Abstract
Idiopathic pulmonary fibrosis (IPF) poses challenges to understanding its underlying cellular and molecular mechanisms and the development of better therapies. Previous studies suggest a pathophysiological role for neuraminidase 1 (NEU1), an enzyme that removes terminal sialic acid from glycoproteins. We observed increased NEU1 expression in epithelial and endothelial cells, as well as fibroblasts, in the lungs of patients with IPF compared with healthy control lungs. Recombinant adenovirus-mediated gene delivery of NEU1 to cultured primary human cells elicited profound changes in cellular phenotypes. Small airway epithelial cell migration was impaired in wounding assays, whereas, in pulmonary microvascular endothelial cells, NEU1 overexpression strongly impacted global gene expression, increased T cell adhesion to endothelial monolayers, and disrupted endothelial capillary-like tube formation. NEU1 overexpression in fibroblasts provoked increased levels of collagen types I and III, substantial changes in global gene expression, and accelerated degradation of matrix metalloproteinase-14. Intratracheal instillation of NEU1 encoding, but not control adenovirus, induced lymphocyte accumulation in bronchoalveolar lavage samples and lung tissues and elevations of pulmonary transforming growth factor-β and collagen. The lymphocytes were predominantly T cells, with CD8+ cells exceeding CD4+ cells by nearly twofold. These combined data indicate that elevated NEU1 expression alters functional activities of distinct lung cell types in vitro and recapitulates lymphocytic infiltration and collagen accumulation in vivo, consistent with mechanisms implicated in lung fibrosis.
Keywords: inflammation, fibrosis, fibroblasts
interstitial lung disease (ILD), comprised of a combination of variable degrees of pulmonary inflammation and fibrosis, may develop for a variety of reasons, including genetic abnormalities, hazardous environmental exposures, disturbances to the immune system, as a complication in systemic and pulmonary diseases, or, for unknown reasons, in patients with idiopathic pulmonary fibrosis (IPF) (75). Pulmonary fibrosis, often a relentless and irreversible feature of ILD, poses a particular problem, because it replaces functional lung parenchyma with scar tissue, causing architectural distortion and functional decline of the lungs. With a limited spectrum of effective therapeutic modalities, ILD is the main cause of death in patients with scleroderma (78). Patients with IPF survive only 2–3 yr after diagnosis, making it the most severe form of pulmonary fibrosis (30, 35, 75). Fibroblastic foci, one of the histopathological hallmarks of IPF, are closely associated with epithelial abnormalities, vascular remodeling, and abnormal wound healing and angiogenesis (17, 20, 23, 66, 68, 91). Numerous gene products, including cell surface receptors, their ligands, and downstream signaling elements, as well as extracellular matrix molecules, have all been implicated in the pathogenesis of IPF (7, 49, 81).
A unified hypothetical model of ILD that incorporates multiple distinct and often dissimilar pathways has not yet been assembled. Although far-reaching advances in our understanding of lung pathobiology, in general, and ILD, in particular, have been made at the protein level, the regulatory role of glycans and, more specifically, sialylation remains poorly developed. A number of sialoproteins reportedly contributing to ILD pathogenesis are neuraminidase 1 (NEU1) substrates, including epidermal growth factor receptor (16, 39, 51, 90); platelet-derived growth factor receptor (1, 9, 25); CD44 (19, 27, 28, 37, 61); toll-like receptor (TLR) 4, TLR7, and TLR9 (2, 3, 8, 54, 72, 84); Fas/FasL (5, 79, 80, 87, 89); integrins (11, 59); and ICAM-2 (88).
Sialic acid (SA) residues often occupy the outermost positions of glycan chains (12, 86). These highly electronegative sugars influence protein tertiary conformation and, in their terminal location, are strategically positioned to influence intermolecular and cell-cell interactions through steric hindrance and/or electrostatic repulsion. The sialylation state of a specific glycoprotein is dynamically and coordinately regulated through the opposing catalytic activities of sialyltransferases (24, 36) and neuraminidases/sialidases (NEU) (55–57). Sialyltransferases catalyze transfer of SA to specific glycans in specific linkages, whereas NEUs hydrolyze the glycosidic linkage between SAs and their underlying subterminal sugars. Four human NEUs have now been identified: NEU1, -2, -3, and -4 (55–57). Changes in the sialylation state of a glycoprotein may mask or unmask cryptic binding sites, leading to functional consequences (36, 55).
Before the cloning and identification of human NEUs, increased sialidase activity was reported in bronchoalveolar lavage (BAL) fluid obtained from patients with IPF (32). Another group reported that inhibition of extracellular sialidase activity reversibly inhibited fibroblast proliferation (85). We asked whether one or more NEUs contribute to the pathogenesis of ILD. Our laboratory previously established NEU1 as the predominant sialidase in two human lung cell types known to be operative in ILD: airway epithelia (38) and lung microvascular endothelia (13). We found that stimulation of epidermal growth factor receptor increased NEU1 association with, and desialylation of, this receptor, which, in turn, counterregulated its tyrosine autotransphosphorylation (38). Relevant to the known disturbances in pulmonary angiogenesis in IPF lungs (23), we found that, in postconfluent human lung microvascular endothelia, when CD31 ectodomains are homophilically engaged, NEU1 associates with and desialylates CD31, disrupting CD31-driven capillary-like tube formation or in vitro angiogenesis (33). NEU1 can also regulate the activities of platelet-derived growth factor receptor (25) and TLRs (2, 3), which are known for their contributions to the mechanisms of ILD (1–3, 8, 9, 25, 54, 72, 84).
These combined reports implicate NEU1 involvement in the pathogenesis of ILD, specifically, IPF. This report conveys new results in support of this notion, obtained from the studies of intact human lung tissues, cultured human lung cells, and in vivo experiments in animals.
MATERIALS AND METHODS
Human lung tissue samples.
The study was approved by the Institutional Review Board at the University of Maryland. Patients were recruited from the University of Maryland Medical Center. Lung explant or video-assisted thoracoscopic biopsy tissue samples were obtained from patients who met established criteria for IPF (69). Normal lung tissue samples were obtained from lungs harvested initially for lung transplantation but ultimately not used. For immunohistochemical staining, 5-μm sections of formalin-fixed, paraffin-embedded human lung tissues were deparaffinized and processed for antigen retrieval (Dako, Carpinteria, CA). Staining for NEU1 was performed using a polyclonal antibody (Ab) from LifeSpan BioSciences (Seattle, WA; catalog no. LS-B393/9172), followed by alkaline phosphatase-labeled goat anti-rabbit Ab and chromogen (both from Vector Laboratories, Burlingame, CA), resulting in a blue appearance of NEU1-positive structures; the tissues were counterstained light pink with eosin-Y alcoholic solution.
Adenoviral constructs.
A previously described (13, 38) human NEU1-encoding replication-deficient adenoviral construct (AdV-NEU1) was used for gene delivery in vivo, compared with a similar noncoding control vehicle (AdV-NULL). These two vectors had enhanced green fluorescence protein (EGFP) encoded in the vector backbone under control of a separate promoter. The vectors were propagated, purified, and quantified by ViraQuest (North Liberty, IA) as previously described for comparable constructs encoding other genes (41, 43, 44, 46, 48, 67). Infectivity of the viruses was confirmed by EGFP fluorescence of infected primary pulmonary human and mouse fibroblasts, as well as human embryonic kidney-293, A549, small airway epithelial, and TC-1 (transformed mouse lung epithelial) cells in culture (all from American Type Culture Collection, Manassas, VA), as well as by ELISA for EGFP (Cell BioLabs, San Diego, CA). Transcription was confirmed by real-time quantitative PCR with primers specific for NEU1 mRNA. Production of NEU1 protein was confirmed by Western blot analyses of cell lysates using a monoclonal Ab from Origene (Rockville, MD; catalog no. TA801668).
Cell cultures and assays.
Primary normal human lung fibroblasts (NHLF) were either derived from the lung tissue samples described above, or purchased from Lonza (Walkersville, MD). Additional primary human lung fibroblast cultures from healthy controls and patients with IPF were a kind gift from Dr. Carol Feghali-Bostwick (Medical University of South Carolina). Human pulmonary microvascular endothelial cells (HPMECs) and human small airway epithelial cells (SAECs) were purchased from Promocell (Heidelberg, Germany). Primary human T lymphocytes were purified to >95% purity from peripheral blood of healthy volunteers, as described (40, 42, 48). All cells were propagated and maintained according to the supplier's recommendations and as described (13, 31, 33, 38, 41, 43, 44, 46, 48, 60, 67). Analyses of mRNA and protein expression levels in cultured cells, including quantitative reverse transcription real-time PCR (RT-qPCR), ELISA, and Western blotting, were also performed as described (13, 31, 33, 38, 41, 43, 44, 46, 48, 60, 67).
Epithelial cell monolayer wounding and proliferation assays.
To assess SAEC migration in wounding assays, cells were cultured to confluence in 24-well plates as described (13, 33). Using a sterile 200-μl pipette tip, a single wound was made across the diameter of each monolayer, after which the cell debris was removed by washing with medium. The wounded monolayers were cultured, and, after increasing times, photomicrographs were taken with an inverted microscope. Wound areas were measured using ImageJ software (74) for comparison with the same wounded monolayer at 0 h as a measure of migration into the wound. To assess SAEC proliferation rates, CellTiter AQueous assays (Promega, Madison, WI) were performed per manufacturer's recommendations.
Transcriptomic profiling of endothelial cells and fibroblasts.
