Abstract
Bronchopulmonary dysplasia (BPD), characterized by impaired alveolarization and vascularization in association with lung inflammation and apoptosis, often occurs after mechanical ventilation with oxygen-rich gas (MV-O2). As heightened expression of the proinflammatory cytokine TNF-α has been described in infants with BPD, we hypothesized that absence of TNF-α would reduce pulmonary inflammation, and attenuate structural changes in newborn mice undergoing MV-O2. Neonatal TNF-α null (TNF-α−/−) and wild type (TNF-α+/+) mice received MV-O2 for 8 h; controls spontaneously breathed 40% O2. Histologic, mRNA, and protein analysis in vivo were complemented by in vitro studies subjecting primary pulmonary myofibroblasts to mechanical stretch. Finally, TNF-α level in tracheal aspirates from preterm infants were determined by ELISA. Although MV-O2 induced larger and fewer alveoli in both, TNF-α−/− and TNF-α+/+ mice, it caused enhanced lung apoptosis (TUNEL, caspase-3/-6/-8), infiltration of macrophages and neutrophils, and proinflammatory mediator expression (IL-1β, CXCL-1, MCP-1) in TNF-α−/− mice. These differences were associated with increased pulmonary transforming growth factor-β (TGF-β) signaling, decreased TGF-β inhibitor SMAD-7 expression, and reduced pulmonary NF-κB activity in ventilated TNF-α−/− mice. Preterm infants who went on to develop BPD showed significantly lower TNF-α levels at birth. Our results suggest a critical balance between TNF-α and TGF-β signaling in the developing lung, and underscore the critical importance of these key pathways in the pathogenesis of BPD. Future treatment strategies need to weigh the potential benefits of inhibiting pathologic cytokine expression against the potential of altering key developmental pathways.
Keywords: tumor necrosis factor, TNF-α, neonatal chronic lung disease, bronchopulmonary dysplasia, mechanical ventilation, newborn mice, lung, TGF-β, apoptosis
chronic lung disease in the preterm infant, bronchopulmonary Dysplasia (BPD), is the most frequent chronic lung disease in infancy. The development of BPD is associated with severe respiratory infections, reactive airway disease, and limitations of pulmonary function that persist into adulthood (7). BPD is characterized by disrupted alveolarization and abnormal development of alveolar capillaries with variable degrees of interstitial cellularity, elastic fiber deposition, and fibroproliferation (19). The characteristic pulmonary inflammatory response induced by mechanical ventilation (MV) and oxygen toxicity is a central contributor to the pathologic changes observed in BPD, as evidenced by an induction of proinflammatory cytokines and an increased influx of macrophages and neutrophils (2, 17, 33). Clinical and experimental studies have shown that the upregulation of proinflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α, correlates with the development of BPD (6, 35), and conversely that patients with BPD demonstrate decreased expression of anti-inflammatory cytokines (e.g., IL-10), and growth factors critical for vascular and alveolar development (e.g., VEGF-A and PDGF-A) (30).
Increased expression of TNF-α plays a key role in severe infections and many inflammatory diseases in children and adults, and therapeutic strategies targeting excess TNF-α have been proven effective for many of these conditions (22, 27). TNF-α signaling activates the proinflammatory nuclear factor kappa-B (NFκB) pathway, resulting in the augmentation and perpetuation of the inflammatory response and an increase in apoptosis (21).
Elevated levels of TNF-α have been found in preterm infants who later developed BPD (2, 17, 30, 35); however, the specific function of this cytokine in the pathogenesis of BPD remains unclear. To investigate TNF-α in the pathophysiologic context of BPD and determine its potential as a therapeutic target, we studied TNF-α expression levels in BPD patients, and evaluated its pathophysiologic consequences in a mouse model of BPD. MV with oxygen-rich gas (MV-O2) triggers the onset and progression of BPD in association with a characteristic inflammatory response; therefore, we studied whether the absence of TNF-α in the newborn mouse lung undergoing MV-O2 decreases inflammation and apoptosis, thereby improving lung structure. In vitro, we performed experiments to study the restoration of TNF-α signaling in mouse primary lung (myo)fibroblasts (MFBs). To translate these findings observed in our experimental models to the clinical setting, we measured TNF-α expression in tracheal aspirates of preterm infants prior to, and during MV-O2, to determine whether MV-O2 in the preterm infant leads to significant changes in TNF-α levels associated with the development of BPD.
METHODS
For a more detailed description of the methods applied, please refer to the Supplemental Material for this article available online at the Journal website.
Newborn Mouse Ventilation
Transgenic mice.
Transgenic mice and wild type (WT) controls were purchased from Jackson laboratories (Bar Harbor, ME), provided by Charles River (Sulzfeld, Germany). TNF-α deficient mice have not been described with an abnormal pulmonary phenotype.
Mechanical ventilation experiments.
Six- to seven-day-old C57B/6J WT (TNF-α+/+) and TNF-α knockout (TNF-α−/−) mice born at term gestation (WT 3.8 ± 0.52 g; TNF-α−/− 4.0 ± 0.37 g body wt) were randomly selected to either receive MV-O2 for 8 h (fiO2 0.4) or to spontaneously breathe 40% O2 for 8 h (4–8 mice per group). Mice selected for ventilation underwent a tracheotomy after sedation with ketamine (∼60 μg/g body wt) and xylazine (∼12 μg/g body wt), followed by MV-O2 at 180 breaths/min from a customized, small animal respirator (MicroVent 848, Harvard Apparatus, Holliston, MA) for 8 h. The ventilation protocol was designed to minimize baro- and volutrauma and thereby mimic clinical settings (mean tidal volume 8.68 μl/g body wt; airway pressures: peak 12–13 cmH2O, mean 11–12 cmH2O). Newborn WT and TNF-α−/− control mice, spontaneously breathing 40% oxygen received sham surgery under mild sedation. The ventilation procedure has been published previously (15). At the end of each study, pups were euthanized with pentobarbital sodium and lungs were harvested for further analysis. All animals were viable with response to tactile stimulation and adequate perfusion at the end of each experiment. All surgical and animal care procedures and experimental protocols were reviewed and approved by the local Institutional Animal Care and Use Committee of the Regierung von Oberbayern.
Tissue Assays
Processing lungs for quantitative histology.
