Abstract
Orgasm and ejaculation are two separate physiological processes that are sometimes difficult to distinguish. Orgasm is an intense transient peak sensation of intense pleasure creating an altered state of consciousness associated with reported physical changes. Antegrade ejaculation is a complex physiological process that is composed of two phases (emission and expulsion), and is influenced by intricate neurological and hormonal pathways. Despite the many published research projects dealing with the physiology of orgasm and ejaculation, much about this topic is still unknown. Ejaculatory dysfunction is a common disorder, and currently has no definitive cure. Understanding the complex physiology of orgasm and ejaculation allows the development of therapeutic targets for ejaculatory dysfunction. In this article, we summarize the current literature on the physiology of orgasm and ejaculation, starting with a brief description of the anatomy of sex organs and the physiology of erection. Then, we describe the physiology of orgasm and ejaculation detailing the neuronal, neurochemical, and hormonal control of the ejaculation process.
Keywords: Erectile function, male sexual function, ejaculation, orgasm
Ejaculatory dysfunction is one of the most common male sexual dysfunctions that is often mis-diagnosed or disregarded. At present, there is no definitive cure for ejaculatory dysfunctions (1). New research on the physiology of ejaculation keeps emerging to identify targets of treatment. However, knowledge about this topic is still lacking. In the present article, we summarize the current literature on the physiology of ejaculation. We describe the anatomy of the organs involved and the erection physiology. We discuss the physiology of orgasm and ejaculation as two separate physiological processes. In addition, we describe the neurochemical and hormonal regulation of the ejaculation process.
FUNCTIONAL ANATOMY OF THE MALE GENITAL ORGANS
The male genital system consists of external and internal reproductive and sexual organs such as the penis, prostate, epididymis, and testes. Figure 1 shows the gross anatomy of the ejaculatory structures. Table 1 provides a summary of the functional anatomy of these organs (2–5).
FIGURE 1.
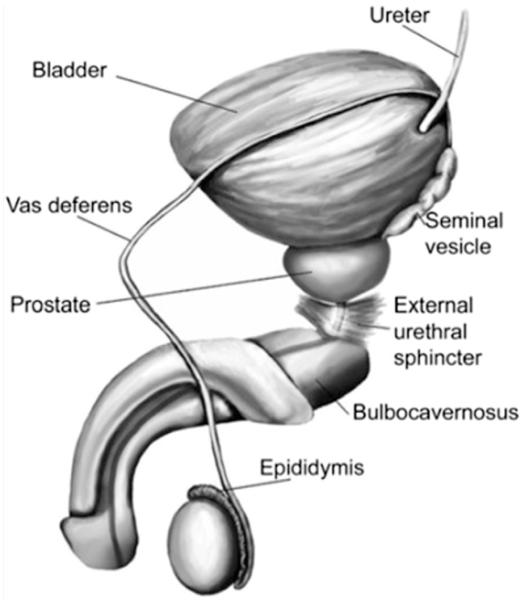
Gross anatomy of the ejaculation structures. (Reprinted with permission from Sheu G, Revenig LM, Hsiao W. Physiology of ejaculation. In: Mulhall JP, Hsiao W, eds. Men's sexual health and fertility: a clinician's guide. New York: Springer; 2014:15.)
TABLE 1.
Summary of the functional anatomy of the male genital organs.
| Organ | Characteristics |
|---|---|
| Penis | Composed of three chambers: paired corpora cavernosa (erectile bodies) and a midline ventral corpus spongiosum (contains urethra) |
| Main blood supply: internal pudendal artery | |
| Somatic sensation: pudendal nerve (S2-S4) | |
| Autonomic nerve fibers: cavernous nerves (pelvic plexus) contain both sympathetic (hypogastric plexus) and parasympathetic nerve fibers (S2-S4) | |
| Urethra | Four segments: prostatic urethra, membranous urethra (passes through the urogenital diaphragm), bulbar urethra, and penile urethra (ends with a small dilatation at the fossa navicularis near the meatus) |
| Cowper's glands: located on both sides of the membranous urethra and open in the bulbar urethra | |
| Veromontanum: small elevation of the posterior wall of the membranous urethra, related to ejaculatory ducts, prostatic utricle, and prostatic ducts | |
| Testis | It is divided by fibrous septa into many lobules containing seminiferous tubules |
| Leydig cells: main source of T production | |
| Seminiferous tubules: contain germ cells and sertoli cells. Forms the rete testis inside the testis mediastinum | |
| Rete testis: gives rise to 15–20 efferent ductules | |
| Epididymis | Posterior and superior to the testicle |
| Composed of head, body, and tail | |
| Efferent ductules unite to form the convoluted duct of the epididymis | |
| Becomes the vas deferens at the end of the tail | |
| Vas deferens | Muscular tube; typically 45 cm long and has a 2.5 mm diameter |
| It is a continuation of the epididymis | |
| Joins the seminal vesicle duct to form the ejaculatory duct, which then drains into the veromontanum | |
| Supplied by the vasal artery, a branch of the inferior vesical artery | |
| Prostate | Surrounds the prostatic urethra |
| Composed of 70% glandular component and 30% fibromuscular component | |
| Arterial supply: inferior vesical and middle rectal arteries | |
| Seminal vesicles |
Paired structures; located lateral to the vas deferens |
| Typically 5 cm long and 1 cm wide | |
| Joins the vas to form the ejaculatory duct | |
| Arterial supply: inferior vesical and middle rectal arteries |
PHYSIOLOGY OF ERECTION
The penile erection results from complex neurovascular mechanisms. Several central and peripheral neurological factors in addition to molecular, vascular, psychological and endocrino-logical factors are involved, and the balance between these factors is what eventually determines the functionality of the penis. In this section, we summarize some of those mechanisms.
Cerebral Control
Cerebrally controlled penile erections are induced through erotic visual stimuli or thoughts. The main cerebral structures involved in erection are contained within the medial preoptic area (MPOA) and paraventricular nucleus (PVN) in the hypothalamus (6). Dopamine is the most important brain neurotransmitter for erection, likely through its stimulation of oxytocin release (7). Another important neurotransmitter is norepinephrine, which is demonstrated through the erectogenic effect of the α-2 agonist (Yohimbine) (8). Several other brain neurotransmitters are involved in the erection process to varying degrees such as nitric oxide (NO), α-melanocyte stimulating hormone (α-MSH), and opioid peptides (9).
Autonomic Control
Parasympathetic stimulation is the main mediator for penile tumescence, although central suppression of the sympathetic nervous system also plays a role. Parasympathetic supply to the penis is derived from the sacral segments S2-S4 (10). However, patients with sacral spinal cord injury still maintain erections through psychogenic stimulation, although of less rigidity than normal. These psychogenic erections do not occur in patients with lesions above T9 (11), suggesting that the main mechanism for these erections is central suppression of sympathetic stimulation (12). Patients with lesions above T9 still may maintain reflexogenic erections. This implies that the main mechanism for reflexogenic tumescence is the preservation of the sacral reflex arc, which mediates erection through tactile penile stimulation (13, 14).
Molecular Mechanisms
The penis at baseline is in a flaccid state maintained by the contraction of corporal smooth muscles and constriction of cavernous and helicine arteries leading to moderate state of hypoxia with partial pressure of oxygen of 30–40 mm Hg (15). During sexual arousal, NO is released from cavernous nerve terminals through the action of neuronal NO synthase (16). The NO activates guanylate cyclase, which in turn converts guanosine triphosphate to cyclic guanosine monophosphate (15, 17), leading eventually to smooth muscle relaxation and vasodilation (18). Although the initiation of tumescence is through neuronal NO synthase, the maintenance of erection is through endothelial NO synthase (19). The eventual smooth muscle relaxation and vasodilation results in blood flowing into the paired corpora and filling of the sinusoids, with increased intracorporal pressure (to >100 mm Hg during full erection) and compression of the subtunical venules, markedly reducing the venous outflow (13).
