Abstract
OBJECTIVE
To critically evaluate the use of uroflowmetry (UF) in a large urethral stricture disease cohort as a means to monitor for stricture recurrence.
MATERIALS AND METHODS
This study included men that underwent anterior urethroplasty and completed a study-specific follow-up protocol. Pre- and postoperative UF studies of men found to have cystoscopic recurrence were compared to UF studies from successful repairs. UF components of interest included maximum flow rate (Qm), average flow rate (Qa), and voided volume, in addition to the novel post-UF calculated value of Qm minus Qa (Qm-Qa). Area under the receiver operating characteristic curves (AUC) of individual UF parameters was compared.
RESULTS
Qm-Qa had the highest AUC (0.8295) followed by Qm (0.8241). UF performed significantly better in men ≤40 with an AUC of 0.9324 and 0.9224 for Qm-Qa and Qm respectively, as compared to 0.7484 and 0.7661 in men >40. Importantly, of men found to have anatomic recurrences, only 41% had a Qm of ≤15 mL/s at time of diagnostic cystoscopy, whereas over 83% were found to have a Qm-Qa of ≤10 mL/s.
CONCLUSION
Qm rate alone may not be sensitive enough to replace cystoscopy when screening for stricture recurrence in all patients, especially in younger men where baseline flow rates are higher. Qm-Qa is a novel calculated UF measure that appears to be more sensitive than Qm when using UF to screen for recurrence, as it may be a better numerical representation of the shape of the voiding curve.
Uroflowmetry (UF) is a simple, noninvasive method to evaluate voiding function in patients experiencing lower urinary tract symptoms.1,2 It is often combined with other metrics, including the International Prostate Symptom Score, in the initial diagnosis and follow-up of benign prostatic hyperplasia (BPH), and other causes of obstruction.3 In patients with urethral stricture disease (USD) who have undergone urethroplasty, UF is one of the most frequently used tests to monitor for stricture recurrence.4 However, UF’s use as a stand-alone tool to screen for recurrence following urethroplasty has never been rigorously validated.
It has been well established that the maximum flow rate (Qm) in patients with USD is significantly diminished relative to age-matched normal controls.5,6 This knowledge has been extrapolated to the post-urethroplasty setting, where commonly used cutpoints of a postoperative Qm of less than 10 mL/s or a postoperative Qm of less than 15 mL/s are used as indicators of urethral stricture recurrence.5–7 Similarly, when UF data are available both pre- and postoperatively, a change in Qm following surgery of less than 10 mL/s has also been suggested as a predictor of recurrence.8 The goal for each of these UF parameters is to minimize the invasiveness of postoperative screening while maximizing the ability to find recurrences.
The purpose of this study is to rigorously evaluate the capability of individual UF parameters, such as Qm and average flow rate (Qa), as well as a novel hybrid measure (Qm-Qa) to monitor for urethral stricture recurrence. Use of Qm-Qa has not been described in prior literature and attempts to provide a simple method to quantify the shape of the voiding curve. The study tested two hypotheses: (1) when compared to the gold-standard cystoscopy, UF parameters will have high test (screening) sensitivity and specificity, and (2) the sensitivity and specificity of UF to screen for stricture recurrence will be diminished in older patients.
MATERIALS AND METHODS
Subjects
The Trauma and Urologic Reconstruction Network of Surgeons (TURNS) is a multi-institutional effort that aims to prospectively monitor urethroplasty outcomes. The shared, centrally located web-based TURNS database was retrospectively queried for all men who had undergone anterior urethroplasty between 2009 and 2014. Data for these men were prospectively collected under Institutional Review Board-approved protocols, with patient consent obtained prior to surgery. Study inclusion criteria included men who had a follow-up cystoscopy at 3, 6, or 12 months postoperatively and had a corresponding same-day UF study. In patients with multiple follow-up cystoscopies/UF studies, the most recent instance was used for analysis. Recurrence was defined as the inability to advance a 17 French cystoscope past the previously reconstructed portion of the urethral lumen with minimal force; neither symptoms nor requirement for secondary operations were considered in this definition.
