Abstract
Impulsive sexual decision-making may underlie sexual risk-taking behavior that contributes to the disproportionately high prevalence of HIV infection among cocaine users. Delay-discounting procedures measure impulsive decision-making and may provide insight into the underlying mechanisms of sexual risk-taking behavior. The anxiolytic drug buspirone reduces delay discounting in rats and blunts the reinforcing effects of cocaine in some preclinical studies suggesting that it might have utility in the treatment of cocaine-use disorders. This study determined whether buspirone mitigates impulsive risky sexual decision-making in cocaine users on a sexual delay-discounting procedure. The effects of buspirone maintenance on the abuse-related and physiological effects of cocaine were also tested. Nine (N = 9) current cocaine users completed a repeated-measures, inpatient protocol in which sexual delay discounting was assessed following three days of maintenance on placebo and buspirone (30 mg/day) in counterbalanced order. The reinforcing, subject-rated, and physiological effects of placebo and intranasal cocaine (15 and 45 mg) were also assessed during buspirone and placebo maintenance. Buspirone increased the likelihood of condom use for hypothetical sexual partners that were categorized as most likely to have a sexually transmitted infection and least sexually desirable. Cocaine functioned as a reinforcer and increased positive subjective effects ratings, but buspirone maintenance did not impact these effects of cocaine. Buspirone was also safe and tolerable when combined with cocaine and may have blunted some its cardiovascular effects. The results from the sexual delay-discounting procedure indicate that buspirone may reduce preference for riskier sex in cocaine users.
Keywords: cocaine, buspirone, HIV risk, delay discounting, self-administration
Introduction
Cocaine-use disorders are closely linked with the transmission of HIV/AIDS and other sexually transmitted infections (STIs). Although injection drug use is a direct route of HIV transmission, cocaine users without a history of injection drug use are still at increased risk for HIV infection through risky sexual practices. Recent epidemiological data indicate that, after controlling for any history of injection drug use, the lifetime prevalence of HIV is more than 20-fold higher among current cocaine users relative to individuals not reporting current use (United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2014). The effective treatment of cocaine-use disorders has been proposed as one approach to mitigate HIV risk (e.g., Metzger, Navaline, & Woody, 1998). Pharmacological interventions have generally demonstrated limited efficacy at reducing cocaine use (Haile, Mahoney, Newton, & De La Garza, 2012; Montoya & Vocci, 2008; Penberthy, Ait-Daoud, Vaughan, & Fanning, 2010), but might improve other treatment outcomes in cocaine-dependent individuals (e.g., Kalechstein, Mahoney, Yoon, Bennett, & De La Garza, 2013). Whether sexual HIV-risk behaviors are amenable to pharmacological manipulation is unknown.
Buspirone (Buspar®) is an anxiolytic medication with low abuse potential (Balster, 1990; Troisi, Critchfield, & Griffiths, 1993) that may beneficially affect cognitive-behavioral processes, specifically impulsivity, associated with cocaine-use disorders. Impulsivity is a complex, multidimensional construct that broadly reflects a tendency to engage in behavior without sufficient planning or adequate consideration of potential risks and consequences (Whiteside & Lynam, 2001). Cocaine users have high trait impulsivity on personality questionnaires (Vonmoos et al., 2013) and often display deficits in inhibitory control on behavioral tasks (e.g., Fillmore & Rush, 2002, 2006). Cocaine use is also associated with impulsive sexual risk-taking behavior that likely contributes to the high prevalence of HIV/AIDS and other STIs among cocaine users (Cavazos-Rehg et al., 2009; Harzke, Williams, & Bowen, 2009; Khan et al., 2013; Nydeggar, Ames, Stacy, & Grenard, 2014). Dopamine (DA) and serotonin (5-HT) neurotransmitter systems are primary neuropharmacological targets of cocaine (e.g., Filip, Frankowska, Zaniewska, Golda, & Przegalinkski, 2005; Kuhar, Ritz, & Boja, 1991), and disruptions to these systems contribute to impulsive behavior (reviewed in Dalley & Roiser, 2012). Buspirone functions as a partial agonist at 5-HT1A receptors (Loane & Politis, 2012) and as an antagonist at DA D2-like receptors (e.g., Dhavalshankh et al., 2007). These receptor subtypes are implicated in cocaine use and impulsive decision-making. This overlapping neuropharmacology suggests that buspirone might reduce drug taking and impulsive behaviors in cocaine users.
Delay-discounting procedures provide insight into impulsive decision-making processes that underlie problematic behaviors like drug addiction (Raineri & Rachlin, 1993; Reynolds, 2006). Johnson and Bruner (2012) recently adapted delay-discounting procedures to assess risky sexual decision-making with hypothetical sexual partners classified based on sexual desirability and perceived STI risk. In this task, participants indicate whether they would be more likely to have unprotected sexual intercourse immediately or wait to have protected sex after some systematically varied delay. Responding that is biased in favor of immediate unprotected sex is conceptualized as more impulsive and risky. In that study, cocaine users reliably indicated that they would be less willing to wait to use a condom with hypothetical sexual partners who were perceived as most sexually desirable and least likely to have a STI. Subsequent research has shown that individuals with cocaine-use disorders discount delayed condom use more than matched controls (Johnson, Johnson, Herrmann, & Sweeny, 2015) and discounting of condom-protected sex correlates with self-reported HIV-risk behavior in the natural environment (e.g., Herrmann, Johnson, & Johnson, 2015; Johnson & Bruner, 2012). Chronic buspirone decreases delay discounting for food in rodents (Liu, Wilkinson, & Robbins, 2004), but there do not appear to be any studies that have determined the effects of buspirone on delay discounting of sexual rewards or other commodities in humans.
Buspirone may also be effective for managing cocaine-use disorders because DA and 5-HT are critically involved in the reinforcing effects of cocaine (e.g., Achat-Mendes et al., 2010; Carey et al., 2005; Heidbreder & Newman, 2010; Le Foll et al., 2014; Müller, Carey, Huston, & De Souza Silva, 2007; Spealman, Bergman, Madras, Kamien, & Melia, 1992). The results of preclinical studies that have determined the impact of buspirone on cocaine self-administration are mixed. In early research, acute administration of buspirone increased cocaine self-administration under a fixed-ratio schedule of reinforcement (Gold & Balster, 1992), as well as response rates under a second-order schedule of cocaine reinforcement in non-human primates (Nader & Barrett, 1990). Repeated acute buspirone treatment did not alter cocaine intake in rhesus monkeys using similar procedures (Gold & Balster, 1992; John, Banala, Newman, & Nader, 2014). By contrast, both acute (0.1 and 0.3 mg/kg im) and chronic (0.32 and 0.56 mg/kg/h iv) buspirone attenuated the reinforcing effects of cocaine in recent non-human primate studies (Bergman et al., 2013; Mello, Fivel, Kohut, & Bergman, 2013). The ability of buspirone to alter cocaine self-administration in humans has yet to be evaluated.
The current study determined the influence of buspirone maintenance on discounting of delayed condom-use under placebo or buspirone maintenance, but prior to acute cocaine administration, with the Sexual Discounting Task (Johnson & Bruner, 2012, 2013). In addition, this study determined the effect of buspirone maintenance on the reinforcing, subject-rated, and physiological effects of intranasal cocaine in current cocaine-users. Buspirone maintenance was hypothesized to decrease delay discounting on the Sexual Discounting Task and reduce the abuse-related behavioral effects of cocaine relative to placebo maintenance.
Method
Participants
Nine (N = 9) non-treatment seeking adult cocaine users participated in this within-subject, double-blind, placebo-controlled, complete-crossover design study. Participants met diagnostic criteria for cocaine abuse or dependence according to a computerized version of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV (American Psychiatric Association, 2000) and recent cocaine use was verified by provision of a benzoylecgonine-positive urine specimen during initial screening. All participants reported using cocaine via the smoked and intranasal routes. One participant also reported intravenous cocaine use. Two participants also met diagnostic criteria for alcohol abuse but agreed to discontinue their alcohol use during participation. Three participants reported drinking 4 or fewer drinks per week, three reported drinking 11–15 drinks per week, and three reported drinking 20–33 drinks per week. All nine participants reported daily tobacco use (i.e., smoking cigarettes or cigarillos) and all but one participant had very low to medium levels of nicotine dependence according to the Fagerström Test for Nicotine Dependence (see Table 1; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). All participants reported using cannabis in their lifetime and all but one participant reported using cannabis in the past month; two reported using cannabis ≤ 5 days, three reported using 12–15 days, and three had used ≥ 25 days in the past month. Participants had experience with a variety of other drugs but did not meet diagnostic criteria for abuse or dependence for additional substances. Other screening procedures and inclusion/exclusion criteria were similar to those used previously (Rush, Stoops, Sevak, & Hays, 2010). Participants were generally in good health with no contraindications to cocaine or buspirone. The Institutional Review Board at the University of Kentucky Medical Center approved the consent document and all study procedures. Participants gave their sober, written informed consent prior to enrollment and were compensated for their participation. Demographic information is presented in Table 1.
