Abstract
Bronchopulmonary dysplasia (BPD) is a leading complication of premature birth and occurs primarily in infants delivered during the saccular stage of lung development. Histopathology shows decreased alveolarization and a pattern of fibroblast proliferation and differentiation to the myofibroblast phenotype. Little is known about the molecular pathways and cellular mechanisms that define BPD pathophysiology and progression. We have developed a novel three-dimensional human model of the fibroblast activation associated with BPD, and using this model we have identified the Notch pathway as a key driver of fibroblast activation and proliferation in response to changes in oxygen. Fetal lung fibroblasts were cultured on sodium alginate beads to generate lung organoids. After exposure to alternating hypoxia and hyperoxia, the organoids developed a phenotypic response characterized by increased α-smooth muscle actin (α-SMA) expression and other genes known to be upregulated in BPD and also demonstrated increased expression of downstream effectors of the Notch pathway. Inhibition of Notch with a γ-secretase inhibitor prevented the development of the pattern of cellular proliferation and α-SMA expression in our model. Analysis of human autopsy tissue from the lungs of infants who expired with BPD demonstrated evidence of Notch activation within fibrotic areas of the alveolar septae, suggesting that Notch may be a key driver of BPD pathophysiology.
Keywords: development, disease modeling, fibrosis, lung prematurity, signaling pathways
bronchopulmonary dysplasia (BPD) is a form of chronic lung disease that is both a leading cause of morbidity in infants born prematurely and the most common chronic respiratory disease of infancy (2, 40). Between 23 and 32 wk gestation, fetal lungs are in the canalicular and saccular stages of development. At this developmental stage, a combination of environmental insults to the lungs of premature infants, including hyperoxia, mechanical ventilation, inflammation, and infection, causes a pattern of lung injury and scarring that is characterized by arrest of alveolarization, vascular hypoplasia, increased proliferation of fibroblasts, and development of fibrosis (34). The cellular mechanisms that promote the development of BPD are not well understood nor are the factors that promote rapid progression of the disease (16). BPD is a complex disorder with clinical heterogeneity: not every extremely premature infant develops BPD, and the pathophysiology likely arises from a process involving gene-environment interaction, in addition to known environmental triggers.
Although there are multiple animal models that approximate BPD by exposing developing newborn animals to hyperoxia, infection, or mechanical ventilation, there is currently no human model of the disease (14, 17). Although much has been learned from the animal models, human lung development has a different trajectory than the mouse, the species used most commonly to model BPD (14). Term mouse pups are born in the saccular stage of lung development but, unlike humans at this stage, they are surfactant sufficient and capable of normal gas exchange (34). This and other interspecies developmental differences underscore the need for a human model of BPD, both for improved understanding of the disease pathophysiology and for the development of targeted disease-modifying therapeutics. The pathogenesis of BPD likely includes multiple cell-cell signaling events and cross-talk between neighboring cells (13). In contrast to 2D traditional cell culture methods, a 3D tissue structure more closely approximates the in vivo architecture of the lung parenchyma and provides cells with the ability to grow in a geometry that mimics their in vivo environment. Our 3D disease model provides the opportunity to observe the development and pathogenesis of myofibroblast differentiation and gene expression changes in a way that more closely models the in vivo cell-cell signaling events that are thought to be involved in disease pathogenesis (33).
The Notch pathway plays an essential role in normal lung development and has been previously described to be upregulated in response to airway injury and in response to oxidative stress in the proximal airways (32, 38). Here we describe a unique human 3D culture system that we have developed to model the changes in fibroblast activation associated with BPD and that we have used to identify the involvement of the Notch developmental pathway in the disease pathophysiology.
METHODS
Human fibroblast isolation and cell culture.
Human fetal lung fibroblasts (FLFs) were isolated from 18- to 20-wk old fetal lungs (Advanced Bioscience Resources). Neonatal skin fibroblasts were isolated from foreskins discarded after the circumcision of former premature infants. Tissues were dissociated and then minced by using 1 mg/ml Collagenase/Dispase (Roche) and 0.1 mg/ml DNase with rotation for 45 min at 37°C. After washing with medium containing 1% fetal bovine serum, a single-cell suspension was generated by use of 100- and 40-μm cell strainers. To remove red blood cells, the suspension was incubated in lysis buffer (BD Pharmingen) for 15 min at room temperature. Cells were then plated in six-well tissue culture plates and cultured in DMEM/F12 containing 10% fetal calf serum.
3D culture on alginate beads and hypoxia-hyperoxia model.
Alginate beads were generated by using an electrostatic droplet generator with 3% alginate (Sigma) in the presence of an electric field of 9,000 V. Alginate beads were functionalized by incubation with high-concentration rat tail collagen 1 (9.37 mg/ml, Corning) for 6 days and then coated with Tris-buffered dopamine hydrochloride (2 mg/ml, Sigma) at pH 8.5 to promote cellular adhesion. A 2-ml rotating bioreactor (Synthecon) was loaded with 0.5 ml of sedimented alginate beads and 2 × 106 fibroblasts. Organoid formation occurred for 96 h, after which time the organoid was removed and divided into two pieces, one of which was grown in normoxic conditions and the other was placed in the hypoxic-hyperoxic incubator (Heracell 150i, Thermo Fisher). We exposed the organoids to alternating hypoxia (10% O2) and hyperoxia (70% O2). We oscillated the organoids between the two conditions every 24 h for a total of 4 days. For the high-throughput (HT) 96-well organoids the same protocol was followed except 150-μl volume of beads and 1 × 105 number of cells were seeded in each well of the 96-well plate.
