Abstract
Agonists of adenosine A2A receptors (A2ARs) suppress the activation of most immune cells and reduce acute inflammatory responses. Asthma is characterized by sensitization in response to initial allergen exposure and by airway hyperreactivity in response to allergen rechallenge. We sought to determine if A2AR activation with CGS-21680 (CGS) is more effective when CGS is administered during sensitization or rechallenge. C57BL/6 wild-type mice and Adora2af/fLysMCre+/− mice, which lack A2ARs on myeloid cells, were sensitized with intranasal ovalbumin (OVA) and LPS. Airway sensitization was characterized by a rapid increase in numbers of IL-6+ and IL-12+ macrophages and dendritic cells in lungs. A2AR activation with CGS (0.1 μg·kg−1·min−1 sc) only during sensitization reduced numbers of IL-6+ and IL-12+ myeloid cells in the lungs and reversed the effects of OVA rechallenge to increase airway hyperresponsiveness to methacholine. CGS treatment during sensitization also reduced the expansion of lung T helper (Th1 and Th17) cells and increased expansion of regulatory T cells in response to OVA rechallenge. Most of the effects of CGS administered during sensitization were eliminated by myeloid-selective A2AR deletion. Administration of CGS only during OVA rechallenge failed to reduce airway hyperresponsiveness. We conclude that myeloid cells are key targets of adenosine during sensitization and indirectly modify T cell polarization. The results suggest that a clinically useful strategy might be to use A2AR agonists to inhibit sensitization to new aeroallergens. We speculate that adenosine production by macrophages engulfing bacteria contributes to the curious suppression of sensitization in response to early-life infections.
Keywords: adenosine, adenosine A2A receptor, asthma, allergen, macrophages
lung inflammation and airway hyperresponsiveness are hallmarks of asthma, which can be treated with anti-inflammatory agents such as corticosteroids and leukotriene D4 antagonists. Either T helper 1/17 (Th1/Th17)- or Th2-dominated immune responses to aeroallergens mediate asthma pathogenesis in different individuals, depending on the degree of co-exposure to LPS from infection or inhalation of dust mites (23). LPS enhances Th1 priming with the production of IFN-γ and IL-1 (14), whereas the absence of LPS favors Th2 priming with the production of IL-4 and IL-13 (26). Hence, atopic asthma is recognized to be a heterogeneous disease with different inflammatory profiles (15). Zhu et al. (48) found in mice that low levels of LPS exposure, along with allergen, induce the production of TNF-α by dendritic cells (DCs), which enhances IL-4 and IL-13 production by mast cells and natural killer T (NKT) cells. These cytokines favor naïve T cell differentiation into Th2 cells and subsequent eosinophilic inflammation. In the presence of high LPS, allergens stimulate macrophages and DCs to produce IL-6 and IL-12, which promote differentiation of naïve T cells into Th1 and Th17 cells, which respond to allergens by producing cytokines such as IFN-γ and IL-17 to promote airway inflammation with neutrophils and macrophages, but not eosinophils (48).
Adenosine activates four G protein-coupled receptors: A1, A2A, A2B, and A3 (29). Activation of the A2A receptor (A2AR), which is expressed on most cells of the immune system, generally acts to suppress Th1 inflammatory processes through Gs-coupled receptors, which elevate cAMP in neutrophils (39, 40), macrophages (35), T cells (28), and NKT cells (27). By reducing inflammation, A2AR activation indirectly influences airway responsiveness (13) in a way that is fundamentally different from direct airway bronchodilation in response to β2-adrenergic agonists (17). In the current study we examined the effects of A2AR activation in a high-LPS mouse model of asthma that is associated with Th1/Th17 priming. The results indicate that A2AR activation effectively prevents airway sensitization but marginally reduces airway hyperresponsiveness when added during allergen rechallenge. Using Adora2af/fLysMCre+/− mice, we demonstrate that suppression of aeroallergen-induced sensitization by 2-[4-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine [CGS-21680 (CGS)] 1) principally depends on myeloid A2ARs, 2) is associated with reduced myeloid production of IL-6 and IL-12 and enhanced production of transforming growth factor (TGF)-β, 3) is associated with airway expansion of regulatory T (Treg) cells, and 4) is associated with reduced production of Th1 and Th17 cells. These findings suggest that it may be therapeutically effective to treat allergic individuals with A2AR agonists during their initial exposures to antigens in a new workplace or habitat.
MATERIALS AND METHODS
Mice.
Eight- to 10-wk-old male C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Adora2af/fLysMCre+/− and Adora2af/fLysMCre−/− littermate controls were generated by crossing C57BL/6 LysMCre+ mice with congenic Adora2af/f mice produced as described elsewhere (8). The Animal Care and Use Committee of the La Jolla Institute approved experiments in accordance with National Institutes of Health guidelines.
Mouse sensitization, rechallenge, and treatment with CGS.
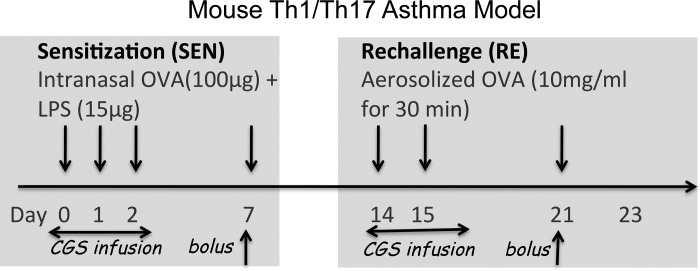
Mice were sensitized by intranasal administration of 100 μg of ovalbumin (OVA) and 15 μg of LPS (OVA-LPS) in a total volume of 20 μl on days 0, 1, 2, and 7. Mice were rechallenged with three exposures for 30 min to aerosolized 1% OVA in PBS on days 14, 15, and 21. This regimen is associated with production of Th1/Th17 cells and neutrophil recruitment into alveoli (24). For evaluation of the in vivo effects of treatment with an A2AR agonist, mice were treated with CGS (21) (100 ng·kg−1·min−1) administered via 3-day Alzet minipumps implanted subcutaneously 5 h before day 0 sensitization and with a bolus (1 μg/kg ip) 30 min before day 7 sensitization. Other mice were treated with CGS only during OVA rechallenge (Fig. 1). CGS treatment via Alzet minipumps was started 24 h before day 14 rechallenge and with a bolus (1 μg/kg ip) 30 min before day 21 rechallenge.