Table 1.
Demographic Characteristics (N = 9)
| M (SD) | Range | |
|---|---|---|
| Age | 39.4 (6.7) | 26–48 |
| Sexa | ||
| Male:Female | 7:2 | |
| Racea | ||
| African-American | 6 | |
| Caucasian | 3 | |
| Years of Educationb | 11.1 (2.1) | 7.0–14.0 |
| Weight (kg) | 74.5 (18.4) | 53.9–106.1 |
| Cocaine Use | ||
| Years used | 17.7 (6.4) | 10–25 |
| Grams used (past month) | 11.0 (9.8) | 1.5–28.0 |
| Days used (past month) | 13.1 (4.5) | 4.0–21.0 |
| DAST score | 9.0 (3.4) | 5.0–13.0 |
| Alcohol Use | ||
| Drinks per week | 13.2 (10.8) | 0.3–33.0 |
| MAST score | 10.3 (7.3) | 1.0–24.0 |
| Past-month Cannabis Use (n = 8) | ||
| Joints used | 39.5 (60.3) | 5.0–186.0 |
| Days used | 16.1 (9.8) | 4.0–31.0 |
| Daily Tobacco Use | ||
| Cigarettes/day (n = 8) | 9.5 (7.6) | 1.0–20.0 |
| Cigarillos/day (n = 1) | 3 | |
| FTND Score | 2.6 (2.2) | 0–6 |
Note. M = Mean. SD = Standard Deviation. DAST = Drug Abuse Screening Test (Skinner, 1982). MAST = Michigan Alcoholism Screening Test (Selzer, 1971). FTND = Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991)
Presented as n.
Participants with < 12 years of education reported having passed the General Education Development (GED) test.
General Procedures
Participants were enrolled as inpatients at the University of Kentucky Chandler Medical Center Clinical Research Development and Operations Center (CRDOC) for up to 15 days. Participants completed one practice and six experimental sessions. Participants were informed that they would receive various drugs, either orally or intranasally, that could include placebo, buspirone, or cocaine. Participants were also told that these drugs could be administered alone or in combination. Beyond this general information, participants were blind to the type or dose of drug administered in any given experimental session. Participants were told that the purpose of the study was to learn more about how drugs affect mood, physiology, and performance on laboratory tasks. Otherwise, participants received no instruction of what they were supposed to do or what outcomes might be expected.
Participants were required to provide a urine sample that was negative for all drug metabolites other than those of cocaine or cannabis on the day of their admission to the CRDOC. Participants were then allowed to acclimate to the CRDOC for one day and were observed for signs of drug or alcohol withdrawal. Withdrawal symptoms were not detected.
Practice session
Following the acclimation period, participants completed a practice session to familiarize them with the experimental session routine and experimental tasks. No medications were administered during the practice session.
Drug maintenance days
Starting on the day after the practice session, participants received maintenance medication (i.e., placebo or 10 mg buspirone) three times each day (i.e., at 0700 hr, 1500 hr, and 2300 hr) for the remainder of their participation with the exception that only the 0700 hr maintenance dose was given on the final experimental session day. The total daily maintenance dose was 0 or 30 mg buspirone. Following three days under the first maintenance condition, participants completed three experimental sessions (see Experimental sessions below). Maintenance continued across experimental session days and the other maintenance condition began on the day following the third experimental session. The order of maintenance conditions was counterbalanced across participants. Four participants were maintained on buspirone first and five participants received placebo first. Medication side effects were monitored once daily at 1500 hr with the Udvalg for Kliniske Undersøgelser (UKU) scale (Lingjærde et al., 1987).
Experimental sessions
Three experimental sessions were completed on consecutive weekdays during each maintenance condition and session start times were separated by at least 24 hr. On each experimental session day, participants received the appropriate maintenance dose at 0700 hr, ate a standard fat-free breakfast, and tobacco users were allowed to smoke one cigarette. Experimental sessions began at 0900 hr and lasted approximately 5 hr. At the outset of each session, participants completed baseline subject-rated measures and the sexual discounting task. Approximately 30 min later, they sampled the cocaine dose (0, 15, or 45 mg) that was available that day. Subject-rated measures were then collected at 15-min intervals for 60 min following administration of the sampling dose (see Subject-Rated Measures below). Participants then completed a Drug Choice Procedure in which they were given the opportunity to choose between the dose of cocaine that was available that day or an alternative reinforcer (see Drug Choice Procedure below). Physiological indices were monitored at regular intervals throughout the session.
Drug and alcohol abstinence were verified prior to each session with urine and expired breath samples. Urine drug screens that preceded experimental sessions were occasionally positive for cannabis because of use prior to enrollment and experimentally administered cocaine. In order to proceed with an experimental session, urine and breath samples were required to be negative for all other drug metabolites and alcohol, respectively, and participants were required to pass a field sobriety test. Urine pregnancy tests were conducted for female participants before each session and all pregnancy screens were negative throughout the study.
Sexual Discounting Task
The effect of buspirone maintenance on sexual risk-taking was determined using the Sexual Discounting Task (Johnson & Bruner, 2012), a reliable index of risky, sexual decision-making (Johnson & Bruner, 2013). In this task, participants selected photographs of individuals with whom they would be willing to engage in casual sex from 60 color photographs of men and women. From this initially selected pool of photographs, participants then identified the hypothetical sexual partner whom they: (a) most wanted to have sex with, (b) least wanted to have sex with, (c) thought was most likely to have a STI, and (d) thought was least likely to have a STI based on appearance. For each of the four partner conditions, participants indicated the likelihood with which they would have unprotected sex immediately or wait to engage in protected sexual intercourse after a specified delay when they would have a condom on a visual analog scale (VAS). Participants were asked to imagine that there was no chance of pregnancy and that they were not in a committed sexual relationship for the purpose of this task. Likelihood of condom use was assessed across eight delays in ascending order: 0 delay (i.e., condom immediately available), 1 hr, 3 hr, 6 hr, 1 day, 1 week, 1 month, and 3 months. Raw delay discounting data were used to generate a series of indifference points, defined as the proportion of the delayed option marked on the VAS. Sexual discounting was assessed approximately 20 min before sampling the cocaine dose during the 1st and 4th experimental sessions (i.e., after three days of maintenance on each buspirone condition but prior to cocaine administration). The same photographs and partner classifications selected by each individual were used during both assessments.
Drug Choice Procedure
The reinforcing effects of cocaine were assessed with a Drug Choice Procedure identical to that used previously (see Stoops, Lile, Glaser, Hays, & Rush, 2012 for further detail). Briefly, participants made six discrete choices, at 30-min intervals, between the dose of cocaine sampled at the beginning of the session and an alternative reinforcer (US $0.25) that were available on independent, concurrent progressive-ratio schedules of reinforcement. This procedure was implemented such that the response requirement for the next choice on a given option systematically increased with each choice of that particular reinforcer (Stoops, Lile, Glaser, Hays, & Rush, 2010). The maximum number of drug choices served as the dependent measure.
Subject-Rated Measures
A battery of subject-rated measures was completed on an Apple Macintosh laptop computer (Apple, Cupertino, CA, U.S.A.) in a fixed order. Subject-rated measures were completed 30 min prior to receiving the sampling dose during each experimental session, immediately after dosing, and then once every 15 min for 45 min. Subject-rated measures were not collected during the Drug Choice Procedure due to the potential for carry-over effects. The measures included were the Adjective Rating Scale (Oliveto et al., 1992) and the Drug-Effect Questionnaire (Rush et al., 2003). These scales are sensitive to the effects of stimulants (e.g., Rush, Stoops, & Hays, 2009).
Physiological Measures
Heart rate, blood pressure, and oral temperature were recorded using an automated digital vital-signs monitor (Dinamap Pro, GE Medical Systems, Milwaukee, WI, U.S.A.) starting 30 min prior to administration of the sampling dose and at 15-min intervals thereafter for the remainder of the 5-hr session. Cardiac rhythmicity was monitored continuously throughout experimental sessions. Specific physiological safety criteria were in place to terminate participation (i.e., systolic or diastolic blood pressure exceeding 180 or 120 mmHg, respectively; heart rate in excess of 130 beats per minute; or clinically significant and/or prolonged electrocardiogram abnormalities) but no participants were excluded for exceeding these parameters and no doses were withheld.