Immunofluorescence.
Immunofluorescence (IF) was performed as described previously (32). Organoid cultures were fixed in 4% paraformaldehyde, washed, and either stained in the well or embedded in Histogel and subsequently paraffin embedded and sectioned (5-μm thickness). IF was done after Tris-EDTA-Tween/citrate buffer-mediated antigen retrieval followed by permeabilization with 0.3% Triton X-100 in protein blocking buffer (Dako) for at least 30 min at room temperature. Sections were incubated with primary antibodies diluted in blocking solution, overnight at 4°C. After several washes in Tris-buffered saline with Tween 20 (TBST), sections were incubated with secondary antibodies for 1–2 h at room temperature or overnight at 4°C, washed, counterstained with DAPI (Vector Laboratories), and placed under a coverslip, and the edges were sealed with nail polish. Slides were then analyzed by fluorescent microscopy with a LSM 780 Zeiss confocal microscope (Carl Zeiss). The following primary antibodies were used: mouse anti-α-smooth muscle actin (α-SMA) (Sigma, A2547), rabbit anti-vimentin (Bioss, bs-0756r), rabbit anti-Notch intracellular domain (NICD) (Abcam, ab8925).
Real-time quantitative PCR.
Total RNA was extracted from the cells around the beads by using the RNeasy micro kit (Qiagen) according to the manufacturer's instructions. DNAse treatment was performed with RQ1 RNase-Free DNase (Promega). Reverse transcription was performed with SuperScript II First Strand Kit (Invitrogen). Real-time quantitative PCR (qPCR) was performed with the TaqMan PCR Master Mix (Applied Biosystems) or SYBR Green Supermix (Bio-Rad) on an Applied Biosystems StepOnePlus Real-Time PCR System. Each RNA sample was reverse transcribed in triplicate, and appropriate negative controls were included in each run. Gene-specific primer pairs and probes were obtained from Applied Biosystems (see Table 1 for probe catalog numbers and primer sequences). For analysis, the ΔCT method was applied with 18S and B2M as endogenous controls. Relative gene expression, presented as a ratio of a target gene to reference control, was used for analysis (32).
Table 1.
Primer and probe details from qPCR experiments
| Gene | Catalog Number |
|---|---|
| 18S | 4318839 |
| JAG1 | hs01070032 |
| HES1 | hs00172878 |
| HEY1 | hs01114113 |
| NQO1 | Hs02512143 |
| Gene | Primer Sequence |
| α-SMA | AAAAGACAGCTACGTGGGTG |
| GCCATGTTCTATCGGGTACTTC | |
| B2M | CGTGTGAACCATGTGACTTTG |
| GCATCTTCAAACCTCCATG | |
| TGFB1 | CAATTCCTGGCGATACCTCAG |
| GCACAACTCCG | |
| TGACATCAA | |
| PDGFRa | TGGCAGTACCCCATGTCTGAA |
| CCAAGACCGTCACAAA | |
| AGGC | |
| PDE5a | GCAGAGTCCTCGTGCAGATAA |
| GTCTAAGAGGCCGGTCAAATTC | |
| LOX | CGGCGGAGGAAAACTGTCT |
| TCGGCTGGGTAAGAAATCTGA | |
| LOXL2 | GGGTGGAGGTGTACTATGATGG |
| CTTGCCGTAGGAGGAGCTG | |
| COL1A1 | GAGCGGTAACAAGGGTGAGC |
| CTTCCCCATTAGGGCCTCTC | |
| ET1 | AGAGTGTGTCTACTTCTGCCA |
| CTTCCAAGTCCATACGGAACAA | |
| COL3A1 | GGAGCTGGCTACTTCTCGC |
| GGGAACATCCTCCTTCAACAG | |
| TNC | TCCCAGTGTTCGGTGGATCT |
| TTGATGCGATGTGTGAAGACA | |
| ELN | GCAGGAGTTAAGCCCAAGG |
| TGTAGGGCAGTCCATAGCCA |
Statistics.
Triplicate samples were used in each experiment. Experiments were repeated a minimum of three times. All values are reported as mean with error bars representing ± SE. Statistical analysis was performed by using Microsoft Excel, with two-tailed Student's t-test being used for two-group comparisons. For all measurements, P values less than 0.05 were considered statistically significant.
Study approval.
Approval for this research was obtained from the UCLA Institutional Review Board.
RESULTS
The 3D lung organoid model created an alveolar template that was scalable for high-throughput applications.
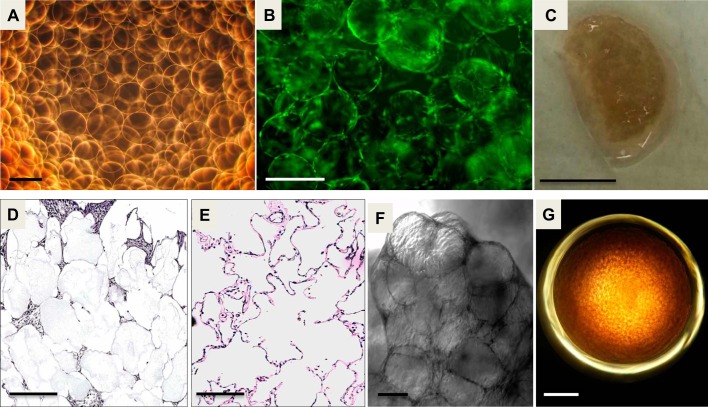
To recapitulate the architecture of distal lung tissue, alginate beads were functionalized with collagen I and poly-dopamine (Fig. 1A) to create alveolar templates for primary human fetal lung cells attachment and growth. FLFs were added to the beads in a rotating 2-ml bioreactor at 37°C, and 24 h later the beads were uniformly coated with the FLFs (Fig. 1B). The beads and cells were spun together in the bioreactor for a total of 4 days at 37°C, generating a lobular organoid structure (Fig. 1C) held together by the mesenchymal cells proliferating on and around the beads. Cross sections of the lung organoid viewed with hematoxylin and eosin staining showed structural homology between the lung organoid and the distal human lung (Fig. 1, D and E). Variable coating and proliferation of fibroblasts around the beads was seen. Some beads were separated by a single cell, and other areas showed proliferation of fibroblasts with multiple cell layers in the interstitium between the beads. The geometry and 3D arrangement of the fibroblasts in our model are important for allowing the cell-coated beads to aggregate together and contract to form the lung organoid (Fig. 1F). We also generated lung organoids in a 96-well plate that were identical in structure to the organoids made in the 2-ml bioreactor to utilize this model for HT applications for BPD drug discovery (Fig. 1G).