Assessment of global gene expression of HPMECs was performed using the Affymetrix (Santa Clara, CA) microarray approach (Genome Explorations, Memphis, TN). Cells were infected in culture with either AdV-NEU1 or, as a control, AdV-NULL. Total RNA was isolated 48 h later using Trizol reagent (Life Technologies, Grand Island, NY) and quality-controlled by capillary electrophoresis. Profiling of gene expression was performed using the GeneChip Human Genome U133 Plus 2.0 Array. Expression data were analyzed following background correction, quantile normalization, and probe set signal summarization by the Robust Multi-array Average method (26). For transcriptomic profiling of primary adult NHLF, cells were infected with AdV-NEU1 or, as a control, AdV-NULL in cultures. Total RNA was isolated 48 h later using Trizol and submitted to Otogenetics (Atlanta, GA) for RNASeq assays. After controlling the integrity and purity of RNA, cDNA was generated, fragmented, and subjected to Illumina library preparation. The library was then submitted for Illumina HiSeq2500 sequencing using Rapid v1 SBS chemistry. Paired-end 106 nucleotide reads were generated and checked for data quality using FASTQC (Babraham Institute, Cambridge, UK). The sequencing data were mapped against human reference genome with STAR (15), and the expression levels of genes and transcripts were measured by Cufflinks (83) by counting the reads mapped on known genes and transcripts regions and normalizing them into fragments per kilobase per million mapped (FPKM). The fold change in FPKM of genes and transcripts across different samples was then analyzed.
Adhesion of T lymphocytes to endothelial cell monolayers.
Microfluidics 48-well plates were used in the BioFlux 200 system (Fluxion Biosciences, San Francisco, CA). The temperature of the plates was maintained at 37°C at all times using the integrated system plate heater. All microfluidics channel washing and cell-loading procedures were performed at a wall shear stress force of 2.0 dyn/cm2. The microfluidic channels were precoated with 100 μg/ml fibronectin for 1 h at room temperature. The channels were washed with cell culture medium, and HPMECs were seeded at 7 × 106 cells/ml and allowed to adhere overnight, resulting in a confluent monolayer. The endothelial cell-lined channels were then pulsed with AdV-NEU1 or AdV-NULL at 4 × 109 viral particles/ml. The plates were incubated at 37°C in humidified 5% CO2 atmosphere for 24 h. Infectivity of HPMECs with AdVs was confirmed to be >90% based on microscopic green fluorescent protein (GFP) fluorescence. The monolayers were washed before pulsing the channels with T cells. Primary human T cells were purified from peripheral blood of healthy volunteers by negative selection using RosetteSep Human T Cell Enrichment Cocktail (StemCell Technologies, Vancouver, BC, Canada) to >97% purity by flow cytometry for cell surface CD3. A suspension of 1 × 107 freshly purified T cells/ml was passed through the microfluidics channels already containing the monolayers of AdV-infected HPMECs. The flow was stopped, and T cells were allowed to adhere to HPMECs for 1 h, resulting in 725–750 T cells per microscopic visual field. The flow of T-cell suspension was then established at a wall shear stress force of 0.06 dyn/cm2, which was determined to be optimal in pilot experiments. Phase-contrast digital images were acquired with a video camera once a minute for 15 min. T cells that remained adherent to the confluent endothelium were counted in each frame using ImageJ software (74).
Endothelial cell capillary-like tube formation assays.
Each well of a 96-well plate was coated with Matrigel (BD Biosciences, San Jose, CA) following the manufacturer's recommendations, and nonmanipulated, AdV-NULL-infected, or AdV-NEU1-infected HPMECs were seeded in triplicate at 1.5 × 104 cells/well. Digital photomicrographs were acquired with an inverted microscope after 6 h of culture. Branches of capillary-like tubes were counted per high-power field.
Plasminogen activator inhibitor-1/luciferase assay for active TGF-β.
PAIL cells (mink lung epithelial cells stably transfected with an expression construct containing a truncated plasminogen activator inhibitor-1 promoter fused to the firefly luciferase reporter gene) were a kind gift of Dr. Daniel B. Rifkin, New York University School of Medicine; the cells were maintained and used as previously described (4). Stimulation with recombinant transforming growth factor (TGF)-β (R&D Systems, Minneapolis, MN) was used as a positive control, revealing a dose-dependent increase in luminescence in the concentration range 0.05–1.00 ng/ml. Luminescence was measured using a luciferase assay system (Promega), according to the manufacturer's recommendations.
In vivo experiments.
The experiments were performed in female C57BL/6 mice aged 10–12 wk (The Jackson Laboratory, Bar Harbor, ME). The animals were treated in accordance with a research protocol approved by the Institutional Animal Care and Use Committee of the University of Maryland. The AdVs were instilled intratracheally, as described in detail (41, 43, 44, 46, 48, 67). Quantitative PCR with primers from SABiosciences-Qiagen (Valencia, CA) was used to confirm overexpression of NEU1 RNA in lung homogenates. Intratracheal instillations, BAL analyses, flow cytometry of BAL cells, preparation and analyses of lung homogenates, and histological and immunohistological analyses of lung sections were performed as described (31, 41, 43, 44, 46, 48, 60, 67). For Western blotting for collagen, lung tissue was homogenized, and equal volumes of lung homogenate samples were used for pepsin/acid-soluble collagen extraction by digesting at 4°C with 0.1 mg/ml pepsin in 0.5 M acetic acid overnight. These samples were loaded in equal total protein amounts on gels, and the transferred blots developed with anti-collagen type I Ab (Rockland, Limerick, PA). Hydroxyproline-based QuickZyme tests were also used, which included complete tissue hydrolysis in 6 M HCl at 95°C, followed by the quantitative determination of collagen based on hydroxyproline levels in the lysates. These assays were calibrated with known amounts of collagen standard, rather than the free amino acid hydroxyproline.
Statistical analyses.
For each assay, data are reported as means ± SD. Differences between groups were evaluated with the Student's two-tailed unequal-variance t-test or the Mann-Whitney U-test using Statistics Toolbox version 9.1 in Matlab version 8.4.0.150421. One-way ANOVAs with pairwise comparisons test were performed in R version 3.1.1.
RESULTS
NEU1 expression is increased in the lungs of patients with IPF.
Initial experiments tested whether NEU1 is expressed in human lungs. Lung sections of three healthy individuals and seven patients with IPF were stained for NEU1, revealing its expression in both healthy and diseased lung tissues (Fig. 1). Examination at lower magnification revealed that nonimmune control IgG did not stain the tissue (Fig. 1A), whereas alveolar structures, bronchial epithelium, and endothelial layers stained similarly for NEU1 in healthy controls and unaffected areas of the lungs from patients with IPF (Fig. 1B). Epithelial and endothelial cells, as well as cells in the disease-affected parenchyma, also stained for NEU1 in the lung sections from patients with IPF, and the intensity of the staining appeared to be consistently higher in the affected areas of the lung compared with healthy controls (Fig. 1C). Due to the major differences in microarchitecture and NEU1 staining patterns between normal (Fig. 1B) and IPF-affected (Fig. 1C) lung tissues, quantitative assessment of the differences in NEU1 staining intensity poses a challenging task. However, histological analyses at higher magnification (Fig. 1, D–I) revealed more pronounced staining of epithelial (Fig. 1, D–F) and endothelial (Fig. 1, G–I) cells in the lungs from patients with IPF compared with that detected in healthy controls. Bronchial epithelium stained for NEU1 in all tested tissue samples, with particularly dense staining in the brush border of epithelial cells. This staining appeared more intense in the bronchial epithelium in all tested lung tissue samples from patients with IPF (Fig. 1, E and F) compared with healthy controls (Fig. 1D). Endothelial cells also appeared NEU1-positive, and, again, the staining was more intense in patients with IPF (Fig. 1, H and I) than was staining in healthy controls (Fig. 1G).
Fig. 1.
Photomicrographs of lung sections from healthy control (Ctrl) volunteers and IPF patients immunohistochemically stained for NEU1 (blue). A: a nonimmune immunoglobulin control was used in the same protocol. Counterstaining with eosin-Y alcoholic solution appears in light pink. A–C: overview of the sections with a ×10 objective, whereas the remaining panels (D–I) provide a more detailed view with a ×40 objective. Note that bronchial epithelium (D–F) shows more intense NEU1 positivity in the brush border in sections from patients with IPF (arrows in E and F) compared with a healthy control (arrowhead in D). Similarly, endothelial cells (G–I) appear to stain more intensely in patients with IPF (arrows in H and I) compared with a healthy control (arrowhead in G).
In patients with IPF, the parenchymal lung tissue appeared less intensely stained, yet lung fibroblasts were NEU1-positive within areas of fibrosis (Fig. 2A). RT-qPCR assays revealed that NEU1 mRNA in human lung fibroblasts is the most abundant among the four known mammalian NEUs (Fig. 2B). To further assess whether NEU1 protein is indeed expressed in pulmonary fibroblasts, primary fibroblast cultures derived from the lungs of an additional seven control individuals and eight additional patients with IPF were lysed and studied with quantitative immunoblotting for NEU1 (Fig. 2, C and D). These assays showed that NEU1 protein was significantly elevated (Fig. 2D) in lung fibroblast cultures from IPF patients (Fig. 2C, lanes 5–8 and 12–15) compared with cultures from healthy controls (Fig. 2C, lanes 1–4 and 9–11). Additionally, fibroblast cultures from 9 healthy individuals and 10 patients with IPF were tested in RT-qPCR assays for NEU1 mRNA steady-state levels (Fig. 2E). NEU1 mRNA normalized to 18S rRNA was elevated (P = 0.024) in pulmonary fibroblast cultures from patients with IPF compared with cultures from healthy controls (Fig. 2E).
Fig. 2.