Drug Administration
All drugs were administered under medical supervision in a double-blind fashion. Buspirone maintenance doses (Teva Pharmaceuticals, Sellersville, PA, U.S.A.) were prepared by over-encapsulating commercially available 10 mg buspirone HCl tablets in size zero, opaque gelatin capsules. Placebo capsules contained only cornstarch. All capsules were administered orally under observation by nursing staff.
Cocaine doses (0 [placebo], 15, and 45 mg) were prepared by combining the appropriate amount of cocaine HCl, USP powder (Medisca, Plattsburg, NY, U.S.A.) with lactose monohydrate powder to yield a total of 60 mg of powder. To administer the cocaine dose, the participant was presented with a mirror, standard razor blade, and a 65-mm plastic straw. A nurse then placed the cocaine dose on the mirror and the participant was instructed to divide the powder into two even “lines” and insufflate one line of powder through each nostril within 2 min.
Data Analysis
Data from the Sexual Discounting Task were analyzed in GraphPad Prism version 6.0e for Mac OS X (GraphPad Software, La Jolla California U.S.A., www.graphpad.com). First, model fits between the hyperbolic (V = A/(1 + kD); Mazur, 1987) and hyperboloid (V = A/(1 + kD)s; Green, Fry, & Myerson, 1994) models were compared using Akaike’s Information Criteria (AICc; e.g., Akaike, 1981; see also Symonds & Moussalli, 2011). The difference in AICc values was 150.7, indicating that the hyperboloid model with “s” as a shared parameter was preferred. Hyperboloid discounting functions (Green et al., 1994) were then fit to raw data from the Sexual Discounting Task using non-linear regression as described previously (Johnson, 2012; Johnson et al., 2015; Motulsky & Christopoulos, 2003). In the function V = A/(1 + kD)s, V is subjective value of condom use, A is an index of the likelihood of using an immediately available condom, and D is delay to condom availability. The k parameter serves to index the rate of condom-use discounting as a function of delay to condom availability and the exponential parameter, s, describes the non-linear scaling of the likelihood of condom use (Green & Myerson, 2004). When the s parameter is equal to 1, the form of the equation reduces to a hyperbola (Mazur, 1987). In the omnibus regression analysis, the A parameter was constrained between 0 and 1, the k parameter was required to be > 0, and s was a shared (global) parameter to control for the interdependency of s and k. This approach permitted an independent assessment of the effects of experimental conditions on likelihood of using an available condom (i.e., A parameter) and rate of condom-use discounting (i.e., k parameter) in a single model without necessitating data transformation.
Comparisons between the hypothetical sexual partner and buspirone maintenance conditions for the likelihood of using an immediately available condom (i.e., A) and the rate of condom-use discounting (i.e., k) were made using extra sums-of-squares F-tests, as described previously (Johnson et al., 2015; Motulsky & Christopoulos, 2003). Briefly, these tests compare the difference in error between two nonlinear regression models; one in which model parameters are shared (i.e., one curve best-fit the data when collapsed across conditions) and another where parameters are free to vary across conditions (i.e., a separate best-fit curve for each condition). If the extra sums-of-squares F-test is significant, it indicates that the groups differ because there is significantly less error when the model parameters are unshared (Johnson et al., 2015). The Bonferroni correction for multiple comparisons was applied to control family-wise error rates with a resulting alpha level of p < .0031. Orderliness of the delay discounting data was judged using an algorithm (Johnson & Bickel, 2008) that has been used with the Sexual Discounting Task previously (Johnson & Bruner, 2012; Johnson et al., 2015).
All other data analyses were performed in IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, U.S.A.). Data from the Drug Choice Procedure were expressed as number of drug choices and analyzed using a two-factor, repeated-measures ANOVA with Cocaine (0, 15, and 45 mg) and Buspirone (0 and 30 mg) as the factors. Raw data from subject-rated and physiological measures that were collected following administration of the sampling dose of cocaine were analyzed as time course data using separate 2 (Buspirone Dose) × 3 (Cocaine Dose) × 5 (Time) repeated-measures ANOVAs. When the assumption of sphericity was violated according to Mauchly’s test, the Greenhouse-Geisser adjustment of the degrees of freedom was used. Interpretation of the subject-rated and physiological data was guided by F-values. Significant effects were followed up with Dunnett’s post hoc tests where appropriate. For brevity, neither main effects of Time nor constituent main effects subsumed under a statistically significant interaction are reported. The alpha level was set at p ≤ .05.
Results
Sexual Discounting Task Performance
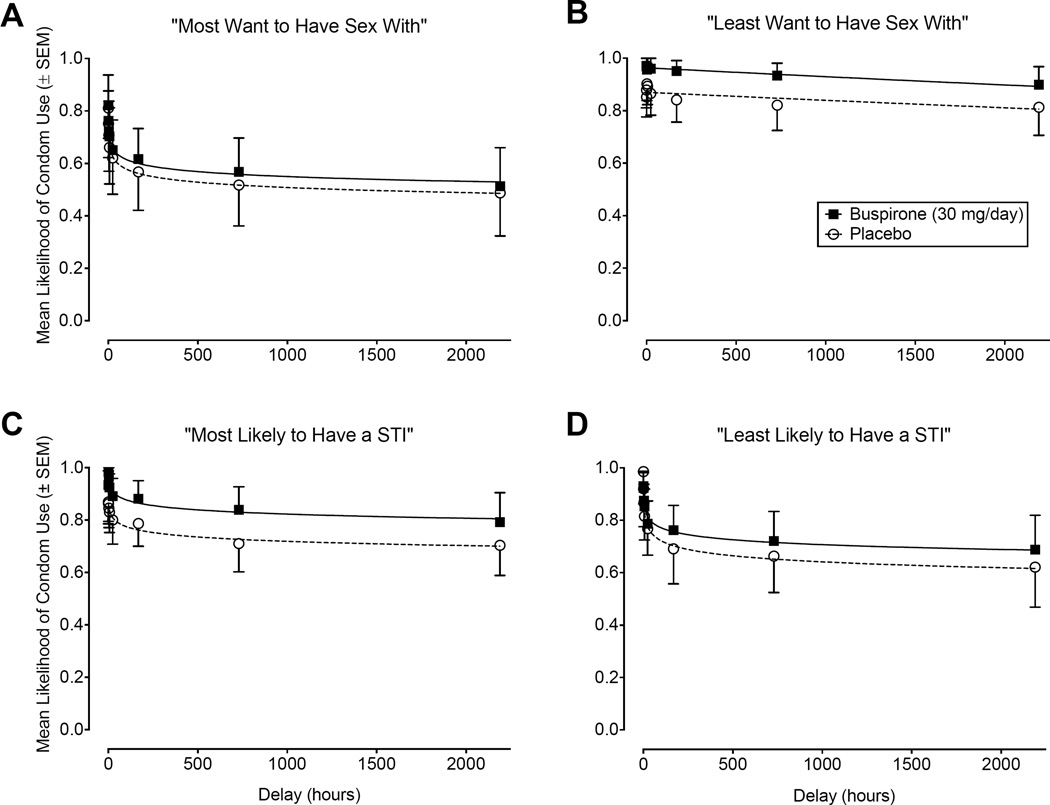
The effect of buspirone maintenance on responses on the Sexual Discounting Task expressed as the mean likelihood of condom use (raw proportion of VAS ± SEM) as a function of delay with hyperboloid best-fit functions is shown for each hypothetical sexual partner condition in Figure 1. Sexual discounting data were generally well described by the hyperboloid model and the likelihood of hypothetical condom use decreased as a function of increasing delay. Goodness-of-fit (r2) indices were > 0.86 with the exception of that for partners rated as “Least Want to Have Sex With” under placebo maintenance (r2 = 0.68). Overall, data from the Sexual Discounting Task were orderly. Only 3 (4%) of the 72 total delay-discounting functions were found to be nonsystematic and, in each case, only one of the eight data points was nonsystematic. Omnibus regression analyses with extra sums-of-squares F-tests revealed significant differences between the conditions for likelihood of using an immediately available condom (i.e., A parameter), F(7, 47) = 26.28, p < .0001, and the rate of discounting of delayed condom use (i.e., k parameter), F(7, 47) = 55.22, p < .0001.
Figure 1.
Data from the Sexual Discounting Task expressed as mean likelihood of condom use (raw proportion of VAS ± SEM) with hyperboloid best-fit functions for each of the four partner categories: Most Want to Have Sex With (Panel A), Least Want to Have Sex With (Panel B), Most Likely to Have a STI (Panel C), and Least Likely to Have a STI (Panel D). Closed squares (solid best-fit function) represent data from when participants were maintained on buspirone and open circles (dashed best-fit function) show data from placebo maintenance.