PHYSIOLOGY OF ORGASM
There is no standard definition of orgasm. Each specialty such as endocrinology or psychology examines this activity from each one’s perspective, making it difficult to reach a consensus on the definition. Orgasm is generally associated with ejaculation, although the two processes are physiologically different (20). Certain physiological features are associated with orgasm, including hyperventilation up to 40 breaths/min, tachycardia, and high blood pressure (21). In fact, faster heart rate was found to be an indicator of “real” male orgasm during intravaginal intercourse, differentiating it from “fake” orgasm (22). Orgasm is also associated with powerful and highly pleasurable pelvic muscle contractions (especially ischiocavernosus and bulbocavernosus) (23), along with rectal sphincter contractions and facial grimacing (21). There is also an associated release and elevation in PRL and oxytocin levels after orgasm; however, the significance of this elevation is not entirely clear (24).
Studies using positron emission tomography, which measures changes in regional cerebral blood flow, have identified areas of activation in the brain during orgasm. Primary intense activation areas are noted to be in the mesodiencephalic transition zones, which includes the midline, the zona incerta, ventroposterior and intralaminar thalamic nuclei, the lateral segmental central field, the suprafascicular nucleus, and the ventral tegmental area. Strong increases were seen in the cerebellum. Decreases were noted at the entorhinal cortex and the amygdale (25).
Quality and intensity of orgasms are variable. For instance, short fast buildup of sexual stimulation toward orgasm is associated with less intense orgasms than slow buildup. Early orgasms are less satisfying than later orgasms in life as the person learns to accept the pleasure associated with orgasms. Lower levels of androgen are associated with weaker orgasms, such as in hypogonadism or in older age (20). It has been suggested that pelvic muscle exercises, particularly the bulbocavernosus and ischiocavernosus muscles, through contracting those muscles 60 times, 3 times daily for 6 weeks will enhance the pleasure associated with orgasm (20). However, the effort and time associated with such exercises prevent their utilization. The orgasm induced through deep prostatic massage is thought to be different from the orgasm associated direct penile stimulation. Although penile stimulation orgasms are associated with 4–8 pelvic muscle contractions, prostatic massage orgasms are associated with 12 contractions. Prostatic massage orgasms are thought to be more intense and diffuse than penile stimulation orgasms, but they require time and practice and are not liked by many men (20, 26, 27).
Following orgasm in men is a temporary period of inhibition of erection or ejaculation called the refractory period. This is a poorly understood phenomenon, with some investigators suggesting a central rather than spinal mechanism causing it (28). Elevated levels of PRL and serotonin after orgasm have been suggested as a potential cause; however, there is much debate about their exact role (29). More research is still needed in the area of male orgasm (20).
PHYSIOLOGY OF EJACULATION
Ejaculation is a physiological process heavily controlled by the autonomic nervous system. It consists of two main phases: emission and expulsion. The main organs involved in ejaculation are the distal epididymis, the vas deferens, the seminal vesicle, the prostate, the prostatic urethra, and the bladder neck (30).
Emission
The first step in the emission phase is the closure of bladder neck to prevent retrograde spillage of the seminal fluid into the bladder. This is followed by the ejection of prostatic secretions (10% of the final semen volume) containing acid phosphatase, citric acid, and zinc, mixed with spermatozoa from the vas deferens (10% of the volume) into the prostatic urethra. Subsequently, the fructose-containing seminal vesicle fluid alkalinizes the final ejaculatory fluid. The seminal vesicle fluid constitutes 75%–80% of the final seminal fluid. Cowper’s glands and periurethral glands produce a minority of the seminal fluid (1, 31). The organs involved in the ejaculation process receive dense autonomic nerve supply, both sympathetic and parasympathetic, from the pelvic plexus. The pelvic plexus is located retroperitoneally on either side of the rectum, lateral and posterior to the seminal vesicle (32). It receives neuronal input from the hypogastric and pelvic nerves in addition to the caudal paravertebral sympathetic chain (33). The sympathetic neurons play the predominant role in the ejaculation process. Their nerve terminals secrete primarily norepinephrine, although other neurotransmitters such as acetylcholine and nonadrenergic/noncholinergic also play important roles (34). The role of the hypogastric plexus in emission is best demonstrated clinically by the loss of emission after non-nerve sparing para-aortic lymph node dissection for testicular cancer (35), and induction of emission in paraplegic men through electrical stimulation of superior hypogastric plexus (35). Input from genital stimulation is integrated at the neural sacral spinal level to produce emission (36). The emission phase of ejaculation is also under a considerable cerebral control, and can be induced through physical or visual erotic stimulation (37).
Expulsion
Expulsion follows emission as the process of ejaculation climaxes, and refers to the ejection of semen through the urethral meatus. The semen is propelled through the rhythmic contractions of the pelvic striated muscles in addition to the bulbospongiosus and ischiocavernosus muscles (23). To achieve antegrade semen expulsion, the bladder neck remains closed, whereas the external urethral sphincter is open. The external sphincter and the pelvic musculature are under somatic control; however, there is no evidence that voluntary control plays a role in the expulsion process (30). The exact trigger for expulsion is unknown. It has been suggested that a spinal center is triggered during emission of seminal fluid into the prostatic urethra (38). However, there is mounting evidence through clinical and experimental studies to suggest that this is not the case. For instance, men can still have rhythmic contractions during orgasm despite “dry ejaculation,” for example, due to prostatectomy (23, 39, 40). This, in addition to the identification of spinal generator for ejaculation (SGE) in rats, led to the postulation that the process of expulsion is a continuum of the process initiated through emission, after reaching a certain spinal activation threshold (30, 41).
NEURONAL CONTROL OF EJACULATION
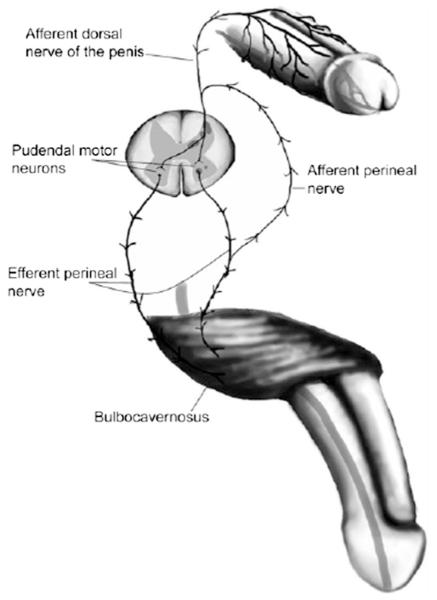
Ejaculation is heavily controlled by the nervous system. Figure 2 summarizes the reflex circuit necessary to elicit ejaculation.
FIGURE 2.
Reflex circuit needed to establish ejaculation. (Reprinted with permission from Sheu G, Revenig LM, Hsiao W. Physiology of ejaculation. In: Mulhall JP, Hsiao W, eds. Men's sexual health and fertility: a clinician's guide. New York: Springer; 2014:18.)
Peripheral Nervous System
Afferents
The main sensory input from the penis comes from the dorsal nerve of the penis, which transmits sensation from the glans, prepuce, and penile shaft. It transmits signals to the upper and lower segments of the sacral spinal cord (42). The glans contains encapsulated nerve endings, termed Krause-Finger corpuscles, whereas the remaining penile shaft contains free nerve endings. Stimulation of these corpuscles potentiated by stimulation from other genital areas, such the perineum, testes, and penile shaft, play an important role in the ejaculation process (43). A secondary afferent route is through the hypogastric nerve, which runs through the paravertebral sympathetic chain to enter the spinal cord through the thoracolumbar dorsal roots (44). The sensory afferents terminate in the medial dorsal horn and the dorsal gray commissure of the spinal cord (45).
