Abstract
Recently approved therapies that modulate CFTR function have shown significant clinical benefit, but recent investigations regarding their molecular mechanism when used in combination have not been consistent with clinical results. We employed micro-optical coherence tomography as a novel means to assess the mechanism of action of CFTR modulators, focusing on the effects on mucociliary clearance. Primary human airway monolayers from patients with a G551D mutation responded to ivacaftor treatment with increased ion transport, airway surface liquid depth, ciliary beat frequency, and mucociliary transport rate, in addition to decreased effective viscosity of the mucus layer, a unique mechanism established by our findings. These endpoints are consistent with the benefit observed in G551D patients treated with ivacaftor, and identify a novel mechanism involving mucus viscosity. In monolayers derived from F508del patients, the situation is more complicated, compounded by disparate effects on CFTR expression and function. However, by combining ion transport measurements with functional imaging, we establish a crucial link between in vitro data and clinical benefit, a finding not explained by ion transport studies alone. We establish that F508del cells exhibit increased mucociliary transport and decreased mucus effective viscosity, but only when ivacaftor is added to the regimen. We further show that improvement in the functional microanatomy in vitro corresponds with lung function benefit observed in the clinical trials, whereas ion transport in vitro corresponds to changes in sweat chloride. Functional imaging reveals insights into clinical efficacy and CFTR biology that significantly impact our understanding of novel therapies.
Keywords: mucociliary transport
cystic fibrosis (CF) is caused by reduced or absent cystic fibrosis transmembrane conductance regulator (CFTR) protein activity, an epithelial anion transporter of chloride and bicarbonate (16, 27, 32). Defective CFTR results in significant morbidity and early mortality in affected individuals by profoundly disrupting mucociliary clearance and airway defense (36). There are >1,900 mutations associated with CFTR-related diseases that can be classified into distinct molecular categories (24). The most common mutation, F508del, has an allelic frequency of 75% and is present in at least one copy in close to 90% of individuals with CF, thus representing an important therapeutic target (38, 45). The mutation results in a misfolded CFTR that is rapidly subjected to endoplasmic reticulum-associated degradation (ERAD), leaving little residual CFTR at the cell surface as its primary abnormality; the minimal protein that escapes ERAD also exhibits defective gating and reduced cell surface residence time (10, 21). In contrast, the G551D CFTR mutation exhibits normal cell surface expression but profoundly diminished open channel probability, severely reducing transepithelial anion transport (4).
New molecular therapies for CF are intended to restore CFTR function by correction of protein misfolding or potentiation of defective channel gating. This has been particularly efficacious for patients with G551D or other closely related gating mutations treated with the CFTR potentiator ivacaftor (VX-770); in this setting, ivacaftor is associated with profound therapeutic benefit (1, 31, 34), including augmentation of mucociliary clearance (34) and rescue of CFTR activity to ∼30–50% of wild-type CFTR function, as estimated in vivo (2, 35) or in vitro (44), respectively. Treatment of F508del CFTR has been much more complex, in part due to its multiple cellular defects, but also because corrector therapy is inherently less efficacious (23, 26, 30). Although corrector monotherapy with the investigational CFTR corrector lumacaftor (formerly VX-809) can restore partial CFTR function in patients homozygous for F508del (9), it does not exhibit therapeutic benefit until the sequential addition of ivacaftor to the regimen (6), suggesting the importance of addressing both prominent cellular abnormalities. Moreover, improved spirometry was only observed with addition of ivacaftor to lumacaftor therapy, even though further improvements in CFTR ion transport were not observed (6). Recently, the potential deleterious effects of ivacaftor on F508del thermal stability and membrane residence time have been described and been postulated to explain suboptimal therapeutic benefits observed with ivacaftor-lumacaftor cotherapy (8, 20, 46). However, these in vitro results relied on measures of ion transport to assess CFTR activity and do not adequately explain apparent clinical findings, which emphasize the benefit of potentiator therapy when added to the regimen (6). Resolving this conundrum is critical, since multiagent CFTR modulator therapy is now approved (47); furthermore, assays that rely on use of primary human epithelial cells have been proposed to help guide use of personalized therapeutic regimens for the individualized treatment of CF, including those with rare genetic alleles.
Functional assessments of the airway surface at the cellular level will be crucial to understanding the full effects of CFTR modulators, especially given inadequacies of ion transport studies as a completely predictive biomarker (8, 46). Given that it is now understood that CFTR exhibits a variety of effects on the airway, including chloride secretion, altered mucus viscosity (3), and adhesion to the gland ducts (13), assays that detect the protean effects of CFTR modulation are needed. To address this, our laboratories have developed micro-optical coherence tomography (μOCT), a high-speed, high-resolution imaging modality that allows for simultaneous evaluation of epithelial function microanatomy in situ and in living tissue (3, 15, 18, 19, 43). This technology enables new assessments of mechanism and may better predict the success of therapeutics that target epithelial function since measurements of mucociliary transport and effective mucus viscosity can be readily acquired, and may dissociate with measures of ion transport alone (3). These analyses provide insight into the mechanisms by which therapeutic benefit of corrector-potentiator therapy is conferred to CF patients, and provide a more complete assessment of complex CFTR modulator regimens, suggesting the potential for predicting clinical response on an individualized basis.
MATERIALS AND METHODS
Primary human bronchial epithelial cell cultures.
Primary human bronchial epithelial (HBE) cells were derived from lung explants obtained from either normal subjects, or patients with cystic fibrosis, as previously described (37, 40, 44). Briefly, first- or second-passage cells underwent expansion, attained confluency, and were seeded on 6.5-mm-diameter permeable supports (0.5 × 106 cells/filter; Corning, Corning, NY) coated with NIH 3T3 fibroblast unconditioned media. Cells were grown in differentiating media for at least 6–8 wk until terminally differentiated before experimental use.
CFTR modulation experiments.
Primary HBE cells were washed 48 h before imaging; 50 μl of warm sterile PBS were added to the apical surface and incubated at 37°C for 15 min, followed by aspiration of the apical fluid. Immediately following wash, CFTR correctors or potentiators were added to the media. Dimethyl sulfoxide (DMSO) was used as a vehicle control. VX-770, C18, and forskolin (Calbiochem, San Diego, CA) were solubilized in DMSO and added to media at a 1:1,000 dilution, for a final concentration of 10 μM VX-770, 3 μM C18, and 20 μM forskolin, as previously published (40). Cells were exposed to treatments for 24–48 h before imaging.