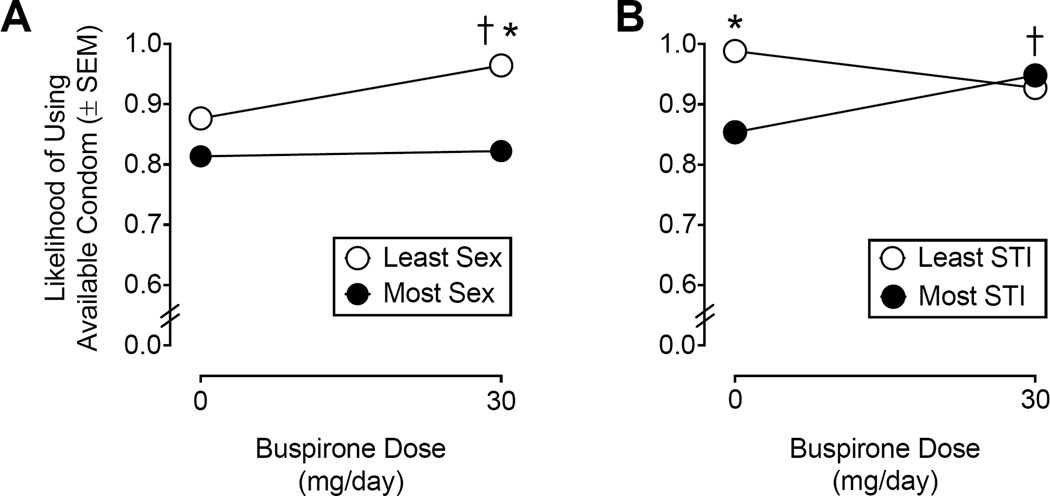
Likelihood of using an immediately available condom (A parameter)
Figure 2 shows hyperboloid best-fit parameter estimates for likelihood of using an immediately available condom (i.e., at the 0-delay condition) as a function of buspirone maintenance condition for hypothetical sexual partners classified based on sexual desirability (panel A) and STI risk (panel B). Relative to responses during placebo maintenance (A = 0.87, SEM = 0.007), buspirone maintenance increased the initial likelihood of condom use (A = 0.96, SEM = 0.007) with hypothetical sexual partners who were classified as “Least Want to Have Sex With”, F(1, 11) = 41.50, p < .0001. Buspirone maintenance did not affect the likelihood of using an immediately available condom (A = 0.82, SEM = 0.017) for hypothetical sexual partners who were classified as “Most Want to Have Sex With” compared to placebo (A = 0.81, SEM = 0.017), F(1, 11) = 0.13. When comparing between hypothetical partners classified based on sexual desirability (i.e., Most Want to Have Sex With vs. Least Want to Have Sex With), participants indicated a greater likelihood of using an immediately available condom with partners classified as “Least Want to Have Sex With” relative to partners classified as “Most Want to Have Sex With” when maintained on buspirone, F(1, 11) = 221.50, p < .0001, but not during placebo maintenance, F(1, 11) = 12.54, p = .0046.
Figure 2.
Hyperboloid best-fit parameter estimates (± SEM) for likelihood of using an immediately available condom (i.e., A parameter) under placebo and buspirone (30 mg/day) maintenance with hypothetical sexual partners classified based on sexual desirability (Panel A) and perceived STI risk (Panel B). An asterisk (*) indicates a statistically significant difference between partner conditions at a given dose of buspirone (p < .003). A dagger (†) indicates a statistically significant difference between Placebo and Buspirone within a given partner condition (p < .003).
For hypothetical sexual partners classified as “Most Likely to Have a STI”, buspirone maintenance increased the likelihood of using an immediately available condom (A = 0.94, SEM = 0.008) relative to placebo maintenance (A = 0.85, SEM = 0.009), F(1, 11) = 17.00, p = .0017. Likelihood of engaging in protected sex did not significantly differ between placebo (A = 0.98, SEM = 0.017) and buspirone maintenance (A = 0.92, SEM = 0.014) with hypothetical partners who were classified as “Least Likely to Have a STI”, F(1, 11) = 5.21, p = .04. When comparing partners classified as most and least likely to have an STI, participants indicated that they would be more likely to use an immediately available condom with hypothetical sexual partners classified as “Least Likely to Have a STI” compared to those classified as “Most Likely to Have a STI” under placebo maintenance, F(1, 11) = 49.14, p < .0001. Buspirone maintenance did not significantly impact the likelihood of initial condom use between hypothetical sexual partners classified based on STI risk, F(1, 11) = 0.63, p = .44.
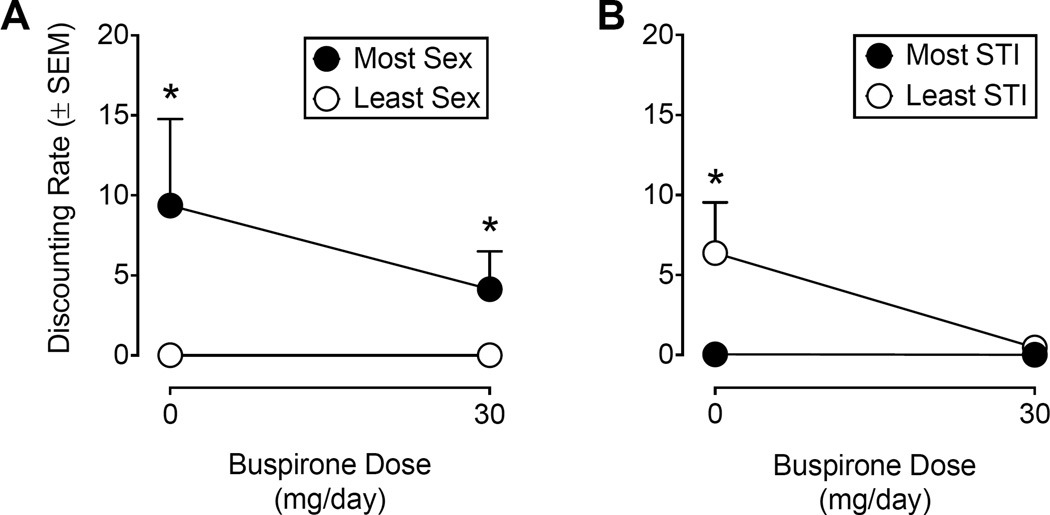
Rate of discounting of delayed condom use (k parameter)
Figure 3 shows hyperboloid best-fit parameter estimates for the rate of discounting of delayed condom use as a function of buspirone maintenance condition for hypothetical sexual partners classified based on sexual desirability (panel A) and STI risk (panel B). For hypothetical partners classified based on sexual desirability, participants were less likely to wait to use a delayed condom with the hypothetical partner that participants identified as the one they most wanted to have sex with (Placebo: k = 9.35, SEM = 5.40; Buspirone: k = 4.13, SEM = 2.36) relative to hypothetical partners with whom they least wanted to have sex (Placebo: k = 0.002, SEM = 0.001; Buspirone: k = 0.001, SEM = 0.0007). The extra sum-of-squares F-tests revealed significant differences between these partner conditions under both placebo, F(1, 11) = 149.30, p < .0001, and buspirone maintenance, F(1, 11) = 491.40, p < .0001. Although the rate of discounting of delayed condom use was numerically lower under buspirone maintenance with hypothetical sexual partners classified as most sexually desirable relative to placebo maintenance, this decrease was not statistically significant, F(1, 11) = 3.25, p = .099. Buspirone maintenance did not alter the rate of discounting of delayed condom use with hypothetical partners that participants least wanted to have sex with, F(1, 11) = 2.08, p = .18.
Figure 3.
Hyperboloid best-fit parameter estimates (± SEM) of the discounting rate parameter (i.e., k parameter) under placebo and buspirone (30 mg/day) maintenance with hypothetical sexual partners classified based on sexual desirability (Panel A) and perceived STI risk (Panel B). Remaining details are the same as those for Figure 2.
For hypothetical partners classified based on STI risk, participants discounted delayed condom use significantly more with hypothetical partners that were identified as “Least Likely to Have a STI” (k = 6.37, SEM = 3.16) relative to hypothetical sexual partners classified as “Most Likely to Have a STI” (k = 0.04, SEM = 0.019) when maintained on placebo, F(1, 11) = 59.81, p < .0001. However, this difference was not statistically significant when participants were maintained on buspirone (Most STI: k = 0.02, SEM = 0.08; Least STI: k = 0.47, SEM = 0.23), F(1, 11) = 13.47, p = .004. The extra sums-of-squares F-tests failed to detect significant differences in the rate of discounting of delayed condom use between placebo and buspirone maintenance for partners selected as “Most Likely to Have a STI”, F(1, 11) = 1.06, p = .32, or “Least Likely to Have a STI”, F(1, 11) = 13.11, p = .004.