Fig. 1.
Development of the 3D lung organoid: A: bright-field micrograph of alginate beads, generated by cross-linking with barium in the presence of an electric field. Scale bar = 200 μm. B: calcein intravital stain of beads after 24 h in the bioreactor showing fetal lung fibroblasts (FLFs) coating the beads. Scale bar = 150 μm. C: photograph of lung organoid generated after 4 days in rotating bioreactor. Scale bar = 1 cm. D: hematoxylin and eosin stain of a cross section of a lung organoid showing FLFs growing around alginate beads. Scale bar = 200 μm. E: hematoxylin and eosin stain of a cross section of normal human lung. Scale bar = 200 μm. F: bright-field microscopy of lung organoid. Scale bar = 100 μm. G: bright-field microscopy of high-throughput (HT) lung organoid growing in a well of a 96-well plate. Scale bar = 1.6 mm.
Exposure of 3D lung organoids to alternating hypoxia and hyperoxia recapitulates the phenotype of fibroblast activation seen in the fibrotic component of human BPD.
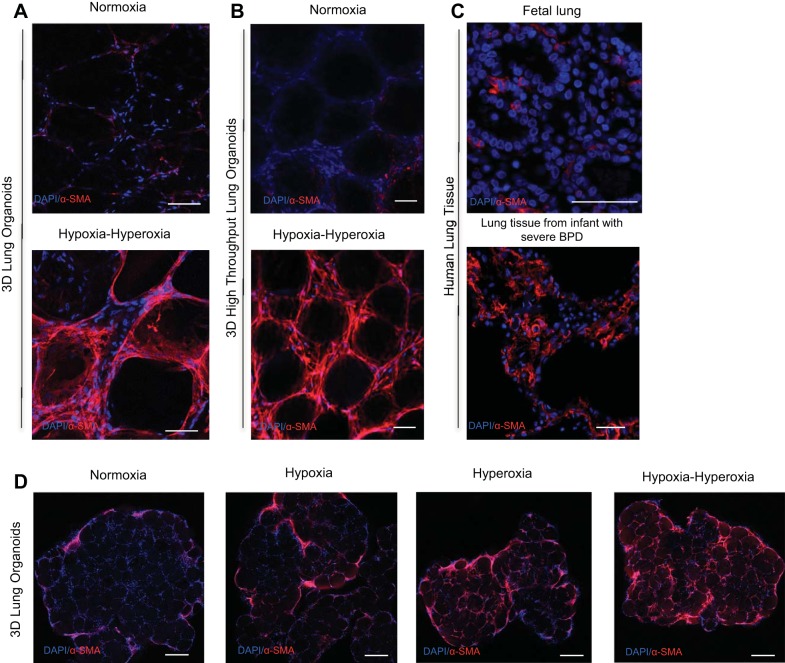
In response to alternating hypoxia-hyperoxia, we saw a phenotype of increased fibroblast activation as demonstrated by markedly increased expression of α-SMA by IF compared with organoids exposed to normoxia (Fig. 2A). This pattern of stress-fiber α-SMA expression strongly resembled that seen in the lungs of human infants who died with BPD (Fig. 2C). Whereas fetal lung (Fig. 2C) and healthy newborn lungs have very little α-SMA expression in the alveolar spaces, the lungs of human infants with BPD have been previously described to have bands of α-SMA expressing fibroblasts at the alveolar septae (3). This bandlike pattern of α-SMA expression in response to hypoxia-hyperoxia is recapitulated in our model both in the large organoids and in the HT organoids made in the 96-well plates (Fig. 2B). When compared with alternating hypoxia-hyperoxia, organoids exposed to hypoxia alone did not demonstrate the same degree of α-SMA expression relative to normoxic controls (Fig. 2D). Exposure to hyperoxia alone showed some increased α-SMA expression relative to normoxia samples, but not to the same degree as the organoids exposed to alternating hypoxia-hyperoxia (Fig. 2D).
Fig. 2.
α-SMA IF of the 3D model of fibroblast activation associated with BPD. A: α-SMA IF of 3D lung organoids exposed to hypoxia-hyperoxia (bottom) vs. normoxia controls (top). B: α-SMA IF of HT 3D lung organoids exposed to hypoxia-hyperoxia (bottom) vs. normoxia controls (top). C: α-SMA IF of fetal lung tissue (top) vs. lung tissue from an infant with severe BPD (bottom). Scale bar = 50 μm D: α-SMA IF of 3D lung organoids exposed to normoxia, hypoxia, hyperoxia, and alternating hypoxia-hyperoxia. Scale bar = 200 μm.