UF
Interpretation of UF readouts was made by the surgeon of record as per study protocol. Basic parameters of UF included Qm, Qa, voided volume (VV), postvoid residual (PVR), and shape of the voiding curve. A novel calculated value was Qm minus Qa (Qm-Qa). The changes (Δ) between pre- and postoperative parameters were also calculated in a subset of men. UF studies with voided volumes of less than 150 mL were discarded from the analysis.
Statistics
Descriptive statistics were first used to characterize the patient demographics, location of urethral stricture, and nature of repair. Men were divided into either a cystoscopic recurrence or successful repair group, and t tests were used to assess the differences in pre- and postoperative UF parameters between the two groups. Receiver operating characteristic (ROC) curves were constructed to determine the predictive value of each UF parameter in diagnosing urethral stricture recurrence relative to the cystoscopic gold standard. Sensitivity, specificity, positive predictive value, and negative predictive value of UF parameters to detect cystoscopic recurrence were calculated using predetermined, commonly cited cutpoints. The patients were further stratified into >40 years or ≤40 years of age, and similar analysis was repeated. Follow-up was determined as the time from surgery to the time of the last objective (UF or cystoscopy) data point. Statistical analysis was completed using SAS® 9.3 (Cary, NC), with statistical significance set at P < .05.
RESULTS
Demographics
Of the 1181 men in the TURNS database, 323 men met study criteria. The majority of men were excluded because of a lack of postoperative cystoscopy data (n = 524) or an absent or poor UF study (n = 334) from the same clinic visit. Urethroplasty was performed by 7 surgeons from different academic institutions. The mean age of included patients was 44.35 ± 15.26 with a mean follow-up time of 12.84 ± 12.38 months. The most common location of stricture repair was the bulbar urethra (n = 272), followed by the penile urethra (n = 27), and the mean intraoperative stricture length was 3.62 ± 2.93 cm. The most common repair was excision and primary anastomosis (n = 139), followed by substitution ventral onlay (n = 55) and substitution dorsal onlay (n = 42). Using cystoscopic criteria, 58 (18%) of the men in the study were noted to have recurrence.
Preoperative UF Data
Preoperative UF studies were available in 189 (59%) of the men. The mean preoperative Qm was 9.44 ± 6.82 mL/s, mean preoperative mean Qa was 5.87 ± 4.40 mL/s, mean VV was 258.12 ± 176.50 mL, and mean PVR was 162.26 ± 198.64 mL. Preoperative UF values were not predictive of operative success nor did they correlate with age, stricture length, or stricture location.
Postoperative UF Data
Comparison of postoperative UF data between men with and without evidence of cystoscopic recurrence is shown in Table 1. The mean postoperative Qm, Qa, and Qm-Qa were significantly different between cohorts; there was no difference in postoperative VV (398.91 ± 204.33 vs 365.33 ± 205.62 mL, P = .2584).
Table 1.
Comparison of UF parameters between successful repair and recurrence groups (ranked by ROC AUC)
| Successful Repair Group
|
Recurrence Group
|
P Value | ROC AUC (vs cystoscopy) | |||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |||
| Postoperative Qm-Qa (mL/s) | 253 | 13.27 ± 8.19 | 57 | 7.42 ± 5.40 | <.0001 | 0.8295 |
| Postoperative Qm (mL/s) | 265 | 28.05 ± 12.52 | 58 | 17.11 ± 8.31 | <.0001 | 0.8241 |
| ΔQm (mL/s) | 157 | 19.88 ± 14.30 | 32 | 8.07 ± 10.57 | <.0001 | 0.7638 |
| Δ(Qm -Qa) (mL/s) | 146 | 10.38 ± 9.14 | 31 | 4.23 ± 6.19 | <.0001 | 0.7531 |
| Postoperative Qa (mL/s) | 253 | 14.84 ± 7.47 | 57 | 9.80 ± 4.51 | <.0001 | 0.7289 |
| ΔQa (mL/s) | 146 | 9.07 ± 8.49 | 31 | 3.74 ± 6.07 | <.0001 | 0.7004 |
| Postoperative PVR (mL) | 244 | 72.64 ± 105.30 | 54 | 136.67 ± 174.00 | .0116 | 0.6296 |
| Postoperative VV (mL) | 265 | 398.91 ± 204.33 | 58 | 365.33 ± 205.62 | .2584 | 0.5647 |
AUC, area under the receiver operating characteristic curves; PVR, postvoid residual; Qa, average flow rate; Qm, maximum flow rate; ROC, receiver operating characteristic; UF, uroflowmetry; VV, voided volume.
