Abstract
Hypokalemia is common during the treatment of diabetic ketoacidosis (DKA); however, severe hypokalemia at presentation prior to insulin treatment is exceedingly uncommon. A previously healthy 8-yr-old female presented with new onset type 1 diabetes mellitus, severe DKA (pH = 6.98), and profound hypokalemia (serum K = 1.3 mmol/L) accompanied by cardiac dysrhythmia. Insulin therapy was delayed for 9 h to allow replenishment of potassium to safe serum levels. Meticulous intensive care management resulted in complete recovery. This case highlights the importance of measuring serum potassium levels prior to initiating insulin therapy in DKA, judicious fluid and electrolyte management, as well as delaying and/or reducing insulin infusion rates in the setting of severe hypokalemia.
Keywords: diabetic ketoacidosis, hypokalemia, insulin, low-dose insulin drip, pediatric
Nearly one third of children with newly diagnosed type 1 diabetes present in diabetic ketoacidosis (DKA). Higher proportions of young children and those from disadvantaged socioeconomic groups present with DKA (1). DKA is the leading cause of mortality among children with diabetes, and electrolyte abnormalities are a recognized complication of DKA contributing to morbidity and mortality (2, 3). Total body potassium deficiency of 3-6 mEq/kg is expected at presentation of DKA due to osmotic diuresis, emesis, and secondary hyperaldosteronism; however, pretreatment serum potassium levels are usually not low due to the extracellular shift of potassium that occurs with acidosis and insulin deficiency (3, 4). After insulin treatment is initiated, potassium shifts intracellularly and serum levels decline. Replacement of potassium in intravenous fluids is the standard of care in treatment of DKA to prevent the potential consequences of hypokalemia including cardiac arrhythmias and respiratory failure. There are several case reports in the literature of severe hypokalemia occurring during the treatment of DKA; however, significant hypokalemia prior to treatment is rare and this case of severe hypokalemia in a child presenting with DKA appears to be a unique addition to the literature with important teaching points to prevent iatrogenic morbidity and mortality (5-8).
In this report, we describe a child with new onset type 1 diabetes mellitus presenting in severe DKA with profound hypokalemia. We discuss the unique challenges in managing this child with respect to cardiac complications of hypokalemia in the setting of severe DKA.
Case history/examination
A previously healthy 8-yr-old, 21 kg female presented to the emergency department with fatigue, 15% weight loss, and altered mental status. Fatigue and weight loss started approximately 4 wk prior to presentation along with viral upper respiratory infection symptoms. Two weeks prior to presentation she was seen at an urgent care and prescribed amoxicillin for acute otitis media. Although polyuria and polydipsia were present at that time, no laboratory evaluation was performed. Two days prior to presentation she developed abdominal pain and vomiting. Subsequently, she developed slurred speech and presented to our pediatric emergency department. With the exception of the recently prescribed antibiotics, she was not taking any medications and had negative past medical, surgical, and family histories. In the emergency department she appeared ill, underweight, and volume depleted with Kussmaul respirations. She was confused by questions and had a delayed response time; Glascow Coma Scale was 14. No focal neurologic deficits were noted. Cardiovascular exam was notable for an inappropriately low heart rate for her hydration status (80 beats/min), blood pressure of 105/72 mmHg (70%ile systolic, 88%ile diastolic), and delayed peripheral capillary refill (4 s). Lungs were clear despite labored respirations with tachypnea and nasal flaring. Her abdomen was soft, non-tender and non-distended, and a hard mobile mass was palpable on the left side consistent with stool.
Investigations
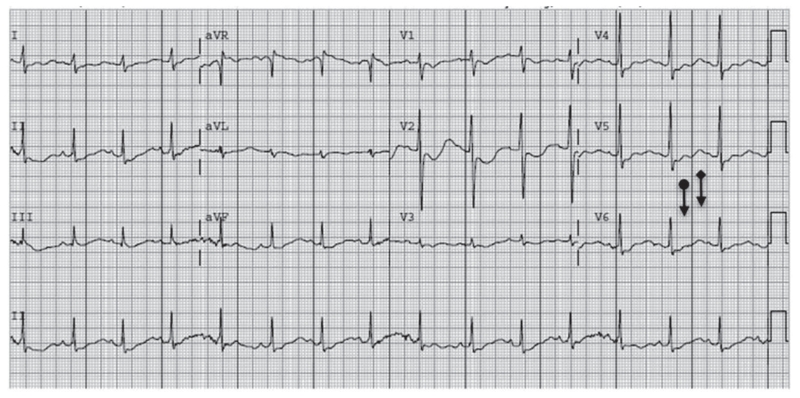
Initial laboratory investigation revealed severe metabolic acidosis (pH = 6.98), hyperglycemia, hyperosmolality, profound hypokalemia (1.3 mmol/L), and renal insufficiency (Table 1). An electrocardiogram (EKG) revealed flattened T waves and upright U waves (Fig. 1).