Lungs (n = 6–8/group) were fixed intratracheally with buffered 4% paraformaldehyde overnight at 20 cmH2O, as previously described (3). Volume of fixed lungs was measured by fluid displacement (28). After paraffin embedding and isotropic uniform random sectioning (28), quantitative assessment of alveolar area and number of incomplete and complete alveolar walls (septal density) was performed in 2–3 independent random tissue sections (4 μm, hematoxylin and eosin) per animal (CAST-Grid 2.1.5, Olympus, Ballerup, Denmark). Radial alveolar counts were assessed ≥30 fields of view in 2–3 independent random tissue sections per animal (13).
Assessment of PDGF-rα positive cells and related apoptosis in distal lung.
Paraformaldehyde-fixed lung tissue sections were stained for PDGF-Rα (C-20) (Santa Cruz Biotechnology, No. sc-338), cleaved Caspase-3 (Cell Signaling Technology, No. 9661S), and DAPI (Sigma Aldrich, No. D8417) in combination. Double-positive cells were quantified in eight different fields of view/animal (×400 magnification) with the Imaris Software (Imaris Software, Zurich, Switzerland).
Protein extraction and immunoblot analysis.
Lungs from 8-h studies (n = 4/group) were excised, weighed, and stored at −80°C for later protein extraction by using high urea buffer (KPO4, Urea, AppliChem, Darmstadt, Germany) and Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, No. 1861280). After measurement of protein concentrations (BCA, No. 23227, Pierce Scientific Rockford, IL), immunoblots were performed using a Bis-Tris (Life Technologies, No. NP0321BOX, Darmstadt, Germany) or a Tris-Acetate (Life Technologies, No. EA0375BOX) gel as published previously (15) using the following antibodies: Caspase-3 (Cell Signaling, No. 9662S), cleaved caspase-3 (Cell Signaling Technology, No. 9661), cleaved caspase-6 (Cell Signaling, No. 9761S), caspas-8 (Bio Vision, 3259-100), pSMAD 2 (Cell Signaling, No. 3101S), SMAD 2/3 (Cell Signaling, No. 3102S), SMAD 7 (Santa Cruz Biotechnology, No. sc-9183) β-actin (Santa Cruz Biotechnology, No. sc-81178); secondary antibody goat anti-mouse IgG (Santa Cruz Biotechnology, No. 2060) secondary antibody goat anti-rabbit IgG (Santa Cruz Biotechnology, No. 2301), or donkey anti-goat IgG-HRP (Santa Cruz Biotechnology, No. 2020) conjugated to horseradish peroxidase. Images were detected by chemiluminescence (GE Healthcare, No. RPN2232, Buckinghamshire, Great Britain) and quantified by densitometry (Bio Rad, Munich, Germany).
RNA extraction and quantitative real-time PCR.
After mRNA extraction (Carl Roth, No. A979.1) and purification (Peqlab, No. 12–6834-01, Erlangen, Germany) quantitative real-time PCR was applied to measure lung mRNA expression of IL-1β, CXCL-1, and MCP-1 using proprietary primer-probes (Eurofins mwg operon, Ebersberg, Germany).
In Vitro Studies
Mouse primary MFBs.
Mouse MFBs were extracted from PBS-flushed lungs of 5- to 7-day-old C57B/6J WT mice and cultured on a petridish (Corning, No. 430167, Tewksbury MA) in media (Gibco, No. 41966-029, Darmstadt, Germany) containing Pen/Strep (Gibco, No. 15140-122) and Gentamycin (Lonza, No. BE02-012E, Basel, Switzerland). FACS analysis of primary mouse lung MFBs showed the following characterization: 77.2 ± 14% PDGF-Rα+Vimentin+, 16.7 ± 12% Vimentin+, 77.6 ± 27% αSMA+, 32 ± 8.6% CD90+, and 8.5 ± 4.5% CD105+. In addition, the analysis showed a negligible amount of leucocytes (0.6 ± 0.5% CD45+).
Mechanical stretch experiments.
Primary mouse lung MFBs were seeded on flexible-bottomed laminin-coated culture plates (Flex Cell International, No. BF-3001L) to undergo in vitro stretch in room air at 70–80% confluence (cyclic strain by vacuum pressure: shape/sine; elongation min 2%, max 8%; frequency 2 Hz; duty cycle 50%; cycles 43,216; duration 24 h) for 24 h. Treatment with TNF-α was performed with 100 ng/ml recombinant TNF-α (Pepro Tech, No. 300-01A). The stretch experiment was started right after adding TNF-α treatment. At the end of each experiment, cells were harvested in 60 μl of RIPA buffer [150 mM NaCl (AppliChem, No. A2942), 10 mM Tris-buffer pH 7.2, (AppliChem, No. A1379), 0.1% SDS, (AppliChem, No. A1502), 1% Triton X 100, (Carl Roth, No. 3051.2), 1% sodium deoxycholate (Sigma, No. D6750), and 5 mM EDTA (AppliChem, No. A3562)] including Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, No. 1861280).
TNF-α Cytokine Levels in Tracheal Aspirate Samples of Preterm Infants
Tracheal aspirates were obtained at birth from preterm infants <29 wk gestational age who required MV-O2 (n = 79) starting the first day of life. BPD was defined according to Jobe and Bancalari (20). Patient characteristics are outlined in Tables 1 and 2. The study was approved by the ethics committee of the Ludwig-Maximilians University in Munich (No. 195-07) and is in accordance with the declaration of Helsinki. Written parental informed consent was obtained in all cases. TNF-α protein expression was determined by using a commercially available ELISA according to the manufacturer's instructions (TNF-α Quantikine ELISA kit, R&D) and standardized to sIgA (Immundiagnostik AG, Bensheim, Germany) to correct for dilution effects from the suctioning procedure.
Table 1.