Efferents
The efferent peripheral nervous system constitutes of sympathetic, parasympathetic, and motor nervous components (46). The soma of the preganglionic sympathetic cell bodies involved in ejaculation are located in the intermedio-lateral cell column and in the central autonomic region of the thoracolumbar segments (T12-L1) (47). The preganglionic sympathetic fibers emerge from the ventral roots of the spinal cord and travel through the paravertebral sympathetic chain to relay either directly through the splanchnic nerve, or through relaying first in the celiac superior mesenteric ganglia and then through the intermesenteric nerve, to the inferior mesenteric ganglia (48). The hypogastric nerve then emanates from the inferior mesenteric ganglia to join the parasympathetic pelvic nerve to form the pelvic plexus, which then sends fibers to the ejaculation structures (49). The preganglionic parasympathetic cell bodies are located in the sacral parasympathetic nucleus. The sacral parasympathetic nucleus neurons travel then in the pelvic nerve to the post-ganglionic parasympathetic cells located in the pelvic plexus. The motor neurons involved in ejaculation are located in Onuf’s nucleus in the sacral spinal cord, which projects fibers through the motor component of the pudendal nerve to reach the pelvic musculature, including the bulbospongiosus, ischiocavernosus, and external urethral sphincter (50).
Central Nervous System
Spinal network
The thoracolumbar sympathetic, sacral parasympathetic (mainly sacral parasympathetic nucleus), and somatic sacral Onuf’s nucleus ejaculatory spinal nuclei play an important role in the integration of peripheral and cerebral input and coordinating output to the pelviperineal structures involved in ejaculation (46). An additional spinal center is the SGE located in laminae X and VII of L3-L4 spinal segments (51). The SGE contains spinal interneurons called lumbar spinothalamic cells, which project fibers to the parvocellular subparafascicular nucleus of the thalamus in addition to preganglionic sympathetic and parasympathetic neurons innervating the pelvis (41). The SGE stimulation elicits a complete ejaculatory response resulting in collection of motile spermatozoa in anesthetized rats (52). Further research on the SGE spinal center is still needed, and it is unclear whether it contains other cells than lumbar spinothalamic cells.
Brain network
Sensory and motor areas in the brain play an important role in the ejaculation, which requires a highly coordinated and integrated central process. The study by Holstege et al. (25) using positron emission tomography showed that certain areas in the brain are activated in the orgasm and ejaculation process. Furthermore, specific areas in the brain have been involved in the ejaculation process, as demonstrated in animal immunohistochemical studies examining Fos protein pattern of expression (53–56), and confirmed using a serotonin 1A subtype receptor agonist proejaculatory pharmacologic agent in rats (57). These are discrete areas within the posteromedial bed nucleus of stria terminalis, the parvicellular part of the subparafascicular thalamus, the posterodorsal preoptic nucleus, and the posterodorsal medial amygdaloid nucleus. There are reciprocal connections that link those areas to the MPOA of the hypothalamus, a brain area with a well-established role in controlling sexual behavior as demonstrated by anatomical and functional studies (54, 55, 58). Electrical or chemical stimulation of the MPOA elicited ejaculation (59–62), whereas an MPOA lesion was shown to abolish both phases of ejaculation (63). No direct connections of MPOA to the spinal centers for ejaculation were found on neuroanatomical studies; however, there are projections of MPOA to other regions in the brain involved in ejaculation, such as PVN, the periaqueductal gray, and the paragigantocellular nucleus (nPGi) (64–66).
The PVN projects to pudendal motor neurons located in the L5-L6 spinal segment in addition to autonomic preganglionic neurons in the lumbosacral spinal cord in rats (45, 67, 68). It also projects to nPGI in the brainstem (69). Bilateral lesions of the PVN with N-methyl-D-aspartate (NMDA) results in a one-third reduction of the seminal ejaculate material weight (70). The parvicellular part of the subparafascicular thalamus was found to send projections to bed nucleus of stria terminalis, medial amygdala (MeA), and MPOA (71, 72) and receives input from lumbar spinothalamic cells (51). The precise role of these regions is still unclear but they are likely involved in relaying genital signals to MPOA (53, 55). The brainstem regions (nPGI and periaqueductal gray) have recently received increasing attention. The nPGI nucleus likely plays an inhibitory role in ejaculation as evidenced through the urethrogenital reflex experimental model, a rat model for the expulsion phase of ejaculation (73, 74). Using the same model, the periaqueductal gray was found to be important for the ejaculation process, likely by acting as a relay between MPOA and nPGI (75). Midbrain structures have a significant role in ejaculation; however, much is still unknown about their exact role and further research is needed. Figure 3 summarizes the putative brain structures involved in ejaculation.
FIGURE 3.
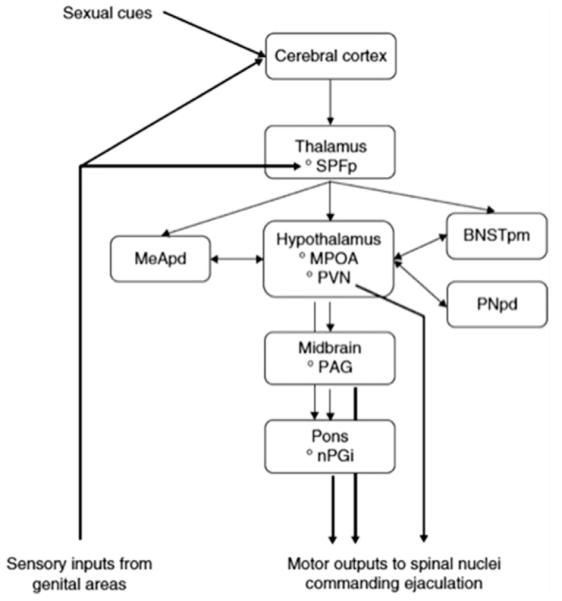
Putative brain structures involved in ejaculation. BNSTpm = posteromedial bed nucleus of stria terminalis; MeApd = posterodorsal medial amygdaloid nucleus; MPOA = medial preoptic area; PAG = periaqueductal gray; nPGi = paragigantocellular nucleus; PNpd = posterodorsal preoptic nucleus; PVN = paraventricular thalamic nucleus; SPFp = parvicellular part of the subparafascicular thalamus. (Reprinted with permission from Clement P, Giuliano F. Physiology of ejaculation. In: Mulhall JP, Incrocci L, Goldstein I, Rosen RC, eds. Cancer and sexual health. New York: Springer; 2011:82.)
NEUROCHEMICAL REGULATION OF EJACULATION
Many neurotransmitters are involved in the ejaculation process. Defining the exact role of these neurotransmitters is difficult given the variety of sexual parameters affected, the different sites of action within the spinal and the supraspinal pathways, and the presence of multiple receptor types. Some of the molecules that received special attention for their role in ejaculation are mentioned later.
Dopaminergic Control
Dopamine is known to be important for normal male sexual response (76, 77). Two families of dopamine receptors exist, D1-like (D1 and D5 receptors) and D2-like (D2, D3, and D4 receptors) (46). In rats, D2-like receptors are known to stimulate ejaculation (78, 79), and trigger ejaculation even in anesthetized rats (80, 81). Systemic injection of the D3 receptor agonist 7-OH-DPAT has been shown to trigger ejaculation in rats without affecting arousal (82, 83). It also triggers ejaculation in anesthetized rats when injected directly into the cerebral ventricles or MPOA with the effect being specifically reversed by the D3, not the D2 antagonist (84, 85). The D3 receptor blockage has been shown to inhibit the expulsion phase of ejaculation and lengthen ejaculation latency in rats (86).
Serotonergic Control
Evidence suggests that serotonin (5HT) inhibits ejaculation (87). Selective serotonin reuptake inhibitors increase 5HT tone resulting in impairment of ejaculation, which led to their clinical use in premature ejaculation. This inhibitory effect is likely to occur in the brain (88), as 5HT effect on ejaculation in the spine is likely stimulatory (89). The amphetamine derivative p-chloroamphetamine leads to a sudden release of 5HT in synaptic clefts triggering ejaculation in anesthetized rats with complete spinal cord lesion (89). Intrathecal serotonin or selective serotonin reuptake inhibitor injection leads to enhancement of the expulsion phase of ejaculation (88). There are 14 receptor subtypes for 5HT, with 1A, 1B, and 2C being the ones involved in ejaculation (90). It is difficult to designate one influence for each receptor subtype, as each receptor could either activate or inhibit ejaculation depending on its location within the central nervous system (46).