Table 1.
Evolution of labs beginning at presentation to the emergency department. Insulin infusion initiated just before 9 h at 0.025 units/kg/h (arrow). Insulin infusion increased to 0.05 units/kg/h around 21 h after presentation (double arrow)
| Lab (normal values) | Initial | 2h | 6h | 8h ↓ 9h | 12h | 18h ↓↓ 24 h | 36 h | 72 h | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Venous pH (7.32–7.42) | 6.98 | 7.01 | 6.99 | 6.96 | 7.14 | 7.15 | 7.26 | 7.34 | 7.38 | |
| Sodium (134–143 mmol/L) | 123 | 125 | 134 | 139 | 144 | 144 | 145 | 147 | 143 | 131 |
| Corrected sodium | 139 | 140 | 146 | 149 | 152 | 151 | 148 | 149 | 145 | 133 |
| Potassium (3.4–4.7 mmol/L) | 1.3 | 1.5 | 1.7 | 2.7 | 2.3 | 2.3 | 2.5 | 2.3 | 2.5 | 3.4 |
| Chloride (96–109 mmol/L) | 89 | 95 | 102 | 107 | 116 | 117 | 119 | 121 | 117 | 102 |
| Bicarbonate (20–31 mmol/L) | 5 | <5 | <5 | 5 | <5 | <5 | 9 | 12 | 16 | 24 |
| Anion gap | 25 | >26 | >28 | 27 | >24 | >23 | 17 | 14 | 10 | 5 |
| Glucose (60–105 mg/dL) | 1112 | 1045 | 851 | 714 | 610 | 504 | 284 | 218 | 192 | 221 |
| Glucose (3.3–5.8 mmol/L) | 62 | 58 | 47 | 40 | 34 | 28 | 16 | 12 | 11 | 12 |
| BUN (7–17 mg/dL) | 24 | 22 | 22 | 21 | 18 | 20 | 21 | 20 | 17 | 20 |
| Creatinine (0.23–0.61 mg/dL) | 1.47 | 1.15 | 1.19 | 1.15 | 0.81 | 0.89 | 0.72 | 0.65 | 0.57 | 0.46 |
| Calcium (8.8–10.1 mg/dL) | 11.6 | 10.2 | 10.6 | 9.1 | 11.0 | 10.5 | 9.8 | 8.7 | 8.3 | |
| Magnesium (1.6–2.3mg/dL) | 3.5 | 3.4 | 3.0 | 2.7 | 2.4 | 2.1 | ||||
| Phosphorus (2.5–6.0 mg/dL) | 5.1 | 5.4 | 5.4 | 4.3 | 4.4 | 4.1 | ||||
| Calculated serum osmolality | 316 | 316 | 323 | 325 | 328 | 323 | 313 | 301 | 303 | 281 |
Fig. 1.
Electrocardiogram (EKG) at presentation (K 1.3) demonstrating flattened T waves (circle) and upright ‘U’ waves (diamond).
