med 55: 368–377, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 25: e119–e122, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bohlen F, Kratzsch J, Mueller M, Seidel B, Friedman-Einat M, Witzigmann H, Teupser D, Koerner A, Storck M, Thiery J. Leptin inhibits cell growth of human vascular smooth muscle cells. Vascul Pharmacol 46: 67–71, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J 13: 1231–1238, 1999. [PubMed] [Google Scholar]
- 10.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496–501, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Chao HH, Hong HJ, Liu JC, Lin JW, Chen YL, Chiu WT, Wu CH, Shyu KG, Cheng TH. Leptin stimulates endothelin-1 expression via extracellular signal-regulated kinase by epidermal growth factor receptor transactivation in rat aortic smooth muscle cells. Eur J Pharmacol 573: 49–54, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology 148: 160–165, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correia ML, Haynes WG. Obesity-related hypertension: is there a role for selective leptin resistance? Curr Hypertens Rep 6: 230–235, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Dong F, Zhang X, Ren J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension 47: 222–229, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Fortuno A, Bidegain J, Baltanas A, Moreno MU, Montero L, Landecho MF, Beloqui O, Diez J, Zalba G. Is leptin involved in phagocytic NADPH oxidase overactivity in obesity? Potential clinical implications. J Hypertens 28: 1944–1950, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Fruhbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes 48: 903–908, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall ME, Smith G, Hall JE, Stec DE. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in Cre-recombinase-mediated cardiotoxicity. Am J Physiol Regul Integr Comp Physiol 303: R1241–R1250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang F, Xiong X, Wang H, You S, Zeng H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta Biochim Biophys Sin (Shanghai) 42: 325–331, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Juan CC, Chuang TY, Lien CC, Lin YJ, Huang SW, Kwok CF, Ho LT. Leptin increases endothelin type A receptor levels in vascular smooth muscle cells. Am J Physiol Endocrinol Metab 294: E481–E487, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Ketonen J, Pilvi T, Mervaala E. Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessels 25: 254–262, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, Moriwaki C, Hano T, Nishio I. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun 273: 745–749, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol 295: H1514–H1521, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F, Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis 28: 15–22, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension 37: 670–676, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Laight DW, Desai KM, Anggard EE, Carrier MJ. Endothelial dysfunction accompanies a pro-oxidant, pro-diabetic challenge in the insulin resistant, obese Zucker rat in vivo. Eur J Pharmacol 402: 95–99, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Lane PW, Dickie MM. The effect of restricted food intake on the life span of genetically obese mice. J Nutr 64: 549–554, 1958. [DOI] [PubMed] [Google Scholar]
- 31.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes 49: 293–297, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Mamputu JC, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes 54: 2227–2234, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Liang CF, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 33: 777–784, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Lob HE, Vinh A, Li L, Blinder Y, Offermanns S, Harrison DG. Role of vascular extracellular superoxide dismutase in hypertension. Hypertension 58: 232–239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens 20: 1245–1250, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Martin SS, Blaha MJ, Muse ED, Qasim AN, Reilly MP, Blumenthal RS, Nasir K, Criqui MH, McClelland RL, Hughes-Austin JM, Allison MA. Leptin and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 239: 67–72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maytin M, Leopold J, Loscalzo J. Oxidant stress in the vasculature. Curr Atheroscler Rep 1: 156–164, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276: 17625–17628, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res 96: 1178–1184, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol 295: R1730–R1736, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naderali EK, Brown MJ, Pickavance LC, Wilding JP, Doyle PJ, Williams G. Dietary obesity in the rat induces endothelial dysfunction without causing insulin resistance: a possible role for triacylglycerols. Clin Sci (Lond) 101: 499–506, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen Dinh CA, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19: 1110–1120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci 47: 141–150, 2001. [PubMed] [Google Scholar]
- 45.Ozumi K, Sudhahar V, Kim HW, Chen GF, Kohno T, Finney L, Vogt S, McKinney RD, Ushio-Fukai M, Fukai T. Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: a key regulator of extracellular superoxide dismutase. Hypertension 60: 476–486, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 13: 18–19, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Qin Z, Gongora MC, Ozumi K, Itoh S, Akram K, Ushio-Fukai M, Harrison DG, Fukai T. Role of Menkes ATPase in angiotensin II-induced hypertension: a key modulator for extracellular superoxide dismutase function. Hypertension 52: 945–951, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quehenberger P, Exner M, Sunder-Plassmann R, Ruzicka K, Bieglmayer C, Endler G, Muellner C, Speiser W, Wagner O. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res 90: 711–718, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, Rader DJ, Kimmel SE. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab 89: 3872–3878, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez A, Gomez-Ambrosi J, Catalan V, Fortuno A, Fruhbeck G. Leptin inhibits the proliferation of vascular smooth muscle cells induced by angiotensin II through nitric oxide-dependent mechanisms. Mediators Inflamm 2010: 105489, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension 43: 1074–1079, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Schinzari F, Tesauro M, Rovella V, Di Daniele N, Mores N, Veneziani A, Cardillo C. Leptin stimulates both endothelin-1 and nitric oxide activity in lean subjects but not in patients with obesity-related metabolic syndrome. J Clin Endocrinol Metab 98: 1235–1241, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Schroeter MR, Schneiderman J, Schumann B, Gluckermann R, Grimmas P, Buchwald AB, Tirilomis T, Schondube FA, Konstantinides SV, Schafer K. Expression of the leptin receptor in different types of vascular lesions. Histochem Cell Biol 128: 323–333, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 31: 409–414, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Shin HJ, Oh J, Kang SM, Lee JH, Shin MJ, Hwang KC, Jang Y, Chung JH. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem Biophys Res Commun 329: 18–24, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clement K, Holvoet P, Tedgui A, Mallat Z. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2691–2698, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Valle JM, Estepa RM, Camacho RM, Estrada RC, Luna FG, Guitarte FB. Endothelial dysfunction is related to insulin resistance and inflammatory biomarker levels in obese prepubertal children. Eur J Endocrinol 156: 497–502, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes 51: 168–173, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Vendrov A, Vendrov K, Smith A, Yuan J, Sumida A, Robiduox J, Runge MS, Madamanchi NR. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid Redox Signal 23: 1389–1409, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Wang H, Luo W, Guo C, Wang J, Chen YE, Chang L, Eitzman DT. Leptin-induced endothelial dysfunction is mediated by sympathetic nervous system activity. J Am Heart Assoc 2: e000299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu-Peng XS, Chua SC Jr, Okada N, Liu SM, Nicolson M, Leibel RL. Phenotype of the obese Koletsky (f) rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor (Lepr): evidence for deficient plasma-to-CSF transport of leptin in both the Zucker and Koletsky obese rat. Diabetes 46: 513–518, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Zeidan A, Purdham DM, Rajapurohitam V, Javadov S, Chakrabarti S, Karmazyn M. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J Pharmacol Exp Ther 315: 1075–1084, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]