Expression of NEU1 in human lung fibroblasts from patients with IPF. A: photomicrograph of a fibroblastic focus area in a lung section from a patient with IPF after immunohistochemical staining for NEU1 (objective ×40). Arrows indicate selected stromal NEU1-positive cells, likely fibroblasts, staining in blue. B: reverse transcriptase-quantitative PCR for NEU1, -2, -3, and -4 mRNAs, normalized to 18S rRNA and further normalized to the average value for the NEU1 mRNA levels. Primary fibroblast cultures from 7 adult healthy controls were tested, each with specific primers for the indicated mRNAs. The pairwise differences between the levels of all tested mRNA species are significant (P < 0.05), except for the NEU2 to NEU4 comparison, with both NEU2 and NEU4 mRNAs expressed at low levels. C: Western blotting (left) for NEU1 and β-actin in cultured primary pulmonary fibroblasts from 7 adult healthy controls (C; lanes 1–4, 9–11) and 8 patients with IPF (P; lanes 5–8, 12–15). D: densitometry of the NEU1 bands normalized to the corresponding β-actin bands in C and further normalized to the average value for healthy control fibroblasts. Average normalized densitometric values in each group are indicated with horizontal bars. The difference in NEU1 protein levels between IPF and control fibroblast cultures is significant (P = 0.047). E: reverse transcriptase-quantitative PCR for NEU1 mRNA normalized to 18S rRNA in cultured primary pulmonary fibroblasts from 9 adult healthy controls and 10 patients with IPF, as indicated. Individual fold differences from the average value for healthy controls are plotted. The mean value in each group is indicated with a horizontal bar. The difference in NEU1 mRNA levels between IPF and control fibroblast cultures is significant (P = 0.024).
Since NEU1 expression in human lung tissues and cultured fibroblasts obtained from IPF patients was elevated, we asked whether this increased expression might contribute to IPF pathophysiology.
Overexpression of NEU1 in cell culture affects cell phenotypes.
The observations of elevated NEU1 expression levels in diverse cell types in the lungs of patients with IPF (Figs. 1 and 2) prompted the subsequent experiments aimed at establishing whether NEU1 overexpression in cultured human cells via gene delivery has a disease-relevant effect on cellular phenotypes seen in IPF.
The effect of NEU1 overexpression on primary human airway epithelial cells.
SAECs were cultured to confluence, infected with AdV-NEU1, or, as a control, AdV-NULL, and tested in scratch wound-healing assays (Fig. 3). Based on EGFP fluorescence (encoded under a separate promoter in the backbone of both AdVs), 75–80% of cells appeared infected. Elevated NEU1 expression restrained epithelial cell migration as early as 2 h, persisting throughout the 24-h study period (Fig. 3). Parallel cell proliferation assays revealed that AdV-NEU1-infected cells proliferated at the same rate as AdV-NULL-infected controls, indicating that SAEC migration rather than proliferation was responsible for the differences observed in wound-healing assays (Fig. 3).
Fig. 3.
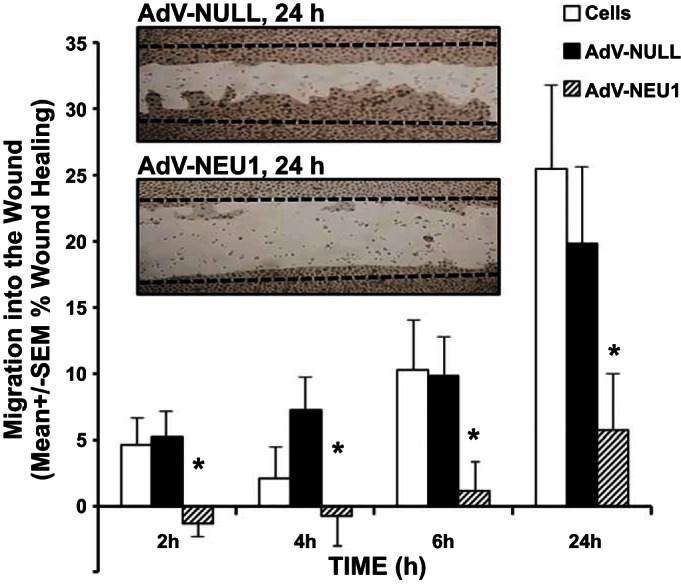
NEU1 impairs SAEC migration into a wound. SAECs infected with AdV-NULL or AdV-NEU1 were cultured to confluence in the wells of 24-well plates, after which a single wound was placed across the diameter of each monolayer. At 0, 2, 4, 6, and 24 h, images of each monolayer were captured, and cell migration into the wound at each time point was calculated relative to that observed at 0 h in the same wounded monolayer. Vertical bars represent mean ± SE percent migration into the wound; n = 8 for each condition. *Significant decreases compared with the simultaneous AdV-NULL-infected controls at P < 0.05.
The effect of NEU1 overexpression on primary human lung microvascular endothelial cells.
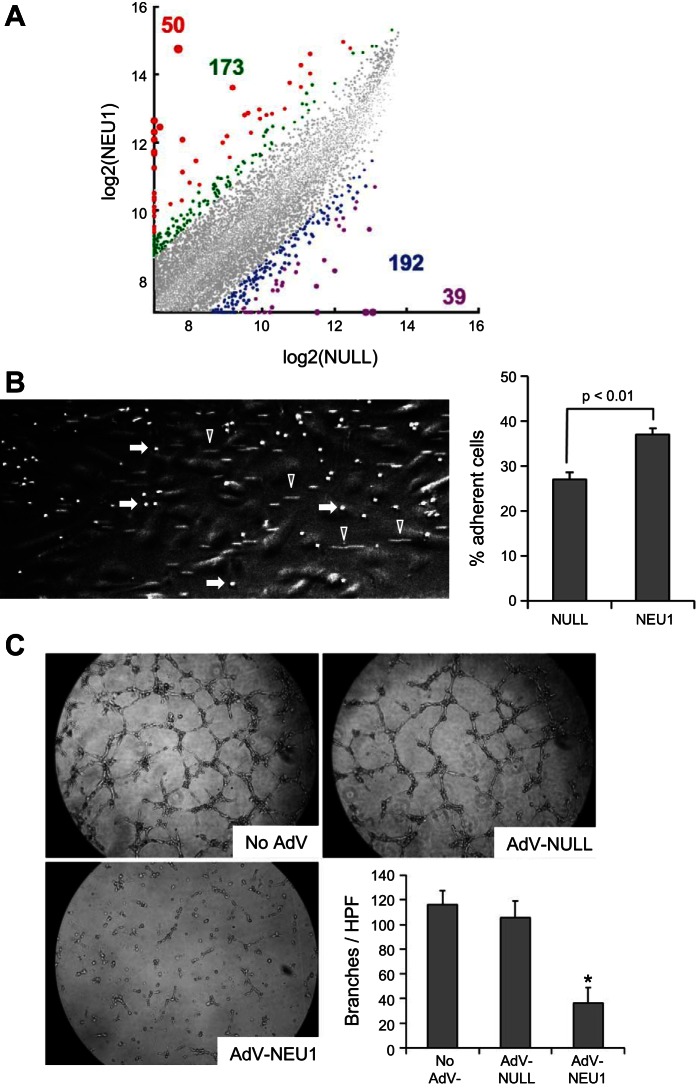
To extend our knowledge about the effects of elevated NEU1 expression on endothelial cells, HPMEC cultures were infected with AdV-NEU1 or AdV-NULL, and, 48 h later, changes in gene expression were assessed using Affymetrix microarray-based profiling of global gene expression (Fig. 4A, left). Gene delivery of NEU1 to HPMECs provoked substantial changes, both increases and decreases, in the expression levels of hundreds of genes compared with AdV-NULL-infected HPMECs. The signals from 50 probe sets were increased and from 39 probe sets decreased greater than fivefold, whereas the signals from 173 probe sets were increased and from 192 probe sets decreased three- to fivefold (Fig. 4A and Supplemental Table S1; Supplemental material for this article is available online at the journal website). For example, the expression of TNFSF15, a known inhibitor of vasculogenesis, was elevated ∼3.7-fold (Supplemental Table S1). This experiment indicates that NEU1 expression causes broad changes in gene expression profile, which in turn provoke changes in the cellular phenotype.
Fig. 4.
NEU1 overexpression alters endothelial cell function. A: Affymetrix microarray-based transcriptomic profiling of HPMECs. Normalized gene expression values in cultured AdV-NEU1-infected HPMECs were plotted vs. AdV-NULL-infected cells. Each dot represents a pair of expression levels of a gene, with the expression level defined as the normalized log base 2 signal value for the gene-specific probe set. The colorized dots represent genes with substantially different expression levels. The genes with fivefold higher (red), fivefold lower (purple), three- to fivefold higher (green), and three- to fivefold lower (blue) expression levels are shown, with the similarly colored numbers representing the counts of the corresponding genes. The corresponding genes are listed in Supplemental Table S1. Fold differences were not considered for low-expressing genes, defined as those with the higher expression level in each pairwise comparison, being in the lower 25th percentile of expression levels of all genes. B: representative microscopic image of a BioFlux 200 microfluidics channel with a clearly visible monolayer of HPMECs (large cells appearing in gray), adherent T lymphocytes (smaller cells appearing as brightly white, selected cells indicated with arrows), and nonadherent T lymphocytes in flow, appearing as cellular tracks (selected tracks are indicated with arrowheads). Adherent T cells were counted in triplicate for each condition in 3 independent experiments (the bar graph on the right, means ± SD are shown), revealing an increase in T lymphocyte adhesion to AdV-NEU1-infected cells (P < 0.01). C: photomicrographs of noninfected HPMECs or HPMECs infected with AdV-NULL or AdV-NEU1, with mean ± SD counts (n = 6 for each condition) of the number of branches of capillary-like tubes per high-power field (HPF). *Significant decrease (P < 0.01) in the number of branches in AdV-NEU1-infected vs. AdV-NULL-infected HPMECs.
To further assess this possibility, the cell adhesion properties of HPMECs were assessed. Considering that ILD is often associated with an increase in pulmonary lymphocytes (14, 47, 52, 58, 63–65, 82), the adhesion of primary human T lymphocytes to AdV-NEU1-infected HPMEC monolayers was measured compared with AdV-NULL-infected HPMEC control monolayers. HPMEC monolayers were infected with these AdV constructs in BioFlux microchannels, and purified primary human T lymphocytes were pulsed through the channels and allowed to adhere for 1 h. After flow through the channels was established, cells that remained firmly adherent to HPMECs were counted in the digital images (Fig. 4B, left). The experiments revealed that nonadherent T cells detached under flow from HPMEC monolayers within seconds, whereas the remaining adherent T cells persisted for 15 min and longer of continued flow. The percentage of cells that remained adherent under flow revealed that NEU1-overexpressing HPMECs had a greater ability to retain T cells (P < 0.01, Fig. 4B, right). Additional experiments addressed the effect of elevated NEU1 expression on capillary-like tube formation by HPMECs (Fig. 4C). The infection of cultured HPMECs with AdV-NEU1 but not AdV-NULL led to a dramatic reduction in HPMEC capillary-like tube formation (Fig. 4C).