Hospital course
In the emergency room she was given a 10 ml/kg normal saline bolus along with an infusion of IV potassium chloride 0.3 mEq/kg over an hour. Insulin infusion was withheld secondary to her profound hypokalemia and she was quickly transferred to the pediatric intensive care unit (PICU). Serum potassium level improved to 1.7 mmol/L following the first infusion. Potassium repletion continued with potassium chloride infusions (increased to 1 mEq/kg over 1 h) and maintenance fluids with maximum potassium amenable to peripheral infusion (30 mEq potassium acetate and 30 mEq potassium phosphate) infused at 90 ml/h (1.5 times her maintenance intravenous fluid rate). She received an additional 5 ml/kg of normal saline fluid bolus. During this time she remained lucid but with delayed mentation. Her corrected sodium level was monitored closely to ensure she did not have a significant drop as this would be concerning for a higher risk of cerebral edema (9). Her heart rate slowly approached a more contextually appropriate rate of >100 beats/min. After receiving a total of 3.4 mEq/kg potassium repletion total over 8 h, her serum potassium reached 2.7 mmol/L with an unchanged anion gap. At this time, insulin infusion was initiated at 0.025 units/kg/h. Over the subsequent 12 h, she received an additional 3.6 mEq/kg potassium. During this time she had improvement in her mental status, acidosis, and anion gap, and her serum potassium remained greater than 2.3 mmol/L (Table 1). Her insulin dose was then increased to 0.05 units/kg/h for the next 24 h with resolution of her acidosis. She was transitioned to subcutaneous insulin dosing and transferred out of the PICU on hospital day 3. She received a total of 15 mEq/kg IV potassium over the first 48 h, followed by enteral potassium chloride supplementation 20 mEq three times daily secondary to persistent hypokalemia. Her serum potassium first reached a normal level of 3.4 mmol/L 72 h after presentation with a total of 18 mEq/kg of supplemental potassium. She was discharged on hospital day 6 on oral potassium supplementation in addition to her insulin regimen.
Outcome and follow-up
Studies were negative for celiac disease, autoimmune thyroid disease, and renal tubular acidosis. Potassium supplementation was discontinued after 2 wk and potassium levels have remained normal (last 4.7 mmol/L). Given the complete resolution of her hypokalemia, we did not pursue further evaluation for rarer underlying causes of hypokalemia. Her HbA1C improved from >14% to 6.8% after 3 months of insulin replacement. She currently has no evidence of cardiac, renal, hepatic, or neurologic sequelae. Her weight has increased to 29.6 kg that corresponds to the 60%ile for age, up from 5%ile at diagnosis and 10%ile at discharge.
Discussion
In this article we present a pediatric patient with new onset type 1 diabetes mellitus with profound hypokalemia upon presentation with severe DKA. Hypokalemia in the setting of pediatric DKA has been reported in the literature as early as the 1940s, although profound hypokalemia (<2.5 mmol/L) is unusual (10). Total body potassium depletion is expected in DKA largely due to osmotic renal losses. Measurement of extracellular serum potassium in DKA patients with severe acidosis greatly underestimates the total body potassium deficit in these patients due to the extracellular potassium shift caused by insulin deficiency and metabolic acidosis. Therefore only 5-10% of patients have hypokalemia at presentation and almost never <2.5 mmol/L (11-13). In normal adults, approximate total body content of potassium is 50 mEq/kg and 98% is contained intracellularly (14). In DKA patients with severe hypokalemia, potassium deficit can reach 10 mEq/kg. Treatment with insulin usually results in a decrease in the measured serum potassium due intracellular potassium shifts and, potentially, an aldosterone-like effect of insulin on the renal tubule that further increases urinary potassium losses (15). In cases of profound hypokalemia prior to insulin therapy, many have an identifiable etiology. Our patient’s history and studies are not consistent with renal tubular acidosis, celiac disease, or nutritional deprivation. Malnutrition alone is unlikely to result in hypokalemia; however, eating disorders or low consumption of potassium-rich foods, such as fruits and vegetables, have been reported to contribute in other cases of DKA-associated severe hypokalemia (14, 16). Because this child was able to discontinue potassium supplementation within 2 wk, we suspect her hypokalemia was most likely secondary to potassium depletion from her prolonged untreated hyperglycemia and hyperosmolar state and/or reduced intake in the days prior to presentation.