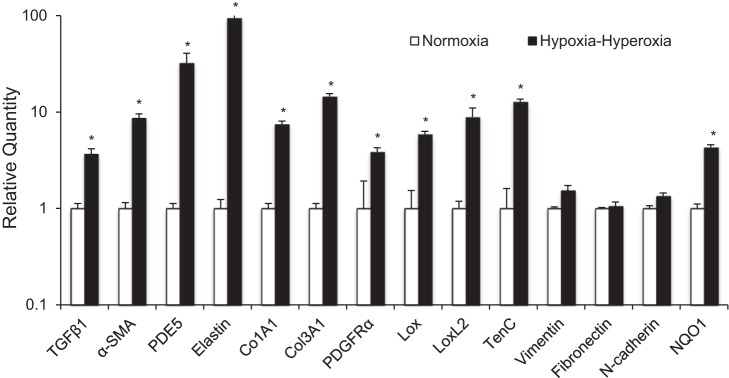
We then used qPCR to examine the expression of 10 genes from multiple gene families previously described to be upregulated either in human infants with BPD or in animal models of BPD (6, 10, 18, 20, 22, 28, 31). We found all 10 genes to have significantly increased transcription in our model compared with the normoxia control (Fig. 3). As a negative control, three genes known to be expressed by fibroblasts but without known increased expression in BPD or in response to oxidative stress, vimentin, fibronectin, and N-cadherin, were analyzed, and these genes all showed no increase in expression in response to hypoxia-hyperoxia (Fig. 3). To ensure that the changes in ambient oxygen in our incubator created changes in oxidative stress in the organoid, we measured expression of NQO1, a phase II enzyme that has been previously shown to be activated by Nrf2 in response to oxidative stress (8). NQO1 showed significantly increased expression in the organoid exposed to hypoxia-hyperoxia compared with normoxic controls, suggesting that the fluctuation in oxygen levels in the incubator was experienced by the cells in the submerged organoid cultures.
Fig. 3.
Validation of the 3D model of fibroblast activation associated with BPD at the level of gene expression. Comparison of gene expression differences of molecular targets known to upregulated in BPD by qPCR in 3D lung organoids grown in normoxia vs. exposed to hypoxia-hyperoxia. Triplicate samples were used in each experiment. Experiments were repeated a minimum of 3 times. *P < 0.001.
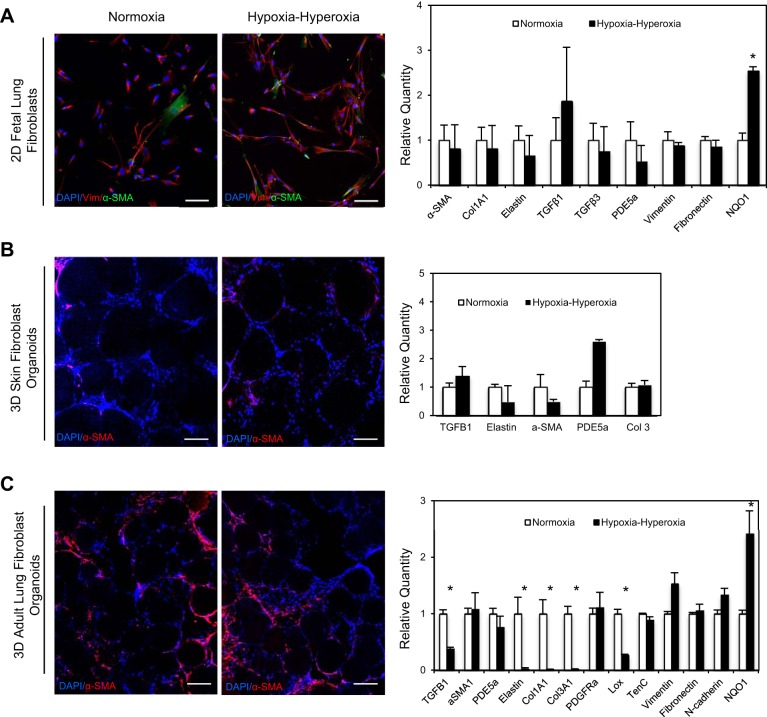
As a control experiment, FLFs were cultured in 2D by standard tissue culture techniques and were exposed to the same pattern of alternating hypoxia and hyperoxia. When comparing the hypoxia-hyperoxia-exposed 2D cells to normoxic controls, we did observe changes in fibroblast morphology including elongation of the cells; however, we saw no changes in α-SMA expression by IF or at the level of gene expression for multiple targets known to be upregulated in BPD (Fig. 4A). We did observe significantly increased expression of NQO1, indicating a cellular response to oxidative stress, and that the 2D cells were exposed to the increased oxygen levels (Fig. 4A). To ensure that the phenotype we saw was both lung specific and not an artifact of the 3D culture method itself, we isolated skin fibroblasts from neonatal foreskin and cultured these cells on the functionalized alginate beads, generating an organoid structure populated by skin fibroblasts. When cells were exposed to alternating hypoxia-hyperoxia, we saw no changes in α-SMA expression by IF or in expression of the panel of BPD-related genes by qPCR (Fig. 4B). Lung organoids comprised of primary adult lung fibroblasts did not demonstrate the pattern of fibroblast activation and proliferation in response to alternating hypoxia and hyperoxia that was observed in the FLFs (Fig. 4C). In fact, the adult lung fibroblast organoids exposed to variable oxygen exposure had significantly decreased expression of extracellular matrix genes such as Elastin, Col1A1, and Col3A1, with no change in α-SMA expression by qPCR or immunofluorescence. Therefore, the fibroblast proliferation and activation seen in the 3D model was specific to FLFs.