Human bronchial explants.
Use of human tissues was approved by the University of Alabama Birmingham Institutional Review Board (IRB). After written informed consent, bronchial samples of lung explants from normal failed donors and subjects with ΔF508/ΔF508 genotype were obtained and placed in minimal essential media (MEM) with 100 mg/ml ceftazidime, 80 mg/ml tobramycin, and 1.25 mg/ml amphotericin B. After receipt, bronchial samples were incised longitudinally to expose the airway lumen, cleared of excessive endogenous sputum or mucus, and incubated at 37°C for 2–4 h to reequilibrate to physiological conditions. Four sections of bronchial tissues were collected from each donor; these were treated with DMSO vehicle, 10 μM VX-770, 3 μM C18, or VX-770 plus C18, and incubated for 2 h with treatment before μOCT imaging.
Short-circuit current measurements.
Short-circuit current (Isc) of HBE monolayers was measured under voltage-clamp conditions using MC8 voltage clamps and P2300 Ussing chambers (Physiologic Instruments, San Diego, CA) as previously described (37, 40). Monolayers were initially bathed on both sides with identical Ringer solutions containing (in mM) 115 NaCl, 25 NaHCO3, 2.4 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, 1.2 MgCl2, and 10 d-glucose (pH 7.4). Bath solutions were vigorously stirred and gassed with 95% O2-5% CO2. Isc measurements were obtained using an epithelial voltage clamp (Physiologic Instruments). A 1-s 3-mV pulse was imposed every 10 s to monitor resistance calculated using Ohm's law. Where indicated, the mucosal bathing solution was changed to a low-Cl− solution containing 1.2 mM NaCl and 115 mM sodium gluconate, and all other components as above. Amiloride (100 μM) was added to block residual Na+ current, followed by the agonists forskolin (20 μM) and ivacaftor as indicated (minimum 5-min observation at each concentration). CFTRInh-172 (10 μM) was added to the mucosal bathing solution at the end of experiments to block CFTR-dependent Isc. All chambers were maintained at 37°C, and agonist stimulation was initiated within 15 min of placement in the chambers.
Airway surface liquid depth measurement by confocal microscopy.
The apical surfaces of HBE cells were washed three times, and then test compounds were added to the basolateral compartment 24–48 h before labeling. Texas red dye (25 μl at 10 mg/ml in FC-70) was added apically, and cells were allowed to equilibrate 2 h at 37°C. Transwell membranes were placed in a sterile glass-bottom dish coated with MEM and imaged with a Carl Zeiss (Peabody, MA) confocal microscope using a ×20 (numerical aperture 0.88, working distance 0.55 mm) air objective lens. Cells were visualized with DIC optics to evaluate cell morphology before initiating fluorescence microscopy. Subsequently, Z-scan confocal fluorescent microscopy images were acquired from the top of the airway surface liquid (ASL) through the top of the cell surface. X-Z-scans were analyzed using Zen2008 software at four regions of interest (ROI) per well each located 1 mm from the filter periphery and at each quadrant; five estimates of ASL depth were taken equally dispersed across each ROI (22, 33). Because baseline ASL depth varies among donors, each experiment was internally controlled using cells derived from a single donor.
Fluorescence recovery after photobleaching.
To measure fluorescence recovery after photobleaching (FRAP) in HBE cells, ASL was stained with FITC (10 μl at 1 mg/ml in PBS) and incubated for 30 min at 37°C. FC-70 (50 μl) was added apically to the monolayer to prevent evaporative loss. Transwell membranes were then placed in a sterile glass dish containing 100 μl MEM to bathe the basolateral compartment. After an initial Z-scan to determine cell height, a height was selected for FRAP imaging ∼8 μm above the cell surface so that viscosity of the ASL could be measured (as opposed to overlying buffer or FC-70). A circular diameter of ∼3–5 μm on the ASL surface was bleached for 50 iterations for 60 s at 100% laser power with a 5-mW argon laser (458 and 488 nm) and 30-mW diode laser (405 nm) to maximize bleach. Images were acquired using an LSM710 Carl Zeiss confocal microscope (Oberkochen, Germany) and a ×20 air objective every 4 s for 90–110 s or until maximal recovery was reached. The fluorescence intensity was normalized using background and unbleached fluorescence images. Normalized intensity values were fitted to the formula I(t) = I0 − I1 × e(−t/τ1)/I0 − I2 × e(−t/τ2). I1 and I2 are the percentage of two mobile fractions with I0 representing the final intensity after bleaching. The recovery curves of normalized values from at least three experimental repeats were plotted using one-phase association equation (Graphpad Prism version 6.0, La Jolla, CA). In these studies, ASL depth was not quantified, since rapid planar imaging (rather than Z-scan reconstructions) is required to measure the rate that the stained ASL fills the bleached area.
Particle-tracking microrheology.
Particle-tracking microrheological techniques were used to measure viscosity of mucus on HBE cell cultures (3, 17). HBE cells were treated with 0.1% benzalkonium chloride (Acros Organics) 1 h at 37°C to disrupt ciliary beating. The cells were then rinsed in media (600 μl) at 37°C for 15 min and then transferred to fresh media (600 μl). Polystyrene (PS) beads were purchased from Molecular Probes (Life Technologies, Grand Island, NY) as red (580/605) fluorescent carboxyl-modified beads. Methoxy-poly(ethylene glycol) (PEG) (mol wt 5,000) was conjugated to the beads through published techniques (24a). PEG-PS beads (2.0 × 1011 particles/ml) were diluted in PBS (1/120) and then 10 μl were loaded on the apical side of cell monolayers and incubated at 37°C 5% CO2. After 3–4 h, the insert was loaded on a glass-bottom culture dish (35 mm) (MatTek, Ashland, MA). Fluorescence time-series images were acquired using an inverted microscope (Nikon Eclipse TE200, Melville, NY) at a frame rate of ∼17 frames/second. Images were analyzed using ImageJ and the SpotTracker plugin (http://bigwww.epfl.ch/sage/soft/spottracker/SpotTrackerX2D_.jar). Resulting particle tracks were analyzed with custom Matlab procedures to compute mean squared displacement (MSD) while subtracting spurious bulk motion common to all tracks. Dynamic viscosity was derived from MSD by application of the generalized Stokes-Einstein relation as described (21a).