ROC analysis was performed comparing UF to cystoscopy (gold standard) (Fig. 1). Postoperative Qm-Qa demonstrated the highest area under the receiver operating characteristic curves (AUC) of 0.8295 (95% confidence interval: 0.7426, 0.9164); postoperative Qm followed closely behind with an AUC of 0.8241 (0.7452, 0.9031). AUC values were not significantly different between Qm-Qa and Qm. Postoperative PVR demonstrated an AUC of 0.6296.
Figure 1.
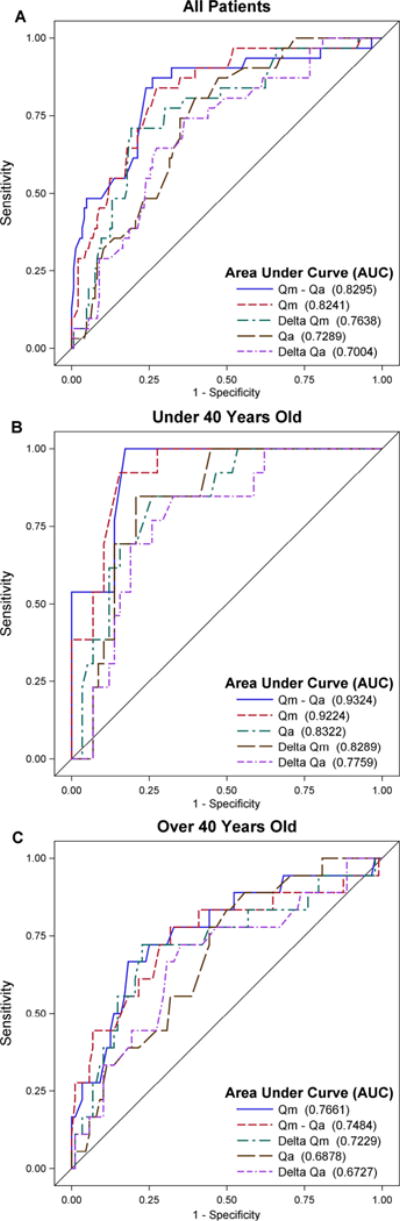
(A–C)—ROC curves of UF parameters predicting cystoscopic urethral stricture recurrence. ROC, receiver operating characteristic; UF, uroflowmetry. (Color version available online.)
Sensitivity tables were constructed with various cutpoints to further evaluate the predictive capabilities of each parameter (Table 2). A commonly used cutpoint of Qm < 10 mL/s had a sensitivity for detecting cystoscopic recurrence of only 21%.7 A postoperative Qm-Qa < 10 mL/s was 83% sensitive and 58% specific.
Table 2.
Sensitivity, specificity, PPV, and NPV of UF parameters to diagnose cystoscopic recurrence of urethral strictures
| Cutpoints (mL/s) | Age | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Postoperative Qm < 10 | All | 21 | 97 | 60 | 85 |
| ≤40 | 21 | 100 | 100 | 86 | |
| >40 | 21 | 95 | 47 | 84 | |
| Postoperative Qm < 15 | All | 41 | 88 | 43 | 87 |
| ≤40 | 33 | 97 | 73 | 87 | |
| >40 | 47 | 81 | 36 | 87 | |
| Postoperative Qm < 20 | All | 67 | 74 | 36 | 91 |
| ≤40 | 58 | 91 | 58 | 91 | |
| >40 | 74 | 61 | 30 | 91 | |
| Postoperative Qm < 25 | All | 84 | 52 | 28 | 94 |
| ≤40 | 79 | 73 | 39 | 94 | |
| >40 | 88 | 37 | 24 | 93 | |
| Postoperative Qm-Qa < 6 | All | 41 | 85 | 38 | 87 |
| ≤40 | 38 | 92 | 50 | 87 | |
| >40 | 44 | 80 | 33 | 87 | |
| Postoperative Qm-Qa < 8 | All | 64 | 70 | 32 | 90 |
| ≤40 | 58 | 79 | 38 | 90 | |
| >40 | 68 | 63 | 29 | 90 | |
| Postoperative Qm-Qa < 10 | All | 83 | 58 | 30 | 94 |
| ≤40 | 83 | 70 | 37 | 95 | |
| >40 | 82 | 49 | 26 | 93 | |
| ΔQm* < 10 | All | 81 | 48 | 26 | 92 |
| ≤40 | 83 | 49 | 26 | 93 | |
| >40 | 79 | 48 | 25 | 91 | |
| ΔQm* < 15 | All | 90 | 37 | 24 | 94 |
| ≤40 | 92 | 42 | 25 | 96 | |
| >40 | 88 | 33 | 23 | 93 |
NPV, negative predictive value; PPV, positive predictive value; other abbreviations as in Table 1.