Fig. 4.
Changes in α-SMA expression and other genes associated with BPD were specific to FLFs cultured in 3D. A: IF for α-SMA and vimentin expression and BPD target gene expression pattern by qPCR in 2D FLF cultured in normoxia and hypoxia-hyperoxia conditions. Scale bar = 50 μm. B: IF for α-SMA and vimentin expression and BPD target gene expression pattern by qPCR in 3D skin fibroblasts cultured in normoxia and hypoxia-hyperoxia conditions. Scale bar = 50 μm. C: IF for α-SMA and vimentin expression and BPD target gene expression pattern by qPCR in 3D adult primary fibroblasts cultured in normoxia and hypoxia-hyperoxia conditions. Scale bar = 50 μm. Triplicate samples were used in each experiment. Experiments were repeated a minimum of 3 times. *P < 0.001.
Activation of the Notch pathway is a key driver of the fibrotic phenotype in our model and is also seen in human infants with BPD.
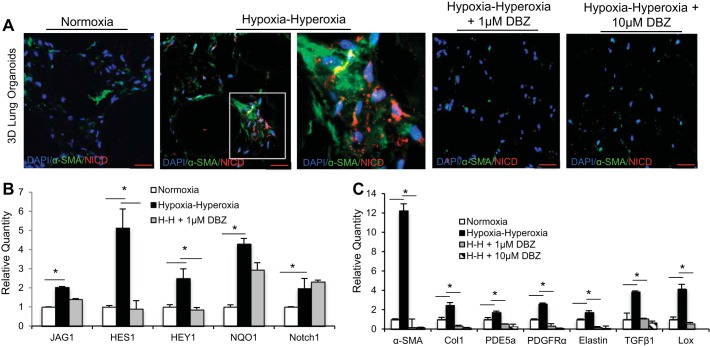
The Notch pathway has been shown by prior studies to play a critical role in lung development and septation, repair after injury to the airway, and oxidative stress response, and therefore we hypothesized that the Notch pathway may be involved in the development of fibroblast activation and differentiation in our model (15, 24, 32, 37, 38). We found that, in response to hypoxia-hyperoxia exposure, the areas of increased α-SMA expression in our model as observed by IF also demonstrated intranuclear staining for the activated form of Notch, NICD (Fig. 5A). When we pretreated organoids with dibenzazepine (DBZ), a γ-secretase-specific inhibitor of Notch activation and signaling, the organoids did not develop increased areas of α-SMA expression following exposure to hypoxia-hyperoxia, as was seen in the organoids that were not treated with DBZ (Fig. 5A). Additionally, the organoids pretreated with DBZ had no NICD demonstrated in the nuclei (Fig. 5A).
Fig. 5.
Notch pathway involvement in BPD pathophysiology in the 3D lung model. A: IF for α-SMA and NICD expression in 3D FLF lung organoids comparing normoxia to hypoxia-hyperoxia conditions, with and without pretreatment with Notch inhibitor DBZ. Examination of α-SMA-positive fibroblasts (inset) showed evidence of intranuclear presence of NICD. Scale bar = 50 μm. B: expression of downstream effectors of the Notch pathway, HES1 and HEY1, upstream targets JAG1 and NOTCH1 and oxidative stress enzyme NQO1 as measured by qPCR in response to hypoxia-hyperoxia and treatment with DBZ, *P < 0.01. C: comparison by qPCR of the gene expression pattern of molecular targets known to be upregulated in BPD between 3D organoids grown in normoxia and organoids exposed to hypoxia-hyperoxia, with and without pretreatment with Notch inhibitor DBZ, *P < 0.01. Triplicate samples were used in each experiment. Experiments were repeated a minimum of 3 times.
We also found evidence of Notch pathway upregulation in our model at the level of gene expression. Analyzing RNA from our model showed significantly increased transcription of NOTCH1 and JAG1 and downstream mediators HES1 and HEY1 (Fig. 5B). HES1, one of the key downstream effectors of Notch signaling, is known to be expressed in fetal lung (19). Treating our lung organoid model with DBZ decreased the expression of HES1 and HEY1 in response to hypoxia-hyperoxia, as would be expected with inhibition of γ-secretase. NOTCH1 and JAG1, which are upstream of this enzyme, and NQO1, a phase II enzyme upregulated in response to oxidative stress also upstream of γ-secretase, did not change their expression in response to DBZ following exposure to hypoxia-hyperoxia (Fig. 5B), which provides evidence that the inhibition by DBZ of the phenotype of fibroblast activation and α-SMA expression was occurring via the Notch pathway and not through off-target effects. Indeed, inhibiting Notch appeared to prevent the development of the hypoxia-hyperoxia phenotype completely, both at the level of α-SMA expression by immunostaining and at the level of gene expression for the panel of genes known to be upregulated in BPD (Fig. 5C). Thus inhibiting Notch signaling altered the FLF myofibroblast differentiation and gene-expression pattern that define phenotype in our model.