Patient characteristics no/mild BPD
| Patient Number | Gestational Age, wk | Birth Weight, g | Gender | Antenatal Steroids | Postnatal Steroids | Intubated, days | Ventilatory Support, days |
|---|---|---|---|---|---|---|---|
| 1 | 25 + 5 | 872 | F | incomplete | yes | 1 | 71 |
| 5 | 27 + 4 | 670 | m | complete | no | 6 | 34 |
| 6 | 24 + 2 | 730 | m | incomplete | yes | 41 | 69 |
| 10 | 28 + 3 | 1,230 | m | complete | no | 6 | 37 |
| 16 | 27 + 4 | 950 | m | incomplete | no | 2 | 24 |
| 19 | 29 + 2 | 1,240 | f | complete | yes | 4 | 20 |
| 24 | 27 + 1 | 950 | m | incomplete | no | 10 | 22 |
| 26 | 26 + 6 | 740 | m | incomplete | yes | 25 | 56 |
| 27 | 24 + 4 | 550 | m | complete | yes | 14 | 61 |
| 31 | 24 + 3 | 740 | f | incomplete | no | 19 | 51 |
| 33 | 28 + 3 | 1,550 | m | incomplete | no | 14 | 19 |
| 34 | 28 + 3 | 1,150 | m | incomplete | no | 1 | 29 |
| 37 | 25 + 3 | 570 | m | incomplete | yes | 35 | 66 |
| 39 | 26 + 5 | 985 | m | complete | no | 2 | 26 |
| 42 | 23 + 5 | 620 | m | incomplete | yes | 31 | 67 |
| 43 | 23 + 5 | 560 | m | incomplete | yes | 39 | 78 |
| 45 | 28 + 0 | 1,050 | m | incomplete | no | 1 | 38 |
| 47 | 24 + 3 | 700 | f | complete | no | 30 | 44 |
| 51 | 25 + 1 | 680 | m | incomplete | no | 16 | 53 |
| 57 | 27 + 2 | 805 | f | complete | yes | 19 | 36 |
| 59 | 28 + 5 | 1,780 | m | complete | no | 8 | 32 |
| 60 | 25 + 6 | 940 | f | complete | yes | 5 | 65 |
| 62 | 26 + 6 | 940 | m | incomplete | yes | 4 | 17 |
| 64 | 28 + 5 | 1,340 | f | incomplete | no | 1 | 13 |
| 67 | 24 + 4 | 640 | f | incomplete | yes | 34 | 53 |
| 72 | 25 + 5 | 840 | m | incomplete | no | 7 | 58 |
| 75 | 26 + 1 | 880 | f | complete | no | 39 | 10 |
| 76 | 28 + 2 | 1,150 | m | complete | yes | 3 | 20 |
| 77 | 28 + 2 | 1,180 | m | complete | no | 1 | 9 |
| 80 | 28 + 2 | 1,130 | f | incomplete | no | 2 | 28 |
| 89 | 24 + 1 | 650 | f | complete | yes | 24 | 38 |
| 92 | 27 + 4 | 1,150 | m | incomplete | no | 1 | 26 |
| 96 | 27 + 3 | 1,135 | f | complete | no | 1 | 17 |
| 100 | 28 + 1 | 1,205 | f | incomplete | no | 6 | 19 |
| 105 | 25 + 5 | 815 | f | incomplete | no | 1 | 31 |
| 107 | 27 + 4 | 1,110 | f | incomplete | no | 1 | 1 |
| 109 | 26 + 4 | 960 | m | incomplete | no | 3 | 42 |
| 110 | 26 + 4 | 765 | m | incomplete | no | 6 | 59 |
| 115 | 25 + 5 | 850 | m | complete | no | 2 | 57 |
Depicted are the clinical characteristics of the cohort fulfilling the diagnostic criteria of no or mild bronchopulmonary dysplasia (BPD) (20). M, male; f, female; incomplete, incomplete course of two dosages of corticosteroids within 48 h; complete, complete course of antenatal steroids; ventilatory support, any form of mechanical ventilator support including continuous positive airway pressure and oxygen therapy. All preterm infants received surfactant therapy.
Table 2.
Patient characteristics moderate/severe BPD
| Patient Number | Gestational Age, wk | Birth Weight, g | Gender | Antenatal Steroids | Postnatal Steroids | Intubated, days | Ventilatory Support, days |
|---|---|---|---|---|---|---|---|
| 7 | 28 + 0 | 860 | f | incomplete | no | 21 | 62 |
| 12 | 23 + 5 | 610 | f | incomplete | yes | 32 | 89 |
| 13 | 26 + 6 | 840 | f | complete | no | 5 | 65 |
| 15 | 27 + 4 | 810 | m | incomplete | no | 5 | 70 |
| 17 | 25 + 6 | 630 | f | incomplete | no | 1 | 81 |
| 18 | 25 + 6 | 560 | f | incomplete | no | 39 | 71 |
| 25 | 27 + 1 | 1,060 | m | incomplete | no | 9 | 70 |
| 28 | 26 + 3 | 905 | m | complete | yes | 50 | 102 |
| 30 | 24 + 6 | 510 | f | incomplete | yes | 68 | 79 |
| 32 | 26 + 6 | 850 | m | complete | yes | 2 | 75 |
| 38 | 26 + 5 | 735 | m | complete | no | 10 | 85 |
| 48 | 24 + 5 | 690 | m | complete | yes | 34 | 79 |
| 49 | 24 + 3 | 600 | f | incomplete | no | 24 | 82 |
| 50 | 24 + 2 | 600 | m | complete | yes | 28 | 85 |
| 54 | 26 + 3 | 770 | m | complete | yes | 30 | 87 |
| 56 | 25 + 1 | 690 | m | incomplete | yes | 45 | 78 |
| 58 | 24 + 2 | 600 | f | incomplete | yes | 99 | 159 |
| 61 | 27 + 0 | 1,050 | m | complete | yes | 22 | 66 |
| 63 | 28 + 4 | 540 | m | incomplete | no | 19 | 111 |
| 68 | 27 + 3 | 850 | m | incomplete | yes | 29 | 76 |
| 69 | 27 + 3 | 850 | m | incomplete | yes | 27 | 76 |
| 70 | 27 + 3 | 680 | m | complete | no | 8 | 64 |
| 71 | 27 + 3 | 790 | f | complete | no | 7 | 60 |
| 73 | 24 + 1 | 550 | m | incomplete | yes | 42 | 98 |
| 74 | 24 + 2 | 650 | m | incomplete | yes | 39 | 83 |
| 78 | 24 + 6 | 810 | m | complete | yes | 28 | 107 |
| 81 | 23 + 6 | 650 | m | incomplete | yes | 39 | 89 |
| 86 | 25 + 4 | 540 | f | incomplete | yes | 19 | 76 |
| 87 | 25 + 4 | 600 | f | incomplete | yes | 31 | 74 |
| 90 | 27 + 2 | 990 | f | incomplete | no | 79 | 79 |
| 93 | 24 + 2 | 485 | m | complete | yes | 37 | 87 |
| 94 | 26 + 1 | 700 | m | complete | yes | 22 | 75 |
| 95 | 26 + 1 | 890 | m | complete | yes | 25 | 70 |
| 99 | 28 + 1 | 1,215 | m | incomplete | no | 16 | 58 |
| 101 | 24 + 1 | 570 | m | incomplete | no | 40 | 91 |
| 103 | 23 + 1 | 510 | f | incomplete | yes | 44 | 98 |
| 104 | 22 + 5 | 530 | f | incomplete | yes | 48 | 122 |
| 108 | 26 + 5 | 315 | m | complete | no | 27 | 133 |
| 111 | 27 + 4 | 960 | f | incomplete | no | 12 | 77 |
| 112 | 23 + 4 | 700 | m | complete | yes | 34 | 98 |
Depicted are the clinical characteristics of the cohort fulfilling the diagnostic criteria of moderate or severe BPD (20). M, male; f, female; incomplete, incomplete course of two dosages of corticosteroids within 48 h; complete, complete course of antenatal steroids; ventilatory support, any form of mechanical ventilator support including continuous positive airway pressure and oxygen therapy. All preterm infants received surfactant therapy.