Drug Choice Procedure
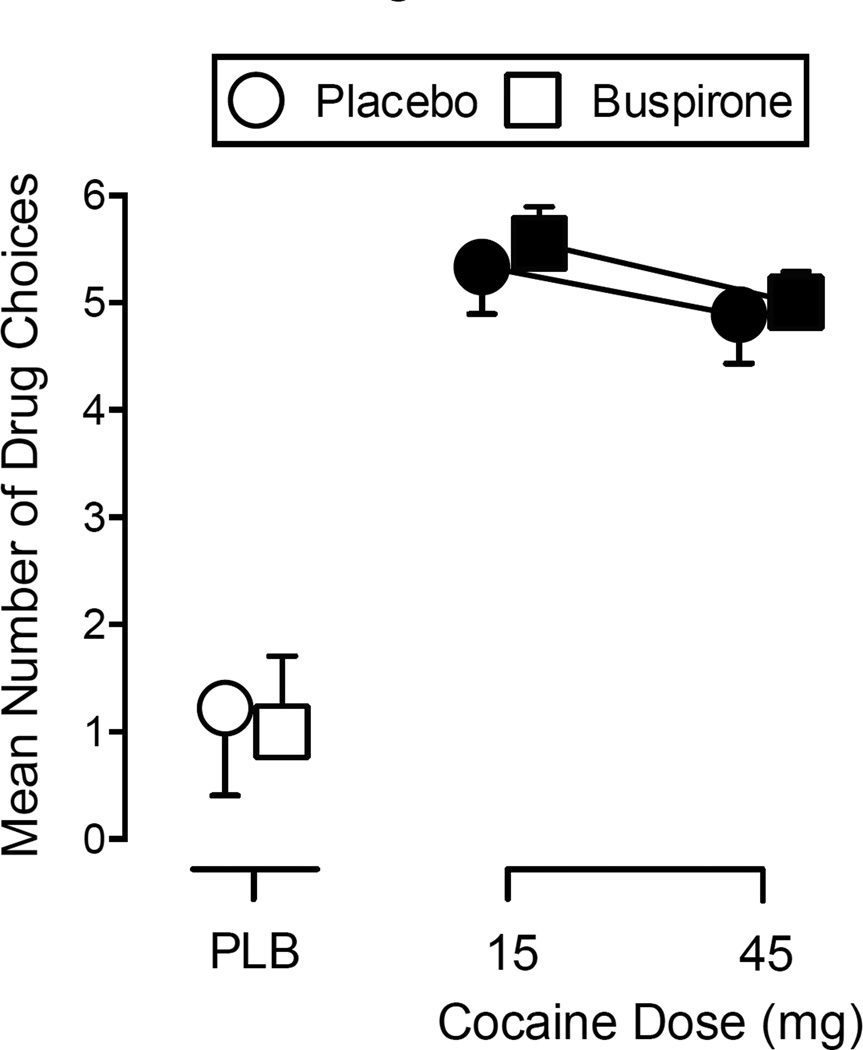
Grouped data from the Drug Choice Procedure (expressed as mean number of drug choices ± SEM) are shown in Figure 4. The two-way, repeated-measures ANOVA revealed only a significant main effect of Cocaine, F(2, 16) = 35.44, p < .001. Post hoc analysis indicated that each dose of cocaine (15 and 45 mg) significantly increased drug choices over placebo, regardless of buspirone maintenance condition.
Figure 4.
Mean number of drug choices (± SEM) for each dose of intranasal cocaine (0, 15, and 45 mg) from the Drug Choice Procedure under placebo or buspirone maintenance. The first drug choice corresponds with a breakpoint of 400 responses. Each subsequent choice required an additional 200 responses. Data points are offset for clarity. Filled symbols indicate a significant difference from Placebo during Placebo maintenance (open circle above PLB; p ≤ .05).
Subject-Rated Measures
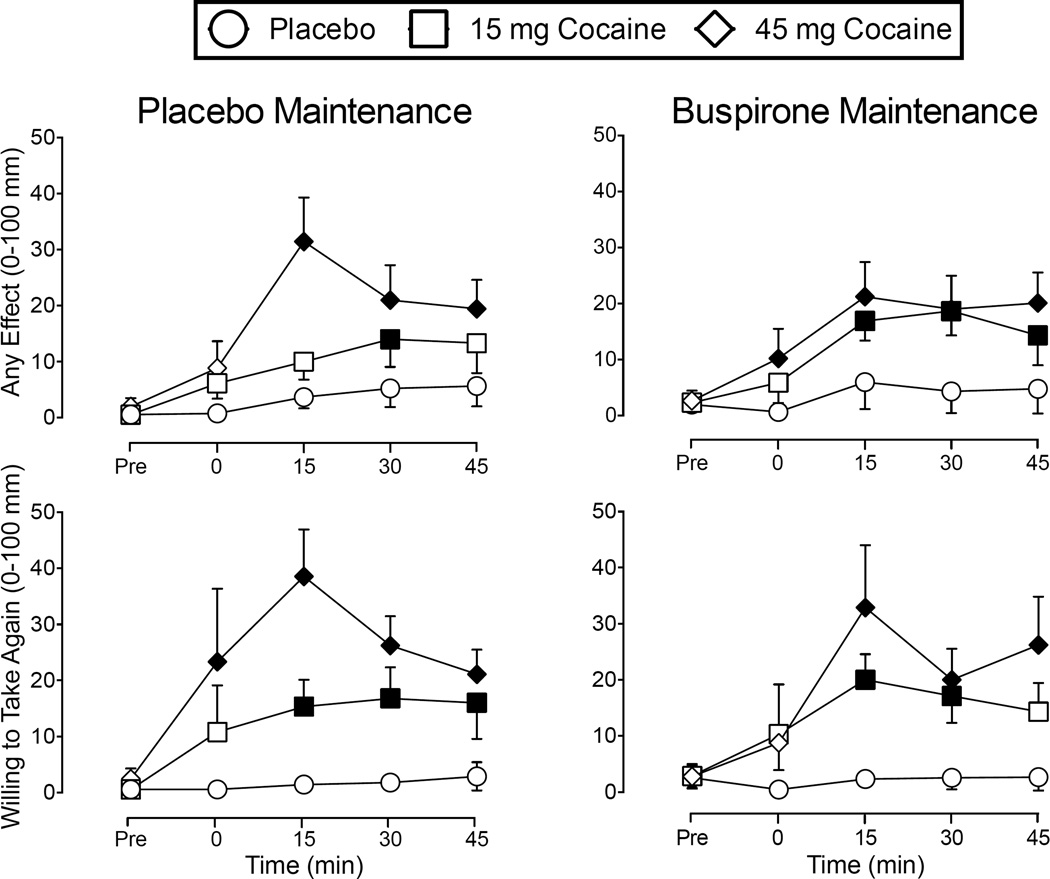
Repeated-measures ANOVAs revealed significant Cocaine × Time interactions for 11 items from the Drug-Effect Questionnaire (F-values ranged from 3.12 to 7.40): Active-Alert-Energetic, Any Effect, Good Effects, High, Irregular Racing Heartbeat, Like Drug, Rush, Shaky-Jittery, Stimulated, Willing to Pay For, and Willing to Take Again. Representative, grouped, time-course data for ratings of Any Effect and Willing to Take Again under placebo and buspirone maintenance are shown in Figure 5. Post hoc tests showed that cocaine significantly increased subject-ratings on each of these measures above placebo in a dose- and time-dependent manner, regardless of buspirone condition. There were also significant main effects of Cocaine on ratings of Nervous-Anxious, Restless, and Talkative-Friendly (F-values ranged from 3.50 to 6.20; data not shown), with similar effects observed to those shown in Figure 5. There were no other statistically significant main effects or interactions, F-values < 3.23.
Figure 5.
Mean time course data (± SEM) for subject ratings of Any Effect and Willing to Take Again from the Drug Effect Questionnaire. Figures on the left represent data collected under placebo maintenance and figures on the right show data when maintained on buspirone (30 mg/day). Filled symbols indicate a significant difference for active cocaine doses (15 and 45 mg) from Placebo during Placebo maintenance (open circles on the left side; p ≤ .05).