μOCT image acquisition.
Measurements of functional microanatomic parameters in in vitro cultured cells and ex vivo tissue were performed using μOCT, a high-speed, high-resolution microscopic reflectance imaging modality. We have previously described μOCT methods to image airway epithelia and the associated quantitative analysis (3, 18, 19). In brief, the μOCT instrument provides cross-sectional images of the airway epithelium at a resolution of ∼1 μm. This resolution is sufficient to directly visualize and quantify microanatomic parameters, including ASL depth, periciliary layer (PCL) depth, ciliary beat frequency (CBF), mucociliary transport (MCT) rate, and mucus gland output without using exogenous dyes or particles. Typical acquisition speed is 20,480 Hz line rate, resulting in 40 frames/s at 512 lines/frame.
μOCT image analysis.
Quantitative analysis of images provided several metrics. ASL and PCL depths were characterized directly by geometric measurement of the respective layers in Image J software. For PCL, measurement time averaging of images over several frames captured the length of fully extended cilia. CBF was quantified by Fourier analysis of the reflectance of beating cilia using custom code in Matlab (Mathworks, Natick, MA). MCT rate was determined using time elapsed and distance traveled of native particulates in the mucus over multiple frames. For each HBE monolayer, images were acquired 1 mm from the filter periphery with a scanning beam parallel to the tangent of the circumference of the filter membrane disc. For trachea, images were acquired at randomly chosen locations on the mucosal surface with the optical beam scanned along the longitudinal direction.
Statistical analysis.
Statistical analysis was performed in GraphPad Prism version 6.0. Inferential statistics (mean, SD, and SE) were computed using ANOVA, unpaired or paired t-test, as appropriate. For multiple comparisons, post hoc testing was applied only if ANOVA was significant. For MCT, nonparametric statistical tests were used, since results were not normally distributed. P values <0.05 were considered significant. Statistics are presented as means ± SE, except as indicated. Unless otherwise indicated, mean values per HBE monolayer or per tissue explant are shown.
Study approvals.
Procedures involving human cells and tissue were approved by the IRB at the University of Alabama Birmingham (IRB nos. X080625002, X110916018, and X101111014) and Massachusetts General Hospital (IRB no. 2008P000178).
RESULTS
Ivacaftor potentiates G551D CFTR in a dose-dependent manner.
Initial experiments investigated the association between ion transport function and ASL depth in HBE monolayers expressing G551D CFTR and the effect of CFTR potentiation on these parameters. Cells derived from G551D/F508del donors were grown until terminally differentiated and serially treated with increasing concentrations of ivacaftor (VX-770). Ivacaftor elicited a clear response in CFTR-dependent Isc (Fig. 1A). Cumulative currents demonstrated a dose-response curve that plateaued at 10 μM (Fig. 1B), a value previously shown to approximate the maximum effective concentration (44). CFTR potentiation caused an increase in ASL depth, even under chronic stimulation conditions; when G551D/F508del HBE cells were treated with ivacaftor to the basolateral compartment for 24 h, ASL depth, indicated by confocal microscopy (Fig. 1C), increased compared with vehicle control. Notably, the response in ASL accumulation to potentiation with ivacaftor was not incremental; ASL depth did not increase until a dose of 100 nM was used (Fig. 1D); ASL depths in response to the highest dose of ivacaftor were comparable to ASL of non-CF donors, at 18.03 ± 1.6 μm (3, 18).
Fig. 1.
Ivacaftor potentiates G551D CFTR in a dose-dependent manner. A: representative short-circuit current (Isc) tracing from G551D/F508del human bronchial epithelial (HBE) cells serially exposed to amiloride (100 μM), forskolin (20 μM), and then VX-770 apically at concentrations indicated. CFTRInh-172 was added to confirm CFTR dependence. B: concentration dependence of VX-770-induced response. C: representative image of the effect of VX-770 on airway surface liquid (ASL, stained with Texas red) on G551D/F508del HBE (stained with CellTracker green). D: quantitative data of ASL height obtained from each concentration of VX-770. E: Isc response to forskolin in the presence and absence of bicarbonate. F: Isc response to CFTRInh-172 in the presence and absence of bicarbonate. n = 4–6 Experiments. *P < 0.05 and ***P < 0.001.
Ivacaftor augments airway functional microanatomy in G551D/F508del HBE.
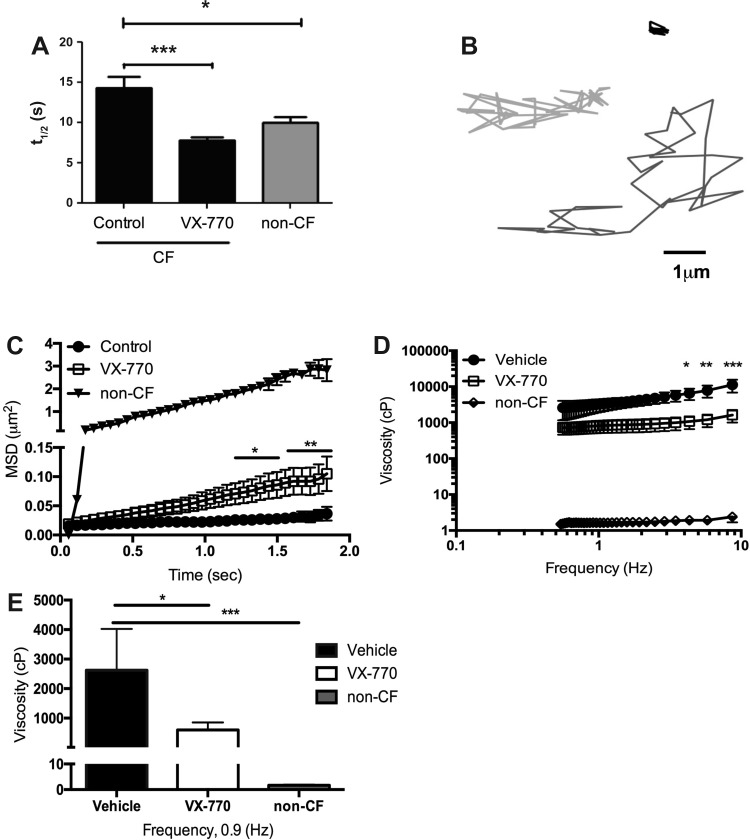
As an archetypal CFTR potentiator, it has been previously demonstrated that ivacaftor acts synergistically with conventional CFTR agonists, such as forskolin (14, 44); however, whether this is important to downstream effects on epithelial function, such as mucociliary transport, has not been evaluated. Accordingly, the basolateral compartment of G551D/F508del HBE cells was treated with forskolin, ivacaftor, or both, for 24 h, and imaged under μOCT. Representative images of cells treated with forskolin only [Fig. 2, A and B, and Supplemental Video S1 (Supplemental data for this article can be found on the journal website.)] compared with cells treated with forskolin and ivacaftor (Fig. 2, B and D, and Supplemental Video S2) showed the effects of activation and potentiation of CFTR. Quantitative data from these videos indicated that combination treatment increased ASL (Fig. 2E), CBF (Fig. 2F), and MCT (Fig. 2G) compared with vehicle or either single treatment alone. To test whether augmented CFTR activity also affected effective viscosity as measured in situ, which can occur independent of ASL depth in the CF situation (3), fluid diffusion in the ASL of G551D/F508del monolayers was estimated by FRAP following chronic agonist administration. The half-life of time to recovery shortened from 12.39 ± 1.3 to 7.57 ± 0.8 s (P < 0.001) in cells treated with ivacaftor for 24 h (Fig. 3A); non-CF data are shown for comparison. These data indicate that ivacaftor-treated G551D/F508del monolayers exhibited a similar effective viscosity to that obtained from non-CF monolayers. To complement these findings, we