Fig. 1.
Scheme for sensitizing mice and for treatment with the adenosine A2A receptor (A2AR) agonist CGS-21680 (CGS) during allergen sensitization (SEN) or rechallenge (RE). Mice were sensitized with 4 intranasal doses of ovalbumin (OVA)-LPS over 7 days and rechallenged with 3 doses of aerosolized OVA on days 14, 15, and 21. For evaluation of the in vivo effects of CGS in response to OVA, mice were treated with vehicle or 100 ng·kg−1·min−1 CGS administered from 3-day Alzet minipumps implanted subcutaneously 5 h before day 0 sensitization and with a bolus injection of CGS (1 μg/kg ip) 30 min before day 7 sensitization. For evaluation of in vivo effects of CGS in response to OVA rechallenge, mice were treated with CGS via Alzet minipump plus a bolus injection during rechallenge, instead of during sensitization. Mice were euthanized on day 3, 21, or 23.
Vascular permeability.
Pulmonary vascular leak was determined by measurement of the extravasation of Evans blue dye (EBD) from the circulation to the lungs. EBD (30 mg/kg body wt in 200 μl) was injected intravenously in mice anesthetized with ketamine-xylazine and allowed to circulate for 30 min. The chest was opened, the inferior vena cava was transected, and the pulmonary vasculature was flushed with 10 ml of saline via the right ventricle to remove intravascular dye. The lung was homogenized and incubated in 100% formamide at 37°C for 24 h to extract EBD. The concentration of dye was determined by spectrophotometry with correction for heme pigments as previously described using the following equation: E620(corrected) = E620 − (1.426 × E740 − 0.03) (45). Data are expressed as micrograms of EBD per gram of lung.
Airway response to methacholine challenge.
After sensitization with intranasal OVA-LPS and rechallenge with aerosolized OVA, mice were treated with aerosolized methacholine at doses of 0, 6.25, 12.5, and 25 mg/ml in PBS. Methacholine-induced changes in enhanced pause (Penh) were determined using noninvasive whole body plethysmography (Buxco Electronic) according to the manufacturer's instructions.
Pulmonary immunohistochemistry and histopathological grading.
Lung inflammation was scored on a scale of 0–4 as described elsewhere (2). After perfusion with PBS, lungs were fixed overnight with 4% paraformaldehyde. Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin.
Staining of immune cells for flow cytometry.
After bronchoalveolar lavage (BAL) to remove alveolar cells, lungs were removed, minced, and incubated in digestion buffer containing 1 mg/ml collagenase type Ia, 60 U/ml hyaluronidase type I-s, and 60 U/ml DNase I for 45 min at 37°C. Single cells were prepared by passage of digested tissue through a 40-μm cell strainer. Cells were resuspended at 2 × 106 cells/ml in RPMI 1640 medium and incubated in 48-well plates with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 h, with the addition of GolgiStop (BD Bioscience) during the last 2 h. Fcγ receptors were blocked, and live cells were stained (LIVE/DEAD Fixable Yellow Dead Cell Stain Kit, Invitrogen) to detect surface markers. Some cells were fixed and permeabilized for intracellular staining of IL-17A, IFN-γ, Foxp3, IL-6, and IL-12p40. Stained cells were analyzed using a flow cytometer (LSR II, BD Bioscience), and data were analyzed using FlowJo version 9.6.4 software (Tree Star).
Cell numbers and cytokines in BAL fluid.
Mice were euthanized using CO2, and tracheae were cannulated. The lungs were flushed three times with 1 ml of PBS. The recovered fluid from the 1st ml was used for cytokine measurements. Total cells in the pooled BALs were counted with a Vi-CELL XR cell counter. To determine the composition of cells in the BAL, ∼2 × 103 cells were spun onto glass slides using a Cytospin cytocentrifuge and stained with a PROTOCOL Hema 3 stain set (Fisher Scientific). At least 300 cells were counted and identified as macrophages, lymphocytes, neutrophils, or eosinophils according to standard morphological criteria. Cytokines in BAL were measured by ELISA (eBioscience) according to the manufacturer's instructions.
Analysis of A2AR responses in spleen-derived Th1 and Th17 cells.
Splenocytes were isolated from 8-wk-old C57BL/6J mice. CD4+/CD62L+ cells were prepared using T Cell Isolation Kit II (Miltenyi Biotech, Auburn, CA). One × 105 cells/well were cultured in 200 μl of RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 mM HEPES in a 96-well plate. Th1-polarizing conditions were produced by coating plates with 3 μg/ml anti-CD3 Ab (BD Pharmingen, San Diego, CA) and then adding 2 μg/ml anti-CD28 Ab (BD Pharmingen), 20 ng/ml IL-2 (R & D Systems, Minneapolis, MN), 20 ng/ml IL-12 (R & D Systems), and 10 μg/ml anti-IL-4 (R & D Systems) to T cell cultures. Th17-polarizing conditions were produced by coating plates with 3 μg/ml anti-CD3 Ab and then adding 2 μg/ml anti-CD28, 5 ng/ml TGF-β (PeproTech, Rocky Hill, NJ), 100 ng/ml IL-6 (R & D Systems), 10 μg/ml anti-IL-4 (R & D Systems), and 10 μg/ml anti-IFN-γ (R & D Systems). After 5 days in culture, cells were washed, and CGS or vehicle was added to the cells 30 min before restimulation with anti-CD3 (3 μg/ml) and anti-CD28 (2 μg/ml). After restimulation for 24 h, supernatants were collected for cytokine measurements.