The effect of NEU1 overexpression on fibroblasts.
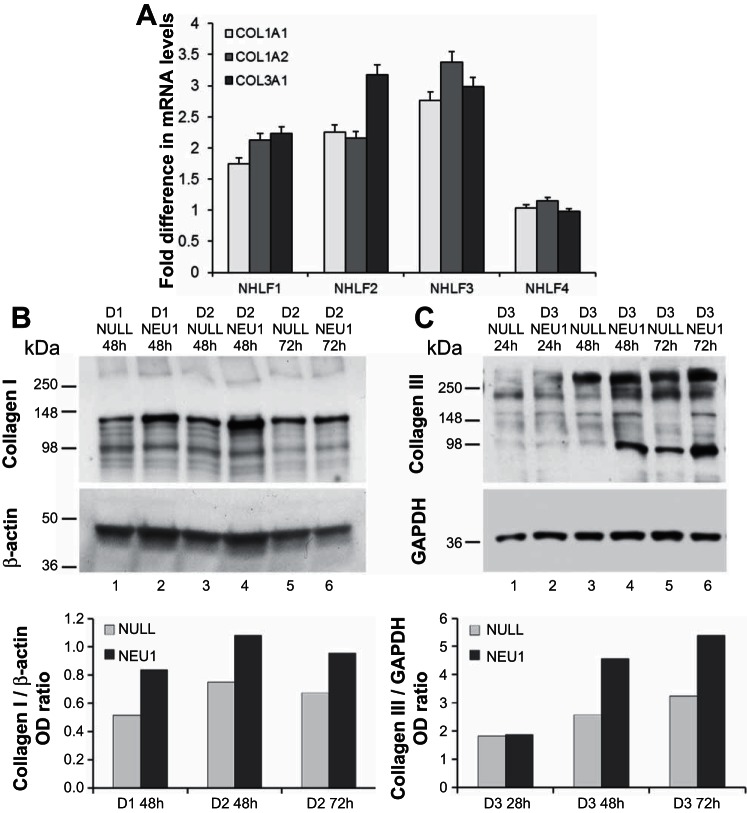
Primary adult NHLF from four different healthy donors were each infected in cell culture with either AdV-NEU1 or AdV-NULL. Steady-state mRNA levels for collagens COL1A1, COL1A2, and COL3A1 were measured by RT-qPCR relative to 18S rRNA levels at 24, 48, and 72 h. Clear but transient increases were observed at 24 h for all three collagen chains in three out of four tested primary NHLF cultures infected with AdV-NEU1 compared with matching cultures infected with AdV-NULL (Fig. 5A). The levels of these collagen chains in the AdV-NEU1-infected cultures decreased to those in AdV-NULL-infected cultures at 48 and 72 h after infection.
Fig. 5.
NEU1 upregulates collagen mRNA and protein expression in cultured normal human lung fibroblasts (NHLF). A: steady-state levels COL1A1, COL1A2, and COL1A3 mRNAs normalized to 18S rRNA in AdV-NEU1- vs. AdV-NULL-infected NHLF cultures from 4 different donors 24 h after infection. Mean fold ratios ± SD of 3 PCR replicates are shown for each collagen chain for each culture. B: Western blotting for collagen type I (top) and β-actin (middle) of cell lysates from NHLF derived from two separate donors (D1, D2) at 48 and 72 h after in-culture cell infection with AdV-NEU1 or AdV-NULL, as indicated. Bottom: the collagen band densities normalized to the densities of the β-actin bands. C: Western blotting for collagen type III (top) and GAPDH (middle) of cell lysates from NHLF derived from a separate donor (D3) at 24, 48, and 72 h after in-culture cell infection with AdV-NEU1 or AdV-NULL, as indicated. Bottom: the collagen band densities normalized to the densities of the corresponding GAPDH bands. OD, optical density.
NHLF from four additional donors were each infected in culture with either AdV-NEU1 or AdV-NULL, and, after confirmation of NEU1 mRNA overexpression by RT-qPCR, Western blot assays were performed at 24, 48, and 72 h after infection to analyze changes in the protein levels of collagen types I and III in cell lysates (examples are shown in Fig. 5, B and C). An increase in collagen levels in NEU1-expressing cells was consistently observed. The quantitative PCR and Western blot findings indicate that NEU1 overexpression transiently increases collagen mRNA and protein levels.
We considered the possibility that NEU1 overexpression leads to elevated collagen levels (Fig. 5) through a TGF-β-dependent mechanism. NHLF were either activated with recombinant human TGF-β for 24 h and 48 h, after which the steady-state mRNA levels of NEU1 were quantified by RT-qPCR, or infected with AdV-NEU1 or AdV-NULL for similar times, and the steady-state mRNA levels of TGF-β1 were measured by RT-qPCR. There was no difference in TGF-β mRNA levels between AdV-NEU1-infected and AdV-NULL-infected cells, nor were there differences in NEU1 mRNA levels between TGF-β-treated and nontreated cells. Another possibility is that NEU1 overexpression affects cellular phenotypes by activating latent TGF-β protein. To address this possibility, experiments with PAIL cells were performed. These cells express luciferase in response to stimulation with active TGF-β (4). In pilot experiments, we observed an increase in luminescence at concentrations of TGF-β as low as 0.05 ng/ml. However, no increase in luminescence was observed in AdV-NEU1-infected PAIL cells compared with AdV-NULL-infected cells. Additional experiments were performed in which nonmanipulated PAIL cells were cocultured with AdV-NEU1- or AdV-NULL-infected primary fibroblasts, again revealing no difference in luminescence. We concluded that the observed increase in collagen in fibroblast cultures (Fig. 5) was unlikely to be mediated by elevated TGF-β.
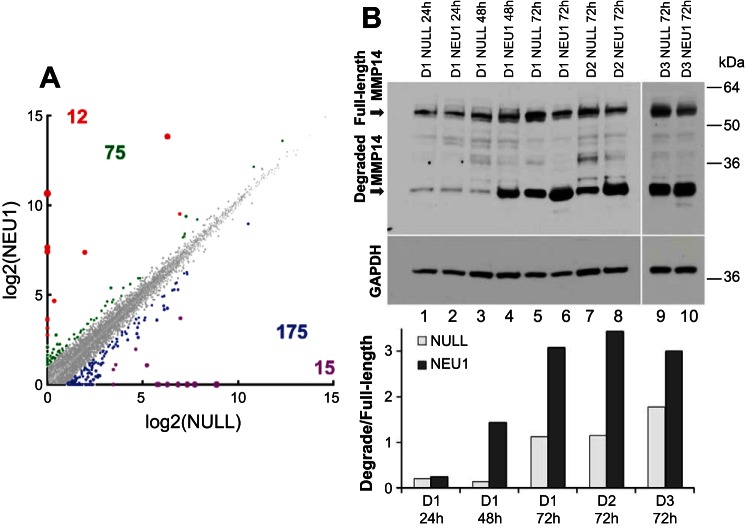
In light of the apparent TGF-β-independent profibrotic effect of NEU1 overexpression on collagen levels in fibroblast cultures, a possibility of collagen regulation at both transcriptional and/or posttranscriptional levels was considered. To address the first possibility, an unbiased approach was utilized, in which primary NHLF were infected with AdV-NEU1 or AdV-NULL, infections confirmed by fluorescent microscopy for EGFP, and comparative RNASeq analyses were performed (Fig. 6A and Supplemental Table S2). The FPKM values for 12 genes were increased and 15 genes decreased greater than fivefold, whereas the values from 75 genes were increased and from 175 genes decreased two- to fivefold (Fig. 6A and Supplemental Table S2). The RNASeq analyses confirmed that there was no increase in the production of TGFβ in response to NEU1 overexpression (∼2.4-fold decrease was observed, Supplemental Table S2). The data also suggested that the observed increase in collagen (Fig. 5) is likely a result of NEU1-driven suppression of antifibrotic mechanisms. More specifically, the level of matrix metalloproteinase-1 (MMP-1), an important collagen-cleaving enzyme, was decreased ∼4.2-fold, and the level of an anti-fibrotic cytokine, hepatocyte growth factor, was decreased ∼2.2-fold.
Fig. 6.
Transcriptional and posttranslational changes induced in cultured primary fibroblasts by NEU1 overexpression. A: RNASeq transcriptomic profiling of NHLFs. FPKM values in cultured AdV-NEU1-infected NHLFs were plotted vs. AdV-NULL-infected NHLF cells. Each dot represents a pair of expression levels of a gene, with the expression level defined as the normalized log base 2 signal value for the gene-specific probe set. The colorized dots represent genes with substantially different expression levels. The genes with fivefold higher (red), fivefold lower (purple), two- to fivefold higher (green), and two- to fivefold lower (blue) expression levels are shown, with the similarly colored numbers representing the counts of the corresponding genes. The corresponding genes are listed in Supplemental Table S2. B: Western blotting for MMP-14 of NHLF from 3 different donors (D1–D3) at 24 h (lanes 1 and 2), 48 h (lanes 3 and 4), or 72 h (lanes 5–10) following infections with AdV-NULL (lanes 1, 3, 5, 7, and 9) or AdV-NEU1 (lanes 2, 4, 6, 8, and 10). The full-length and degraded MMP-14 forms are indicated. The membrane was stripped of the antibody and reprobed for GAPDH to demonstrate the loading. The bar graph shows the ratio of optical densities of the degraded and full-length bands of MMP-14 in the tested samples.