Data Analysis
Data are given as means ± SD. Two-way analysis of variance and the Bonferroni post hoc test were performed to compare two groups of controls and two groups of mechanically ventilated WT (TNF-α+/+) and knockout (TNF-α−/−) newborn mice. To compare datasets from two groups of mice (immunoblot analysis), Student's unpaired t-test, or the nonparametric Mann-Whitney test (for datasets with a skewed distribution) were performed. This analysis was used as well to analyze data from patient material. Statistical analysis was done with Prism 5 software package (GraphPad, San Diego, CA) and Sigma Plot v12.3 (Systat Software, San Jose, CA). Differences were considered statistically significant when the P value was <0.05.
RESULTS
MV-O2 Induces Similar Impairments in the Alveolar Development in WT and TNF-α Null Mice
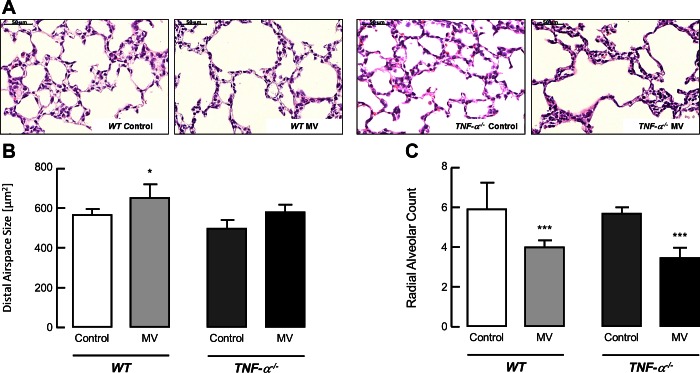
Using quantitative morphometry, we found that exposing mice to MV-O2 for 8 h impaired lung structure in both groups, resulting in a similar increase in distal airspace size and a decrease in radial alveolar counts (a measure of alveolar number) in both WT (TNF-α+/+) and TNF-α−/− mice (Fig. 1, A–C).
Fig. 1.
Mechanical ventilation with oxygen rich gas (MV-O2) impairs alveolar structure in TNF-α−/− and wild type (WT) mice. MV-O2 for 8 h increased airspace size and decreased alveolar number in both newborn TNF-α−/− as well as WT mice. A: representative lung tissue sections (×200) from 6- to 7-day-old WT and TNF-α−/− mice after MV-O2 for 8 h, showing increased air space size in both groups when compared with unventilated controls that breathed 40% O2 for 8 h. B: summary data (means and SD) for alveolar area, assessed by quantitative image analysis of lung tissue sections, showed an increase of alveolar area after MV-O2 of TNF-α−/− mice for 8 h compared with respective controls, whereas there was no significant change in lungs of WT littermates compared with TNF-α−/− mice upon MV-O2 for 8 h. Significant difference between groups, *P < 0.05; n = 4–8/group. C: summary data (means and SD) for radial alveolar counts, an index of alveolar number, in lung tissue sections from WT and TNF-α−/− mice after 8 h of MV-O2, compared with unventilated controls spontaneously breathing 40% O2 for 8 h. Significant difference between groups, ***P < 0.001; n = 4–8/group.
MV-O2 Induces a Greater Degree of Apoptosis and Inflammation in the Lungs of TNF-α Null vs. WT mice
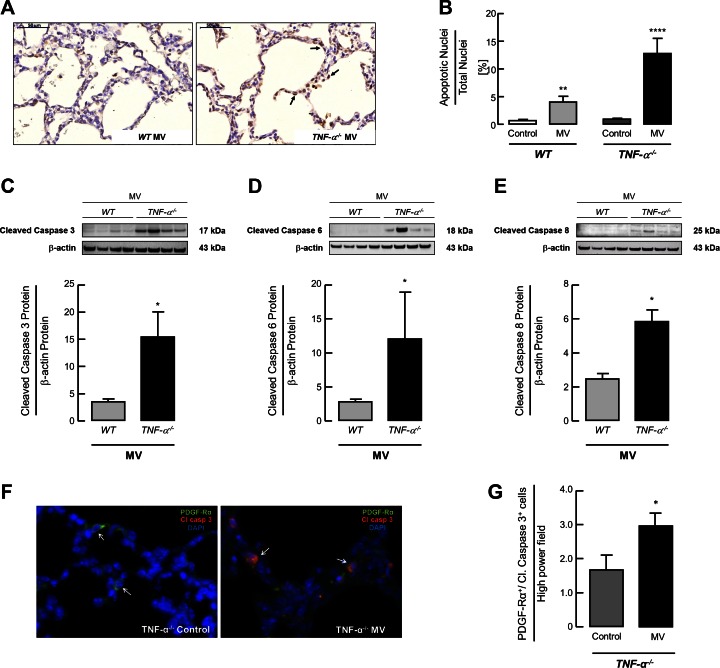
Semiquantitative analysis of TUNEL-positive cells in the lungs of ventilated newborn mice following MV-O2 for 8 h showed a threefold increase in apoptosis in TNF-α−/− mice subjected to MV-O2 compared with ventilated WT pups (Fig. 2, A and B). These differences were further supported by the analysis of caspase protein expression showing a significant two to threefold increase in cleaved caspase-3, cleaved caspase-6 and cleaved caspase-8 in the lungs of ventilated TNF-α−/− mice compared with ventilated WT mice (Fig. 2, C–E). Dual staining for Caspase-3 and PDGF-Rα showed a significant increase in apoptotic MFBs in the lungs of ventilated TNF-α−/− pups compared with unventilated control animals (Fig. 2, F and G).