ΔQm = change in maximum flow rate following urethroplasty.
Subgroup analysis stratified men into cohorts of ≤40 and >40 years of age. The recurrence rates were similar between the groups (17% vs 18%, respectively, P = .9016). Men ≤40 years had a higher postoperative mean Qm (31.45 ± 13.60 mL/s) compared to men >40 years (22.18 ± 10.16 mL/s, P < .0001). ROC analysis revealed significantly higher AUC in men ≤40 years compared to men >40 for both Qm-Qa (0.9324 vs 0.7484) and Qm (0.9224 vs 0.7661).
Men with preoperative UF studies available were used as a urethral stricture test cohort to validate the sensitivities of the cutpoints. A Qm-Qa < 10 mL/s was 94% sensitive (ie, 169 of 179 patients with preoperative UF had a Qm-Qa of <10 mL/s). Only 60% (115/189) of patients had a Qm of <10 mL/s and 84% (158/189) had a Qm of <15 mL/s.
DISCUSSION
The purpose of this study was to critically evaluate and compare the ability of UF parameters to independently identify cystoscopic recurrence of urethral strictures following urethroplasty. Of specific interest were the commonly cited cutpoints of postoperative Qm < 10 mL/s, postoperative Qm < 15 mL/s, and ΔQm < 10 mL/s as indicators of recurrence. In this study cohort, we did not find that these generic cutpoints were sensitive enough for use as reliable screening thresholds. In general, UF parameters did not demonstrate a high-enough sensitivity/specificity as a standalone screening test relative to cystoscopy. However, UF did appear to be a more useful screener in the younger patient population (≤40 years old).
UF is commonly used to assess bladder outlet obstruction in the context of BPH. Two studies evaluating the ability of Qm to predict bladder outlet obstruction reported sensitivities of only 39% and 47% when a Qm <10 mL/s cutpoint was used.3,9 Although UF on its own does not appear to have adequate diagnostic capability in BPH to replace urodynamic studies or imaging, its ability to provide objective measurements in conjunction with other tests contributes to optimal patient management.10 In USD, UF has taken on a similar role. Meeks et al estimated that 56% of urologists currently use UF as one of several primary tests to monitor for urethral stricture recurrence after urethroplasty.4 Despite this, there is no consensus as to which UF parameters have the most diagnostic value and when they should be used. Validation and incorporation of UF into a standardized screening protocol have the potential to limit the need for invasive cystoscopies.
A postoperative Qm < 10 mL/s was previously reported to have a sensitivity of only 54% in detection of recurrence.5 If this cutpoint had been used in this population, a sensitivity of only 21% would have been achieved and 46 of the 58 recurrences would have been missed. A postoperative Qm < 15 mL/s performed similarly poorly with a sensitivity of only 41%. Although Qm alone is typically the parameter of interest when interpreting UF, its usage as a screening tool is hampered by the wide distribution of Qm in the recurrence group (17.11 ± 8.31 mL/s), likely the result of heterogeneous effects from bladder dysfunction and prostatic size/obstruction. An improvement can be seen with ΔQm, which allows for an individually normalized value. A prior study reported that an improvement of ΔQm < 10 mL/s had a sensitivity of 94% with a specificity of 78%.8 In this population, a similar improvement in sensitivity to 81% and specificity to 48% was seen with this threshold.