Failure to acknowledge and properly manage hypokalemia in DKA can result in severe, symptomatic hypokalemia with detrimental effects on the neuromuscular and cardiopulmonary systems. Serum potassium levels <2.5 mmol/L have been associated with muscle necrosis and levels <2 mmol/L can result in ascending paralysis (8). Importantly, severe hypokalemia has been associated with respiratory compromise in adult patients with DKA (14, 17). Hypokalemia also causes cardiac myocyte hyperpolarization leading to increased excitability and delayed repolarization. Notable EKG findings in hypokalemic patients include early T-wave flattening with subsequent ST-T depression, U waves, and prolongation of the QU or QT intervals. Another EKG feature of severe hypokalemia is a prolonged PR interval, which was the upper end of normal for age in our patient (0.15 s, normal 0.09–0.17 s) (18). These electrophysiological changes can result in atrial and ventricular arrhythmias (14, 19). Although uncommon in patients without underlying heart disease, hypokalemic cardiac arrest following bicarbonate therapy has been described in a previously healthy adult patient with DKA (5). Importantly, bicarbonate therapy was not given to this patient despite the prolonged and severe acidosis as this therapy is not recommended in childhood DKA and has the potential for complications, including exacerbation of hypokalemia (3). The potential cardiac complications of hypokalemia in DKA necessitate close monitoring in an ICU setting with telemetry capabilities. Another important consideration in DKA management is to closely follow effective serum osmolality and serum sodium corrected for hyperglycemia (3).
The American Diabetes Association (ADA) and International Society for Pediatric and Adolescent Diabetes (ISPAD) consensus statements for managing DKA recommend checking serum potassium prior to insulin treatment and ongoing monitoring of serum potassium levels with replacement in intravenous fluids (3, 4). As our case demonstrates, even with aggressive potassium supplementation, it may take 9–12 h to return to safe serum potassium levels. Of note, potassium replacement could have been more aggressive according to ISPAD recommendations of up to 0.5 mmol/kg/h, however we were limited due to hospital potassium infusion policies for peripheral lines. Alternative management options could have included placement of a central line or increased fluid volume, however both carry significant risks in the setting of severe DKA with abnormal mental status. Our choice of potassium salts for her intermittent infusions was limited to potassium chloride due to the national short-age of potassium acetate and potassium phosphate at the time; however, we recognize this directly contributed to her subsequent hyperchloremic non-anion gap metabolic acidosis. When the patient’s serum potassium levels were consistently rising, we initiated insulin therapy at a very low rate of 0.025 units/kg/h, 25% of the usual rate to avoid precipitating further decline in the serum potassium levels (20). Delaying insulin therapy inevitably prolongs the acidotic state as insulin is required to halt ketosis; however, the immediate dangers posed by profound hypokalemia potentially leading to death from fatal arrhythmias or respiratory failure outweigh the risks of prolonged metabolic acidosis. Literature on lower insulin infusion rates is scant, but some evidence suggests a lower insulin rate may be as effective and potentially reduce complications, particularly hypokalemia, when compared to the most commonly used rate of 0.1 units/kg/h (20-22). Recent guidelines now recommend insulin infusion rates of 0.05–0.1 units/kg/h and note lower doses may be needed for special circumstances such as hypokalemia or hypoglycemia (3).
Profound hypokalemia (<2.5 mmol/L) in untreated DKA is extremely rare and necessitates potassium replacement with delay of insulin therapy until serum potassium levels are > 2.5 mmol/L (4, 14) to avoid the risk of cardiopulmonary and neuromuscular compromise. These children require meticulous care by a team with expertise in both pediatric endocrinology and critical care. Potassium levels should always be checked prior to initiating insulin therapy and when hypokalemia is present, potassium supplementation should be given promptly and delayed administration and/or reduction of the insulin infusion rate may be necessary.