Statistical analysis.
Prism version 6 software (GraphPad) was used for statistical analysis. Data are presented as means ± SE. Unpaired Student's t-test, one-way ANOVA with Tukey's post test, or two-way ANOVA with Bonferroni's post test was used as appropriate to compare experimental groups. P < 0.05 was considered to be significant.
RESULTS
Airway disease was produced by sensitization of mice with intranasal OVA (100 μg) in combination with a high dose of LPS (15 μg) administered four times over 7 days (24). This was followed by rechallenge with aerosolized OVA (10 mg/ml for 30 min) on days 14, 15, and 21 (Fig. 1). In prior studies, similar mouse models of asthma provoked the rapid accumulation of pulmonary macrophages and DCs. Antigen rechallenge in such models causes an expansion of Th1 and Th17 cells and an increase in airway responsiveness to methacholine challenge (24, 25). Our strategy for studying the effects of CGS on the allergic disease process is illustrated in Fig. 1. Since innate immune responses occur rapidly, we measured the effects of CGS on lung myeloid cells and myeloid cytokine accumulation in the BAL acutely, on day 3 following OVA-LPS sensitization on days 0, 1, and 2. Because adaptive immune responses are manifest more slowly, T cell accumulation in the lung and lymphokine accumulation in the BAL were sampled on day 21, following OVA rechallenge on days 14, 15, and 21. Airway hyperresponsiveness and lung inflammation were measured on day 23 following OVA rechallenge on days 14, 15, and 21, based on prior reports suggesting that this corresponds to the time of peak responses.
A2ARs are expressed on multiple cells of the immune system, including macrophages, DCs, neutrophils, and T cells. Since macrophages and DCs present antigens to T cells and produce cytokines that influence T cell polarization, we reasoned that A2AR signaling in myeloid cells might influence OVA sensitization. To test this hypothesis, myeloid-selective A2AR deletion was produced in Adora2af/fLysMCre mice as described elsewhere (7).
Effects of CGS on pulmonary myeloid cells during sensitization.
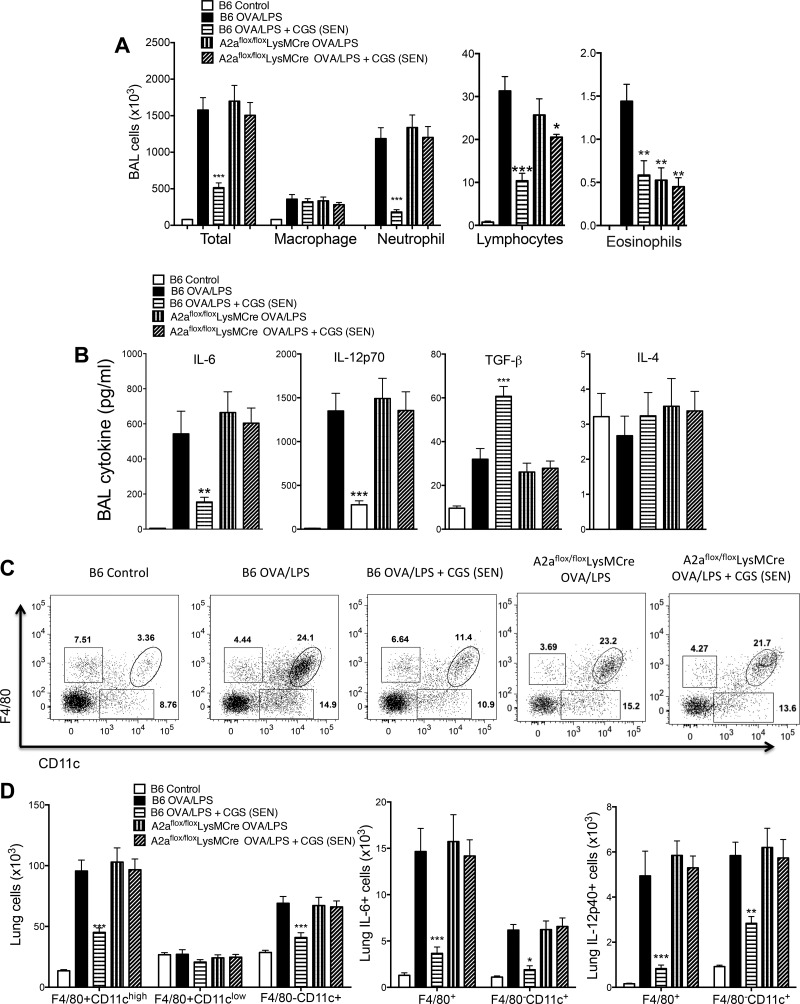
Airway sensitization following three exposures to intranasal OVA-LPS triggered a marked increase in inflammatory cells in the BAL on day 3 (Fig. 2A). CGS significantly inhibited the accumulation of total cells, neutrophils, lymphocytes, and eosinophils in the BAL. The total number of macrophages in the BAL was not significantly affected by CGS. In a similar mouse model, Bonneau et al. (5) also found that, among cells that accumulate in the BAL in response to OVA, only macrophage numbers are not affected by intranasal CGS. These findings suggest that factors produced in acutely inflamed alveoli that are chemotactic to circulating monocytes or lung macrophages are not strongly influenced by CGS. In Adora2af/fLysMCre+ mice in which A2ARs are deleted from macrophages and neutrophils (7), the effect of CGS on total cells and neutrophil accumulation into the BAL was abolished (Fig. 2A), suggesting that neutrophil chemotaxis is controlled by A2ARs on myeloid cells and, perhaps, in part by cell-intrinsic A2ARs (12). BAL lymphocyte accumulation in response to CGS was attenuated by myeloid-selective A2AR deletion (Fig. 2A), suggesting that lymphocyte chemotaxis is partly controlled by myeloid A2ARs and partly by T cell-intrinsic receptors. Only very small numbers of eosinophils accumulated in the BAL as expected with this asthma model. The chemotaxis of eosinophils was inhibited by CGS, and this inhibition was not affected by myeloid-selective A2AR deletion (Fig. 2A), suggesting that eosinophil chemotaxis may be controlled by eosinophil-intrinsic A2AR signaling or chemotactic cytokine production by nonmyeloid cells (1).