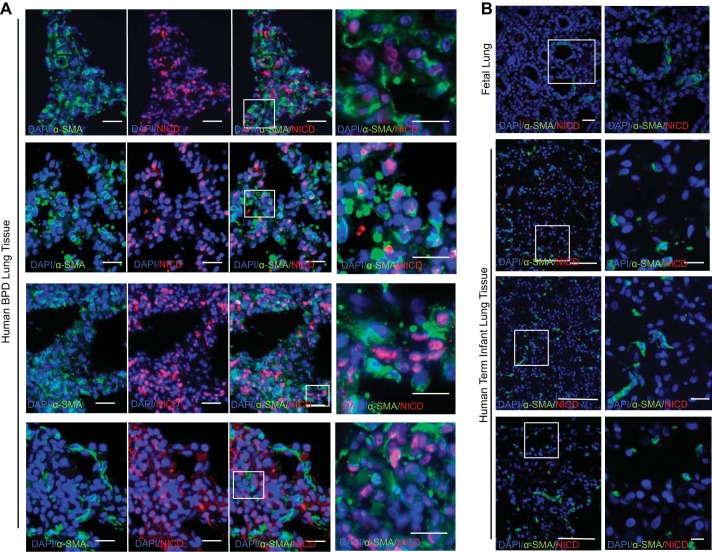
To determine whether the Notch pathway identified in our model was of clinical relevance, we examined NICD expression in fetal lung tissue and in lung tissue taken at autopsy from four infants who died with BPD. In the autopsy tissue from infants with BPD, we observed Notch activation, as evidenced by nuclear NICD expression by IF in the alveolar septae in cells that were also expressing α-SMA (Fig. 6A). Examination of fetal lung tissue by IF showed very little baseline expression of α-SMA and no intranuclear NICD (Fig. 6B), which suggests that the FLF used in our model do not have increased expression of α-SMA or Notch signaling prior to exposure to hypoxia-hyperoxia. As a histological comparison, we examined the lungs from term infants who were stillborn and found lower levels of α-SMA expression compared with the BPD patients and no evidence of Notch activation since there was no intranuclear expression of NICD observed (Fig. 6C).
Fig. 6.
Notch pathway involvement in patient samples. A: IF for α-SMA and NICD expression in autopsy tissue from the lungs of 4 human infants who died with BPD. Scale bar = 25 μm. B: IF for baseline α-SMA and NICD expression in fetal lung. Scale bar = 20 μm. C: IF for α-SMA and NICD expression in autopsy tissue from the lung of 3 stillborn term human infants. Scale bar = 25 μm.
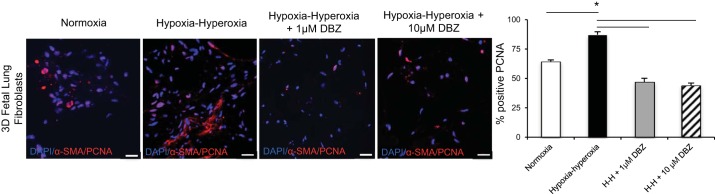
Another important aspect of the BPD phenotype in human infants is increased fibroblast proliferation (6). By PCNA staining, organoids exposed to hypoxia-hyperoxia demonstrated increased cellular proliferation compared with normoxic controls (Fig. 7). DBZ inhibition of Notch signaling altered the cellular proliferation of FLFs exposed to hypoxia-hyperoxia as evidenced by PCNA staining of the BPD lung organoids cultured with and without DBZ (Fig. 7). In response to hypoxia-hyperoxia exposure, lung organoids showed an increase in mitotic activity that decreased even below normoxia controls when the organoids were treated with DBZ (Fig. 7).
Fig. 7.
Inhibition of Notch decreased myofibroblast differentiation as well as cellular proliferation as measured by intranuclear PCNA expression. PCNA and α-SMA IF of organoids cultured in normoxia and hypoxia-hyperoxia exposure, with and without pretreatment with Notch inhibitor DBZ. Scale bar = 20 μm. We counted 200 cells in triplicate for each condition and reported as average % positive for each group, *P < 0.01. Triplicate samples were used in each experiment. Experiments were repeated a minimum of 3 times.
DISCUSSION
Much of our understanding of BPD pathophysiology comes from several highly developed animal models, including sheep, rat, mouse, rabbit, pig, and baboon, that are exposed to various environmental stimuli thought to be implicated in BPD, including hyperoxia, mechanical ventilation, and maternal infection (1, 4, 7, 11, 17, 30, 39). Although these animal models have taught us a great deal about BPD, there are key differences between other mammalian species and humans; for example, mice are born at term with lungs fully functional for gas exchange while still in the saccular stage of lung development, unlike their human counterparts (17). Of additional note, several targeted therapies have shown promise in animal models but have not shown benefit in humans, likely in part because of these developmental differences (17), which underscores the need for a human, in vitro model for the disease.
To create a human model of the mesenchymal changes associated with BPD, we used a new 3D primary cell lung organoid model developed by our laboratory to create a cellular scaffold that replicates the geometry of the interstitial region between alveoli. In this model, alternating hypoxia and hyperoxia resulted in fibroblast proliferation, myofibroblast differentiation, and gene expression changes that replicate many of the changes seen in human infants with BPD. Alternating hypoxia and hyperoxia has been used in prior animal models (4) and appears consistent with the clinical course of premature infants, who are exposed to widely variable oxygen saturations, especially in the initial period after birth (21). The observed changes in α-SMA gene expression and protein expression by IF in response to hypoxia-hyperoxia are found in human infants with BPD and in animal models of BPD, thus validating the phenotypic response seen in our model in response to hypoxia-hyperoxia as a model of the mesenchymal component of BPD (3, 10, 18, 20, 28).