Fig. 2.
MV-O2 increases apoptosis in lungs of TNF-α−/− compared with WT mice. A: TUNEL staining of lung tissue sections, showing an increased number of apoptotic cells (black arrows) in the lungs of 6- t 7-day-old TNF-α−/− pups after 8 h of MV-O2 compared with ventilated WT mice. B: quantitative image analysis of TUNEL-positive cells indicating a significant increase in apoptosis in lungs of TNF-α−/− mice compared with WT littermates after 8 h of MV-O2. Significant difference between groups, **P < 0.01, ****P < 0.0001; n = 4–7/group. Immunoblot for protein expression showed a significant increase of pulmonary caspase-3 (C), caspase-6 (D), and caspase-8 (E) protein expression in newborn TNF-α−/− mice compared with WT littermates after 8 h of MV-O2. Significant difference between groups, *P < 0.05; n = 4/group. F: immunofluorescence image of lung tissue (×400, merged) showed increased dual staining for cleaved caspase-3 (red) and PDGF-Rα (green) in the lungs of 6- to 7-day-old TNF-α−/− mice after 8 h MV-O2 (panel at right) compared with unventilated controls (panel at left); white arrows indicate single (left) and dual (right) positive cells; nuclear counterstain with DAPI (blue). G: quantification of the images indicated an increase in dual positive cells per high-power field in 6- to 7-day-old TNF-α−/− mice after 8 h MV-O2. Significant difference between groups, *P < 0.05; n = 4/group; 10 high-power fields analyzed per mouse.
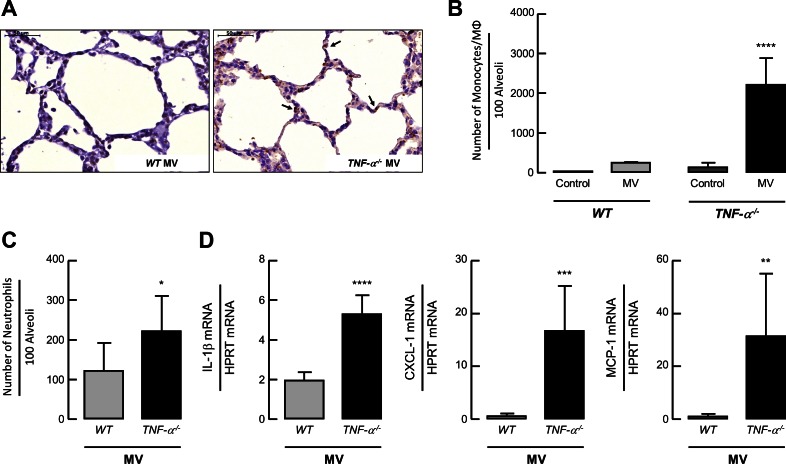
With respect to pulmonary inflammation, the number of monocytes/macrophages and neutrophils were increased in the lungs of newborn TNF-α−/− pups after 8 h of MV-O2 (Fig. 3, A–C) accompanied by an increase in IL-1β, CXCL-1, and MCP-1 mRNA expression compared with ventilated WT newborn mice (Fig. 3C).
Fig. 3.
MV-O2 increases number of infiltrating monocytes and heightens cytokine expression in lungs of TNF-α−/− compared with WT mice. A: F4/80 immunhistochemistry in paraformaldehyde-fixed lung sections, showing increased pulmonary infiltration of macrophages (black arrows) in TNF-α−/− newborn mice after MV-O2 for 8 h compared with WT mice. Quantitative image analysis of F4/80-positive cells (B) and number of neutrophils per 100 alveoli (C) demonstrated a significant increase in numbers of monocytes/macrophages and neutrophils in the lungs of TNF-α−/− compared with WT mice. Significant difference between groups, *P < 0.05, ****P < 0.0001; n = 3–5/group. D: in line with this, pulmonary mRNA expression of IL-1β, chemokine (C-X-C motif) ligand-1 (CXCL-1), and monocyte chemotactic protein 1 (MCP-1) were increased in newborn TNF-α−/− mice upon MV-O2 for 8 h in contrast to WT pups. Significant difference between groups, **P < 0.01, ***P < 0.001. ****P < 0.0001; n = 4/group.
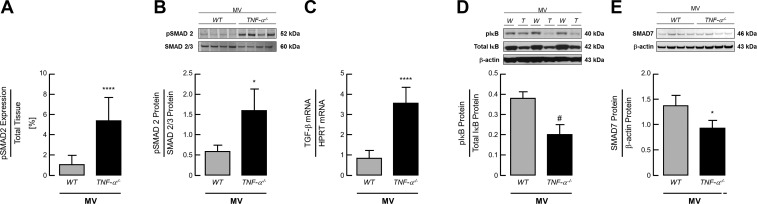
MV-O2 Increases Activation of TGF-β Signaling and Decreases NFκB Activation and SMAD7 Expression in Lungs of TNF-α−/− Compared with WT Mice
Analysis of TGF-β signaling in the lungs of ventilated newborn mice showed a significant threefold increase in pSMAD 2/3 expression in newborn TNF-α−/− mice compared with WT pups (Fig. 4, A and B), in line with a significant increase in TGF-β mRNA expression in these lungs after 8 h MV-O2 (Fig. 4C). These findings were associated with a significant decrease in IκB phosphorylation, indicating a reduction in NF-κB activation in the lungs of newborn TNF-α−/− pups after 8 h of MV-O2 compared with ventilated WT mice (Fig. 4D). Analysis of the TGF-β inhibitor SMAD-7 showed a significant reduction of its protein expression in the lungs of ventilated TNF-α−/− compared with WT pups (Fig. 4E).
Fig. 4.
MV-O2 increases activation of transforming growth factor-β (TGF-β) signaling and decreases NFκB activation and SMAD7 expression in lungs of TNF-α−/− compared with WT mice. A: quantitative image analysis of pSMAD2 staining per total tissue indicated a significant increase of pSMAD2 expression in the lung periphery of TNF-α−/− mice compared with WT littermates after 8 h of MV-O2. Significant difference between groups, ****P < 0.0001; n = 5–6/group. B: these results were confirmed by immunoblot analysis showing a significant increase of pSMAD2 protein expression in the lungs of newborn TNF-α−/− mice undergoing MV-O2 in contrast to WT pups. C: MV-O2 for 8 h resulted in increased TGF-β mRNA expression. Significant difference between groups, *P < 0.05, ****P < 0.0001; n = 4/group. D: downstream, MV-O2 for 8 h reduced the expression of phosphorylated IκB, indicating a reduced activation of NF-κB in the lungs of newborn TNF-α−/− mice compared with WT pups. Significant difference between groups, #P = 0.0501; n = 3/group. E: these results were accompanied by a significant decrease in SMAD7 protein expression in the lungs of newborn TNF-α−/− mice compared with WT pups. Significant difference between groups, *P < 0.05; n = 4/group.