Nitric Oxide
The role of NO in ejaculation has received special attention after the introduction of type-5 phosphodiesterase (PDE5) inhibitors and using them for premature ejaculation. Nitric oxide has an inhibitory role on the ejaculation process (1). Centrally, intrathecal sildenafil results in elevation of NO and cyclic guanosine monophosphate levels in MPOA causing a decreased peripheral sympathetic tone and inhibition of ejaculation (91). N-Nitro-l-arginine methyl-ester injection, an NO synthase inhibitor, was shown to increase the number of seminal emissions and reduce latency to first seminal emission in rats (92). Peripherally, nitronergic innervation and NO synthase were found in the seminal vesicle, vas deferens, prostate, and urethra (93–97). Therefore, drugs such as PDE5 inhibitors or NO donors are associated with reduced seminal vesicle contraction and inhibit seminal emission (92). The administration of NO inhibitors, such as l-nitroarginine-methylester, diminishes human seminal vesicle contraction (98), inhibits vasal contraction in guinea pigs (99), and decreases latency to ejaculation in rats (100). Furthermore, reduced latency to emission was found in knockout mice for the gene encoding endothelial NO synthase compared with their wild-type counterparts (101).
HORMONAL REGULATION OF EJACULATION
Although male sexual function is heavily regulated by the hormonal system, there are few clinical studies performed to evaluate hormonal regulation of ejaculation, and the knowledge about hormonal effect on ejaculation is still lacking. We discuss some of the studies examining the effect of different hormones on ejaculation.
Oxytocin
Oxytocin is an oligopeptide synthesized in the supraoptic and PVN of the hypothalamus and released from the posterior pituitary gland. Oxytocin serum level increases after male ejaculation to levels ranging from 20%–360% of normal levels before reaching baseline at 10 minutes after ejaculation (102). Pharmacologic oxytocin administration in humans and animals results in increased ejaculated spermatozoa (103), confirming that oxytocin has a role in male genital tract motility. It was specifically found to augment powerful epididymal contractions and sperm motility (104), an important effect blunted by pretreatment with the oxytocin antagonist (des Gly–NH2d(CH2)5–[d-Tyr2,Thr4] ornithine vasotocin) (105). Peripheral oxytocin receptors were found to be highly expressed in the epididymis and tunica albuginea (in smooth muscles more than epithelial cells), and to a lesser extent in the vas deferens and seminal vesicle (104). Oxytocin has a synergistic action on the epididymis with endothelin-1, where they augment epididymal contraction and propel spermatozoa forward (102, 106). Injection of oxytocin into the cerebral ventricles in male rats facilitated ejaculation by shortening the ejaculation latency and postejaculatory refractory periods (107), whereas these effects were curbed using the oxytocin receptor antagonist (d(CH2)5–Tyr(Me)–[Orn8]vasotocin) injected into the cerebral ventricles (108). Despite these encouraging findings and some anecdotal evidence suggesting that intranasal oxytocin can facilitate orgasm in an anorgasmic male (109), a double-blind placebo-controlled clinical study (110) failed to demonstrate an effect of intranasal oxytocin on sexual behavior.
Prolactin
Hyperprolactinemia has a marked inhibitory effect on male sexual desire (111). A modest increase in serum PRL levels (15–20 ng/mL) has been detected in men after orgasm, and could be contributing to the after-orgasm refractory period (112). Some investigators have hypothesized that a low PRL level is a cause of premature ejaculation, where PRL levels were similarly low in those men with lifelong or acquired premature ejaculation (113). Further research is needed on this issue.
Thyroid Hormones
The relationship between thyroid hormonal abnormalities and ejaculatory dysfunction has been well documented (114–116). In rats, l-thyroxin administration has been shown to increase bulbospongiosus contractile activity and seminal vesicle contraction frequency (117). Clinically, the prevalence of suppressed TSH, which is a marker of hyperthyroidism, was found to be twofold higher in patients with premature ejaculation than in patients who reported normal ejaculatory timing (118). In the first prospective multicenter study (114) on the topic, half of hyperthyroidism patients had premature ejaculation, whereas only 15% reported this symptom after cure of their thyroid dysfunction. Another single-center prospective study by Cihan et al. (116) demonstrated a prevalence of 72% of premature ejaculation in hyperthyroidism, which was reduced after treatment. It also identified a positive correlation of TSH with intravaginal ejaculation latency time. Öztürk et al. (119) found similar results. However, Waldinger et al. (120) found no correlation between TSH and intravaginal ejaculation latency time in a cohort of Dutch men with lifelong premature ejaculation. A meta-analysis by Corona et al. (102) demonstrated a threefold increase of hyperthyroidism in patients with premature ejaculation compared with controls, a finding that was more pronounced in patients with acquired rather than lifelong premature ejaculation. They also showed an increase in intravaginal ejaculation latency time by 84.6 ± 34.2 seconds (P=.001) upon treatment of hyperthyroidism. These findings suggest that thyroid hormones do not only affect the ankle reflex, but also the ejaculatory reflex, and screening patients with ejaculatory dysfunction for thyroid hormone abnormalities is warranted (102).
Glucocorticoids
Cortisol (F) levels in several animal studies were found to be elevated during arousal and ejaculation (121–123). In horses and donkeys, F was elevated 30 minutes after ejaculation, with unknown significance of this finding (124, 125). In addition, F levels were sharply elevated after electroejaculation in several anesthetized animal studies (126, 127). In humans, however, there was no change in F levels whether during sexual stimulation or orgasm (128–131). Although hypercortisolism in men was associated with reduced libido, no effect was identified on orgasm or ejaculation (132). Replacement of F in Addison disease was associated with improvement in overall sexual function including orgasm (133). Data in humans are still too preliminary to draw final conclusions, and further research is needed.
Estrogens
Estradiol plays an important role in the regulation of the emission phase of ejaculation through the regulation of epididymal contractility, luminal fluid reabsorption, and sperm concentration (134, 135). This role in the epididymis is the reason for recommending Tamoxifen as a first-line treatment for idiopathic oligospermia by the World Health Organization (136). Finkelstein et al. (137) showed that E2 deficiency, along with androgen deficiency, contributes to decreased libido and erectile function.
Androgens
Testosterone, through its central and peripheral androgen receptors, has a well-known role on male sexual function, particularly on libido (138). Low T levels are associated with delayed ejaculation, whereas high levels were associated with premature ejaculation (102). This is likely because the emission phase of the ejaculation relies on the NO-PDE5 system, which is influenced by T (138, 139). Testosterone facilitates the control of the ejaculatory reflex through its androgen receptors in the MPOA and other areas in the central nervous system (140). Furthermore, pelvic floor muscles involved in ejaculation are androgen dependent (141). There are likely multiple mechanisms involved in T action and further research is needed to identify specific targets for treatment in the ejaculatory reflex. Table 2 summarizes the neurochemical and hormonal regulation of ejaculation.
TABLE 2.
Neurochemical and hormonal regulation of ejaculation.
| Neurotransmitter/hormone | Effect |
|---|---|
| Dopamine | Stimulates ejaculation through D2-like receptors (D2, D3, and D4 receptors, mainly D3) |
| Serotonin | Inhibits ejaculation in the brain and stimulates it in the spine through the receptors 5HT, with 1A, 1B, and 2C |
| Nitric oxide | Inhibits ejaculation through reduction of seminal vesicle contraction and seminal emission |
| Oxytocin | Synthesized in the supraoptic and PVN of the hypothalamus and released from the posterior pituitary gland |
| Augments powerful epididymal contractions and sperm motility | |
| Acts in the CNS to stimulate ejaculation | |
| Prolactin | Secreted from the pituitary gland |
| Hyperprolactinemia has a marked inhibitory effect on male sexual desire, through inhibition of GnRH (therefore T production) and dopamine production | |
| Thyroid hormones | Hypothyroidism and hyperthyroidism are associated with delayed and premature ejaculation, respectively |
| Glucocorticoids | Cortisol levels are elevated after ejaculation in animal studies |
| No change in cortisol levels in humans | |
| Replacement of cortisol in Addison disease improves sexual function including orgasm | |
| Estrogens | Regulates the emission phase of ejaculation through the regulation of epididymal contractility, luminal fluid reabsorption, and sperm concentration |
| Androgens | Low levels are associated with delayed ejaculation, whereas high levels are associated with premature ejaculation |
| Facilitates the control of the ejaculatory reflex through its androgen receptors in the MPOA and other areas in the CNS | |
| Pelvic floor muscles involved in ejaculation are androgen dependent |
Note: CNS = central nervous system; MPOA = medial preoptic area; PVN = paraventricular nucleus.