assessed effective mucus viscosity in situ using particle-tracking microrheology of G551D monolayers, again following 24 h chronic administration. Representative tracings from particles added to G551D/F508del monolayers treated with either vehicle or ivacaftor (Fig. 3B) indicated that the potentiator therapy decreased effective viscosity. Graphical representation of MSD (Fig. 3C) confirmed that particles applied to treated monolayers moved more freely than particles tracked in the CF control mucus, and more resembles particle movement in non-CF mucus. This is demonstrated in the full viscoelastic curve (Fig. 3E), in which the viscosity of the potentiator-treated cell mucus layer was lower than control at all frequencies; effective viscosity of cells treated with ivacaftor (600 cP) was significantly lower than control (2,600 cP, P < 0.05) at the physiological frequency of 0.9 Hz (Fig. 3F). These data indicate that activation of mutant CFTR is required to provide sufficient activity to augment ASL depth and reduce CF mucus viscosity, and that the doses producing measurable improvement to the two parameters can be distinct. Although the viscosity of treated CF mucus is not entirely normalized to non-CF values, the mucus is thinned enough to augment MCT rates. This suggests a mechanistic distinction between these properties, as also seen among changes in different clinical endpoints (i.e., sweat chloride vs. nasal potential difference vs. lung function) with ivacaftor therapy in G551D patients (2, 34).
Fig. 2.
Ivacaftor augments airway functional anatomy in G551D/F508del HBE. Representative micro-optical coherence tomography (μOCT) images of G551D/F508del HBE treated with forskolin alone (A) or forskolin + ivacaftor (VX-770; C). Text indicates the cross-sectional view of the filter membrane (Filter), the epithelial cell monolayer (ep), and the air interface (air). The ASL layer depth is demarked (orange bar). Mucociliary transport (MCT) of forskolin alone (B) or forskolin + VX-770 (D) is represented by the vectoral transport (red arrow) of mucus particles over time (time shown on the y-axis); slope of diagonal streak indicates motion speed, with horizontal lines being faster and vertical lines being static. Scale (white bar) = 5 μm. E–G: quantitative analysis showing that combinations of forskolin and VX-770 treatment increase ASL, ciliary beat frequency (CBF), and MCT compared with each agent alone. n = 4–6. *P < 0.05 and τP = 0.06.
Fig. 3.
Ivacaftor treatment in G551D/F508del HBE decreases effective viscosity of the mucus layer. A: half-life of recovery curves from VX-770-treated cystic fibrosis (CF) cells returns to normal donor levels. B: representative tracings from particle-tracking microrheology of CF cells treated with vehicle control (black), VX-770 (light gray), and non-CF (dark gray) cells. C: movement of each particle was quantified to give mean-squared displacement (MSD). Statistics reported for VX-770 compared with vehicle. D: viscoelastic curves of particles applied to the mucus layer of vehicle-, VX-770-, and non-CF-treated monolayers. Statistics reported for VX-770 compared with vehicle. E: effective viscosity of the mucus layers at 0.9 Hz. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Chronic treatment with ivacaftor and C18 in F508del/F508del HBE decreases CFTR function.
Recent reports have shown that chronic exposure to ivacaftor decreases the thermal stability of F508del-CFTR at the cell surface when administered chronically (i.e., 24–48 h in vitro) as opposed to its acute effects, which do not alter surface expression. This effect results in a relatively diminished Isc response in cells treated with ivacaftor and lumacaftor combined for 24 h or more compared with cells treated with lumacaftor alone (8, 20, 46). To confirm these studies, F508del homozygous primary HBE monolayers were treated basolaterally with ivacaftor (VX-770), C18 (a lumacaftor homolog), or the combination for 48 h to achieve chronic exposure to these drugs. Representative Isc tracings showed that cells treated with the combination of these modulators exhibit an altered response to acute addition of the CFTR agonists forskolin (10 μM) and ivacaftor (10 μM) compared with cells treated with the F508del corrector C18 alone (Fig. 4A). In particular, while forskolin response was augmented in C18-treated cells with (5.52 ± 1.5 μA/cm2) or without (5.04 ± 0.4 μA/cm2) the presence of ivacaftor pretreatment, ivacaftor pretreatment did not potentiate this effect (Fig. 4, A and B); furthermore, the subsequent response to acute addition of ivacaftor was dramatically blunted by ivacaftor pretreatment (8.46 ± 0.5 μA/cm2 C18 alone vs. −0.83 ± 0.2 μA/cm2 C18 + ivacaftor; Fig. 4, A and C), reflecting the deleterious effects of ivacaftor on F508del stability shown previously (8, 20, 46). The result was that total stimulated current (forskolin + acute ivacaftor response) was significantly diminished in cells treated chronically with the combination of C18 and ivacaftor [4.69 ± 1.6 μA/cm2 compared with C18 alone (14.41 ± 0.9 μA/cm2)], which was augmented over the untreated control (9.5 ± 1.2 μA/cm2; Fig. 4, A and D). The responses to the CFTR-specific inhibitor CFTRInh-172 were similar, and indicated that F508del-CFTR is less active in F508del-corrected cells exposed to chronic ivacaftor (Fig. 4E). These data corroborate recent reports (8, 20, 46), but do not reflect responses observed in the clinic, which demonstrate sequential addition of ivacaftor to lumacaftor-treated F508del homozygous patients was necessary to confer improved lung function (6, 47). This is also evident in vivo by assessing the change in sweat chloride concentrations after 2 wk of treatment with placebo, lumacaftor, or lumacaftor plus ivacaftor (6, 31, 47). Treatment with the corrector alone decreased sweat chloride concentrations by 6.8 ± 2.0 mmol/l (Fig. 4F); however, this effect was not increased in subjects who received combination therapy (decreased concentrations by 9.3 ± 2.8 mmol/l). These data indicate that measures of ion transport alone, whether in vitro or in the clinic, do not reflect the beneficial effects of adding ivacaftor to corrector treatment, and suggest the need for alternative measures of airway function to understand clinical benefit.