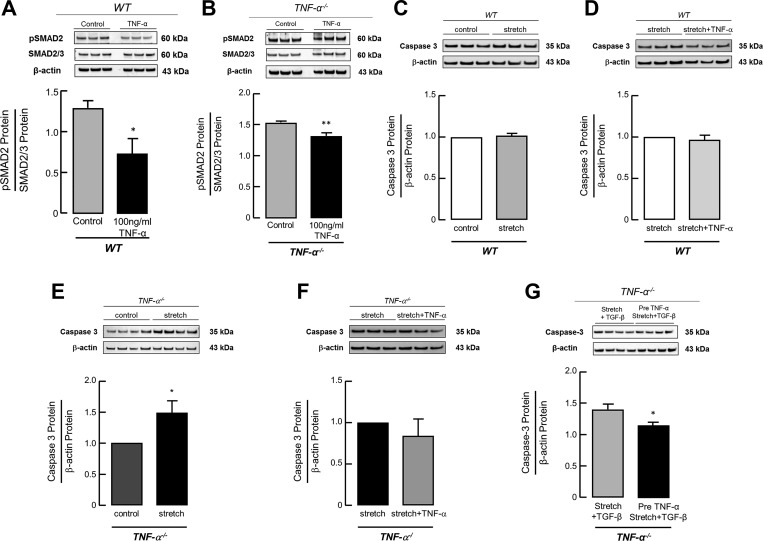
TNF-α Treatment Successfully Decreases TGF-β Activation and Stretch-induced Caspase-3 Expression in Primary Lung MFBs from WT and TNF- α−/− mice
The cross talk between the TGF-β and the TNF-α pathway was confirmed by reduced pSMAD2 protein expression in MFBs derived from WT as well as TNF-α−/− mice upon treatment with TNF-α (100 ng/ml) (Fig. 5, A and B). Stretching primary lung MFBs in vitro at room air significantly increased cleaved caspase-3 protein expression in cells derived from newborn TNF-α−/− pups (Fig. 5E) but not in MFBs derived from newborn WT pups (Fig. 5C). This increase in caspase-3 expression was prevented by the supplementation of TNF-α (100 ng/ml) prior to mechanical stretch (Fig. 5F) or stretch along with TGF-β (Fig. 5G) in MFBs derived from newborn TNF-α−/− mice, whereas TNF-α treatment (100 ng/ml) in WT cells had no significant effect on the expression level of cleaved caspase-3 (Fig. 5C).
Fig. 5.
TNF-α treatment decreases TGF-β activation and stretch-induced caspase-3 expression in primary lung (myo)fibroblasts (MFBs) from WT and TNF-α−/− mice. Confirming the interaction between the TNF-α and the TGF-β pathway, immunoblot analysis showed a reduction in pSMAD2 protein expression after TNF-α treatment (100 ng/ml TNF-α in H2O + 0.1% BSA) in primary lung MFBs isolated from WT (A) and TNF-α−/− (B) mice compared with untreated MFBs. Significant difference between groups, *P < 0.05, **P < 0.01; n = 3 mice/group. In line with this, primary lung MFBs derived from TNF-α−/− mice revealed a significant increase of caspase-3 protein expression upon mechanical stretch (E), reversed by TNF-α treatment (at the onset of stretch) (F) in contrast to the effect in MFBs isolated from WT mice, where caspase-3 expression remained unchanged (C, D). Significant difference between groups, *P < 0.05; n = 3–4/group. Likewise, TNF-α treatment in primary lung MFBs from TNF-α−/− mice prior to TGF-β application along with in vitro stretch successfully reduced caspase-3 expression compared with untreated cells (G). Significant difference between groups, *P < 0.05; n = 4 mice/group.
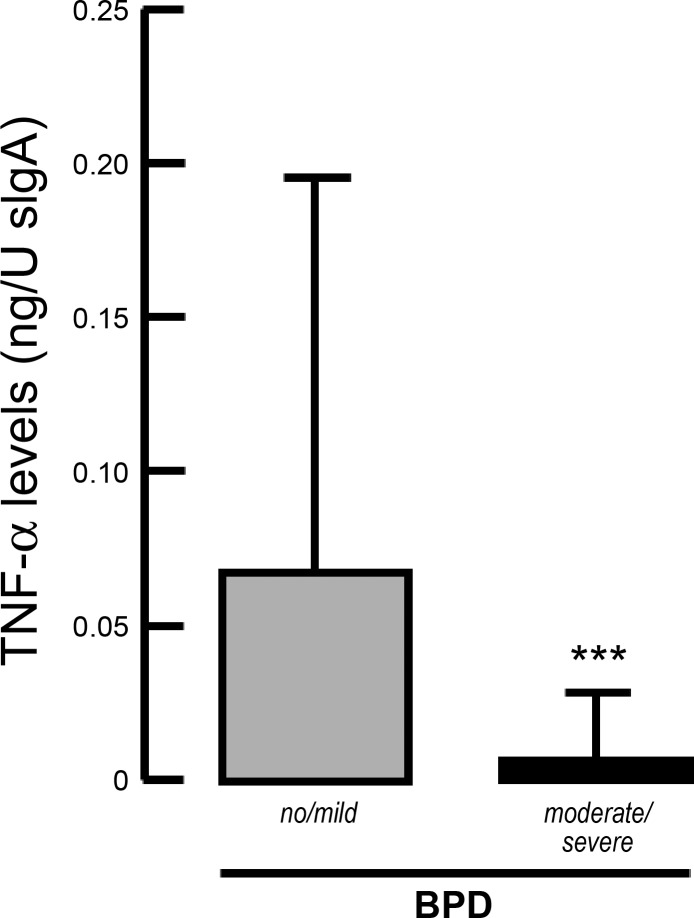
TNF-α Levels in Tracheal Aspirate in Preterm Infants with and Without BPD
To substantiate the experimental findings in a cohort of preterm infants, we analyzed TNF-α level in tracheal aspirates at the onset and during prolonged MV-O2. In line with our findings, TNF-α levels were significantly reduced in tracheal aspirate samples obtained at birth from preterm infants who later developed moderate or severe BPD, compared with preterms with no or mild disease (Fig. 6).