To address the possibility of collagen regulation by NEU1 at the posttranscriptional level, the well-known importance of the ever-present balance between collagen protein production and degradation was considered. One collagen-degrading enzyme, MMP-14, also known as MT1-MMP, is a membrane-tethered matrix metalloprotease expressed by pulmonary fibroblasts that exerts collagenolytic activity (71). This enzyme degrades itself autolytically, with full-length and degraded forms readily identifiable by Western blotting (70). Importantly, sialylation counterregulates MMP-14 proteolysis, and treatment with NEU permits its degradation (70). Comparative Western blotting analyses revealed that cultured NHLF express increasing amounts of MMP-14, both full-length and degraded forms, with time, but the degraded form is strongly elevated in AdV-NEU1-infected fibroblasts compared with AdV-NULL-infected cells from three separate donors (Fig. 6B). These data indicate that NEU1-driven autolytic degradation of MMP-14 decreases its capacity to degrade collagen, thereby shifting the balance between collagen production and turnover toward net collagen accumulation. In combination with RNASeq data (Fig. 6A), it appears that NEU1 regulates collagen levels through several molecular pathways without directly utilizing TGF-β.
Taken together, these results indicate that NEU1 overexpression in the same cell types found to express higher NEU1 levels in the lungs of patients with IPF (epithelial and endothelial cells, fibroblasts; Figs. 1 and 2) influence cellular behavior potentially contributing to IPF pathophysiology. The subsequent experiments aimed to establish whether NEU1 gene delivery to mouse lungs in vivo provokes changes relevant to IPF pathogenesis.
Gene delivery of NEU1 into mouse lungs alters lymphocyte recruitment and collagen deposition in vivo.
Mice were intratracheally infected with AdV-NEU1 or the AdV-NULL control, as previously described (13, 31, 33, 38, 41, 43, 44, 46, 48, 67). In vivo infections with AdV were confirmed by strong expression of EGFP protein (encoded in the viral backbones of both AdV-NEU1 and AdV-NULL constructs), similar to that previously reported (Fig. 2 in Ref. 46). No detectable EGFP was observed in lung homogenates of unmanipulated or intratracheally PBS-challenged mice, whereas strong expression of EGFP was observed in the lungs of AdV-NEU1- or AdV-NULL-challenged mice. As expected, by contrast to EGFP expression driven by both AdVs, infections with AdV-NEU1 but not AdV-NULL caused elevated expression of human NEU1 mRNA in lung homogenates (Fig. 7A). NEU1 mRNA was maximal on day 3, with time-dependent decrease on days 7 and 14.
Fig. 7.
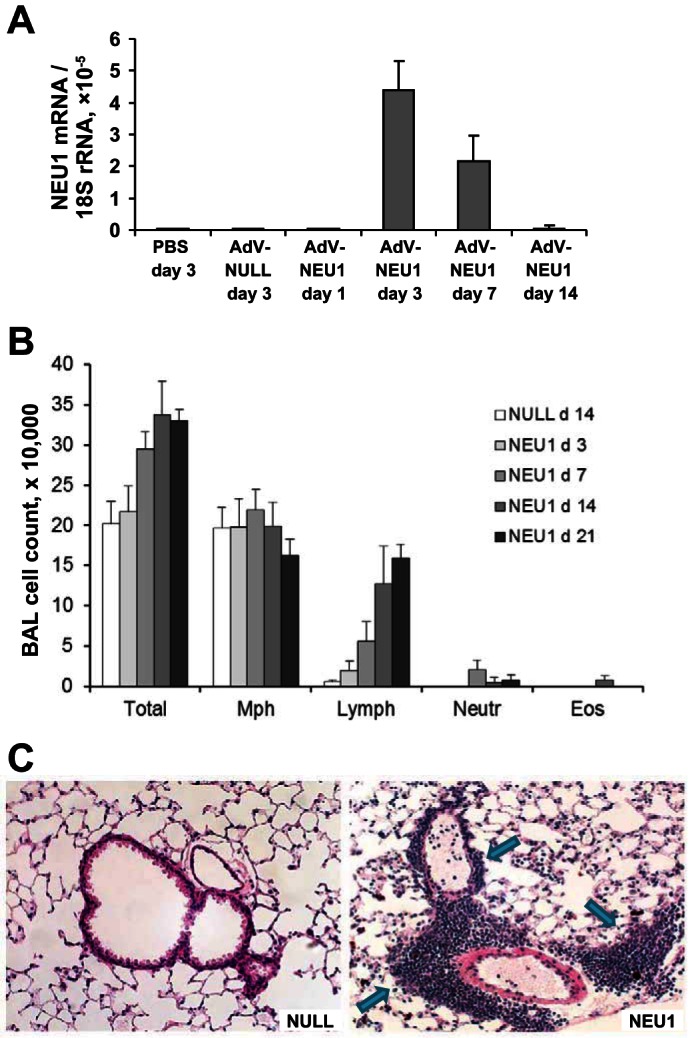
Gene delivery of NEU1 to mouse lungs. A: quantitative reverse transcriptase-polymerase chain reaction for human NEU1 mRNA normalized to 18S rRNA using the 2−ΔΔCt method in mRNA purified from mouse lung homogenates. Three mice in each group were intratracheally administered the indicated adenoviral constructs or PBS and sacrificed at the indicated times. Vertical bars represent the mean NEU1 mRNA-to-18S rRNA ratio × 10−5 ± SD. The PCR primers from SABiosciences (Frederick, MD) were highly specific for human NEU1 and did not cross-react with mouse NEU1. B: changes in the total cell counts and cellular composition of BAL induced by gene delivery of NEU1. Vertical bars represent mean counts ± SD; 3–5 mice per group at the indicated times after intratracheal instillation of AdV-NULL or AdV-NEU1. Note the time-dependent increase in total BAL cellularity and in the number of pulmonary lymphocytes in NEU1-expressing mice; P < 0.05 on days 7–21, one-way ANOVA. C: hematoxylin and eosin staining of lung sections from mice 14 days after intratracheal instillation of AdV-NULL or AdV-NEU1, as indicated. Note the cellular infiltrates in NEU1-expressing mice (arrows).
Mice infected with recombinant AdVs showed no signs of morbidity, such as body weight loss, ruffled fur, dehydration, diarrhea, hunched posture, or decreased motor activity, at any time postinfection. Total and differential BAL cell counts revealed a time-dependent elevation in lymphocytes in AdV-NEU1-infected, but not AdV-NULL-infected mice (Fig. 7B). Consistent with these observations in BAL, histological evaluation revealed minimal, if any, changes in the lungs of AdV-NULL-infected mice (Fig. 7C, left). By contrast, large perivascular and peribronchial lymphocytic infiltrates were evident, as well as patchy areas of diffuse parenchymal lymphocytic infiltrates, in the lungs of AdV-NEU1-infected mice (Fig. 7C, right).
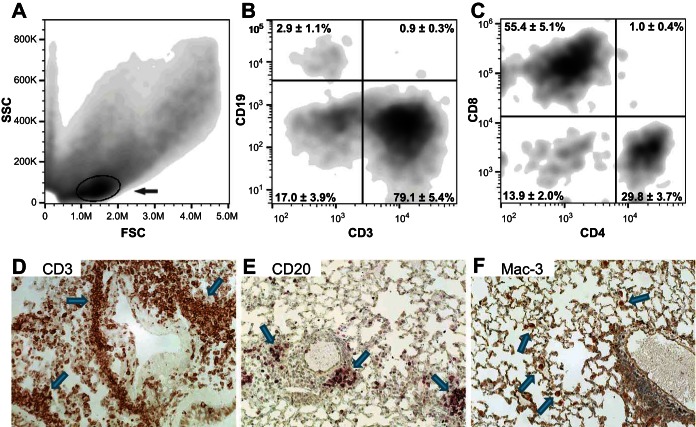
For a more detailed phenotypic characterization of the infiltrating cells, flow cytometry analyses of BAL cells (Fig. 8, A–C) and immunohistochemical analyses (Fig. 8, D–F) of pulmonary sections were performed. Forward- vs. side-scatter plots revealed a large cell population of BAL cells present in NEU1-expressing (circled in Fig. 8A) but not AdV-NULL-infected or nonmanipulated control mice (43–45, 48). This cell population was overwhelmingly composed of T cells (CD3+), with minimal B cells present (CD19+, Fig. 8B). Among the BAL T cells, the number of CD8+ lymphocytes exceeded the number of CD4+ cells (Fig. 8C). In agreement with these observations, the lung-infiltrating cells were overwhelmingly CD3+ T cells (Fig. 8D), with occasional CD20+ B cells present in the infiltrates (Fig. 8E) and scattered Mac-3+ macrophages occurring in the parenchyma but not in the peristructural infiltrates (Fig. 8F).
Fig. 8.
Flow cytometry of BAL cells (A–C) and immunohistochemistry of lung sections (D–F) from mice overexpressing human NEU1; 3–5 mice per group. Forward- (FSC) vs. side-scatter (SSC) analyses of BAL cells from NEU1-expressing mice reveal a population of cells that is not present in control mice (gated and indicated with an arrow in A). B: cell surface staining with anti-CD3 and anti-CD19 antibodies reveals that the majority of these cells are T lymphocytes, with only occasional B lymphocytes present. C: further gating of CD3+ cells reveals a predominance of CD8+ T cells. D: immunohistochemical analyses, with positivity for the tested markers appearing in brown color (selected cells and cell clusters indicated with arrows), show a similar abundance of T cells in the lung tissue, particularly in the infiltrates. By contrast, CD20+ B cells are substantially less abundant (E), whereas Mac3+ macrophages are not associated with infiltrates and occur only occasionally in the lung parenchyma (F).