Fig. 2.
Effects of A2AR activation on pulmonary myeloid responses during OVA-LPS sensitization. C57BL/6J wild-type mice (B6 Control) or mice with myeloid-selective A2AR deletion [Adora2af/fLysMCre (A2aflox/floxLysMCre)] were treated with vehicle or CGS only during sensitization (SEN). Bronchoalveolar lavage (BAL) cells or cells harvested from digested lung after BAL (lung cells) were prepared on day 3 (see Fig. 1). A: numbers of various cells in BAL as determined after centrifugation (Cytospin) by morphology and staining with the PROTOCOL Hema 3 stain set. B: BAL cytokine concentrations. C: representative fluorescein-activated cell sorting (FACS) plots of lung cells stained for F4/80 and CD11c. D: effects of CGS treatment and myeloid A2AR deletion on numbers of lung myeloid cells. Values are means ± SE (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. B6 OVA/LPS (by 1-way ANOVA and Tukey's multiple comparison tests).
We also examined the effects of CGS on the acute accumulation of cytokines in the BAL in response to OVA-LPS (Fig. 2B). CGS treatment during sensitization significantly blunted the accumulation of IL-6 and IL-12 and enhanced the production of TGF-β. The effects of CGS were reversed completely in Adora2af/fLysMCre+ mice with myeloid-selective A2AR deletion, suggesting that the source of these cytokines is macrophages that are regulated by A2AR signaling.
We next examined populations of myeloid cells derived from enzymatically dispersed lungs following BAL to remove most immune cells from alveoli. As shown in Fig. 2, C and D, CGS administered during OVA-LPS sensitization significantly reduced the rapid (day 3) accumulation of F4/80+/CD11c+ (composed of activated macrophages) and F4/80− CD11c+ (composed of DCs) cells. Increases in numbers of these myeloid cells were significantly reduced by A2AR activation with CGS administered during sensitization. Among the myeloid cells that accumulated in the lungs in response to OVA sensitization were IL-6+ and IL-12+ cells. CGS treatment reduced the numbers of lung myeloid cells expressing these cytokines. The effects of CGS were completely eliminated in Adora2af/fLysMCre+ mice lacking myeloid A2ARs.
Effects of CGS on pulmonary T cell polarization.
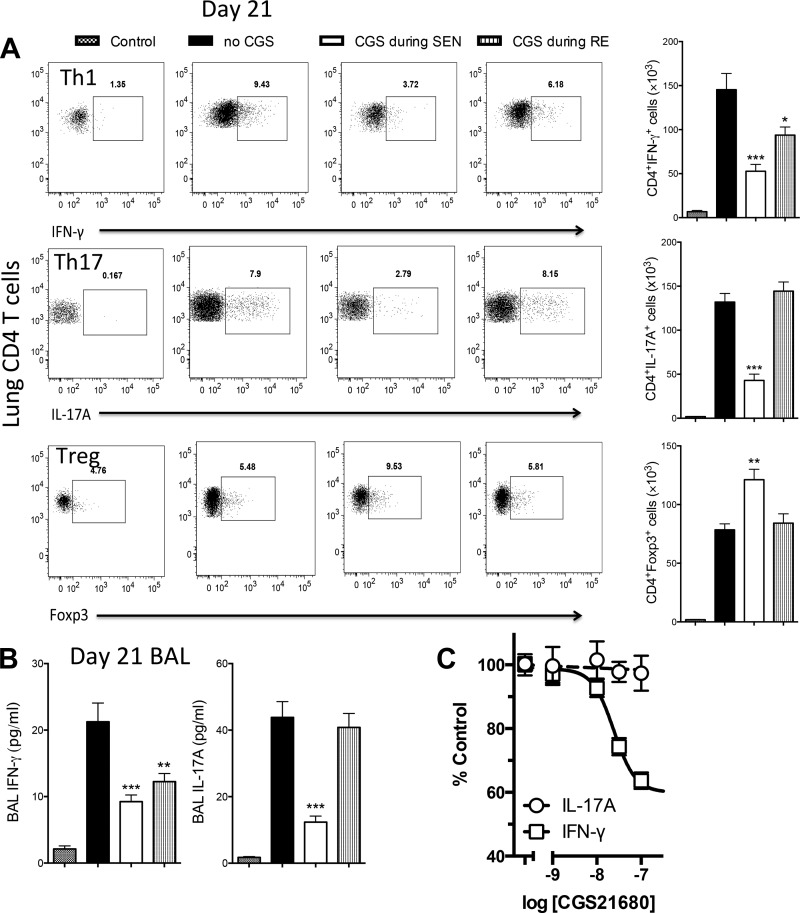
We next sought to determine if CGS added only during OVA sensitization or only during OVA rechallenge produced any modification of pulmonary T cells that appear in the lung during OVA rechallenge. Numbers of T cell subsets in enzymatically dispersed lungs (after washout of BAL cells) were measured on day 21 (Fig. 1). Intracellular staining for IFN-γ, IL-17A, and Foxp3 was used to define Th1, Th17, and Treg cells, respectively. As shown in Fig. 3A, OVA sensitization and rechallenge resulted in substantial increases in numbers of Th1, Th17, and Treg cells in the lung. Addition of CGS only during OVA-LPS sensitization reduced the numbers of Th1 and Th17 cells and significantly increased the number of Treg cells. Addition of CGS only during OVA rechallenge modestly reduced the number of Th1 cells but failed to influence the number of Th17 or Treg cells. BAL cytokines paralleled these responses: CGS treatment during sensitization reduced BAL IFN-γ and IL-17 (measured on day 21), whereas CGS added only during rechallenge reduced BAL IFN-γ, but not IL-17 (Fig. 3B). These findings suggest that A2AR activation reduces the polarization of naïve T cells to Th17 cells that occurs during sensitization but does not influence IL-17 release from effector Th17 cells during OVA rechallenge. To investigate this further, we measured the acute effects of CGS directly on spleen-derived Th1 and Th17 cells in vitro. As illustrated in Fig. 3C, Th1, but not Th17, cells are inhibited by A2AR activation.