Fig. 6.
TNF-α levels in tracheal aspirates associated with the development of BPD. Significantly reduced TNF-α levels at birth in tracheal aspirates obtained from preterm infants later developing moderate or severe BPD compared with infants with no or mild disease (20). Significant difference between groups, ***P < 0.001; n = 79 preterm infants.
DISCUSSION
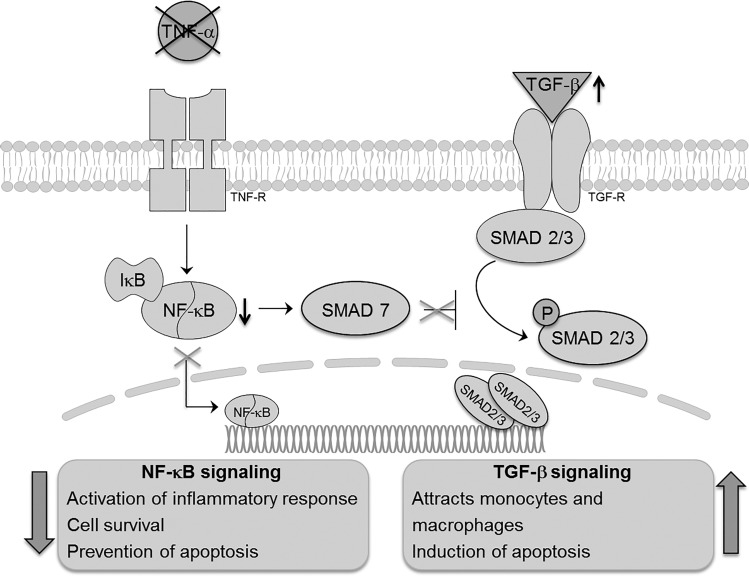
Clinical and experimental evidence has identified an association between increased levels of TNF-α in the lung undergoing MV-O2 and the development of BPD, suggesting that heightened TNF-α expression may be a harbinger of BPD development (2, 17, 30, 35). Although loss of TNF-α signaling had not been previously reported to affect normal lung development at any stage, the present study demonstrates that the absence of TNF-α in the developing lung undergoing MV-O2 results in an increase in apoptosis and inflammation associated with increased TGF-β signaling (Fig. 7).
Fig. 7.
Schematic model of the anticipated pathophysiologic process derived from the results of the experimental studies.
TNF-α, the best studied cytokine of the TNF family, is well known for its characteristic proinflammatory activity in the context of different diseases (4, 9). The successful amelioration of both infectious as well as noninfectious inflammatory diseases by the inhibition of TNF-α in adult and pediatric patient cohort provided the rationale for the current study (25).
TNF-α signaling induces and perpetuates the inflammatory response, and also invokes cell death by promoting binding of the TNF receptor 1 (TNFR1) to the associated death domain proteins (1, 5, 12, 29). On the other hand, TNF-α mediated downstream activation of the NF-κB pathway may result in prosurvival functions that have been reported by other investigators (5, 31, 32).
Here, we demonstrate in a unique in vivo model that the absence of TNF-α is associated with excess activation of TGF-β signaling, increased inflammatory mediator expression, accentuated apoptosis, and reduced NF-κB activity in the ventilated newborn lung.
As indicated by costaining experiments, the process of apoptosis induced by MV-O2 affects the PDGF-Rα positive pulmonary MFB, driving the process of alveolar septation. We therefore undertook in vitro experiments at room air to study the cell-specific response to stretch with or without the additional application of TGF-β. In line with our in vivo findings, the in vitro analysis showed an increase in caspase expression upon mechanical stretch in primary lung MFBs derived from neonatal TNF-α−/− in contrast to pulmonary MFBs from WT mice. Verifying the cross talk between the TNF-α and the TGF-β pathway in primary cells derived from the newborn mouse lung, TNF-α treatment successfully reversed both and increased TGF-β activation as well as excess caspase expression induced by in vitro stretch.
Previous studies in mice and in humans have shown that MV-O2 increases TGF-β signaling in the lung (15, 16, 26). In these studies, heightened activation of TGF-β augments pulmonary inflammation by enhancing monocyte recruitment to the lung and increasing apoptosis. As recent studies have demonstrated important anti-inflammatory, cell survival, and developmental functions of NF-κB in the newborn lung (18, 23, 24), the decrease in NF-κB activity we observed may further enhance the proapoptotic effects of heightened TGF-β signaling. Furthermore, the decrease in NF-κB activity in our model was accompanied by a reduction of SMAD-7 protein levels. Cross talk between the NF-κB and the TGF-β pathways has been previously demonstrated (14), with suppression of NFκB resulting in an excess in TGF-β activation, thus augmenting the recruitment of inflammatory cells and promoting proinflammatory cytokine production and cell death induction (15, 16). Therefore, in our model reduced NF-κB activity could not only impede the development of the newborn lung and affect cell survival (18) but may also promote excessive TGF-β activation, which in turn further enhances the recruitment of inflammatory cells to the lung (34).
In concert with the effects on apoptosis and inflammation, the absence of TNF-α did not result in an improvement in lung structure in neonatal mice undergoing MV-O2. Whether the increase in cell death in MFBs as well as in other cell types relevant for lung development is or is not related to impaired long-term pulmonary outcome needs to be addressed in future studies.
TNF-α level obtained from tracheal aspirates in preterm infants support the hypothesis derived from the experimental studies and allow the translation of the findings into the human system. Here, lower TNF-α levels at birth in infants that later develop BPD may explain activation of the TGF-β pathway, reported by a variety of studies (15, 16, 26). Besides the immediate detrimental effects of TGF-β with the induction of apoptosis and acute inflammation, the perpetuation of the inflammatory response enhanced by TGF-β may account for the adverse long-term effects of MV-O2 following conventional or new ventilation protocols (8, 11).
Taken together, the data presented unravel the complexity of TNF-α function in the developing lung undergoing MV-O2 and contribute to a broader understanding of the heterogeneous impact of constitutive and induced TNF-α levels in normal and abnormal lung development. Moreover, these results suggest that upper and lower threshold levels need to be defined in keeping with the Goldilocks principle. With respect to previous studies, the unfavorable effects of TNF-α withdrawal in the developing lung may relate to the adverse reactions observed in adult patients treated with TNF-α inhibitors (10).