Prior work has demonstrated that pulmonary mesenchyme is a critical driver of normal lung development (25). Specifically, mesenchymal cells in the developing lung have been shown to direct the processes of branching morphogenesis, epithelial differentiation, pulmonary vascular development, and secondary septation (3, 25–27). During the saccular stage of lung development, the alveolar septae are thickened and enriched in fibroblasts, and signaling events from these fibroblasts drive the process of alveolarization (5). Pulmonary fibroblasts are important cells in the pathogenesis of BPD, since the fibroblasts are thought to respond to environmental stress through paracrine signaling, which drives their proliferation, activation to myofibroblasts, migration into the alveolar septae, and deposition of extracellular matrix (3, 23). In developing our human model of the histopathological changes in BPD, we looked to the FLF as the cell type that could best generate an observable myofibroblast phenotype in response to variable oxygen concentrations.
One limitation of our model is that it includes a single mesenchymal cell type with the notable absence of the contribution from alveolar epithelial and endothelial cells. However, by focusing on the contribution of one isolated cell type, our 3D lung model allowed us to specifically assess the mesenchymal component of the disease as well as the role of pulmonary mesenchyme in expressing various genes that contribute to BPD pathophysiology, such as PDGFRα, α-SMA, and TGFβ. In the future, we plan to exploit the modularity of this model and incorporate additional lung cell types to study their interactions, e.g., mesenchymal cells, endothelial cells, and epithelial cells, to gain insight into how communication between different cell types can drive the pathophysiology of this disease.
Our lung organoid recreates the 3D niche of FLFs growing in close proximity in the alveolar interstitium. One possible reason for differences between the results in the cells cultured in 3D compared with 2D is that the 3D culture system likely creates a microenvironment that allows the FLFs to engage in cell signaling by direct contact between adjacent cells, and through activation via paracrine signaling (33), which we speculate drives the pathophysiology both in the model of the disease and in vivo (33, 35). For Notch signaling to occur, the cell expressing Notch ligand must be in direct contact with a neighboring cell expressing the Notch receptor. Our 3D model affords cells the opportunity to grow in close contact in the anatomically correct locations that allow this specific cell-cell contact and therefore these signaling events to take place.
Although the creation of this 3D microenvironment in our organoid was necessary to develop the mesenchymal phenotype in response to hypoxia-hyperoxia, it was not sufficient. The FLF cell type grown in the organoid was critical for differentiation to myofibroblasts and expression of genes known to be upregulated in BPD because we did not observe this phenotype with neonatal skin fibroblasts or adult pulmonary fibroblasts. This is consistent with the development of BPD occurring only during a specific window in development in preterm infants and is congruous with the observation that adults exposed to hyperoxia and mechanical ventilation do not develop the same pathology as premature infants with the same exposures (9, 12). It is also consistent with the pathogenesis of BPD being dependent on developmental pathways. In addition, the recreation of the 3D fibroblast microenvironment in our model is scalable to a 96-well format, which provides a critical advance for developing potential future applications, such as a HT drug screen to prevent differentiation to myofibroblasts. These HT organoids also can provide a platform for the future study of multiple molecular pathways.
Although major advances in lung cellular biology and development have been made over the past 50 years, the precise cellular pathways and properties that regulate the pathophysiology of BPD remain elusive (13). Since BPD is defined by a developmental arrest in alveolar septation, we were interested in examining our model to see whether there were developmental programs involved in the mechanism of the development of the fibrotic phenotype as well. It has been previously shown that, in response to oxidative stress, Nrf2 activates the Notch pathway in other cell types in the lung (32) and liver (36, 37) by binding to antioxidant response elements in the Notch promoter and also that the Notch pathway is known to be activated in response to airway injury (38); overexpression of Notch is also known to inhibit alveolar septation (38), a process that is disrupted in BPD (2). Moreover, the Notch pathway has also been implicated in myofibroblast differentiation and the development of fibrosis in other pulmonary diseases (24). We found that the Notch pathway drove the cellular proliferation and differentiation to α-SMA-positive myofibroblasts in our model and also demonstrated evidence for increased activated Notch expression by myofibroblasts in the lungs of human infants with BPD, and we did not see the presence of activated Notch in the lungs of term infants. The identification of the Notch pathway in BPD is significant; although Notch pathway expression in response to airway injury in the proximal airways has been well-described (29), Notch has not previously been identified in BPD pathophysiology and presents a new opportunity to look for druggable targets that interact with this pathway and its downstream effectors.
In summary, we have developed an in vitro 3D human model of fibroblast activation associated with BPD in a novel culture system that replicates the alveolar architecture found in human distal lung. The phenotypic changes in our model have been validated by comparison with human BPD lung autopsy tissue and qPCR for specifically upregulated genes in BPD. Using our model, we have been able to augment our understanding of BPD pathophysiology, identifying the Notch pathway as being a key driver of the development of the phenotype of myofibroblast differentiation and gene expression in our model. The model has allowed us to identify that the Notch pathway is activated in the distal lung in BPD and may be a driver of the pathophysiology seen in BPD. Since we are now able to generate HT organoids, a future step would be to increase the scale of our investigation to identify additional molecular pathways and targets that may be perturbed in the clinical setting of changes in oxygen tension. By creating this HT human in vitro model of the disease we hope to create a 3D HT drug screen that may foster the discovery of novel therapies to improve the survival and outcomes for premature infants.