In conclusion, ejaculation is a complex process involving several anatomical structures and under extensive neurochemical and hormonal regulation. Orgasm, although associated with ejaculation, is a distinct physiological process, different from ejaculation. Many aspects of these physiological processes are still unknown and further research is needed to identify treatments for ejaculatory dysfunction.
Footnotes
A.A. has nothing to disclose. B.N.B. has nothing to disclose. T.F.L. has nothing to disclose.
REFERENCES
- 1.Sheu G, Revenig LM, Hsiao W. Physiology of ejaculation. In: Mulhall JP, Hsiao W, editors. Men's sexual health and fertility. Springer Science; New York: 2014. pp. 13–29. [Google Scholar]
- 2.Bella AJ, Shamloul R. Functional anatomy of the male sex organs. In: Mulhall JP, Incocci L, Goldstein I, Rosen R, editors. Cancer and sexual health. Springer Science; New York: 2011. pp. 3–12. [Google Scholar]
- 3.Meacham R, Lipshultz L, Howards S. Male infertility. In: Gillenwater JY, Grayhack JT, Howards S, Duckett JW, editors. Adult and pediatric urology. Mosby; St. Louis: 1996. pp. 1747–802. [Google Scholar]
- 4.Hinman F. Normal surgical anatomy. In: Thomas Thomas AJ, Nagler HN, editors. Atlas of surgical management of male infertility. William & Wilkins; New York: 1995. pp. 9–20. [Google Scholar]
- 5.Romanes G. The pelvis and perineum. In: Romanes G, Cunningham D, editors. Cunningham's manual of practical anatomy. 13th ed Oxford University Press; London, UK: 1975. pp. 199–240. [Google Scholar]
- 6.Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–54. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- 7.Danjou P, Lacomblez L, Warot D, Puech AJ. Assessment of erectogenic drugs by numeric plethysmography. J Pharmacol Methods. 1989;21:61–9. doi: 10.1016/0160-5402(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 8.Clark JT, Smith ER, Davidson JM. Testosterone is not required for the enhancement of sexual motivation by yohimbine. Physiol Behav. 1985;35:517–21. doi: 10.1016/0031-9384(85)90133-7. [DOI] [PubMed] [Google Scholar]
- 9.Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63:811–59. doi: 10.1124/pr.111.004515. [DOI] [PubMed] [Google Scholar]
- 10.Lue TF, Zeineh SJ, Schmidt RA, Tanagho EA. Neuroanatomy of penile erection: its relevance to iatrogenic impotence. J Urol. 1984;131:273–80. doi: 10.1016/s0022-5347(17)50344-4. [DOI] [PubMed] [Google Scholar]
- 11.Paick JS, Lee SW. The neural mechanism of apomorphine-induced erection: an experimental study by comparison with electrostimulation-induced erection in the rat model. J Urol. 1994;152(6 Pt 1):2125–8. doi: 10.1016/s0022-5347(17)32336-4. [DOI] [PubMed] [Google Scholar]
- 12.Chapelle PA, Durand J, Lacert P. Penile erection following complete spinal cord injury in man. Br J Urol. 1980;52:216–9. doi: 10.1111/j.1464-410x.1980.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 13.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–95. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtois FJ, Charvier KF, Leriche A, Raymond DP. Sexual function in spinal cord injury men. I. Assessing sexual capability. Paraplegia. 1993;31:771–84. doi: 10.1038/sc.1993.120. [DOI] [PubMed] [Google Scholar]
- 15.Sattar AA, Salpigidis G, Schulman CC, Wespes E. Relationship between intrapenile O2 lever and quantity of intracavernous smooth muscle fibers: current physiopathological concept. Acta Urol Belg. 1995;63:53–9. [PubMed] [Google Scholar]
- 16.Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2007;20:17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- 17.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–50. [PubMed] [Google Scholar]
- 18.Walsh MP. The Ayerst Award Lecture 1990. Calcium-dependent mechanisms of regulation of smooth muscle contraction. Biochem Cell Biol. 1991;69:771–800. doi: 10.1139/o91-119. [DOI] [PubMed] [Google Scholar]
- 19.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci. 2002;99:4061–6. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin R. Physiology of orgasm. In: Mulhall JP, Incocci L, Goldstein I, Rosen R, editors. Cancer and sexual health. Springer Science; New York: 2011. pp. 35–48. [Google Scholar]
- 21.Masters W, Johnson V. Human sexual response. Little Brown; Boston: 1966. [Google Scholar]
- 22.Levin R, editor. Heart rate responses can be used to differentiate simulated from real orgasms in the human male: a pilot study. Proceedings of the first conference on orgasm. VRP Publishers; Bombay: 1991. [Google Scholar]
- 23.Gerstenberg TC, Levin RJ, Wagner G. Erection and ejaculation in man—assessment of the electromyographic activity of the bulbocavernosus and ischiocavernosus muscles. Br J Urol. 1990;65:395–402. doi: 10.1111/j.1464-410x.1990.tb14764.x. [DOI] [PubMed] [Google Scholar]
- 24.Levin R. Is prolactin the biological ‘off switch’ for human sexual arousal? Sex Relat Ther. 2003;18:289–343. [Google Scholar]
- 25.Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AS. Brain activation during human ejaculation. J Neurosci. 2003;23:9185–93. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hite S. The Hite report on male sexuality. Ballantine Books; New York: 1981. [Google Scholar]
- 27.Perry JF. Do men have a G-spot? Aust Forum. 1988;2:37–41. [Google Scholar]
- 28.Levin R. Revisiting post-ejaculatory refractory time—what we know and what we do not know in males and females. J Sex Med. 2009;6:2376–89. doi: 10.1111/j.1743-6109.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- 29.Turley KR, Rowland DL. Evolving ideas about the male refractory period. BJU Int. 2013;112:442–52. doi: 10.1111/bju.12011. [DOI] [PubMed] [Google Scholar]
- 30.Giuliano F, Clement P. Physiology of ejaculation: emphasis on serotonergic control. Eur Urol. 2005;48:408–17. doi: 10.1016/j.eururo.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Master VA, Turek PJ. Ejaculatory physiology and dysfunction. Urol Clin North Am. 2001;28:363–75. doi: 10.1016/s0094-0143(05)70145-2. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol. 1987;16:46–60. doi: 10.1016/s0022-5347(17)43655-x. [DOI] [PubMed] [Google Scholar]
- 33.Keast JR. Pelvic ganglia. In: McLahlan EM, editor. Autonomic ganglia. Harwood Academic; Luxemberg: 1995. pp. 445–79. [Google Scholar]
- 34.Dail WG, Moll MA. Localization of vasoactive intestinal polypeptide in penile erectile tissue and in the major pelvic ganglion of the rat. Neuroscience. 1983;10:1379–86. doi: 10.1016/0306-4522(83)90119-7. [DOI] [PubMed] [Google Scholar]
- 35.Brindley GS, Sauerwein D, Hendry WF. Hypogastric plexus stimulators for obtaining semen from paraplegic men. Br J Urol. 1989;64:72–7. doi: 10.1111/j.1464-410x.1989.tb05526.x. [DOI] [PubMed] [Google Scholar]
- 36.Ver Voort SM. Ejaculatory stimulation in spinal-cord injured men. Urology. 1987;29:282–9. doi: 10.1016/0090-4295(87)90072-0. [DOI] [PubMed] [Google Scholar]
- 37.Comarr A. Sexual function among patients with spinal cord injury. Urol Int. 1970;25:134–68. doi: 10.1159/000279669. [DOI] [PubMed] [Google Scholar]
- 38.McKenna KE, Chung SK, McVary KT. A model for the study of sexual function in anesthetized male and female rats. Am J Physiol. 1991;261:R1276–85. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- 39.Bergman B, Nilsson S, Petersen I. The effect on erection and orgasm of cystectomy, prostatectomy and vesiculectomy for cancer of the bladder: a clinical and electromyographic study. Br J Urol. 1979;51:114–20. doi: 10.1111/j.1464-410x.1979.tb02843.x. [DOI] [PubMed] [Google Scholar]