The novel Qm-Qa parameter may be superior to Qm or ΔQm in monitoring for stricture recurrence (Fig. 2). ROC AUC for Qm-Qa (0.8295) was similar to Qm (0.8241, P = .8089) but higher than ΔQm (0.7638, P = .0492). Using a cutpoint of Qm-Qa < 10 mL/s, a sensitivity of 83% and a specificity of 58% were seen. Unlike Qm alone, the Qm-Qa is able to capture the overall shape of the curve by factoring in Qa. A patient with a cystoscopic recurrence on the higher end of the Qm spectrum may have a flow of 20 to 25 mL/s, yet still present with a flat voiding curve. Whereas the typical cutpoints of Qm will fail to capture this patient, the Qm-Qa is more likely to identify the recurrence. Had a Qm-Qa of <10 mL/s been used as a standalone method to screen for recurrence in this population, 154 fewer cystoscopies would have been performed, but 10 strictures would have been missed. If the entire cohort was preoperatively considered as a group of strictures, the preoperative Qm-Qa < 10 mL/s would have identified 94% of strictures compared to only 60% for Qm < 10 mL/s.
Figure 2.
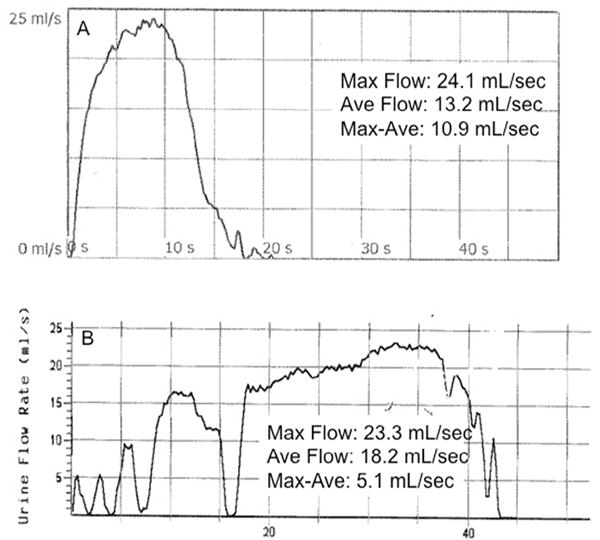
Uroflowmetry tracings from two postoperative patients with high (normal) maximum flow rates. Patient A had a normal cystoscopy, whereas Patient B was found to have recurrence. Note the differences in the Qm-Qa between the two patients.
The value of Qm correlates inversely with age, especially in the population over age 50, where there is a sharp drop off regardless of VV.11,12 Younger patients demonstrate more robust flow due to stronger bladder contractions and less prostatic obstruction. In this study, both Qm and Qm-Qa demonstrated superior predictive capability in detecting stricture recurrence in patients ≤40 years of age (AUC of 0.9224 and 0.9324, respectively) compared to patients >40 years of age (AUC of 0.7661 and 0.7484, respectively). The stronger flow of healthy younger men allows for better discrimination between a patent and strictured urethra. In an older individual, this difference may be less pronounced. Overall, UF appears to have better predictive value in the younger patient population and thus, it may be a more useful stand-alone tool for stricture monitoring in the younger group. In older individuals, where UF parameters are more profoundly affected by the size of the prostate, monitoring of patient-specific subjective measures will likely always remain important.
Limitations to the study include the strict interpretation of a urethral stricture recurrence. This study focused on the anatomical recurrence, which was specifically defined as the inability to advance a standard 17 French cystoscope past the previously reconstructed portion of the urethral lumen with minimal force. Although this is an objective measure, it does not consider the functional outcome (ie, urinary symptoms, quality of life) for the patient. For example, some patients noted as recurrences in this study were relatively asymptomatic and did not undergo secondary repair. Currently, the clinical significance of asymptomatic stricture is unknown, and thus so is the clinical utility of diagnosing them. A second limitation is that the degree of stricture was not graded in this study; longer and tighter strictures likely have a stronger correlation with impaired flow. Finally, a large number of men were excluded from analysis, most of whom had inadequate UF studies. Whereas this exclusion does not diminish the studies’ ability to test UF as a stand-alone measure for diagnosing recurrence, it does highlight the fact that UF can oftentimes be difficult to administer in a busy clinic in which many men arrive with empty bladders. Thus, the clinical practicality of using UF alone must be studied further.