Fig. 4.
Chronic treatment with ivacaftor and C18 in F508del/F508del HBE decreases CFTR function. A: representative tracings of F508del/F508del HBE monolayers treated with vehicle, C18, or C18 + VX-770. B: change in Isc responses to forskolin stimulation. C: change in Isc in response to acute application of VX-770. D: cumulative change in Isc response to both agonists. E: change in Isc after addition of CFTRInh-172. F: change in sweat chloride concentration in subjects treated with VX-809 or VX-809 and VX-770. n = 4–6. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Chronic treatment with ivacaftor and C18 increases epithelial function of the airway surface.
To establish the mechanism responsible for the therapeutic benefit of ivacaftor addition to lumacaftor therapy in CF patients homozygous for F508del, we examined primary HBE cells treated chronically with ivacaftor, C18, or the combination, in the presence of forskolin, for 48 h using μOCT, which can reveal multiple aspects of the functional microanatomy simultaneously and noninvasively (3). Representative images are shown in Fig. 5, A–D, and quantitative data are summarized in Fig. 5, E–H. Results demonstrate that monotherapy with either ivacaftor or C18 did not result in any appreciable changes to mucociliary transport or other functional parameters of the airway surface compared with cells treated with vehicle control (Fig. 5, A, B, E, and G), with or without forskolin stimulation. However, a dramatic increase in ASL depth was observed in F508del/F508del cells treated with the combination of ivacaftor and C18 with forskolin (23.4 ± 2.6 vs. 9.01 ± 1.4 μm C18 + forskolin, 10.99 ± 1.7 μm ivacaftor + forskolin, 13.03 ± 2.8 μm forskolin alone, and 12.53 ± 2.3 μm vehicle, P < 0.05; Fig. 5, C–E). Likewise, MCT rates were dramatically increased after the combination therapy (4.24 ± 1.6 mm/min vs. 0.38 ± 1.4 C18, 0.22 ± 0.08 mm/min ivacaftor, and 1.9 ± 0.07 mm/min vehicle, P < 0.05; Fig. 5, D and G). Quantitative analysis showed a significant improvement in the functional microanatomy in response to chronic combination therapy as measured by ASL depth (Fig. 5E) and MCT (Fig. 5G), whereas monotherapy with either compound alone did not significantly alter these parameters. Interestingly, unlike measures of ion transport, these data reflect changes in lung function observed in clinical studies, as measured by the change in percent forced expiratory volume in 1 s predicted after treatment with placebo, lumacaftor alone, or lumacaftor plus ivacaftor for 14 days (6, 47). Evaluating data from the highest lumacaftor dose group (600 mg one time daily), lumacaftor alone did not elicit a significant change in percent predicted forced expiratory volume in 1 s, whereas, upon addition of ivacaftor to the regimen, lung function significantly improved (Fig. 5H). These data indicate that the changes in the mucociliary transport apparatus, measured by ASL depth, CBF, and MCT rate, better reflect clinical benefit as measured by change in lung function of combination treatment with corrector-potentiator therapy, such as ivacaftor and lumacaftor. Because we have previously demonstrated that altered effective viscosity is highly sensitive to CFTR function and can be a dominant factor toward governing mucociliary transport, we next assessed whether effective mucus viscosity was also affected by addition of ivacaftor to corrector therapy. As shown by representative tracings from particle-tracking microrheology (Fig. 6A) of F508del homozygous monolayers, effective mucus viscosity in situ was reduced by 48 h treatment with CFTR modulator therapy, but only when the combination of ivacaftor and C18 was employed. MSD (Fig. 6B) showed a significant increase in particle movement in monolayers treated with the combination compared with control; monotreatments did not alter MSD measurements significantly. This corresponds to lower viscosity measurements across all frequencies tested with combination therapy (Fig. 6, C and D). These data demonstrate that, while F508del-CFTR may exhibit reduced thermal stability upon treatment with chronic ivacaftor that partially abrogates beneficial effects on ion transport of corrector-potentiator combination therapy (as measured by Isc), the drug combination is absolutely required to induce rescue of the mucociliary clearance apparatus, including changes in effective mucus viscosity, even under chronic administration conditions. One possible explanation could lie in the mechanism for mucin release and formation, which is primarily mediated by bicarbonate concentrations (12, 28, 29). We considered the possibility that ivacaftor may be preferentially correcting bicarbonate transport over chloride, but this was not found to be the case in G551D-F508del HBE cells (Fig. 1, E and F).
Fig. 5.
Chronic treatment with ivacaftor and C18 increases epithelial function of the airway surface. Representative images of F508del homozygous HBE cells treated with forskolin alone (A) or forskolin + C18 and VX-770 (C); text indicates the filter, the epithelial cell monolayer, the air interface, and the ASL layer (orange bar). Scale (white bar) = 5 μm. MCT of forskolin alone (B) or forskolin + C18 and VX-770 (D) is represented by the vectoral transport of mucus particles over time (red arrow), as shown in Fig. 2. E–G: quantitative measurements obtained from μOCT imaging of ASL, CBF, and MCT rates. H: change in %predicted forced expiratory volume in 1 s (FEV1) in subjects treated with VX-809 or VX-809 and VX-770. n = 4–6. *P < 0.05.