Future studies should address the potential for preterm infants undergoing MV-O2 to benefit from determination of TNF-α level at birth to determine their risk for BPD development and consecutive treatment modifications. Considering the complexity of pulmonary TNF-α signaling and NF-κB activity at birth, TNF-α blockade as a targeted treatment option to reduce ventilator-induced lung damage and BPD needs to be carefully reevaluated. Special consideration should be drawn to the right timing and dosing to prevent an imbalance of physiologic NF-κB and TGF-β signaling in the context of TNF-α abundance.
GRANTS
This study was supported by the Friedrich-Baur-Stiftung (40/2010) (to H. Ehrhardt) and the Young Investigator Grant NWG VH-NG-829 by the Helmholtz Gemeinschaft and the Helmholtz Zentrum Muenchen, Germany (to A. Hilgendorff), as well as support from National Heart, Lung, and Blood Institute Grant R01 HL-122918-01 (to C. Alvira), the Tashia and John Morgridge Faculty Scholar Fund, and the Stanford Child Health Research Institute (to C. Alvira).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.E., T.P., C.M.A., and A.H. conception and design of research; H.E., T.P., P.O., K.F., and A.H. interpreted results of experiments; H.E., T.P., P.O., M. Kossert, L.B., and K.F. prepared figures; H.E., T.P., C.M.A., and A.H. drafted manuscript; H.E., T.P., C.M.A., and A.H. edited and revised manuscript; H.E. and A.H. approved final version of manuscript; P.O., M. Kossert, L.B., K.F., M. Koschlig, and C.M.A. performed experiments; P.O., M. Kossert, L.B., K.F., and M. Koschlig analyzed data.
ACKNOWLEDGMENT
We gratefully acknowledge sample collection by the whole staff from the neonatal intensive care unit at the perinatal center in Munich Grosshadern.
REFERENCES
- 1.Baader E, Toloczko A, Fuchs U, Schmid I, Beltinger C, Ehrhardt H, Debatin KM, Jeremias I. Tumor necrosis factor-related apoptosis-inducing ligand-mediated proliferation of tumor cells with receptor-proximal apoptosis defects. Cancer Res 65: 7888–7895, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari V. Hyperoxia-derived lung damage in preterm infants. Sem Fetal Neonatal Med 15: 223–229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol 294: L3–L14, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JR. TNF-mediated inflammatory disease. J Pathol 214: 149–160, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 15: 362–374, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 301: L917–L926, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Carraro S, Filippone M, Da Dalt L, Ferraro V, Maretti M, Bressan S, El Mazloum D, Baraldi E. Bronchopulmonary dysplasia: the earliest and perhaps the longest lasting obstructive lung disease in humans. Early Hum Dev 3: S3–5, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Claure N, Bancalari E. New modes of mechanical ventilation in the preterm newborn: evidence of benefit. Arch Dis Child Fetal Neonatal Ed 92: F508–512, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damas P, Reuter A, Gysen P, Demonty J, Lamy M, Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med 17: 975–978, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Day R. Adverse reactions to TNF-α inhibitors in rheumatoid arthritis. Lancet 359: 540–541, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Eber E, Zach MS. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 56: 317–323, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-κB. Oncogene 22: 3842–3852, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child 35: 544–547, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, Yang X, Chen Z, Van Waes C. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32: 1549–1559, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilgendorff A, Parai K, Ertsey R, Jain N, Navarro EF, Peterson JL, Tamosiuniene R, Nicolls MR, Starcher BC, Rabinovitch M, Bland RD. Inhibiting lung elastase activity enables lung growth in mechanically ventilated newborn mice. Am J Respir Crit Care Med 184: 537–546, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thebaud B, Tamosiuniene R, Jain N, Navarro EF, Starcher BC, Nicolls MR, Rabinovitch M, and Bland RD. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol 303: L215–L227, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 176: 575–581, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iosef C, Alastalo TP, Hou Y, Chen C, Adams ES, Lyu SC, Cornfield DN, Alvira CM. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol 302: L1023–L1036, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr 23: 167–172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-κB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res 154: 228–240, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Lv S, Han M, Yi R, Kwon S, Dai C, Wang R. Anti-TNF-α therapy for patients with sepsis: a systematic meta-analysis. Int J Clin Pract 68: 520–528, 2014. [DOI] [PubMed] [Google Scholar]
- 23.McKenna S, Michaelis KA, Agboke F, Liu T, Han K, Yang G, Dennery PA, Wright CJ. Sustained hyperoxia-induced NF-κB activation improves survival and preserves lung development in neonatal mice. Am J Physiol Lung Cell Mol Physiol 306: L1078–L1089, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelis KA, Agboke F, Liu T, Han K, Muthu M, Galambos C, Yang G, Dennery PA, Wright CJ. IκBβ-mediated NF-κB activation confers protection against hyperoxic lung injury. Am J Respir Cell Mol Biol 50: 429–438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol 27: 55–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morty RE, Konigshoff M, Eickelberg O. Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 607–613, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Rizzo AN, Sammani S, Esquinca AE, Jacobson JR, Garcia JG, Letsiou E, Dudek SM. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 309: L1294–L1304, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26: 57–60, 1970. [PubMed] [Google Scholar]
- 29.Sedger LM, McDermott MF. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants—past, present and future. Cytokine Growth Factor Rev 25: 453–472, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol 26 Suppl 1: S57–62; discussion S63-64, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Suominen JS, Wang Y, Kaipia A, Toppari J. Tumor necrosis factor-alpha (TNF-α) promotes cell survival during spermatogenesis, and this effect can be blocked by infliximab, a TNF-α antagonist. Eur J Endocrinol 151: 629–640, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, Clark RA, Chandrasekar B. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-α (TNF-α)-stimulated cardiac fibroblast proliferation but inhibits TNF-α-induced cardiomyocyte death. J Biol Chem 284: 14414–14427, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods SJ, Waite AA, O'Dea KP, Halford P, Takata M, Wilson MR. Kinetic profiling of in vivo lung cellular inflammatory responses to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 308: L912–L921, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics 128: 111–126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-α, interleukin-1 β, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 177: 825–830, 1997. [DOI] [PubMed] [Google Scholar]