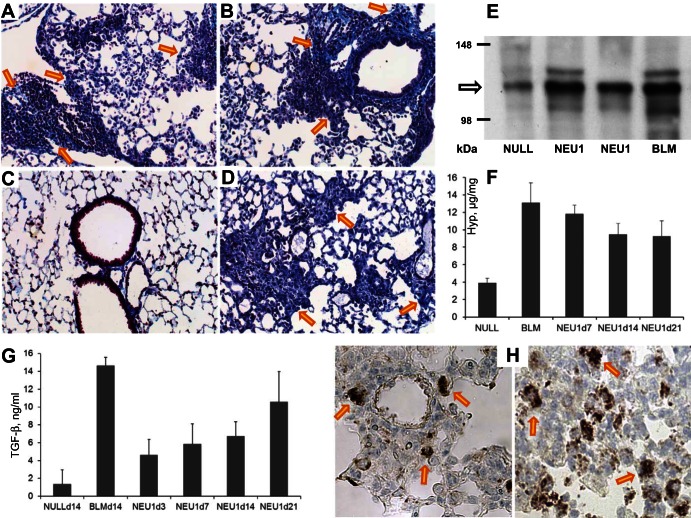
Masson's trichrome staining of the lung sections revealed abundant collagen deposition in association with cellular infiltrates in NEU1-expressing (Fig. 9, A and B) but not AdV-NULL-infected (Fig. 9C) mice. For comparison, a trichrome-stained lung section of a mouse challenged with intratracheal bleomycin is shown in Fig. 9D. To further assess the levels of pulmonary collagen, Western blotting for collagen type I (Fig. 9E) and measurements of hydroxyproline content in lung homogenates (Fig. 9F) were performed. These analyses confirmed that collagen levels were substantially elevated in NEU1-expressing compared with AdV-NULL-infected mice (Fig. 9E). Quantitative, hydroxyproline-based measurements revealed that, again, the collagen content was increased in AdV-NEU1-infected compared with AdV-NULL-infected mice (Fig. 9F). The levels of total TGF-β, a potent profibrotic cytokine, in lung homogenates were also time-dependently elevated (Fig. 9G). The latter observation was inconsistent with the observations in cell cultures, in which no increase in TGF-β was observed. To identify the source of TGF-β in vivo, immunohistochemical staining of lung sections was performed, revealing that, in NEU1-overexpressing mice, TGF-β-expressing cells, likely macrophages (based on cytomorphology), were commonly present in association with the inflammatory infiltrates (Fig. 9H); such cells were not observed in the lungs of AdV-NULL-infected mice.
Fig. 9.
Accumulation of collagen in mouse lungs in response to AdV-mediated NEU1 gene delivery. A–D: Masson's trichrome staining of representative lung sections from two AdV-NEU1-challenged mice on day 14, objective ×20 (A and B). Note the accumulation of collagen fibers appearing in blue in peristructural areas and in association with inflammatory cellular infiltrates (arrows point to selected areas). C: by contrast, similarly stained lung sections from AdV-NULL-challenged mice did not have collagen deposits at levels above the expected presence of collagen in the alveolar walls. D: a positive control section from a bleomycin (BLM)-challenged mouse on day 14 after intratracheal instillation of BLM is shown. E: Western blotting for type I collagen of lung homogenates from an AdV-NULL-infected, two AdV-NEU1-infected, and a BLM-treated mice, as indicated, on day 14 after intratracheal instillations. F: mean ± SD collagen levels, in micrograms per milligram wet lung tissue, from 3–4 mice per group, challenged with AdV-NULL, AdV-NEU1, or BLM (positive control). These measurements were based on hydroxyproline content in QuickZyme assays. The increases are significant (P < 0.01) in BLM-challenged (two-tailed t-test) and NEU1-expressing (one-way ANOVA) mice. G: mean ± SD levels of total TGF-β in lung homogenates of mice infected with AdV-NULL challenged with BLM or infected with AdV-NEU1 at the indicated times (days). The increases are significant in BLM-challenged (P < 0.01, two-tailed t-test) and NEU1-expressing (P < 0.05, one-way ANOVA) mice; 3–4 animals per group were tested. H: immunohistochemical staining for TGF-β of two representative lung sections from NEU1-overexpressing mice, objective ×40. Selected dark brown TGF-β-expressing cells are indicated with arrows.
Combined, these observations indicate that NEU1 gene delivery in vivo causes pulmonary infiltration of T lymphocytes, with greater accumulation of CD8+ T cells as well as elevated levels of pulmonary collagen.
DISCUSSION
The results of these studies suggest that NEU1 is expressed in human pulmonary airway epithelial and microvascular endothelial cells and fibroblasts, as well as in the lungs of patients with IPF (Figs. 1 and 2). The exact quantitative comparison of NEU1 expression levels between healthy and IPF lungs, by either immunohistochemistry or Western blotting of tissue homogenates, is hindered by the profound differences in tissue cellular, biochemical, and structural composition between IPF and normal lungs. Such analyses are further compromised by the selective, localized NEU1 expression in distinct cell types (Figs. 1 and 2). Nevertheless, NEU1 expression levels appeared higher in pulmonary cells from patients with IPF than in healthy controls, based on the intensity of staining of the NEU1-positive areas of the tissue (Figs. 1 and 2) and the Western blotting and RT-qPCR observations in primary fibroblast cultures (Fig. 2). Consistent with our observations, a previous study (32) found elevated sialidase activity in BAL samples from patients with IPF. In combination with an early report on chemical inhibition of extracellular sialidase activity causing an attenuation of fibroblast proliferation (85), these observations suggested that NEU1 expression may contribute to IPF pathogenesis. While the elevations in NEU1 expression in pulmonary epithelial and endothelial cells and fibroblasts in patients with IPF are admittedly relatively modest, the contributions of such elevations to the disease process may be substantial, because these very same cell types are established contributors to disease pathogenesis. By impacting multiple, pathophysiologically important cell types, such chronic, albeit modest in amplitude, elevation in NEU1 levels may have pronounced effects on the overall disease process.
To establish whether elevated NEU1 expression leads to functional consequences in these cell types, recombinant adenovirus-mediated gene delivery of NEU1 to cultured epithelial (Fig. 3) and endothelial (Fig. 4) cells and fibroblasts (Fig. 5) was performed. Such delivery of NEU1, compared with AdV-NULL-infected cells, attenuated epithelial cell migration in wounding assays (Fig. 3). This observation is consistent with the well-known abnormalities in pulmonary epithelium repair contributing to the pathophysiology of pulmonary fibrosis in IPF and other ILDs (7, 10, 30, 66, 68, 76, 81, 91). In endothelial cells, NEU1 delivery caused substantial changes in gene expression (Fig. 4A and Supplemental Table S1), elevated adhesion of T lymphocytes to endothelial monolayers (Fig. 4B), and disrupted endothelial cell capillary-like tube formation (Fig. 4C). Transcriptomic profiling of NEU1-overexpressing endotheliocytes did not reveal changes in the expression levels of T-cell adhesion molecules, yet T-cell adhesion to NEU1-overexpressing endothelia was increased (Fig. 4B). These findings are consistent with the notion that, not only the expression levels, but also the sialylation state of the operative adhesion molecules on either endothelial and/or T cells is important. For example, the degree of sialylation of adhesion molecules CD44 (19, 27, 28, 37, 61) and ICAM-2 (88) is known to substantially affect the adhesion of T cells. These findings are consistent with the already established roles for endothelial dysfunction in IPF and other ILDs (17, 20, 23).
NEU1-overexpressing fibroblasts produced more collagen in culture (Fig. 5), suggesting a direct contribution to collagen deposition. RT-qPCR experiments revealed no increase in TGF-β mRNA levels in NEU1-overexpressing fibroblasts, nor was there an increase in TGF-β activation based on the results obtained with TGF-β-sensitive PAIL cells. Although TGF-β is the strongest known profibrotic mediator, others have reported that fibrosis may occur in an entirely TGF-β-independent fashion (29), and we have described a profibrotic mechanism that requires basal activity of TGF-β signaling without enhancement (50). RNASeq experiments (Fig. 6A and Supplemental Table S2) revealed that NEU1 overexpression causes substantial transcriptomic changes in cultured fibroblasts, without increasing the level of TGF-β mRNA. Interestingly, the level of mRNAs for a collagen-degrading enzyme, MMP-1, was decreased, as was the level of mRNA for an antifibrotic cytokine, hepatocyte growth factor. At the posttranslational level, NEU1 overexpression strongly induced degradation of another collagen-degrading enzyme, MMP-14 (Fig. 6B), in agreement with a previously reported mechanism (70). It appears that NEU1 expression upregulates collagen levels not by stimulating its production but by attenuating collagen turnover. These findings provide initial insight into potential NEU1-dependent mechanisms contributing to ILD. Furthermore, these observations suggest a novel mechanistic perspective on pulmonary pathology at the cellular level, by implicating NEU1 as an upstream master regulator of numerous cell types, epithelial and endothelial cells and fibroblasts, that have already been suggested as critical players in pulmonary inflammation and fibrosis (49).
To assess the effects of elevated NEU1 on the lungs in the complex in vivo environment, AdV-mediated gene delivery of NEU1 to mouse lungs was introduced (Figs. 7–9). A profound increase in pulmonary lymphocytes (Fig. 7), particularly T cells, with a predominance of CD8+ cells (Fig. 8), was notable. Accumulation of CD8+ cells has been previously observed in several ILDs, including IPF, with suggested pathophysiological implications (6, 14, 18, 21, 40, 63, 73). Our results show that pulmonary overexpression of NEU1 recapitulates this known association between predominant accumulation of CD8+ cells in the lung and pulmonary fibrosis (Fig. 9). Reciprocal to our findings of lung fibrosis in the NEU1-overexpressing mice, NEU1-deficient mice display impaired alveolarization and an emphysematous phenotype (77). Similarly, human galactosialidosis with a secondary deficiency of NEU1 results in emphysema (34). These emphysematous changes in mice and humans have been attributed to abnormalities in elastin fiber assembly (34, 77). Emphysema is considered histomorphologically opposite to fibrosis in the sense that the amount of matrix declines in the former and increases in the latter. The effects of NEU1 expression levels on connective tissue homeostasis appear to be organ specific. NEU1-deficient mice, in addition to basal muscular atrophy, demonstrate an impaired growth rate of regenerating muscle fibers following injury; simultaneously, these mice accumulate collagen type III in the endomysium space (62). Differential cellularity, histoarchitecture, and metabolic profile of different tissues are thus likely to contribute to NEU1-driven regulation of connective tissue.