- 40.Holmes GM, Sachs BD. The ejaculatory reflex in copulating rats: normal bulbospongiosus activity without apparent urethral stimulation. Neurosci Lett. 1991;125:195–7. doi: 10.1016/0304-3940(91)90026-p. [DOI] [PubMed] [Google Scholar]
- 41.Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–9. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- 42.Nunez R, Gross GH, Sachs BD. Origin and central projections of rat dorsal penile nerve: possible direct projection to autonomic and somatic neurons by primary afferents of nonmuscle origin. J Comp Neurol. 1986;247:417–29. doi: 10.1002/cne.902470402. [DOI] [PubMed] [Google Scholar]
- 43.Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res. 1986;371:205–30. doi: 10.1016/0006-8993(86)90357-4. [DOI] [PubMed] [Google Scholar]
- 44.Baron R, Janig W. Afferent and sympathetic neurons projecting into lumbar visceral nerves of the male rat. J Comp Neurol. 1991;314:429–36. doi: 10.1002/cne.903140302. [DOI] [PubMed] [Google Scholar]
- 45.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–49. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 46.Clement P, Giuliano F. Physiology of ejaculation. In: Mulhall JP, Incrocci L, Goldstein I, editors. Cancer and sexual health. Springer Science; New York: 2011. pp. 77–89. [Google Scholar]
- 47.Morgan C, de Groat WC, Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986;243:23–40. doi: 10.1002/cne.902430104. [DOI] [PubMed] [Google Scholar]
- 48.Owman C, Stjernquist M. The peripheral nervous system. In: Bjorklund A, Hokfelt T, Owman C, editors. Handbook of chemical neuroanatomy. Elsevier Science; Amsterdam, The Netherlands: 1988. pp. 445–544. [Google Scholar]
- 49.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984;226:238–45. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- 50.Schroder HD. Anatomical and pathoanatomical studies on the spinal efferent systems innervating pelvic structures. 1. Organization of spinal nuclei in animals. 2. The nucleus X-pelvic motor system in man. J Auton Nerv Syst. 1985;14:23–48. doi: 10.1016/0165-1838(85)90123-7. [DOI] [PubMed] [Google Scholar]
- 51.Coolen LM, Veening JG, Wells AB, Shipley MT. Afferent connections of the parvocellular subparafascicular thalamic nucleus in the rat: evidence for functional subdivisions. J Comp Neurol. 2003;463:132–56. doi: 10.1002/cne.10739. [DOI] [PubMed] [Google Scholar]
- 52.Borgdorff AJ, Bernabé J, Denys P, Alexandre L, Giuliano F. Ejaculation elicited by microstimulation of lumbar spinothalamic neurons. Eur Urol. 2008;54:449–56. doi: 10.1016/j.eururo.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 53.Hamson DK, Watson NV. Regional brainstem expression of Fos associated with sexual behavior in male rats. Brain Res. 2004;1006:233–40. doi: 10.1016/j.brainres.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 54.Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–58. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- 55.Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397:421–35. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 56.Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 57.Borgdorff AJ, Bernabé J, Denys P, Alexandre L, Giuliano F. Demonstration of ejaculation-induced neural activity in the male rat brain using 5-HT1A agonist 8-OH-DPAT. Physiol Behav. 1997;62:881–91. doi: 10.1016/s0031-9384(97)00258-8. [DOI] [PubMed] [Google Scholar]
- 58.Meisel R, Sachs B. The physiology of male sexual behavior. In: Knobil E, Neill J, editors. The physiology of reproduction. Raven; New York: 1994. pp. 3–105. [Google Scholar]
- 59.Pehek EA, Thompson JT, Hull EM. The effects of intracranial administration of the dopamine agonist apomorphine on penile reflexes and seminal emission in the rat. Brain Res. 1989;500:325–32. doi: 10.1016/0006-8993(89)90328-4. [DOI] [PubMed] [Google Scholar]
- 60.Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA, Loucks JA. Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: implications for copulation. Life Sci. 1992;51:1705–13. doi: 10.1016/0024-3205(92)90299-5. [DOI] [PubMed] [Google Scholar]
- 61.Marson L, McKenna KE. Stimulation of the hypothalamus initiates the urethrogenital reflex in male rats. Brain Res. 1994;638:103–8. doi: 10.1016/0006-8993(94)90638-6. [DOI] [PubMed] [Google Scholar]
- 62.Larsson K, van Dis H. Seminal discharge following intracranial electrical stimulation. Brain Res. 1970;23:381–6. doi: 10.1016/0006-8993(70)90064-8. [DOI] [PubMed] [Google Scholar]
- 63.Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–54. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 64.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–42. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 65.Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: A WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- 66.Murphy AZ, Rizvi TA, Ennis M, Shipley MT. The organization of preoptic medullary circuits in the male rat: evidence for interconnectivity of neural structures involved in reproductive behavior, antinociception and cardiovascular regulation. Neuroscience. 1999;91:1103–16. doi: 10.1016/s0306-4522(98)00677-0. [DOI] [PubMed] [Google Scholar]
- 67.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–12. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 68.Luiten PG, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–8. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 69.Bancila M, Verge D, Rampin O, Backstrom JR, Sanders-Bush E, McKenna KE, et al. 5-Hydroxytryptamine2C receptors on spinal neurons controlling penile erection in the rat. Neuroscience. 1999;92:1523–37. doi: 10.1016/s0306-4522(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 70.Ackerman AE, Lange GM, Clemens LG. Effects of paraventricular lesions on sex behavior and seminal emission in male rats. Physiol Behav. 1997;63:49–53. doi: 10.1016/s0031-9384(97)00386-7. [DOI] [PubMed] [Google Scholar]
- 71.Yasui Y, Saper CB, Cechetto DF. Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol. 1991;308:293–310. doi: 10.1002/cne.903080212. [DOI] [PubMed] [Google Scholar]
- 72.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–45. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 73.Marson L, McKenna KE. A role for 5-hydroxytryptamine in descending inhibition of spinal sexual reflexes. Exp Brain Res. 1990;88:313–20. doi: 10.1007/BF02259106. [DOI] [PubMed] [Google Scholar]
- 74.Marson L, McKenna KE. The identification of a brainstem site controlling spinal sexual reflexes in male rats. Brain Res. 1990;515:303–8. doi: 10.1016/0006-8993(90)90611-e. [DOI] [PubMed] [Google Scholar]
- 75.Marson L. Lesions of the periaqueductal gray block the medial preoptic area-induced activation of the urethrogenital reflex in male rats. Neurosci Lett. 2004;367:278–82. doi: 10.1016/j.neulet.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 76.Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 77.Peeters M, Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci Biobehav Rev. 2007;32:438–53. doi: 10.1016/j.neubiorev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Ferrari F, Giuliani D. The selective D2 dopamine receptor antagonist eticlopride counteracts the ejaculatio praecox induced by the selective D2 dopamine agonist SND 919 in the rat. Life Sci. 1994;55:1155–62. doi: 10.1016/0024-3205(94)00244-4. [DOI] [PubMed] [Google Scholar]