Fig. 6.
Chronic treatment with ivacaftor and C18 decreases effective viscosity of the mucus layer of homozygous F508del monolayers. A: representative particle tracings of F508del/F508del HBE monolayers treated with vehicle (black) and C18 + VX-770 (gray). B: movement of each particle was quantified to give MSD of cells treated with vehicle, C18, VX-770, or the combination. C: viscoelastic curves of particles applied to the mucus layer of monolayers. D: effective viscosity of the mucus layers at 0.9 Hz. G551D/F508del HBE cells treated with VX-770 demonstrate similar CFTR activity with or without bicarbonate transport measured by the change in Isc in response to forskolin (E) or CFTRInh-172 (F). n = 4–6. *P < 0.05.
Combination CFTR modulator therapy of bronchial tissue from an F508del homozygous donor increases mucus transport disproportionately to other effects on ASL depth.
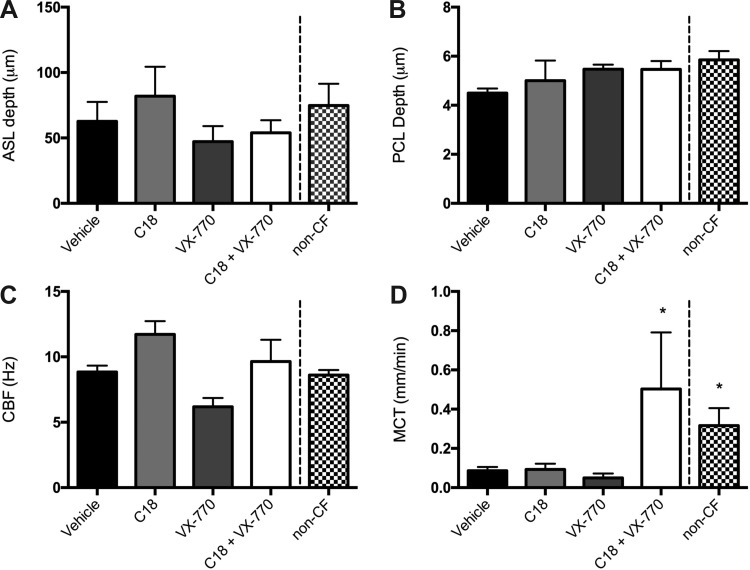
While primary HBE monolayers demonstrated a clear distinction between monotherapy and cotreatment, the simplified nature of the model may not be fully representative of the condition in the patients, such as the contribution of airway glands. Therefore, we treated human bronchial samples freshly obtained from an F508del homozygous donor immediately after explanation to test the effect of CFTR modulators. μOCT imaging indicated that basolateral treatment ex vivo with the combination of C18 and ivacaftor for 4 h had a pronounced increase in MCT rate (0.5 ± 0.2 mm/min, P < 0.05) compared with monotherapy in cell culture with either agent alone [0.09 ± 0.02 mm/min C18, 0.05 ± 0.02 mm/min ivacaftor, 0.08 ± 0.02 mm/min vehicle (Fig. 7D), and 0.32 ± 0.08 mm/min non-CF]. In contrast, no treatment appreciably altered ASL depth (Fig. 7A), PCL depth (Fig. 7B), or CBF (Fig. 7C). Given the lack of change in airway liquid layers, the increased MCT emphasizes the importance of mucus viscosity in an intact tissue model, consistent with prior studies of porcine trachea (3). This confirms the beneficial effects of combination CFTR modulator therapy in a more sophisticated model system, and indicated that the addition of a CFTR potentiator alters the rheological properties of the mucus even in the absence of increased fluid accumulation in situ in a manner that cannot be predicted by measures of ion transport alone.
Fig. 7.
Combination CFTR modulator therapy increases mucus transport in bronchial tissue from a F508del homozygous donor. Explanted bronchial tissue obtained from a F508del homozygous donor was treated basolaterally for 4 h with vehicle, C18, VX-770, or C18 + VX-770. Normal failed donor explant is included for comparison. Quantitative measurements obtained from μOCT imaging demonstrate no change to ASL (A), PCL (B), or CBF (C) but does increase MCT rates (D). *P < 0.05; n = 10 regions of interest derived from 2 donors/condition.
DISCUSSION
Investigation of the functional parameters and characteristics of the epithelial surface and its associated mucus layer demonstrated clear efficacy of ivacaftor in cells with the G551D-CFTR mutation. Measurements of efficacy in vitro for this mutation group have been relatively straightforward, because the molecular defect underlying the G551D gating mutation is less complex than that posed by F508del. Analysis of the functional microanatomy in general, and effective viscosity measurements in particular, indicated a largely normalized phenotype in response to treatment with this potentiator and was consistent with increased Isc in polarized monolayers, as also observed previously (44). These results corroborate observed benefits in ion transport and clinical response (1, 31, 34).
While CFTR activation data showed F508del-CFTR instability with chronic ivacaftor, similar to previous reports (8, 20, 46), downstream function at the epithelial surface was only rescued in response to combination therapy. Although the F508del-CFTR exhibited decreased stimulated currents on Ussing chamber analysis with chronic exposure to ivacaftor, functional imaging clearly demonstrated that CFTR achieves a threshold of activity sufficient to improve mucus clearance. This suggests that, while there may be less overall CFTR activity in F508del cells or tissues when treated with ivacaftor and a CFTR corrector (as estimated by Ussing chamber analysis of intact monolayers, a measure of average CFTR function across a monolayer), the addition of a potentiator that augments channel gating is absolutely required to promote mucus transport. This may be because highly active (i.e., potentiated) CFTRs are necessary to reduce mucus adhesion to the epithelial surface, as estimated by effective viscosity, at local sites near initial expulsion of mucus from the epithelium, which may overcome relatively diminished CFTR function in other areas (13). Data from tissues, which include contribution of the glandular epithelium in addition to the airway surface, clearly demonstrated that mucus viscosity can be dominant over the effects on airway hydration (as measured by ASL or PCL) alone (13). This is consistent with prior observation of the CF mucociliary transport defect in excised swine trachea (3, 13). The degree of functional correction required in specific compartments to restore mucus clearance may have implications for gene therapy, which can have heterogeneous effects on various epithelial cells (5). The discord between CFTR protein activity and downstream functional markers is a finding of importance from these studies and may signify that more in-depth study of the consequences of CFTR correction is needed. One challenge with these sorts of comparisons, which also impacted our study, is that the degree of differentiation can vary between different HBE cell donors, which can significantly impact absolute MCT rates; well-controlled procedures and relative comparisons when baseline MCT rates vary widely in the untreated condition may be more appropriate under these circumstances.