Fig. 3.
Effects of CGS treatment on lung and spleen T cells and BAL cytokines. Mice were treated with CGS only during OVA-LPS sensitization (SEN) or only during OVA rechallenge (RE). CD4+ T cells were prepared from lungs on day 21 (see Fig. 1) and stained for intracellular cytokines or Foxp3. A: representative FACS plots and average total numbers of lung IFN-γ+, IL-17A+, and Foxp3+ cells gated on CD4+ pulmonary lymphocytes derived from enzymatically dispersed lungs after BAL. B: effects of CGS added only during OVA sensitization or only during OVA rechallenge on concentrations of IL-17A and IFN-γ in BAL measured by ELISA on day 21. C: naïve T cells purified from the spleen were differentiated into Th1 or Th17 cells in vitro (see materials and methods). Cells were then activated for 24 h with various amounts of CGS, and IFN-γ (Th1) and IL-17A (Th17) in the supernatants were measured by ELISA. CGS did not affect IL-17A production but inhibited IFN-γ production, with an IC50 of 24 nM. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control (by 1-way ANOVA and Tukey's multiple comparison tests).
Effects of CGS on airway responsiveness to methacholine, vascular leak, and inflammation.
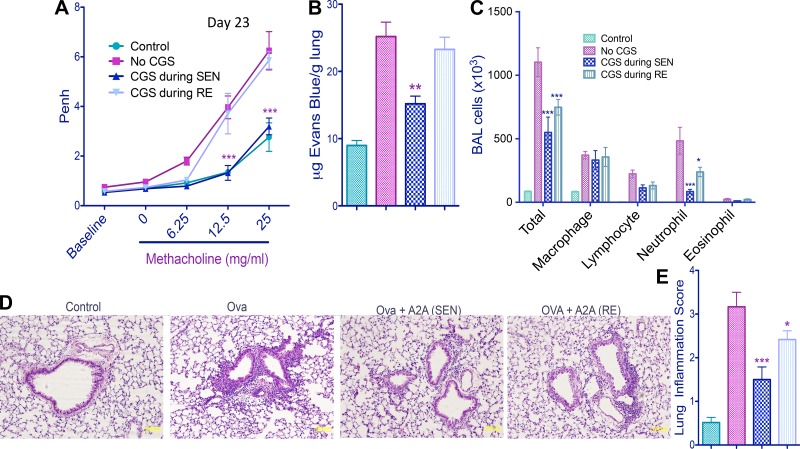
We next sought to determine how exposure to CGS during OVA-LPS sensitization affects pulmonary hyperresponsiveness to methacholine and inflammatory responses during subsequent OVA rechallenge. As shown in Fig. 4A, exposure to CGS only during OVA-LPS sensitization nearly completely prevented airway hyperresponsiveness to methacholine following rechallenge with aerosolized OVA. In contrast, exposure to CGS only during rechallenge with OVA produced a small statistically insignificant reduction in responsiveness to methacholine. Airway inflammation in response to OVA-LPS sensitization and rechallenge was also inhibited much more effectively by CGS added only during sensitization than only during rechallenge. These inflammatory responses include pulmonary vascular leak (Fig. 4B), neutrophil accumulation in the BAL (Fig. 4C), and lung inflammation (Fig. 4, D and E).
Fig. 4.
Effects of CGS treatment only during OVA-LPS sensitization (SEN) or only during OVA rechallenge (RE) on airway responsiveness to methacholine and lung inflammation. Control mice were not exposed to OVA or LPS. Other mice were challenged with OVA and LPS (see Fig. 1). Data were collected from C57BL/6J mice (n = 6) on day 23. A: dose-dependent changes in enhanced pause (Penh) in response to methacholine. ***P < 0.001 (by repeated-measures ANOVA and Bonferroni's multiple comparison test). B: pulmonary vascular leak assessed by accumulation of Evans blue dye uptake into the lung. C: accumulation of various cells into the BAL as determined after centrifugation (Cytospin) by morphology and staining with the PROTOCOL Hema 3 stain set. D: representative images of hematoxylin-eosin-stained lungs. “A2A” indicates A2AR activation by CGS-21680. Original magnification ×20. E: inflammation scores (see materials and methods) calculated by analysis of data from hematoxylin-eosin-stained mouse lungs. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. OVA/LPS (by 1-way ANOVA and Tukey's multiple comparison tests).