- 79.Ferrari F, Giuliani D. Sexual attraction and copulation in male rats: effects of the dopamine agonist SND 919. Pharmacol Biochem Behav. 1995;50:29–34. doi: 10.1016/0091-3057(94)00226-9. [DOI] [PubMed] [Google Scholar]
- 80.Clément P, Bernabé J, Kia HK, Alexandre L, Giuliano F. D2-like receptors mediate the expulsion phase of ejaculation elicited by 8-hydroxy-2-(di-N-propylamino) tetralin in rats. J Pharmacol Exp Ther. 2006;316:830–4. doi: 10.1124/jpet.105.092411. [DOI] [PubMed] [Google Scholar]
- 81.Stafford SA, Coote JH. Activation of D2-like receptors induces sympathetic climactic-like responses in male and female anaesthetised rats. Br J Pharmacol. 2006;148:510–6. doi: 10.1038/sj.bjp.0706763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrari F, Giuliani D. Behavioral effects induced by the dopamine D3 agonist 7-OH-DPAT in sexually-active and -inactive male rats. Neuropharmacology. 1996;35:279–84. doi: 10.1016/0028-3908(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 83.Ahlenius S, Larsson K. Effects of the dopamine D3 receptor ligand 7-OH-DPAT on male rat ejaculatory behavior. Pharmacol Biochem Behav. 1995;51:545–7. doi: 10.1016/0091-3057(94)00390-5. [DOI] [PubMed] [Google Scholar]
- 84.Clement P, Bernabe J, Denys P, Alexandre L, Giuliano F. Ejaculation induced by i.c.v. injection of the preferential dopamine D(3) receptor agonist 7-hydroxy-2-(di-N-propylamino)tetralin in anesthetized rats. Neuroscience. 2007;145:605–10. doi: 10.1016/j.neuroscience.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Kitrey ND, Clément P, Bernabé J, Alexandre L, Giuliano F. Microinjection of the preferential dopamine receptor D3 agonist 7-OH-DPAT into the hypothalamic medial preoptic area induced ejaculation in anesthetized rats. Neuroscience. 2007;149:636–41. doi: 10.1016/j.neuroscience.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 86.Clément P, Pozzato C, Heidbreder C, Alexandre L, Giuliano F, Melotto S. Delay of ejaculation induced by SB-277011, a selective dopamine D3 receptor antagonist, in the rat. J Sex Med. 2009;6:98–108. doi: 10.1111/j.1743-6109.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- 87.Giuliano F. 5-hydroxytryptamine in premature ejaculation: opportunities for therapeutic intervention. Trends Neurosci. 2007;30:79–84. doi: 10.1016/j.tins.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Clément P, Bernabé J, Gengo P, Denys P, Laurin M, Alexandre L, et al. Supraspinal site of action for the inhibition of ejaculatory reflex by dapoxetine. Eur Urol. 2007;51:825–32. doi: 10.1016/j.eururo.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Stafford SA, Bowery NG, Tang K, Coote JH. Activation by p-chloroamphetamine of the spinal ejaculatory pattern generator in anaesthetized male rats. Neuroscience. 2006;140:1031–40. doi: 10.1016/j.neuroscience.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 90.Giuliano F, Clement P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol. 2006;50:454–66. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 91.Sato Y, Zhao W, Christ GJ. Central modulation of the NO/cGMP pathway affects the MPOAinduced intracavernous pressure response. Am J Physiol Regul Integr Com Physiol. 2001;281:R269–78. doi: 10.1152/ajpregu.2001.281.1.R269. [DOI] [PubMed] [Google Scholar]
- 92.Hull EM, Lumley LA, Matuszewich L, Dominguez J, Moses J, Lorrain DS. The roles of nitric oxide in sexual function of male rats. Neuropharmacology. 1994;33:1499–504. doi: 10.1016/0028-3908(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 93.Dixon JS, Jen PY. Development of nerves containing nitric oxide synthase in the human male urogenital organs. Br J Urol. 1995;76:719–25. doi: 10.1111/j.1464-410x.1995.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 94.Hedlund P, Ekström P, Larsson B, Alm P, Andersson KE. Heme oxygen-ase and NO-synthase in the human prostate—relation to adrenergic, cholinergic and peptide-containing nerves. J Auton Nerv Syst. 1997;63:115–26. doi: 10.1016/s0165-1838(96)00139-7. [DOI] [PubMed] [Google Scholar]
- 95.Jen PY, Dixon JS, Gosling JA. Co-localization of nitric oxide synthase, neuropeptides and tyrosine hydroxylase in nerves supplying the human postnatal vas deferens and seminal vesicle. Br J Urol. 1997;80:291–9. doi: 10.1046/j.1464-410x.1997.00219.x. [DOI] [PubMed] [Google Scholar]
- 96.Kaminski HJ, Andrade FH. Nitric oxide: biologic effects on muscle and role in muscle diseases. Neuromuscul Disord. 2001;11:517–24. doi: 10.1016/s0960-8966(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 97.Ückert S, Bazrafshan S, Scheller F, Mayer ME, Jonas U, Stief CG. Functional responses of isolated human seminal vesicle tissue to selective phosphodiesterase inhibitors. Urology. 2007;70:185–9. doi: 10.1016/j.urology.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 98.Bultmann R, Klebroff W, Starke K. Nucleotide-evoked relaxation of rat vas deferens: possible mechanisms. Eur J Pharmacol. 2002;436:135–43. doi: 10.1016/s0014-2999(01)01620-x. [DOI] [PubMed] [Google Scholar]
- 99.Kato K, Furuya K, Tsutsui I, Ozaki T, Yamagishi S. Cyclic AMP-mediated inhibition of noradrenaline-induced contraction and Ca2+ in flux in guinea-pig vas deferens. Exp Physiol. 2000;85:387–98. [PubMed] [Google Scholar]
- 100.Bialy M, Beck J, Abramczyk P, Trzebskj A, Przybylski J. Sexual behavior in male rats after nitric oxide synthesis inhibition. Physiol Behav. 1996;60:139–43. doi: 10.1016/0031-9384(95)02272-4. [DOI] [PubMed] [Google Scholar]
- 101.Kriegsfeld LJ, Demas GE, Huang PL, Burnett AL, Nelson RJ. Ejaculatory abnormalities in mice lacking the gene for endothelial nitric oxide synthase (eNOS) Physiol Behav. 1999;67:561–6. doi: 10.1016/s0031-9384(99)00100-6. [DOI] [PubMed] [Google Scholar]
- 102.Corona G, Jannini EA, Vignozzi L, Rastrelli G, Maggi M. The hormonal control of ejaculation. Nat Rev Urol. 2012;9:508–19. doi: 10.1038/nrurol.2012.147. [DOI] [PubMed] [Google Scholar]
- 103.Maggi M, Kassis S, Malozowski S. Identification and characterization of a vasopressin isoreceptor in porcine seminal vesicles. Proc Natl Acad Sci. 1986;83:8824–8. doi: 10.1073/pnas.83.23.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Filippi S, Vannelli GB, Granchi S. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol Cell Endocrinol. 2002;193:89–100. doi: 10.1016/s0303-7207(02)00101-6. [DOI] [PubMed] [Google Scholar]
- 105.Nicholson HD, Parkinson TJ, Lapwood KR. Effects of oxytocin and vasopressin on sperm transport from the cauda epididymis in sheep. J Reprod Fertil. 1999;117:299–305. doi: 10.1530/jrf.0.1170299. [DOI] [PubMed] [Google Scholar]
- 106.Einspanier A, Ivell R. Oxytocin and oxytocin receptor expression in reproductive tissues of the male marmoset monkey. Biol Reprod. 1997;56:416–22. doi: 10.1095/biolreprod56.2.416. [DOI] [PubMed] [Google Scholar]
- 107.Arletti R, Bazzani C, Castelli M. Oxytocin improves male copulatory performance in rats. Horm Bev. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- 108.Argiolas A, Collu M, d'Aquila P, Gessa GL, Melis MR, Serra G. Apomorphine stimulation of male copulatory behavior is prevented by the oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin in rats. Pharmacol Biochem Behav. 1988;33:81–3. doi: 10.1016/0091-3057(89)90433-4. [DOI] [PubMed] [Google Scholar]
- 109.Ishak WW, Berman DS, Peters A. Male anorgasmia treated with oxytocin. J Sex Med. 2008;5:1022–4. doi: 10.1111/j.1743-6109.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 110.Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intra-nasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Buvat J. Hyperprolactinemia and sexual function in men: a short review. Int J Impot Res. 2003;15:373–7. doi: 10.1038/sj.ijir.3901043. [DOI] [PubMed] [Google Scholar]