Bicarbonate transport has been shown to impact mucus clearance in regard to both airway hydration and the content of submucosal gland secretions (7, 12, 29, 39). However, the effect of ivacaftor or other novel modulators on bicarbonate transport have not been tested. Here we show that ivacaftor exerts proportionate effects on both chloride and bicarbonate transport in HBE cells (Fig. 1). This likely explains why the increased ASL depth in the ivacaftor-treated G551D/F508del cells mirrors the decrease in viscosity in these cells (Figs. 2 and 3) with similar effects seen in the combination-treated F508del/F508del cells (Figs. 5 and 6).
Mucociliary transport and viscosity measurements as in vitro biomarkers are in agreement with clinical results among F508del homozygotes (6, 9, 47), in contrast to previously published in vitro data that focused on CFTR expression and activity alone (8, 20, 46), and shed light on an important finding. Prior in vitro studies demonstrated a diminished response upon addition of ivacaftor to corrector therapy (8, 46), whereas, in CF patients, ivacaftor was absolutely required to establish clinical benefit in lung function (in fact, decrements in lung function have been observed with the sole use of corrector therapy) (6, 47). Notably, the effects on sweat chloride, an indicator of ion transport, matched in vitro findings of stimulated currents in primary human airway monolayers but did not correlate with improved spirometry; in contrast, the effects on the functional microanatomy shown here matched the benefit observed in the clinic (6, 9, 47). This demonstrated the importance of downstream markers of epithelial function, such as those elucidated by μOCT, which can be distinct from measures of ion transport, and points toward the increasing complex relationship between epithelial anion transport and the genesis and transport of mucus.
Overall these studies indicate that preclinical evaluation of CFTR modulator therapy may require more complex and thorough studies than previously suggested (25). While protein stability and ion transport function are clearly important, these parameters alone may not fully identify the potential of modulators to affect function at the epithelial surface. These data suggest that new therapies should be evaluated for more than chloride transport in cell culture. Rather, examination of the effects on airway hydration, ciliary function, and in particular mucus transport and viscosity may be vital components to the evaluation of new therapeutics and allow improved predictions regarding therapeutic efficacy, particularly as increasing complex combinations of CFTR modulators are advanced.
Combination therapy with a corrector and potentiator has recently been approved for the treatment of CF patients with F508del CFTR, although the long-term effects of single-agent therapy have not been directly compared (9, 47). The data presented here support combination treatment of ivacaftor with CFTR corrector therapy for patients homozygous for F508del CFTR over and above either agent alone. Monotherapy with either agent exhibited little effect on mucociliary transport even when ion transport was augmented. It is important to note that not all measures of CFTR function demonstrate equal magnitudes of effect or the same dose response. However, the beneficial and combinatorial effects of correctors and potentiators have been highlighted in the present study (a point debated intensely while ivacaftor-lumacaftor was being considered by the Food and Drug Administration this year), increasing confidence in combination treatment strategy for patients with multiple types of mutations that confer defects in CFTR expression, processing, and/or function.
In addition to improved groupwise comparisons, changes in functional microanatomy may also serve to predict clinical response on a individual basis, since sweat chloride has not performed well for this purpose (11, 34). Primary airway cells, such as those acquired from the nasal epithelia, and used in combination with μOCT imaging and ion transport, may be an excellent tool for predicting response to personalized therapeutic regimens (41). Studies such as those presented here have the potential to evaluate various agents alone and in combination, facilitating prioritization for clinical testing or pilot administration. Such a tool may become increasingly important as new CFTR modulators are discovered and could address complexities posed by exceedingly rare mutations or those that are poorly characterized.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute [R01-HL-116213 (G. J. Tearney and S. M. Rowe) and 5T32-HL-105346-04 (S. E. Birket)] and the National Institute of Diabetes and Digestive and Kidney Diseases [P30-DK-072482 (E. J. Sorscher)], the Research Development Program (R464-CF), the Mucociliary Clearance Consortium (G. J. Tearney and S. M. Rowe) of the Cystic Fibrosis Foundation, the Flatley Foundation (K. K. Chu, G. J. Tearney, and S. M. Rowe), and the Dixon Foundation (G. H. Houser).
DISCLOSURES
LL, GHH, KKC, EJS, GJT, and SMR hold a patent on the use of μOCT for pharmaceutical drug discovery. The other authors have no other competing financial interests to disclose.
AUTHOR CONTRIBUTIONS
S.E.B., K.K.C., G.H.H., L.L., G.M.S., S.S., J.H., W.E.G., E.J.S., G.J.T., and S.M.R. conception and design of research; S.E.B., K.K.C., G.H.H., L.L., C.M.F., G.M.S., V.L., S.S., M.M., P.S., G.J.T., and S.M.R. performed experiments; S.E.B., K.K.C., L.L., C.M.F., G.M.S., V.L., S.S., P.S., J.H., W.E.G., E.J.S., G.J.T., and S.M.R. analyzed data; S.E.B., K.K.C., G.H.H., L.L., C.M.F., G.M.S., S.S., J.H., W.E.G., E.J.S., G.J.T., and S.M.R. interpreted results of experiments; S.E.B., L.L., C.M.F., G.M.S., S.S., and S.M.R. prepared figures; S.E.B., K.K.C., L.L., G.J.T., and S.M.R. drafted manuscript; S.E.B., K.K.C., G.H.H., L.L., C.M.F., G.M.S., V.L., S.S., M.M., P.S., J.H., W.E.G., E.J.S., G.J.T., and S.M.R. edited and revised manuscript; S.E.B., K.K.C., G.H.H., L.L., C.M.F., G.M.S., S.S., M.M., P.S., J.H., W.E.G., E.J.S., G.J.T., and S.M.R. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Arianne Fulce, Kathy Sexton, Thurman Richardson, and the Tissue Collection and Banking Facility at the University of Alabama at Birmingham (UAB) for services related to airway tissue procurement. The authors are grateful to Heather Hathorne for regulatory support for work with human subjects and the patients who donated their organs for these experiments. We acknowledge assistance from the UAB Imaging Core Facility and Mei Wu's laboratory in the Wellman Center of Photomedicine, Massachusetts General Hospital.