Blockade of airway hyperresponsiveness by CGS is mediated by A2ARs on myeloid cells.
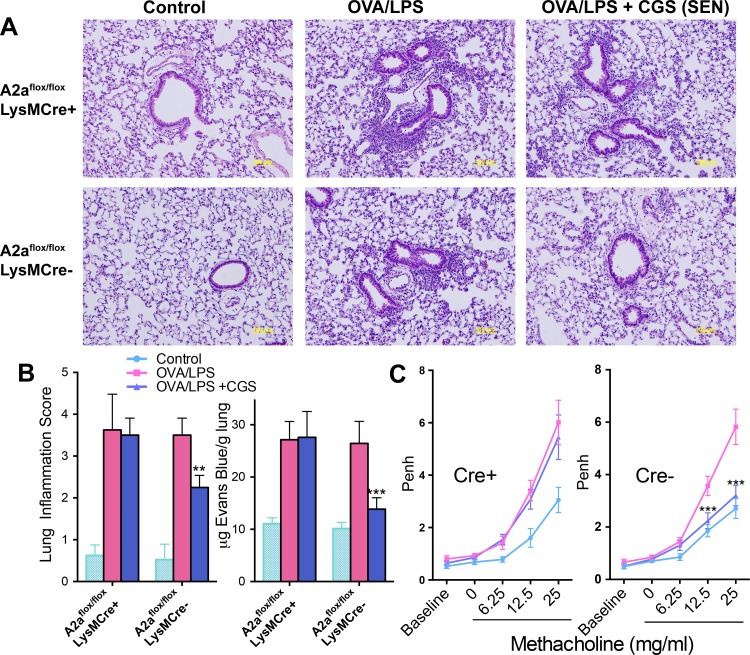
As shown in Fig. 5, the effects of CGS administered during OVA-LPS sensitization to reduce lung inflammation, vascular leak, and respiratory hyperresponsiveness to methacholine were absent in mice with myeloid-selective A2AR deletion. These findings suggest that A2ARs on myeloid cells are primarily responsible for inhibition of aeroallergen sensitization by A2AR activation.
Fig. 5.
Effect of myeloid-selective deletion of A2ARs on the anti-inflammatory effects of CGS added only during OVA sensitization. Control mice were not treated with OVA or LPS. Adora2af/fLysMCre+/− mice and Adora2af/fLysMCre−/− littermate controls (n = 4) were sensitized with OVA-LPS and evaluated on day 23, after OVA rechallenge (see Fig. 1). A: representative images of hematoxylin-eosin-stained lungs. Original magnification ×20. B: inflammation scores (see materials and methods). C: methacholine-induced Penh in mice after allergen rechallenge of Adora2af/fLysMCre+ mice (Cre+) and Cre− littermate controls. **P < 0.01 and ***P < 0.001 vs. OVA/LPS (by 1-way ANOVA and Tukey's multiple comparison tests).
DISCUSSION
Anti-inflammatory effects of A2AR activation are mediated by suppression of the activation of most immune cells, including NKT cells, T cells, macrophages, DCs, and neutrophils (10, 19, 27, 28, 37). A2AR activation reduces LPS-induced lung inflammation and injury by activating receptors on myeloid cells (37). Although adenosine A2A agonists can inhibit inflammatory responses in the lung as well as in other tissues, clinical trials with inhaled A2A agonists such as GW328267X (31) and UK-432097 (ClinicalTrials.gov NCT00430300) to treat asthma have not been successful. Moreover, A2AR activation with CGS administered intranasally to sensitized mice was found to reduce allergen-driven cell influx into the airway, but not to affect airway hyperreactivity (5). The current study demonstrates that the A2AR agonist CGS activates A2ARs on myeloid cells to inhibit airway hyperresponsiveness much more effectively when given during aeroallergen sensitization than during allergen rechallenge. The results indicate that myeloid cells play a central role in controlling airway inflammation and hyperresponsiveness. These findings provide an explanation for the lack of clinical response to A2AR agonist therapy in established asthmatic patients and suggest that such therapy might be useful to blunt airway disease if administered during the initial exposure of an allergic individual to a new antigen, as might occur as a result of moving to a new habitat or changing workplaces.
Myeloid cells as targets of A2AR signaling.
Numerous studies have shown that A2AR stimulation acutely inhibits airway inflammation (20, 34, 42, 44). However, it has not been clear which cells are most important for mediating anti-inflammatory pulmonary responses. During allergen sensitization, one effect of adenosine is to influence the polarization of naïve T cells. In models of autoimmunity, A2AR activation was shown to inhibit Th1 and Th17 effector cell generation (47). This could occur because adenosine has a direct effect on T cells or because adenosine indirectly influences cytokine production by macrophages and other antigen-presenting cells (APCs) to indirectly influence T cell polarization. Both the accumulation of Th1/Th17 cells in the lungs and the accumulation of IFN-γ and IL-17A in the BAL in response to antigen sensitization and rechallenge were inhibited by CGS treatment during sensitization, while BAL TGF-β and numbers of Foxp3+ Treg cells in the lungs were increased. These findings are consistent with prior reports in other disease settings demonstrating that adenosine can increase numbers of Treg cells and promote their immunosuppressive activity (36). Changes in T cell polarization in response to adenosine or CGS have been attributed to modified myeloid production of IL-6, IFN-γ, and TGF-β (46). TGF-β is thought to attenuate allergen-induced airway hyperresponsiveness by increasing airway Treg cells (6). The data in the current study indicate that the effects of CGS are mediated by A2ARs on myeloid cells, thus confirming that the activation state of myeloid cells plays a critical role in the regulation of effector and Treg cell development. These findings are consistent with prior reports that suggest a role for APCs in determining whether the response to allergens will be tolerogenic or inflammatory (16) and further suggest that other agents that modify allergen presentation and cytokine production by APCs may be effective in modifying T cell polarization.