References
- 1.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133:e938–e945. doi: 10.1542/peds.2013-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfsdorf JI, Allgrove J, Craig M, et al. Hyperglycemic crises in pediatric patients with diabetes: a consensus statement from the International Society for Pediatric and Adolescent Diabetes. Pediatr Diabetes. 2014;15(S20):154–179. [Google Scholar]
- 4.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(Suppl 1):S94–S102. doi: 10.2337/diacare.27.2007.s94. [DOI] [PubMed] [Google Scholar]
- 5.Abdulaziz S, Dabbagh O, Al Daker MO, Hassan I. Hypokalaemia and refractory asystole complicating diabetic ketoacidosis, lessons for prevention. BMJ Case Rep. 2012;2012:1–3. doi: 10.1136/bcr-2012-007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer DS, Nichol BA. Intraventricular conduction defect and respiratory tract paralysis in diabetic ketoacidosis. Am J Med. 1963;35:123–129. doi: 10.1016/0002-9343(63)90169-4. [DOI] [PubMed] [Google Scholar]
- 7.Krentz AJ, Ryder RE. Hypokalemia-induced respiratory failure complicating treatment of diabetic ketoacidosis. J Diabetes Complications. 1994;8:55–56. doi: 10.1016/1056-8727(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 8.Vishnu VY, Kattadimmal A, Rao SA, Kadhiravan T. Sporadic hypokalemic paralysis caused by osmotic diuresis in diabetes mellitus. J Clin Neurosci. 2014;21:1267–1268. doi: 10.1016/j.jocn.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Watts W, Edge JA. How can cerebral edema during treatment of diabetic ketoacidosis be avoided? Pediatr Diabetes. 2014;15:271–276. doi: 10.1111/pedi.12155. [DOI] [PubMed] [Google Scholar]
- 10.Greenman L, Mateer FM, Gow RC, Peters JH, Danowski TS. Some observations on the development of hypokalemia during therapy of diabetic acidosis in juvenile and young adult subjects. J Clin Invest. 1949;28:409–414. doi: 10.1172/JCI102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora S, Cheng D, Wyler B, Menchine M. Prevalence of hypokalemia in ED patients with diabetic ketoacidosis. Am J Emerg Med. 2012;30:481–484. doi: 10.1016/j.ajem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Hanas R, Lindgren F, Lindblad B. Diabetic ketoacidosis and cerebral oedema in Sweden – a 2-year paediatric population study. Diabet Med. 2007;24:1080–1085. doi: 10.1111/j.1464-5491.2007.02200.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanwal SK, Bando A, Kumar V. Clinical profile of diabetic ketoacidosis in Indian children. Indian J Pediatr. 2012;79:901–904. doi: 10.1007/s12098-011-0634-3. [DOI] [PubMed] [Google Scholar]
- 14.Murthy K, Harrington JT, Siegel RD. Profound hypokalemia in diabetic ketoacidosis: a therapeutic challenge. Endocr Pract. 2005;11:331–334. doi: 10.4158/EP.11.5.331. [DOI] [PubMed] [Google Scholar]
- 15.Carlotti AP, George-Hyslop C, Bohn D, Halperin ML. Hypokalemia during treatment of diabetic ketoacidosis: clinical evidence for an aldosterone-like action of insulin. J Pediatr. 2013;163:207–212. e201. doi: 10.1016/j.jpeds.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Moulik NR, Jayashree M, Singhi S, Bhalla AK, Attri S. Nutritional status and complications in children with diabetic ketoacidosis. Pediatr Crit Care Med. 2012;13:e227–e233. doi: 10.1097/PCC.0b013e31823c9a11. [DOI] [PubMed] [Google Scholar]
- 17.Dorin RI, Crapo LM. Hypokalemic respiratory arrest in diabetic ketoacidosis. JAMA. 1987;257:1517–1518. [PubMed] [Google Scholar]
- 18.Sharieff GQ, Rao SO. The pediatric ECG. Emerg Med Clin North Am. 2006;24:195–208. doi: 10.1016/j.emc.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. 2011;7:75–84. doi: 10.1038/nrneph.2010.175. [DOI] [PubMed] [Google Scholar]
- 20.Wolfsdorf JI. The International Society of Pediatric and Adolescent Diabetes guidelines for management of diabetic ketoacidosis: do the guidelines need to be modified? Pediatr Diabetes. 2014;15:277–286. doi: 10.1111/pedi.12154. [DOI] [PubMed] [Google Scholar]
- 21.Puttha R, Cooke D, Subbarayan A, et al. Low dose (0.05 units/kg/h) is comparable with standard dose (0.1 units/kg/h) intravenous insulin infusion for the initial treatment of diabetic ketoacidosis in children with type 1 diabetes-an observational study. Pediatr Diabetes. 2010;11:12–17. doi: 10.1111/j.1399-5448.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- 22.Kapellen T, Vogel C, Telleis D, Siekmeyer M, Kiess W. Treatment of diabetic ketoacidosis (DKA) with 2 different regimens regarding fluid substitution and insulin dosage (0.025 vs. 0.1 units/kg/h) Exp Clin Endocrinol Diabetes. 2012;120:273–276. doi: 10.1055/s-0031-1299706. [DOI] [PubMed] [Google Scholar]