REFERENCES
- 1.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordonez CL, Campbell PW, Ashlock MA, Ramsey BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 363: 1991–2003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, Rowe SM, Clancy JP, Konstan MW, Hoch HE, Heltshe SL, Ramsey BW, Campbell PW, Ashlock MA. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros 13: 139–147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birket SE, Chu KK, Liu L, Houser GH, Diephuis BJ, Wilsterman EJ, Dierksen G, Mazur M, Shastry S, Li Y, Watson JD, Smith AT, Schuster BS, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 190: 421–432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bompadre SG, Sohma Y, Li M, Hwang TC. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol 129: 285–298, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher RC, Knowles MR, Johnson LG, Olsen JC, Pickles R, Wilson JM, Engelhardt J, Yang Y, Grossman M. Gene Therapy for Cystic Fibrosis Using E1-Deleted Adenovirus: A Phase I Trial in the Nasal Cavity. Chapel Hill, NC: The University of North Carolina at Chapel Hill Human Gene Therapy; 1994, vol. 5, p. 615–639. [DOI] [PubMed] [Google Scholar]
- 6.Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, Waltz D, Patel NR, Rodman D, and group VXs. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2: 527–538, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299: L542–L549, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med 6: 246–296, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW, Deboeck K, Donaldson SH, Dorkin HL, Dunitz JM, Durie PR, Jain M, Leonard A, McCoy KS, Moss RB, Pilewski JM, Rosenbluth DB, Rubenstein RC, Schechter MS, Botfield M, Ordonez CL, Spencer-Green GT, Vernillet L, Wisseh S, Yen K, Konstan MW. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67: 12–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol 118: 551–559, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest 143: 14–18, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jih KY, Hwang TC. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc Natl Acad Sci USA 110: 4404–4409, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, Zhou W, Nellis JR, Stroebele EK, Chu KK, Tearney GJ, Stevens MJ, Harris JK, Rowe SM, Engelhardt JF. Defective innate immunity and hyper-inflammation in newborn CFTR-knockout ferret lungs. Am J Respir Cell Mol Biol 52: 683–694, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 245: 1073–1080, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Advan Drug Del Rev 61: 86–100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Chu KK, Houser GH, Diephuis BJ, Li Y, Wilsterman EJ, Shastry S, Dierksen G, Birket SE, Mazur M, Byan-Parker S, Grizzle WE, Sorscher EJ, Rowe SM, Tearney GJ. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS ONE 8: e54473, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Shastry S, Byan-Parker S, Houser G, Chu K, Birket SE, Fernandez CM, Gardecki JA, Grizzle W, Wilsterman EJ, Sorscher EJ, Rowe SM, Tearney GJ. An autoregulatory mechanism governing mucociliary transport is sensitive to mucus load. Am J Respir Cell Mol Biol 51: 485–493, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Dawson DC. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiators protect G551D but not DeltaF508 CFTR from thermal instability. Biochemistry 53: 5613–5618, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, Grinstein S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem 268: 21592–21598, 1993. [PubMed] [Google Scholar]
- 21a.Mason KG, van Zanten JH, Wirtz D, Kuo SC. Particle tracking microrheology of complex fluids. Phys Rev Lett 79: 1997. [Google Scholar]
- 22.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest 102: 1125–1131, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza JL, Schmidt A, Li Q, Nuvaga E, Barrett T, Bridges RJ, Feranchak AP, Brautigam CA, Thomas PJ. Requirements for efficient correction of DeltaF508 CFTR revealed by analyses of evolved sequences. Cell 148: 164–174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moskowitz SM, Chmiel JF, Sternen DL, Cheng E, Gibson RL, Marshall SG, Cutting GR. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet Med 10: 851–868, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med 4: 149ra119, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuberger T, Burton B, Clark H, Van Goor F. Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol 741: 39–54, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, Lukacs GL. Mechanism-based corrector combination restores DeltaF508-CFTR folding and function. Nat Chem Biol 9: 444–454, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91: 5340–5344, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Quinton PM. Role of epithelial HCO3 transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol 299: C1222–C1233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabeh WM, Bossard F, Xu H, Okiyoneda T, Bagdany M, Mulvihill CM, Du K, di Bernardo S, Liu Y, Konermann L, Roldan A, Lukacs GL. Correction of both NBD1 energetics and domain interface is required to restore DeltaF508 CFTR folding and function. Cell 148: 150–163, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365: 1663–1672, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Rollins BM, Burn M, Coakley RD, Chambers LA, Hirsh AJ, Clunes MT, Lethem MI, Donaldson SH, Tarran R. A2B adenosine receptors regulate the mucus clearance component of the lung's innate defense system. Am J Respir Cell Mol Biol 39: 190–197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW. Clinical mechanism of the CFTR potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 190: 175–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe SM, Liu B, Hill A, Hathorne H, Cohen M, Beamer JR, Accurso FJ, Dong Q, Ordonez CL, Stone AJ, Olson ER, Clancy JP, Group VXS. Optimizing nasal potential difference analysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D-CFTR mutation. PLoS ONE 8: e66955, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Rowe SM, Pyle LC, Jurkevante A, Varga K, Collawn J, Sloane PA, Woodworth B, Mazur M, Fulton J, Fan L, Li Y, Fortenberry J, Sorscher EJ, Clancy JP. DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers. Pulm Pharmacol Ther 23: 268–278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe SM, Verkman AS. Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Med 3: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shamsuddin AK, Quinton PM. Native small airways secrete bicarbonate. Am J Respir Cell Mol Biol 50: 796–804, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloane PA, Rowe SM. Cystic fibrosis transmembrane conductance regulator protein repair as a therapeutic strategy in cystic fibrosis. Curr Opin Pulm Med 16: 591–597, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol 50, Suppl 40: S3–S13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, Chambers A, Ji D, Gamber K, Chu KK, Tearney G, Tang LP, Fortenberry JA, Du M, Cadillac JM, Bedwell DM, Rowe SM, Sorscher EJ, Fanucchi MV. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS ONE 9: e91253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 106: 18825–18830, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA 108: 18843–18848, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, Hegedus T, Verkman AS, Lukacs GL. Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci Transl Med 6: 24–297, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP, Group TS, Group TS. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373: 220–231, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.