Physiological Measures
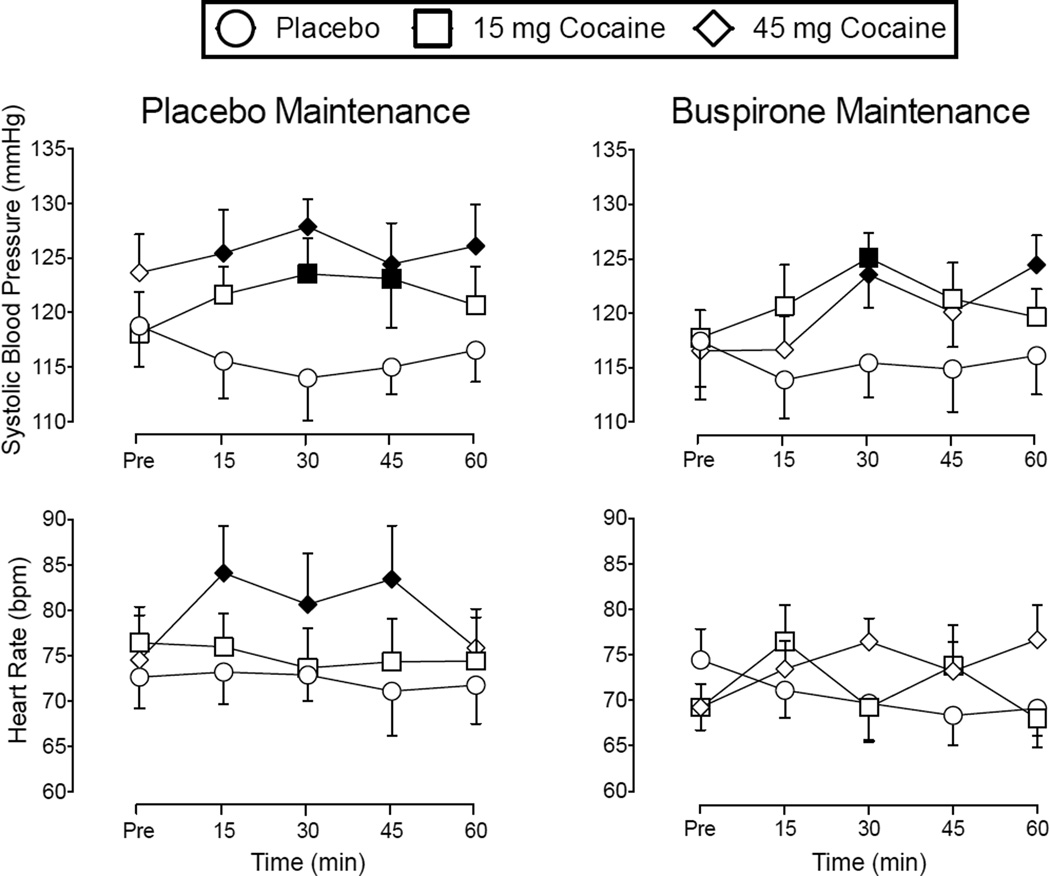
Repeated-measures ANOVAs revealed significant Cocaine × Time interactions for systolic blood pressure, F(8, 64) = 2.36, p < .05, and heart rate, F(8, 64) = 2.27, p < .05. Grouped time course data for systolic blood pressure and heart rate under placebo or buspirone maintenance are shown in Figure 6. Overall, cocaine significantly increased these cardiovascular outcomes in a dose- and time-dependent fashion regardless of buspirone dose. Although there were no significant Cocaine × Buspirone or Cocaine × Buspirone × Time interactions, post hoc analysis revealed fewer statistically significant cocaine-induced increases on cardiovascular measures under buspirone maintenance relative to placebo maintenance (see Figure 6). Buspirone maintenance (M = 97.92, SEM = 0.11) significantly decreased oral temperature relative to placebo maintenance (M = 98.14, SEM = 0.14), F(1, 8) = 7.07, p < .05 (data not shown).
Figure 6.
Mean time course data (± SEM) for Systolic Blood Pressure and Heart Rate. Remaining details are the same as those for Figure 5.
Discussion
This study determined the effects of buspirone maintenance on risky, sexual decision-making on the Sexual Discounting Task and the abuse-related and physiological effects of intranasal cocaine. Participants discounted delayed condom use to a greater degree (i.e., made more risky decisions) with hypothetical sexual partners that they found most sexually desirable and least likely to have a STI. Although it did not significantly alter the rate of discounting of delayed condom use, buspirone maintenance increased the likelihood that participants would use an immediately available condom with hypothetical sexual partners rated as least sexually desirable and most likely to have a STI. Cocaine maintained a higher number of drug choices than placebo, but buspirone maintenance did not attenuate the reinforcing effects of cocaine. Cocaine also increased ratings on questionnaire items that included positive, abuse-related subjective effects and produced prototypical, time-dependent cardiovascular effects. Overall, buspirone did not alter the subject-rated effects of intranasal cocaine but appeared to blunt some of its cardiovascular effects.
Participants discounted delayed condom use to a greater extent with hypothetical sexual partners who were perceived as being most sexually desirable and least likely to have a STI in the current study. These findings parallel those of a previous study that showed cocaine users were less likely to wait to use a condom with hypothetical sexual partners identified as the ones they most wanted to have sex with and that they perceived as being at low risk for having a STI (Johnson & Bruner, 2012). The current findings expand the extant literature by demonstrating that buspirone reduced risky sexual decision-making under certain conditions on a sexual delay-discounting task in cocaine users. Buspirone maintenance significantly increased the probability that participants would choose to engage in protected sexual intercourse with hypothetical partners that were considered most likely to have a STI and least sexually desirable when a condom was immediately available. This outcome suggests that buspirone may have some utility to reduce sexual risk-taking in cocaine users. However, buspirone did not affect the likelihood of condom use with hypothetical partners that were deemed most sexually desirable and least likely to have a STI in the current study. Higher doses of buspirone and longer maintenance periods coupled with additional counseling and behavioral training might be necessary to develop the skills to alter risk-taking behavior when the magnitude of the reinforcer is high (e.g., a highly desired sexual partner) and/or the perceived risks are low. That hypothetical decision-making on the sexual discounting task is correlated with self-reported sexual risk-taking behavior in the natural environment (e.g., Herrmann et al., 2015; Johnson & Bruner, 2012) suggests that improved decision-making in this task might translate to reductions in sexual HIV-risk behavior outside the laboratory. Although buspirone did not consistently improve willingness to wait to use a condom across all partner conditions, these findings collectively suggest that buspirone could improve sexual health outcomes and reduce the spread of STIs like HIV/AIDS in cocaine users. Additional research is needed to determine the mechanism(s) that mediate(s) the reduction in impulsive decision-making by buspirone.
This is the first human laboratory study to systematically evaluate the effect of buspirone maintenance on cocaine self-administration. Three previous preclinical studies determined the effects of repeated buspirone treatment on cocaine self-administration in rhesus monkeys. Gold and Balster (1992) showed that repeated administration of buspirone (0.1 mg/kg intravenous) for 10 days did not affect cocaine intake in daily self-administration sessions. In a similar study, repeated buspirone (0.01–1.0 mg/kg intramuscular) increased cocaine choice at low doses but did not alter cocaine self-administration at higher unit doses nor did it change daily cocaine intake across the range of cocaine doses tested (John et al., 2014). Failure of buspirone to change cocaine-taking behavior in these studies may be due to the relatively short duration of action of buspirone following acute administration (e.g., Gold & Balster, 1992). Mello, Fivel, Kohut, & Bergman (2013) recently showed that intravenous buspirone (0.32 and 0.56 mg/kg/h for 23 hr/day over 7–10 days) shifted the cocaine dose-response curve downward but did not affect food-maintained responding. Because the intravenous buspirone dosing regimen used in that study more likely maintained steady-state plasma levels relative to oral dosing, those findings suggest that maintaining relatively constant buspirone concentrations might more effectively and selectively reduce the reinforcing effects of cocaine.
Differences in the buspirone dose and administration regimen are most likely to account for the incongruent findings between the study by Mello et al. (2013) and those obtained in the current study. First, human participants received 30 mg of buspirone per day (10 mg 3×/day) whereas the cumulative daily dose of buspirone that successfully attenuated cocaine self-administration in monkeys ranged from ~44–129 mg/day. Second, buspirone was administered orally in the current study but was continuously administered via the intravenous route in the previous study with monkeys by Mello and colleagues. Buspirone quickly reaches peak plasma concentrations following oral administration and is rapidly cleared from the body in humans (t1/2 = 2.5 hr; Mahmood & Sahajwalla, 1999). Mello et al. (2013) reported plasma concentrations following 48 hr of continuous exposure that were approximately 24-fold higher than the peak plasma concentrations reported following 20 mg oral buspirone in humans (Mahmood & Sahajwalla, 1999). Plasma concentrations of buspirone were not measured in the current study, so comparisons to those studies are not possible, though peak concentrations lower than those observed in the monkey study are likely, based on prior clinical pharmacokinetics studies. Existing clinical investigations of buspirone as a cocaine pharmacotherapy therefore have been hampered by the difficulty of maintaining steady-state levels of buspirone in plasma. Maintaining higher plasma concentrations of buspirone or its active metabolites (e.g., 1-pyramidinylpiperazine) may be necessary to attenuate the reinforcing effects of cocaine in humans.