Of note, the levels of TGF-β, a potent profibrotic cytokine, were elevated in NEU1-overexpressing mice (Fig. 9G). In combination with the findings in fibroblast cell culture indicative of TGF-β-independent increase in collagen in NEU1-overexpressing fibroblasts (Figs. 5 and 6), the observed elevation in pulmonary TGF-β is certain to further contribute to fibrosis in NEU1-overexpressing mice. The in vivo increase in pulmonary TGF-β was inconsistent with the findings in cell cultures, in which the levels of TGF-β were not elevated. One possible explanation for this discrepancy is that cell cultures represent a simpler system, which was tested shortly after NEU1 overexpression, whereas the measurements of TGF-β in the complexity of the in vivo environment were performed at later times, after the onset of immune inflammation. The in vivo increase in TGF-β may well be part of the ongoing inflammatory response. The immunohistochemical staining for TGF-β (Fig. 9H) confirmed that the expressing cells, likely macrophages, were indeed associated with the inflammatory infiltrates; such cells were not observed in the lungs of AdV-NULL-infected mice.
The observed in vivo effects were specifically induced by NEU1 overexpression and were not artifacts induced by the AdV vehicle or immune response to a foreign protein. First, mice instilled with AdV-NULL showed minimal, if any, infiltration of cells in the lungs, suggesting that the response to infection with the administered amount of nonreplicating AdV was minimal within the timeframe of the experiments. Second, both AdV-NULL and AdV-NEU1 constructs encode a foreign protein (GFP) in their backbones, leading to strong GFP expression in cells infected with these viruses. AdV-NULL-infected mice strongly overexpressed GFP in their lungs (see Fig. 2 in Ref. 46), but showed minimal if any pulmonary response in this and previous studies (31, 41, 43–46, 48, 67), suggesting that overexpression of a foreign protein has a minimal effect on the lung within the experimental time frame. Third, by contrast to the unremarkable effects of AdV-mediated gene delivery of GFP, similar gene delivery of a functionally active protein, whether human or mouse, to mouse lungs had profound effects on pulmonary cellularity and collagen accumulation; these effects were different from those observed in response to NEU1 overexpression and specific to the factor delivered, whether CCL18 (45, 48, 67), splice variants of IL-4 (43, 44), or isoforms of IL-33 (31, 41, 46). These observations indicate that the changes in the lungs were driven by NEU1 overexpression and were different from nonspecific effects of the replication-deficient AdV vehicle or from nonspecific immune/inflammatory responses. Nevertheless, a limitation of the in vivo model utilized in this study is that gene delivery to the lung through intratracheal instillation of AdVs selectively infects and increases gene expression in pulmonary epithelial cells, but not in other cell types (22, 53). Therefore, the model did not fully represent the elevated expression of NEU1 observed in diverse cell types in patients with IPF (Figs. 1 and 2).
This study aimed to assess the role of NEU1 in regulating the cellular mechanisms of ILD. The results form the basis for further research, which will focus on molecular mechanisms through which elevated NEU1 expression affects the phenotypes of epithelial and endothelial cells and fibroblasts. Also, mechanisms controlling the levels of NEU1 expression remain enigmatic and need to be clarified.
In summary, these results, taken together, suggest a role for elevated NEU1 expression in the pathogenesis of IPF and, possibly, other ILDs. In patients with IPF, pulmonary expression of NEU1 is elevated in epithelial and endothelial cells and fibroblasts, which are the cell types known for their participation in the mechanisms of pulmonary fibrosis and inflammation. Overexpression of NEU1 strongly attenuates epithelial cell migration into a scratch wound, changes expression levels of hundreds of genes in endothelial cells, enhances adhesion of T cells to endothelial cell monolayers, and disrupts endothelial cell capillary-like tube formation. In fibroblasts, NEU1 overexpression elevates collagen protein levels. In vivo gene delivery of NEU1 causes overt pulmonary lymphocytosis with a predominance of CD8+ T cells and lung fibrosis. Such an association between CD8+ T cells and fibrosis has been noted in the past in IPF and other ILDs. By controlling these multiple cell types known for their contributions to the pathophysiology of pulmonary fibrosis, NEU1 may be an important orchestrator of disease mechanisms in IPF and other ILDs.
GRANTS
This study was funded in parts by Veterans Affairs Merit Awards I01CX000101, I01BX002352, and I01BX002499, and National Heart, Lung, and Blood Institute Grant R01-HL-126897.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.G.L., S.E.G., and S.P.A. conception and design of research; I.G.L., V.L., S.W.H., P.K., P.H.K., Z.N., A.L., C.L., A.M.-R., and N.W.T. performed experiments; I.G.L., E.P.L., S.E.G., and S.P.A. analyzed data; I.G.L., S.E.G., and S.P.A. interpreted results of experiments; I.G.L. and S.P.A. prepared figures; I.G.L. and S.P.A. drafted manuscript; I.G.L., E.P.L., N.W.T., S.E.G., and S.P.A. edited and revised manuscript; I.G.L., V.L., S.W.H., P.K., P.H.K., Z.N., A.L., E.P.L., C.L., A.M.-R., N.W.T., S.E.G., and S.P.A. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Paul Todd for expert editorial support.
REFERENCES
- 1.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, Huber PE. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 201: 925–935, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, Gilmour A, Guzzo C, Gee K, Beyaert R, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for Toll-like receptor activation and cellular signaling. J Biol Chem 286: 36532–36549, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdulkhalek S, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk regulates nucleic acid-induced endosomal TOLL-like receptor-7 and -9 activation, cellular signaling and pro-inflammatory responses. Cell Signal 25: 2093–2105, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Ajayi IO, Sisson TH, Higgins PD, Booth AJ, Sagana RL, Huang SK, White ES, King JE, Moore BB, Horowitz JC. X-linked inhibitor of apoptosis regulates lung fibroblast resistance to Fas-mediated apoptosis. Am J Respir Cell Mol Biol 49: 86–95, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, Alms WJ, White B. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum 42: 1168–1178, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7 New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol 306: C987–C996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya S, Kelley K, Melichian DS, Tamaki Z, Fang F, Su Y, Feng G, Pope RM, Budinger GR, Mutlu GM, Lafyatis R, Radstake T, Feghali-Bostwick C, Varga J. Toll-like receptor 4 signaling augments transforming growth factor-beta responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol 182: 192–205, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15: 255–273, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc 12, Suppl 1: S16–S20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiodelli P, Urbinati C, Mitola S, Tanghetti E, Rusnati M. Sialic acid associated with alphavbeta3 integrin mediates HIV-1 Tat protein interaction and endothelial cell proangiogenic activation. J Biol Chem 287: 20456–20466, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M, Varki A. The sialome–far more than the sum of its parts. OMICS 14: 455–464, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Cross AS, Hyun SW, Miranda-Ribera A, Feng C, Liu A, Nguyen C, Zhang L, Luzina IG, Atamas SP, Twaddell WS, Guang W, Lillehoj EP, Puche AC, Huang W, Wang LX, Passaniti A, Goldblum SE. NEU1 and NEU3 sialidase activity expressed in human lung microvascular endothelia: NEU1 restrains endothelial cell migration, whereas NEU3 does not. J Biol Chem 287: 15966–15980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniil Z, Kitsanta P, Kapotsis G, Mathioudaki M, Kollintza A, Karatza M, Milic-Emili J, Roussos C, Papiris SA. CD8+ T lymphocytes in lung tissue from patients with idiopathic pulmonary fibrosis. Respir Res 6: 81, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duchesne L, Tissot B, Rudd TR, Dell A, Fernig DG. N-glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding. J Biol Chem 281: 27178–27189, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, Sasano H, Kondo T, Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 169: 1203–1208, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Emad A, Emad Y. Increased in CD8 T lymphocytes in the BAL fluid of patients with sulfur mustard gas-induced pulmonary fibrosis. Respir Med 101: 786–792, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Faller CE, Guvench O. Terminal sialic acids on CD44 N-glycans can block hyaluronan binding by forming competing intramolecular contacts with arginine sidechains. Proteins 82: 3079–3089, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 45: 1–15, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA Jr. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 65: 236–246, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauldie J, Graham F, Xing Z, Braciak T, Foley R, Sime PJ. Adenovirus-vector-mediated cytokine gene transfer to lung tissue. Ann N Y Acad Sci 796: 235–244, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest 142: 200–207, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology 15: 805–817, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Hinek A, Bodnaruk TD, Bunda S, Wang Y, Liu K. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am J Pathol 173: 1042–1056, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Katoh S, Maeda S, Fukuoka H, Wada T, Moriya S, Mori A, Yamaguchi K, Senda S, Miyagi T. A crucial role of sialidase Neu1 in hyaluronan receptor function of CD44 in T helper type 2-mediated airway inflammation of murine acute asthmatic model. Clin Exp Immunol 161: 233–241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh S, Miyagi T, Taniguchi H, Matsubara Y, Kadota J, Tominaga A, Kincade PW, Matsukura S, Kohno S. Cutting edge: an inducible sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J Immunol 162: 5058–5061, 1999. [PubMed] [Google Scholar]
- 29.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol 173: 4020–4029, 2004. [DOI] [PubMed] [Google Scholar]