CONCLUSION
UF is a widely used test to monitor the integrity of the reconstructed urethra after urethroplasty, but the findings from this study suggest that when used alone, the sensitivity is unacceptably low to detect recurrences. Whereas UF appears to perform better in patients under 40 years old, utilization of a standard “cutpoint” (e.g. Qm < 15 ml/s) for all patients performed poorly in this group of individuals. A refined approach will likely need to include patient-specific UF parameters that monitor Qm over time, Qm-Qa values (which may be a novel way to numerically describe the shape of the voiding curve), and the addition of patient-reported outcomes measures. If a standard, noninvasive approach to monitoring the urethra is adopted widely, as has been proposed by many, further refinement will be required.4,13,14
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Bloom DA, Foster WD, McLeod DG, Mittemeyer BT, Stutzman RE. Cost-effective uroflowmetry in men. J Urol. 1985;133:421–424. doi: 10.1016/s0022-5347(17)49003-3. [DOI] [PubMed] [Google Scholar]
- 2.Alyami F, Farhat W, Figueroa VH, Romao RL. Utility and cost-effectiveness of uroflowmetry in a busy pediatric urology practice. Can Urol Assoc J. 2014;8:E615–E618. doi: 10.5489/cuaj.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynard JM, Yang Q, Donovan JL, et al. The ICS-‘BPH’ Study: uroflowmetry, lower urinary tract symptoms and bladder outlet obstruction. Br J Urol. 1998;82:619–623. doi: 10.1046/j.1464-410x.1998.00813.x. [DOI] [PubMed] [Google Scholar]
- 4.Meeks JJ, Erickson BA, Granieri MA, Gonzalez CM. Stricture recurrence after urethroplasty: a systematic review. J Urol. 2009;182:1266–1270. doi: 10.1016/j.juro.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Erickson BA, Breyer BN, McAninch JW. The use of uroflowmetry to diagnose recurrent stricture after urethral reconstructive surgery. J Urol. 2010;184:1386–1390. doi: 10.1016/j.juro.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessels SG, Heyns CF. Prospective evaluation of a new visual prostate symptom score, the international prostate symptom score, and uroflowmetry in men with urethral stricture disease. Urology. 2014;83:220–224. doi: 10.1016/j.urology.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Heyns CF, Marais DC. Prospective evaluation of the American Urological Association symptom index and peak urinary flow rate for the followup of men with known urethral stricture disease. J Urol. 2002;168:2051–2054. doi: 10.1016/S0022-5347(05)64293-0. [DOI] [PubMed] [Google Scholar]
- 8.Erickson BA, Breyer BN, McAninch JW. Changes in uroflowmetry maximum flow rates after urethral reconstructive surgery as a means to predict for stricture recurrence. J Urol. 2011;186:1934–1937. doi: 10.1016/j.juro.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belal M, Abrams P. Noninvasive methods of diagnosing bladder outlet obstruction in men. Part 2: noninvasive urodynamics and combination of measures. J Urol. 2006;176:29–35. doi: 10.1016/S0022-5347(06)00570-2. [DOI] [PubMed] [Google Scholar]
- 10.Barry MJ, Girman CJ, O’Leary MP, et al. Using repeated measures of symptom score, uroflowmetry and prostate specific antigen in the clinical management of prostate disease. Benign Prostatic Hyperplasia Treatment Outcomes Study Group. J Urol. 1995;153:99–103. doi: 10.1097/00005392-199501000-00036. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen JB, Jensen KM, Bille-Brahe NE, Morgensen P. Uroflowmetry in asymptomatic elderly males. Br J Urol. 1986;58:390–395. doi: 10.1111/j.1464-410x.1986.tb09092.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Dhabalia JV, Nelivigi GG, Punia MS, Suryavanshi M. Age, gender, and voided volume dependency of peak urinary flow rate and uroflowmetry nomogram in the Indian population. Indian J Urol. 2009;25:461–466. doi: 10.4103/0970-1591.57912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibold J, Werther M, Alloussi S, et al. Urethral ultrasound as a screening tool for stricture recurrence after oral mucosa graft urethroplasty. Urology. 2011;78:696–700. doi: 10.1016/j.urology.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 14.Okorie CO, Pisters LL, Ndasi HT, Fekadu A. A simplified protocol for evaluating and monitoring urethral stricture patients minimizes cost without compromising patient outcome. Trop Doct. 2010;40:134–137. doi: 10.1258/td.2010.090415. [DOI] [PubMed] [Google Scholar]