Weak cell-intrinsic effects of A2AR activation on Th17 cells.
During allergen rechallenge, adenosine can act directly on T effector cells to suppress cytokine release. However, the results of the current study suggest that A2AR signaling does not suppress the activation of T effector cells enough to significantly inhibit pulmonary inflammation or hyperresponsiveness to methacholine. CGS added during OVA rechallenge partially reduced IFN-γ release from Th1 cells, but CGS had no effect on the number of Th17 cells or IL-17 in the BAL. We also found that CGS applied to Th1 cells in vitro inhibited the release of IFN-γ. In contrast, Th17 cells in vitro were insensitive to CGS. The results suggest that once Th17 cells are produced during sensitization, they cannot be directly inhibited by A2AR activation during aeroallergen rechallenge. A human population of Th17 cells that express CD39 also are resistant to the effects of adenosine as a consequence of low expression of A2ARs (30). Moreover, IL-17 may be particularly important for controlling airway hyperresponsiveness (43). The findings also demonstrate that CGS administered at a dose of 100 ng·kg−1·min−1 does not have significant direct bronchodilator effects. In this regard, CGS differs from epinephrine and selective β2-adrenergic agonists (17).
Influence of environmental factors on allergic responses.
Studies in children indicate that the inner-city environment can promote allergic disease and wheezing. Curiously, early-life exposure to certain allergens together with bacteria has been counterintuitively associated with significant reductions in airway hyperresponsiveness (32). In high-allergen environments, enhancement of microbial exposure has been observed to be more effective than allergen abatement in prevention of hypersensitivity reactions (32). One factor produced in response to microbial infection that prevents airway hyperresponsiveness is adenosine. Moreover, bacteria or macrophages that have engulfed bacteria were found to release adenosine, which influenced cytokine release from mast cells (33). We speculate that adenosine produced by bacteria or by macrophages engulfing bacteria also influences allergen sensitization by effects on APCs.
Since myeloid cells express adenosine A2B receptors (A2BRs) as well as A2ARs, the activation of A2BRs on myeloid cells may also influence allergen sensitization by adenosine. In fact, A2BR blockade has been found to enhance macrophage-mediated bacterial phagocytosis (4). A2BR activation by adenosine is also used by Leishmania parasites within DCs to inhibit their function and evade immune responses (11). Nevertheless, A2BR activation should probably be avoided as a strategy to treat asthma, because A2BR activation stimulates the release of Th2 cytokines and degranulation of mast cells (3, 18, 38).
A2AR agonists for immunotherapy?
Subcutaneous or sublingual allergen-specific immunotherapy is sometimes used effectively to treat allergic asthma (22). Immunotherapy induces desensitization and long-term allergen-specific immune tolerance, as well as suppression of allergic inflammation in affected tissues. Since CGS was found in the current study to enhance immune tolerance, e.g., by increasing Foxp3+ Treg cell polarization, it will be of interest to determine if co-administration of A2AR agonists will improve the effectiveness of immunotherapies that appear to be of some use for the treatment of allergic asthma and rhinitis.
Effects of changing environment on asthmatic individuals.
One strategy used by asthmatic individuals to relieve symptoms of seasonal asthma or allergic rhinitis is change in habitat to avoid exposure of aeroallergens. However, the benefit of such relocation may be short-lived due to high sensitivity of these individuals to novel allergens in the new environment. Also, certain occupational environments produce a high incidence of allergy (41). The data in the current study suggest that treatment of allergic individuals with A2AR agonists during their initial exposure to a new allergen, such as highly allergenic pollen or workplace allergen, might be a useful strategy to produce tolerance.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01 HL-073361.
DISCLOSURES
J. Linden owns equity in Adenosine Therapeutics, LLC, and Lewis and Clark Pharmaceuticals. These companies manufacture drugs targeting adenosine receptors.
AUTHOR CONTRIBUTIONS
H.P. performed the experiments; H.P. analyzed the data; H.P. and J.L. drafted the manuscript; J.L. developed the concept and designed the research; J.L. interpreted the results of the experiments; J.L. prepared the figures; J.L. edited and revised the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Michael Croft (La Jolla Institute) for advice about mouse asthma models.