- 30.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Kopach P, Lockatell V, Pickering EM, Haskell RE, Anderson RD, Hasday JD, Todd NW, Luzina IG, Atamas SP. IFN-gamma directly controls IL-33 protein level through a STAT1- and LMP2-dependent mechanism. J Biol Chem 289: 11829–11843, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambre CR, Pilatte Y, Le Maho S, Greffard A, De Cremoux H, Bignon J. Sialidase activity and antibodies to sialidase-treated autologous erythrocytes in bronchoalveolar lavages from patients with idiopathic pulmonary fibrosis or sarcoidosis. Clin Exp Immunol 73: 230–235, 1988. [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C, Liu A, Miranda-Ribera A, Hyun SW, Lillehoj EP, Cross AS, Passaniti A, Grimm PR, Kim BY, Welling PA, Madri JA, DeLisser HM, Goldblum SE. NEU1 sialidase regulates the sialylation state of CD31 and disrupts CD31-driven capillary-like tube formation in human lung microvascular endothelia. J Biol Chem 289: 9121–9135, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehman A, Mattman A, Sin D, Pare P, Zong Z, d'Azzo A, Campos Y, Sirrs S, Hinek A. Emphysema in an adult with galactosialidosis linked to a defect in primary elastic fiber assembly. Mol Genet Metab 106: 99–103, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol 5: 483–492, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol 94: 887–905, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillehoj EP, Hyun SW, Feng C, Zhang L, Liu A, Guang W, Nguyen C, Luzina IG, Atamas SP, Passaniti A, Twaddell WS, Puche AC, Wang LX, Cross AS, Goldblum SE. NEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signaling. J Biol Chem 287: 8214–8231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, Juan YH, Chen CH, Khoo KH, Yu CJ, Yang PC, Hsu TL, Wong CH. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci U S A 108: 11332–11337, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luzina IG, Atamas SP, Wise R, Wigley FM, Choi J, Xiao HQ, White B. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum 48: 2262–2274, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Luzina IG, Kopach P, Lockatell V, Kang PH, Nagarsekar A, Burke AP, Hasday JD, Todd NW, Atamas SP. Interleukin-33 potentiates bleomycin-induced lung injury. Am J Respir Cell Mol Biol 49: 999–1008, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luzina IG, Lockatell V, Lavania S, Pickering EM, Kang PH, Bashkatova YN, Andreev SM, Atamas SP. Natural production and functional effects of alternatively spliced interleukin-4 protein in asthma. Cytokine 58: 20–26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzina IG, Lockatell V, Todd NW, Highsmith K, Keegan AD, Hasday JD, Atamas SP. Alternatively spliced variants of interleukin-4 promote inflammation differentially. J Leukoc Biol 89: 763–770, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luzina IG, Lockatell V, Todd NW, Keegan AD, Hasday JD, Atamas SP. Splice isoforms of human interleukin-4 are functionally active in mice in vivo. Immunology 132: 385–393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP. Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis Rheum 54: 2643–2655, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW, Papadimitriou JC, McKenzie AN, Atamas SP. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol 189: 403–410, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol 83: 237–244, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Luzina IG, Todd NW, Nacu N, Lockatell V, Choi J, Hummers LK, Atamas SP. Regulation of pulmonary inflammation and fibrosis through expression of integrins alphaVbeta3 and alphaVbeta5 on pulmonary T lymphocytes. Arthritis Rheum 60: 1530–1539, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luzina IG, Todd NW, Sundararajan S, Atamas SP. The cytokines of pulmonary fibrosis: much learned, much more to learn. Cytokine 74: 88–100, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Luzina IG, Tsymbalyuk N, Choi J, Hasday JD, Atamas SP. CCL18-stimulated upregulation of collagen production in lung fibroblasts requires Sp1 signaling and basal Smad3 activity. J Cell Physiol 206: 221–228, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol 46: 380–388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol 176: 5735–5739, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Mastrangeli A, Danel C, Rosenfeld MA, Stratford-Perricaudet L, Perricaudet M, Pavirani A, Lecocq JP, Crystal RG. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest 91: 225–234, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meneghin A, Choi ES, Evanoff HL, Kunkel SL, Martinez FJ, Flaherty KR, Toews GB, Hogaboam CM. TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem Cell Biol 130: 979–992, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22: 880–896, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Monti E, Bonten E, D'Azzo A, Bresciani R, Venerando B, Borsani G, Schauer R, Tettamanti G. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem 64: 403–479, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Monti E, Preti A, Venerando B, Borsani G. Recent development in mammalian sialidase molecular biology. Neurochem Res 27: 649–663, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Moore BB, Fry C, Zhou Y, Murray S, Han MK, Martinez FJ, Flaherty KR; The COMET Investigators. Inflammatory leukocyte phenotypes correlate with disease progression in idiopathic pulmonary fibrosis. Front Med 1: 56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3: a005017, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nacu N, Luzina IG, Highsmith K, Lockatell V, Pochetuhen K, Cooper ZA, Gillmeister MP, Todd NW, Atamas SP. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J Immunol 180: 5036–5044, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakano T, Matsui T, Ota T. Benzyl-alpha-GalNAc inhibits sialylation of O-glycosidic sugar chains on CD44 and enhances experimental metastatic capacity in B16BL6 melanoma cells. Anticancer Res 16: 3577–3584, 1996. [PubMed] [Google Scholar]
- 62.Neves Jde C, Rizzato VR, Fappi A, Garcia MM, Chadi G, van de Vlekkert D, d'Azzo A, Zanoteli E. Neuraminidase-1 mediates skeletal muscle regeneration. Biochim Biophys Acta 1852: 1755–1764, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papiris SA, Kollintza A, Karatza M, Manali ED, Sotiropoulou C, Milic-Emili J, Roussos C, Daniil Z. CD8+ T lymphocytes in bronchoalveolar lavage in idiopathic pulmonary fibrosis. J Inflamm 4: 14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papiris SA, Kollintza A, Kitsanta P, Kapotsis G, Karatza M, Milic-Emili J, Roussos C, Daniil Z. Relationship of BAL and lung tissue CD4+ and CD8+ T lymphocytes, and their ratio in idiopathic pulmonary fibrosis. Chest 128: 2971–2977, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Parra ER, Kairalla RA, Ribeiro de Carvalho CR, Eher E, Capelozzi VL. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration 74: 159–169, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, Whitsett JA. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax 66: 651–657, 2011. [DOI] [PubMed] [Google Scholar]
- 67.Pochetuhen K, Luzina IG, Lockatell V, Choi J, Todd NW, Atamas SP. Complex regulation of pulmonary inflammation and fibrosis by CCL18. Am J Pathol 171: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prasad S, Hogaboam CM, Jarai G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrogenesis Tissue Repair 7: 7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Remacle AG, Chekanov AV, Golubkov VS, Savinov AY, Rozanov DV, Strongin AY. O-glycosylation regulates autolysis of cellular membrane type-1 matrix metalloproteinase (MT1-MMP). J Biol Chem 281: 16897–16905, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Rowe RG, Keena D, Sabeh F, Willis AL, Weiss SJ. Pulmonary fibroblasts mobilize the membrane-tethered matrix metalloprotease, MT1-MMP, to destructively remodel and invade interstitial type I collagen barriers. Am J Physiol Lung Cell Mol Physiol 301: L683–L692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samara KD, Antoniou KM, Karagiannis K, Margaritopoulos G, Lasithiotaki I, Koutala E, Siafakas NM. Expression profiles of Toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int J Oncol 40: 1397–1404, 2012. [DOI] [PubMed] [Google Scholar]
- 73.Sauty A, Rochat T, Schoch OD, Hamacher J, Kurt AM, Dayer JM, Nicod LP. Pulmonary fibrosis with predominant CD8 lymphocytic alveolitis and anti-Jo-1 antibodies. Eur Respir J 10: 2907–2912, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz MI, King TE. Interstitial Lung Disease. Shelton, CT: People's Medical Publishing House-USA, 2010. [Google Scholar]
- 76.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Starcher B, d'Azzo A, Keller PW, Rao GK, Nadarajah D, Hinek A. Neuraminidase-1 is required for the normal assembly of elastic fibers. Am J Physiol Lung Cell Mol Physiol 295: L637–L647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 66: 940–944, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki O, Nozawa Y, Abe M. Sialic acids linked to glycoconjugates of Fas regulate the caspase-9-dependent and mitochondria-mediated pathway of Fas-induced apoptosis in Jurkat T cell lymphoma. Int J Oncol 23: 769–774, 2003. [PubMed] [Google Scholar]
- 80.Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem 286: 22982–22990, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5: 11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todd NW, Scheraga RG, Galvin JR, Iacono AT, Britt EJ, Luzina IG, Burke AP, Atamas SP. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J Inflamm Res 6: 63–70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trujillo G, Meneghin A, Flaherty KR, Sholl LM, Myers JL, Kazerooni EA, Gross BH, Oak SR, Coelho AL, Evanoff H, Day E, Toews GB, Joshi AD, Schaller MA, Waters B, Jarai G, Westwick J, Kunkel SL, Martinez FJ, Hogaboam CM. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med 2: 57ra82, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Usuki S, Hoops P, Sweeley CC. Growth control of human foreskin fibroblasts and inhibition of extracellular sialidase activity by 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. J Biol Chem 263: 10595–10599, 1988. [PubMed] [Google Scholar]
- 86.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest 87: 851–857, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallach-Dayan SB, Elkayam L, Golan-Gerstl R, Konikov J, Zisman P, Dayan MR, Arish N, Breuer R. Cutting edge: FasL(+) immune cells promote resolution of fibrosis. J Autoimmun 59: 67–76, 2015. [DOI] [PubMed] [Google Scholar]
- 88.Weber KS, Alon R, Klickstein LB. Sialylation of ICAM-2 on platelets impairs adhesion of leukocytes via LFA-1 and DC-SIGN. Inflammation 28: 177–188, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, Cosgrove GP, Redente EF, Bamberg A, Brown KK, Reisdorph N, Keith RC, Frankel SK, Riches DW. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J Immunol 187: 527–537, 2011. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y, Lee JY, Lee CM, Cho WK, Kang MJ, Koff JL, Yoon PO, Chae J, Park HO, Elias JA, Lee CG. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. J Biol Chem 287: 41991–42000, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zoz DF, Lawson WE, Blackwell TS. Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci 341: 435–438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.