- 112.Exton MS, Krüger TH, Koch M, Paulson E, Knapp W, Hartmann U, et al. Coitus-induced orgasm stimulates prolactin secretion in healthy subjects. Psychoneuroendocrinology. 2001;26:31–44. doi: 10.1016/s0306-4530(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 113.Corona G, Mannucci E, Jannini EA, Lotti F, Ricca V, Monami M, et al. Hypoprolactinemia: a new clinical syndrome in patients with sexual dysfunction. J Sex Med. 2009;6:1457–66. doi: 10.1111/j.1743-6109.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 114.Carani C, Isidori AM, Granata A, Carosa E, Maggi M, Lenzi A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab. 2005;90:6472–9. doi: 10.1210/jc.2005-1135. [DOI] [PubMed] [Google Scholar]
- 115.Corona G, Mannucci E, Petrone L, Fisher AD, Balercia G, Scisciolo G, et al. Psychobiological correlates of delayed ejaculation in male patients with sexual dysfunctions. J Androl. 2006;27:453–8. doi: 10.2164/jandrol.05154. [DOI] [PubMed] [Google Scholar]
- 116.Cihan A, Demir O, Demir T, Aslan G, Comlekci A, Esen A. The relationship between premature ejaculation and hyperthyroidism. J Urol. 2009;181:1273–80. doi: 10.1016/j.juro.2008.10.150. [DOI] [PubMed] [Google Scholar]
- 117.Cihan A, Demir O, Demir T, Aslan G, Comlekci A, Esen A. Investigation of the neural target level of hyperthyroidism in premature ejaculation in a rat model of pharmacologically induced ejaculation. J Sex Med. 2011;8:90–6. doi: 10.1111/j.1743-6109.2010.02042.x. [DOI] [PubMed] [Google Scholar]
- 118.Corona G, Ricca V, Bandini E, Rastrelli G, Casale H, Jannini E, et al. SIEDY Scale 3, a new instrument to detect psychological component in subjects with erectile dysfunction. J Sex Med. 2012;9:2017–26. doi: 10.1111/j.1743-6109.2012.02762.x. [DOI] [PubMed] [Google Scholar]
- 119.Öztürk MI, Koca O, Tüken M, Keleş MO, Ilktac A, Karaman MI. Hormonal evaluation in premature ejaculation. Urol Int. 2011;88:454–8. doi: 10.1159/000336137. [DOI] [PubMed] [Google Scholar]
- 120.Waldinger MD, Zwinderman AH, Olivier B, Schweitzer DH. Thyroid-stimulating hormone assessments in a Dutch cohort of 620 men with lifelong premature ejaculation without erectile dysfunction. J Sex Med. 2005;2:865–70. doi: 10.1111/j.1743-6109.2005.00142.x. [DOI] [PubMed] [Google Scholar]
- 121.Rabb MH, Thompson DL, Barry BE, Colborn DR, Garza F, Hehnke KE. Effects of sexual stimulation, with and without ejaculation, on serum concentrations of, LH, FSH, testosterone, cortisol and prolactin in stallions. J Anim Sci. 1989;67:2724–9. doi: 10.2527/jas1989.67102724x. [DOI] [PubMed] [Google Scholar]
- 122.Borg KE, Esbenshade KL, Johnson BH. Cortisol, growth hormone, and testosterone concentrations during mating behavior in the bull and boar. J Anim Sci. 1991;69:3230–40. doi: 10.2527/1991.6983230x. [DOI] [PubMed] [Google Scholar]
- 123.Bishop JD, Malven PV, Singleton WL, Weesner GD. Hormonal and behavioural correlates of emotional states in sexually trained boars. J Anim Sci. 1999;77:3339–45. doi: 10.2527/1999.77123339x. [DOI] [PubMed] [Google Scholar]
- 124.Veronesi MC, Tosi U, Villani M, Govoni N, Faustini M, Kindahl H, et al. Oxytocin, vasopressin, prostaglandin F(2α), luteinizing hormone, testosterone, estrone sulfate, and cortisol plasma concentrations after sexual stimulation in stallions. Theriogenology. 2010;73:460–7. doi: 10.1016/j.theriogenology.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 125.Veronesi MC, de Amicis I, Panzani S, Kindahl H, Govoni N, Probo M, et al. PGF(2α), LH, testosterone, oestrone sulphate, and cortisol plasma concentrations around sexual stimulation in jackass. Theriogenology. 2011;75:1489–98. doi: 10.1016/j.theriogenology.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 126.Wildt DE, Phillips LG, Simmons LG, Chakraborty PK, Brown JL, Howard JG, et al. A comparative analysis of ejaculate and hormonal characteristics of the captive male cheetah, tiger, leopard, and puma. Biol Reprod. 1988;38:245–55. doi: 10.1095/biolreprod38.2.245. [DOI] [PubMed] [Google Scholar]
- 127.Brown JL, Wildt DE, Phillips LG, Seidensticker J, Fernando SB, Miththapala S, et al. Adrenal–pituitary–gonadal relationships and ejaculate characteristics in captive leopards (Panthera pardus kotiya) isolated on the island of Sri Lanka. J Reprod Fertil. 1989;85:605–13. doi: 10.1530/jrf.0.0850605. [DOI] [PubMed] [Google Scholar]
- 128.Carani C, Bancroft J, Del Rio G, Granata ARM, Facchinetti F, Marrama P. The endocrine effects of visual erotic stimuli in normal men. Psychoneuroendocrinology. 1990;15:207–16. doi: 10.1016/0306-4530(90)90031-4. [DOI] [PubMed] [Google Scholar]
- 129.Krüger T, Exton MS, Pawlak C, von zur Mühlen A, Hartmann U, Schedlowski M. Neuroendocrine and cardiovascular response to sexual arousal and orgasm in men. Psychoneuroendocrinology. 1998;23:401–11. doi: 10.1016/s0306-4530(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 130.Exton NG, Truong TC, Exton MS, Wingenfeld SA, Leygraf N, Saller B, et al. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology. 2000;25:187–99. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 131.Ismail AA, Davidson DW, Loraine JA. Relationship between plasma cortisol and human sexual activity. Nature. 1972;237:288–9. doi: 10.1038/237288a0. [DOI] [PubMed] [Google Scholar]
- 132.Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, et al. The European Registry on Cushing's syndrome: 2-year experience. Baseline demographic and clinical characteristics. J Endocrinol. 2011;165:383–92. doi: 10.1530/EJE-11-0272. [DOI] [PubMed] [Google Scholar]
- 133.Granata A, Tirabassi G, Pugni V, Arnaldi G, Boscaro M, Carani C, et al. Sexual dysfunctions in men affected by autoimmune addison's disease before and after short-term gluco- and mineralocorticoid replacement therapy. J Sex Med. 2013;10:2036–43. doi: 10.1111/j.1743-6109.2012.02673.x. [DOI] [PubMed] [Google Scholar]
- 134.Vignozzi L, Filippi S, Morelli A, Luconi M, Jannini E, Forti G, et al. Regulation of epididymal contractility during semen emission, the first part of the ejaculatory process: a role for estrogen. J Sex Med. 2008;5:2480. doi: 10.1111/j.1743-6109.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- 135.Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52–66. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rowe P, Comhaire F. WHO manual for the standardized investigation, diagnosis and management of the infertile male. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- 137.Finkelstein JS, Lee H, Burnett-Bowie SAM, Pallais JC, Yu EW, Borges LF, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–22. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Corona G, Maggi M. The role of testosterone in erectile dysfunction. Nat Rev Urol. 2010;7:46–56. doi: 10.1038/nrurol.2009.235. [DOI] [PubMed] [Google Scholar]
- 139.Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, Marini M, et al. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145:2253–63. doi: 10.1210/en.2003-1699. [DOI] [PubMed] [Google Scholar]
- 140.Swaab DF. Sexual differentiation of the brain and behavior. Best Pract Res Clin Endocrinol Metab. 2007;21:431–44. doi: 10.1016/j.beem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 141.Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154:502–21. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]