REFERENCES
- 1.Akahira-Azuma M, Szczepanik M, Tsuji RF, Campos RA, Itakura A, Mobini N, McNiff J, Kawikova I, Lu B, Gerard C, Pober JS, Askenase PW. Early delayed-type hypersensitivity eosinophil infiltrates depend on T helper 2 cytokines and interferon-γ via CXCR3 chemokines. Immunology 111: 306–317, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med 202: 829–839, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol 52: 846–860, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol 186: 2444–2453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonneau O, Wyss D, Ferretti S, Blaydon C, Stevenson CS, Trifilieff A. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol 290: L1036–L1043, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol 296: L307–L319, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 74: 7250–7259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cekic C, Sag D, Day YJ, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med 210: 2693–2706, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen SB, Leo BM, Baer GS, Turner MA, Beck G, Diduch DR. An adenosine A2A receptor agonist reduces interleukin-8 expression and glycosaminoglycan loss following septic arthrosis. J Orthop Res 23: 1172–1178, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Deurloo DT, van Oosterhout AJ. Role of T cell co-stimulation in murine models of allergic asthma. Clin Exp Allergy 34: 17–25, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo AB, Serafim TD, Marques-da-Silva EA, Meyer-Fernandes JR, Afonso LC. Leishmania amazonensis impairs DC function by inhibiting CD40 expression via A2B adenosine receptor activation. Eur J Immunol 42: 1203–1215, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Fortin A, Harbour D, Fernandes M, Borgeat P, Bourgoin S. Differential expression of adenosine receptors in human neutrophils: up-regulation by specific Th1 cytokines and lipopolysaccharide. J Leukoc Biol 79: 574–585, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Fozard JR, Ellis KM, Villela Dantas MF, Tigani B, Mazzoni L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur J Pharmacol 438: 183–188, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, Liu AH. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet 355: 1680–1683, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PG. Tackling asthma phenotypes in community studies. Thorax 64: 369–370, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol 129: 889–901, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Hardy CC, Bradding P, Robinson C, Holgate ST. The combined effects of two pairs of mediators, adenosine with methacholine and prostaglandin D2 with histamine, on airway calibre in asthma. Clin Sci (Lond) 71: 385–392, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Holgate ST. The identification of the adenosine A2B receptor as a novel therapeutic target in asthma. Br J Pharmacol 145: 1009–1015, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt PG, Upham JW. The role of dendritic cells in asthma. Curr Opin Allergy Clin Immunol 4: 39–44, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Impellizzeri D, Di Paola R, Esposito E, Mazzon E, Paterniti I, Melani A, Bramanti P, Pedata F, Cuzzocrea S. CGS 21680, an agonist of the adenosine (A2A) receptor, decreases acute lung inflammation. Eur J Pharmacol 668: 305–316, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist, directly labels A2 receptors in rat brain. J Pharmacol Exp Ther 251: 888–893, 1989. [PubMed] [Google Scholar]
- 22.Jutel M. Allergen-specific immunotherapy in asthma. Curr Treat Options Allergy 1: 213–219, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SH, Shin SY, Lee KH, Kim SW, Cho JS. Long-term effects of specific allergen immunotherapy against house dust mites in polysensitized patients with allergic rhinitis. Allergy Asthma Immunol Res 6: 535–540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol 178: 5375–5382, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Hong SW, Choi JP, Shin TS, Moon HG, Choi EJ, Jeon SG, Oh SY, Gho YS, Zhu Z, Kim YK. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol 183: 5113–5120, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuipers H, Hijdra D, De Vries VC, Hammad H, Prins JB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Lipopolysaccharide-induced suppression of airway Th2 responses does not require IL-12 production by dendritic cells. J Immunol 171: 3645–3654, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203: 2639–2648, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J Immunol 174: 1073–1080, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Linden J, Cekic C. Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol 32: 2097–2103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A, Quintana FJ, Robson SC. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLos One 9: e87956, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luijk B, van den Berge M, Kerstjens HA, Postma DS, Cass L, Sabin A, Lammers JW. Effect of an inhaled adenosine A2A agonist on the allergen-induced late asthmatic response. Allergy 63: 75–80, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, O'Connor GT, Sandel MT, Calatroni A, Matsui E, Johnson CC, Lynn H, Visness CM, Jaffee KF, Gergen PJ, Gold DR, Wright RJ, Fujimura K, Rauch M, Busse WW, Gern JE. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 134: 593–601, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma YJ, Kim CH, Ryu KH, Kim MS, So YI, Lee KJ, Garred P, Lee BL. Adenosine derived from Staphylococcus aureus-engulfed macrophages functions as a potent stimulant for the induction of inflammatory cytokines in mast cells. BMB Rep 44: 335–340, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol 293: L753–L761, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-κB in A2A adenosine receptor induction. Biochem J 391: 575–580, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol 5: 304, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol 179: 1254–1263, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol 180: 7212–7220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan GW, Linden J, Buster BL, Scheld WM. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J Infect Dis 180: 1550–1560, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A2A receptor agonists. Br J Pharmacol 132: 1017–1026, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teranishi H, Uchida M, Hayashi S, Yamada N. Allergenic pollens and spores in the working environment of Japanese pear farmers. Int J Immunopathol Pharmacol 20: 65–67, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Trevethick MA, Mantell SJ, Stuart EF, Barnard A, Wright KN, Yeadon M. Treating lung inflammation with agonists of the adenosine A2A receptor: promises, problems and potential solutions. Br J Pharmacol 155: 463–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsvetkova-Vicheva VM, Gecheva SP, Komsa-Penkova R, Velkova AS, Lukanov TH. IL-17 producing T cells correlate with polysensitization but not with bronchial hyperresponsiveness in patients with allergic rhinitis. Clin Transl Allergy 4: 3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood 116: 5010–5020, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang E, Ouellet N, Simard M, Fillion I, Bergeron Y, Beauchamp D, Bergeron MG. Pulmonary and systemic host response to Streptococcus pneumoniae and Klebsiella pneumoniae bacteremia in normal and immunosuppressed mice. Infect Immun 69: 5294–5304, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson JM, Kurtz CC, Black SG, Ross WG, Alam MS, Linden J, Ernst PB. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J Immunol 186: 6746–6752, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111: 251–259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Z, Oh SY, Zheng T, Kim YK. Immunomodulating effects of endotoxin in mouse models of allergic asthma. Clin Exp Allergy 40: 536–546, 2010. [DOI] [PubMed] [Google Scholar]