The current findings are congruent with the results of a recent multi-site randomized pilot clinical trial with buspirone in treatment-seeking cocaine users. Winhusen et al. (2014) found that buspirone (60 mg/day) did not prevent relapse to cocaine use relative to placebo in cocaine-dependent individuals across a 15-week treatment period. That study reported that buspirone-treated patients did not have more days of continuous cocaine abstinence (39.7 days) relative to patients who received placebo (42.1 days; Winhusen et al., 2014). Although two consistent negative findings are not sufficient to draw firm translational conclusions, the current findings are generally in agreement with the results of this larger pilot clinical trial and may suggest that human drug self-administration techniques effectively model drug-taking behavior under more naturalistic conditions.
The present study also provides important information regarding the safety and tolerability of buspirone in combination with intranasal cocaine. Buspirone did not produce significant side effects and was safely co-administered with cocaine. This finding is in line with clinical trial data showing that illicit cocaine use did not produce untoward effects in participants maintained on 60 mg/day of buspirone (Winhusen et al., 2014). Although there were not statistically significant main or interaction effects of buspirone on cardiovascular outcomes in the current study, fewer significant increases were observed following active cocaine doses during maintenance on buspirone relative to placebo maintenance (see Figure 6). These findings expand upon the clinical trial data to suggest that buspirone maintenance may lessen the cardiovascular risks of cocaine use, at least to some degree. Given that higher doses of buspirone or longer maintenance periods may be necessary to attenuate the reinforcing effects of cocaine, these findings collectively suggest that higher doses of buspirone could be safely co-administered with cocaine without clinically significant cardiovascular side effects.
Despite several novel and valuable contributions to the extant literature on the potential clinical utility of buspirone as a putative pharmacotherapy for cocaine-use disorders, the current study has several limitations that should be considered. First, participants were maintained on a relatively low dose of buspirone (30 mg/day). Even though this dose attenuated risky sexual decision-making under certain conditions and reduced some of the cardiovascular effects of cocaine, future studies should test higher doses with a dosing regimen designed to maintain stable levels of buspirone in plasma. Second, differences in alcohol or other drug use (e.g., cannabis) across subjects could have impacted the results but this seems unlikely because a within-subject design was employed. Third, symptoms of nicotine withdrawal were not measured and some participants may have experienced nicotine withdrawal symptoms near the conclusion of experimental sessions (Hughes, Higgins, & Bickel, 1994). The impact of these symptoms was likely minimal because most participants were not highly nicotine dependent. Fourth, delay discounting of monetary outcomes was not assessed, which may have provided more general information about impulsive decision-making in our sample. Although cocaine users generally discount money to a greater extent than controls (e.g., Coffey, Gudleski, Saladin, & Brady, 2003; Heil, Johnson, Higgins, & Bickel, 2006), previous findings indicate that discounting of condom-protected sex and monetary outcomes are not significantly correlated in cocaine users (Johnson & Bruner, 2013). Finally, the Sexual Discounting Task was only administered following buspirone and placebo maintenance periods and not following acute cocaine administration. The ecological validity of the Sexual Discounting Task would be enhanced if sexual discounting were assessed under conditions where high-risk sexual behaviors are more likely to occur in natural settings (i.e., during acute cocaine intoxication). Emerging evidence suggests that acute stimulant administration negatively impacts impulsive sexual decision-making on this task. For example, acute oral methamphetamine significantly increased discounting of delayed condom use but only for individuals who reported that it increased their level of sexual desire (Johnson, 2015; M. W. Johnson, personal communication, March 3, 2016). Based upon these preliminary findings, cocaine administration might also negatively impact sexual risk-taking intent on this task. Future studies are needed to test the hypothesis that buspirone maintenance would also mitigate possible deleterious effects of intranasal cocaine on sexual risk-taking outcomes.
Conclusions
The current study determined the effects of buspirone on risky sexual decision-making in the Sexual Discounting Task and the reinforcing and abuse-related behavioral effects of intranasal cocaine. Buspirone maintenance reduced risky sexual decision-making and blunted some of the cardiovascular effects of cocaine in current cocaine users. In line with recent clinical trial data, buspirone maintenance did not attenuate the abuse-related behavioral effects of cocaine in the current study. Although buspirone did not reduce cocaine self-administration in the current study, preclinical data (i.e., Mello et al., 2013) suggest that higher doses of buspirone and/or more intensive maintenance regimens might be necessary to reduce cocaine use. On the other hand, given that an extended-release formulation of buspirone is not currently available, a more intensive dosing regimen could potentially reduce medication adherence in a clinical setting. The effects of putative pharmacotherapies may also be increased in participants who are motivated to discontinue their drug use (Perkins et al., 2008). Further research is needed to address these methodological limitations, before buspirone is dismissed as a pharmacotherapy for cocaine-use disorders.
Preliminary evidence that a putative pharmacotherapy may reduce sexual risk-taking behavior in individuals who are disproportionately affected by STIs such as HIV/AIDS is of critical importance. Buspirone also has low abuse potential, a favorable side-effect profile, and might have beneficial effects on outcomes other than cocaine use and sexual risk-taking behavior. Specifically, buspirone is indicated for the treatment of anxiety and there is an association between cocaine/stimulant use and co-morbid anxiety disorder (e.g., Bolton & Sareen, 2006; see Vorspan et al., 2015 for review), so it could be useful in that regard as well. Whether or not to prescribe buspirone to an individual with cocaine-use disorder in the absence of demonstrated efficacy at reducing cocaine use, however, is a clinical decision that would need to be made in consultation with the patient and guided by a careful assessment of the potential risks and probable benefits. Lastly, the effects of cocaine and other psychostimulants on sexual motivation and sexual risk-taking in humans is greatly understudied and the development of interventions to reduce the spread of HIV/AIDS and other STIs in cocaine users would represent significant progress in addressing the overall public health impact of cocaine-use disorders.
Public Health Statement.
Cocaine use disorder remains a significant public health concern for which no pharmacotherapy has been identified. This human laboratory experiment conducted an initial evaluation of the pharmacotherapeutic potential of buspirone for treating cocaine use disorder. Buspirone reduced risky sexual decision making in cocaine users, but generally did not change the pharmacodynamic effects of cocaine, suggesting that buspirone may be useful for decreasing cocaine-associated risk taking.
Acknowledgments
The authors gratefully acknowledge research support from the National Institute on Drug Abuse (R21 DA034095, T32 DA035200, and K02 DA031766) and National Center for Advancing Translational Sciences (TL1 TR000115 and UL1 TR000117) of the National Institutes of Health. These funding agencies had no role in study design, data collection or analysis, or preparation and submission of the manuscript. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Drs. Stoops, Rush, and Lile designed the study. Dr. Bolin coordinated all aspects of data collection, management, and statistical analysis; undertook graphical representation of the data; performed and managed the primary literature search; and composed the initial draft of the manuscript. Drs. Marks and Beckmann assisted with data analysis and manuscript preparation. All authors edited, substantially revised, and have read and approved the content of the submitted manuscript.
The authors would also like to thank the staff at the University of Kentucky Laboratory of Human Behavioral Pharmacology for their expert medical and technical assistance.
Katherine R. Marks is now at the Department of Behavioral Science, University of Kentucky.
Footnotes
Disclosures
The authors have no real or potential conflicts of interest to disclose that are relevant to this research.