GRANTS
This work was supported by a Clinical Fellowship Training Grant from the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research and the California Institute of Regenerative Medicine and through the Scholars in Translational Medicine Program at the Broad Stem Cell Research Center (J. M. S. Sucre). The work was also supported by R01 GM114259-01 and California Institute for Regenerative Medicine (CIRM) 12-02 (B. N. Gomperts) and NSF DGE-1144087 (D. Wilkinson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.S.S., D.W., P.V., M.K.P., B.D., J.A.A.-O., and B.N.G. conception and design of research; J.M.S.S., D.W., P.V., M.K.P., and J.A.A.-O. performed experiments; J.M.S.S., P.V., M.K.P., and J.A.A.-O. analyzed data; J.M.S.S., D.W., P.V., M.K.P., J.A.A.-O., and B.N.G. interpreted results of experiments; J.M.S.S. prepared figures; J.M.S.S. drafted manuscript; J.M.S.S., P.V., J.A.A.-O., and B.N.G. edited and revised manuscript; B.N.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Ved Londhe, April Pyle, and Sherin Devaskar for helpful discussions about our model and experiments and to Brian Hackett for careful and thoughtful review of the manuscript.
REFERENCES
- 1.Albertine KH. Utility of large-animal models of BPD: chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol 308: L983–L1001, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946–1955, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol 292: L550–L558, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 307: L936–L947, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Tornell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 57: 38R–46R, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Caminita F, van der Merwe M, Hance B, Krishnan R, Miller S, Buddington K, Buddington RK. A preterm pig model of lung immaturity and spontaneous infant respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 308: L118–L129, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med 38: 325–343, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cuna A, Halloran B, Faye-Petersen O, Kelly D, Crossman DK, Cui X, Pandit K, Kaminski N, Bhattacharya S, Ahmad A, Mariani TJ, Ambalavanan N. Alterations in gene expression and DNA methylation during murine and human lung alveolar septation. Am J Respir Cell Mol Biol 53: 60–73, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Angio CT, Ryan RM. Animal models of bronchopulmonary dysplasia. The preterm and term rabbit models. Am J Physiol Lung Cell Mol Physiol 307: L959–L969, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Datta A, Kim GA, Taylor JM, Gugino SF, Farrow KN, Schumacker PT, Berkelhamer SK. Mouse lung development and NOX1 induction during hyperoxia are developmentally regulated and mitochondrial ROS dependent. Am J Physiol Lung Cell Mol Physiol 309: L369–L377, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadchouel A, Franco-Montoya ML, Delacourt C. Altered lung development in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100: 158–167, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol 50: 233–245, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu B, Wu Z, Bai D, Liu T, Ullenbruch MR, Phan SH. Mesenchymal deficiency of Notch1 attenuates bleomycin-induced pulmonary fibrosis. Am J Pathol 185: 3066–3075, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100: 145–157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2: 49, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaarteenaho-Wiik R, Kinnula VL, Herva R, Soini Y, Pollanen R, Paakko P. Tenascin-C is highly expressed in respiratory distress syndrome and bronchopulmonary dysplasia. J Histochem Cytochem 50: 423–431, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Katoh M, Katoh M. Integrative genomic analyses on HES/HEY family: Notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. Int J Oncol 31: 461–466, 2007. [PubMed] [Google Scholar]
- 20.Kumarasamy A, Schmitt I, Nave AH, Reiss I, van der Horst I, Dony E, Roberts JD Jr, de Krijger RR, Tibboel D, Seeger W, Schermuly RT, Eickelberg O, Morty RE. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med 180: 1239–1252, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakshminrusimha S, Manja V, Mathew B, Suresh GK. Oxygen targeting in preterm infants: a physiological interpretation. J Perinatol 35: 8–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KJ, Berkelhamer SK, Kim GA, Taylor JM, O'Shea KM, Steinhorn RH, Farrow KN. Disrupted pulmonary artery cyclic guanosine monophosphate signaling in mice with hyperoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 50: 369–378, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Li Y, He H, Liu C, Li W, Xie L, Zhang Y. Csk/Src/EGFR Signaling regulates migration of myofibroblasts and alveolarization. Am J Physiol Lung Cell Mol Physiol 310: L562–L571, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol 174: 1745–1755, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev 32: 98–105, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGowan SE. Paracrine cellular and extracellular matrix interactions with mesenchymal progenitors during pulmonary alveolar septation. Birth Defects Res A Clin Mol Teratol 100: 227–239, 2014. [DOI] [PubMed] [Google Scholar]
- 27.McGowan SE, McCoy DM. Fibroblast growth factor signaling in myofibroblasts differs from lipofibroblasts during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol 309: L463–L474, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan SE, McCoy DM. Fibroblasts expressing PDGF-receptor-alpha diminish during alveolar septal thinning in mice. Pediatr Res 70: 44–49, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Varelas X, Herrick DB, Schwob J, Zhang H, Cardoso WV. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development 142: 258–267, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Reilly M, Thebaud B. Animal models of bronchopulmonary dysplasia. The term rat models. Am J Physiol Lung Cell Mol Physiol 307: L948–L958, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos P, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, Alva-Ornelas JA, Gomperts BN. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 15: 199–214, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD. 3D cell culture systems: advantages and applications. J Cell Physiol 230: 16–26, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Silva DM, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 309: L1239–L1272, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles SA. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 10: 29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakabayashi N, Chartoumpekis DV, Kensler TW. Crosstalk between Nrf2 and Notch signaling. Free Radic Biol Med 88: 158–167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi N, Skoko JJ, Chartoumpekis DV, Kimura S, Slocum SL, Noda K, Palliyaguru DL, Fujimuro M, Boley PA, Tanaka Y, Shigemura N, Biswal S, Yamamoto M, Kensler TW. Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol Cell Biol 34: 653–663, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol 727: 89–98, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Yoder BA, Coalson JJ. Animal models of bronchopulmonary dysplasia. The preterm baboon models. Am J Physiol Lung Cell Mol Physiol 307: L970–L977, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia — trends over three decades. Paediatr Child Health 18: 86–90, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]