References
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. The Journal of Pharmacology and Experimental Therapeutics. 2010;334(2):556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Likelihood of a model and information criteria. Journal of Econometrics. 1981;16:3–14. [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders 4th ed. Text Revision. Washington D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Balster RL. Abuse potential of buspirone and related drugs. Journal of Clinical Psychopharmacology. 1990;10(3 Supplement):31S–37S. doi: 10.1097/00004714-199006001-00007. [DOI] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P. Modification of cocaine self-administration by buspirone (Buspar®): potential involvement of D3 and D4 dopamine receptors. The International Journal of Neuropsychopharmacology. 2013;16(2):445–458. doi: 10.1017/S1461145712000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JM, Sareen J. Lifetime mood, anxiety, and drug use disorders are common in the United States population. Evidence-Based Mental Health. 2006;9(4):113. doi: 10.1136/ebmh.9.4.113. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E, Shanahan A, Müller CP, Huston JP. Evidence that the 5-HT1A autoreceptor is an important pharmacological target for the modulation of cocaine behavioral stimulant effects. Brain Research. 2005;1034(1–2):162–171. doi: 10.1016/j.brainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Cavazos-Rehg PA, Spitznagel EL, Schootman M, Strickland JR, Afful SE, Cottler LB, Bierut LJ. Risky sexual behaviors and sexually transmitted diseases: a comparison study of cocaine-dependent individuals in treatment versus a community-matched sample. AIDS Patient Care and STDS. 2009;23(9):727–734. doi: 10.1089/apc.2008.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11(1):18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Dhavalshankh AG, Jadhav SA, Gaikwad RV, Gaonkar RK, Thorat VM, Balsara JJ. Effects of buspirone on dopamine dependent behaviours in rats. Indian Journal of Physiology and Pharmacology. 2007;51(4):375–386. [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacological Reports. 2005;57(6):685–700. [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. Journal of Psychopharmacology. 2006;20(1):24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Effects of buspirone and gepirone on i.v. cocaine self-administration in rhesus monkeys. Psychopharmacology. 1992;108(3):289–294. doi: 10.1007/BF02245114. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of Delayed Rewards - a Life-Span Comparison. Psychological Science. 1994;5(1):33–36. [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Mahoney JJ, 3rd, Newton TF, De La Garza R., 2nd Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacology & Therapeutics. 2012;134(2):260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzke AJ, Williams ML, Bowen AM. Binge use of crack cocaine and sexual risk behaviors among African-American, HIV-positive users. AIDS and Behavior. 2009;13(6):1106–1118. doi: 10.1007/s10461-008-9450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of the New York Academy of Sciences. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors. 2006;31(7):1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Johnson PS, Johnson MW. Examining delay discounting of condom-protected sex among men who have sex with men using crowdsourcing technology. AIDS Behav. 2015;19(9):1655–1665. doi: 10.1007/s10461-015-1107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89(11):1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- John WS, Banala AK, Newman AH, Nader MA. Effects of buspirone and the dopamine D receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology. 2014;232(7):1279–1289. doi: 10.1007/s00213-014-3760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW. An efficient operant choice procedure for assessing delay discounting in humans: initial validation in cocaine-dependent and control individuals. Experimental and Clinical Psychopharmacology. 2012;20(3):191–204. doi: 10.1037/a0027088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Experimental and Clinical Psychopharmacology. 2008;16(3):264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR. The Sexual Discounting Task: HIV risk behavior and the discounting of delayed sexual rewards in cocaine dependence. Drug and Alcohol Dependence. 2012;123(1–3):15–21. doi: 10.1016/j.drugalcdep.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR. Test-retest reliability and gender differences in the sexual discounting task among cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2013;21(4):277–286. doi: 10.1037/a0033071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Johnson PS, Herrmann ES, Sweeney MM. Delay and probability discounting of sexual and monetary outcomes in individuals with cocaine use disorders and matched controls. PLoS One. 2015;10(5):e0128641. doi: 10.1371/journal.pone.0128641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW. Behavioral economics of sexual HIV risk behavior in humans: Sexual discounting; Paper presented at the 2015 Meeting of Applied Behavioral Analysis International; San Antonio, TX. May, 2015. Abstract retrieved from https://www.abainternational.org/media/94998/2015abaiprogbk_web.pdf. [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd, Yoon JH, Bennett R, De la Garza R., 2nd Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology. 2013;64:472–478. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Khan MR, Berger A, Hemberg J, O’Neill A, Dyer TP, Smyrk K. Non-injection and injection drug use and STI/HIV risk in the United States: the degree to which sexual risk behaviors versus sex with an STI-infected partner account for infection transmission among drug users. AIDS and Behavior. 2013;17(3):1185–1194. doi: 10.1007/s10461-012-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends in Neurosciences. 1991;14(7):299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Collo G, Rabiner EA, Boileau I, Merlo Pich E, Sokoloff P. Dopamine D3 receptor ligands for drug addiction treatment: update on recent findings. Progress in Brain Research. 2014;211:255–275. doi: 10.1016/B978-0-444-63425-2.00011-8. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatrica Scandinavica Supplementum. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Liu YP, Wilkinson LS, Robbins TW. Effects of acute and chronic buspirone on impulsive choice and efflux of 5-HT and dopamine in hippocampus, nucleus accumbens and prefrontal cortex. Psychopharmacology. 2004;173(1-2):175–185. doi: 10.1007/s00213-003-1726-1. [DOI] [PubMed] [Google Scholar]
- Loane C, Politis M. Buspirone: what is it all about? Brain Research. 2012;1461:111–118. doi: 10.1016/j.brainres.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Mahmood I, Sahajwalla C. Clinical pharmacokinetics and pharmacodynamics of buspirone, an anxiolytic drug. Clinical Pharmacokinetics. 1999;36(4):277–287. doi: 10.2165/00003088-199936040-00003. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The Effect of Delay and of Intervening Events on Reinforcement Value. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1987. pp. 55–73. [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Bergman J. Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology. 2013;38(3):455–467. doi: 10.1038/npp.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DS, Navaline H, Woody GE. Drug abuse treatment as AIDS prevention. Public Health Reports. 1998;113(Suppl 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Montoya ID, Vocci F. Novel medications to treat addictive disorders. Current Psychiatry Reports. 2008;10(5):392–398. doi: 10.1007/s11920-008-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky HJ, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting. San Diego, CA: GraphPad Software, Inc; 2003. Retrieved from http://www.mcb5068.wustl.edu/MCB/Lecturers/Baranski/Articles/RegressionBook.pdf. [Google Scholar]
- Müller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A–receptors. Progress in Neurobiology. 2007;81(3):133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Nader MA, Barrett JE. Effects of chlordiazepoxide, buspirone, and serotonin receptor agonists and antagonists on responses of squirrel monkeys maintained under second-order schedules of intramuscular cocaine injection or food presentation. Drug Development Research. 1990;20:5–17. [Google Scholar]
- Nydegger LA, Ames SL, Stacy AW, Grenard JL. Response inhibition moderates the association between drug use and risky sexual behavior. Substance Use & Misuse. 2014;49(11):1457–1464. doi: 10.3109/10826084.2014.912230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. The Journal of Pharmacology and Experimental Therapeutics. 1992;261(3):885–894. [PubMed] [Google Scholar]
- Penberthy JK, Ait-Daoud N, Vaughan M, Fanning T. Review of treatment for cocaine dependence. Current Drug Abuse Reviews. 2010;3(1):49–62. doi: 10.2174/1874473711003010049. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer M, Fonte CA, Briski JL, Scott JA, Chengappa KN. Development of procedures for early screening of smoking cessation medications in humans. Clinical Pharmacology and Therapeutics. 2008;84(2):216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Raineri A, Rachlin H. The effect of temporal constraints on the value of money and other commodities. Journal of Behavioral Decision Making. 1993;6(2):77–94. [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR. Cocaine effects during D-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug and Alcohol Dependence. 2009;99(1–3):261–271. doi: 10.1016/j.drugalcdep.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. The Journal of Pharmacology and Experimental Therapeutics. 2003;306(1):195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. Journal of Clinical Psychopharmacology. 2010;30(2):152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. The American Journal of Psychiatry. 1971;127(12):1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7183189. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Bergman J, Madras BK, Kamien JB, Melia KF. Role of D1 and D2 dopamine receptors in the behavioral effects of cocaine. Neurochemistry International. 1992;20(Supplement):147S–152S. doi: 10.1016/0197-0186(92)90228-j. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. European Journal of Pharmacology. 2010;644(1–3):101–105. doi: 10.1016/j.ejphar.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Influence of acute bupropion pre-treatment on the effects of intranasal cocaine. Addiction. 2012;107(6):1140–1147. doi: 10.1111/j.1360-0443.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds MRE, Moussalli A. A brief guide to model selection, multimodal inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology. 2011;65(1):13–21. [Google Scholar]
- Troisi JR, 2nd, Critchfield TS, Griffiths RR. Buspirone and lorazepam abuse liability in humans: behavioral effects, subjective effects and choice. Behavioural Pharmacology. 1993;4(3):217–230. [PubMed] [Google Scholar]
- United States Department of Health and Human Services. National Survey on Drug Use and Health, 2013. ICPSR35509-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2014-11-18; Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. http://doi.org/10.3886/ICPSR35509.v1. [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Schulz C, Baumgartner MR, Quednow BB. Differences in self-reported and behavioral measures of impulsivity in recreational and dependent cocaine users. Drug and Alcohol Dependence. 2013;133(1):61–70. doi: 10.1016/j.drugalcdep.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Vorspan F, Mehtelli W, Dupuy G, Bloch V, Lepine JP. Anxiety and substance use disorders: co-occurrence and clinical issues. Current Psychiatry Reports. 2015;17(2):4. doi: 10.1007/s11920-014-0544-y. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Winhusen TM, Kropp F, Lindblad R, Douaihy A, Haynes L, Hodgkins C, Brigham GS. Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. The Journal of Clinical Psychiatry. 2014;75(7):757–764. doi: 10.4088/JCP.13m08862. [DOI] [PMC free article] [PubMed] [Google Scholar]