Abstract
Objectives
(1) To analyze the sensitivity and specificity of fine-needle aspiration (FNA) in distinguishing benign from malignant parotid disease. (2) To determine the anticipated posttest probability of malignancy and probability of non-diagnostic and indeterminate cytology with parotid FNA.
Data Sources
Independently corroborated computerized searches of PubMed, Embase, and Cochrane Central Register were performed. These were supplemented with manual searches and input from content experts.
Review Methods
Inclusion/exclusion criteria specified diagnosis of parotid mass, intervention with both FNA and surgical excision, and enumeration of both cytologic and surgical histopathologic results. The primary outcomes were sensitivity, specificity, and posttest probability of malignancy. Heterogeneity was evaluated with the I2 statistic. Meta-analysis was performed via a 2-level mixed logistic regression model. Bayesian nomograms were plotted via pooled likelihood ratios.
Results
The systematic review yielded 70 criterion-meeting studies, 63 of which contained data that allowed for computation of numerical outcomes (n = 5647 patients; level 2a) and consideration of meta-analysis. Subgroup analyses were performed in studies that were prospective, involved consecutive patients, described the FNA technique utilized, and used ultrasound guidance. The I2 point estimate was >70% for all analyses, except within prospectively obtained and ultrasound-guided results. Among the prospective subgroup, the pooled analysis demonstrated a sensitivity of 0.882 (95% confidence interval [95% CI], 0.509–0.982) and a specificity of 0.995 (95% CI, 0.960–0.999). The probabilities of nondiagnostic and indeterminate cytology were 0.053 (95% CI, 0.030–0.075) and 0.147 (95% CI, 0.106–0.188), respectively.
Conclusion
FNA has moderate sensitivity and high specificity in differentiating malignant from benign parotid lesions. Considerable heterogeneity is present among studies.
Keywords: parotid, fine-needle aspiration, sensitivity, specificity
Salivary gland tumors make up 3% of all head and neck tumors. Of these, approximately 85% originate in the parotid gland,1,2 and the majority of these tumors are benign.3,4 To definitively diagnose a tumor as benign or malignant, parotidectomy can be performed with histopathologic examination. However, parotidectomy is associated with a risk of injury to the facial nerve, along with additional potential surgical complications. A less invasive initial method of diagnosis is therefore often preferred, as surgery may be avoided if the tumor is benign on the basis of cytology.
Fine-needle aspiration (FNA) has become a commonly performed diagnostic test in the initial evaluation of a parotid mass. The advantage of this technique is that it can be performed in the outpatient setting with minimal recovery time and low risk of complications. A potential disadvantage is that it has been associated with variable sensitivity and specificity in distinguishing malignant from benign disease. Furthermore, high rates of nondiagnostic aspirations have been reported in the literature.5 An open excisional biopsy is one theoretical option but is not advised because of the risk of tumor spillage, facial nerve injury, scarring, and fistula formation.6 As such, it has largely been abandoned as a primary means of diagnosing parotid masses. More recently, ultrasound-guided core biopsies (USCBs) have been described as a third option.7–11 The use of large-bore needles in core biopsies, however, has been associated with tumor seeding along the needle tract in some reports,12–14 again making FNA a more attractive option.
Given the ongoing significant role of this diagnostic test, our objective was to perform a systematic review and meta-analysis of the utility of FNA in distinguishing between benign and malignant parotid gland masses. Specifically, we analyzed the sensitivity and specificity of parotid FNA, its anticipated posttest probability of malignancy, and the probability of nondiagnostic and indeterminate cytology.
Methods
Search Strategy and Study Selection
In accordance with PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses), a systematic search was performed independently by 2 reviewers (C.C.L., A.R.J.). The PubMed and Embase databases were searched with the Medical Subject Headings terms parotid mass, parotid nodule, parotid tumor, and parotid gland. The result from this search was then cross-searched with the Medical Subject Headings terms biopsy and fine needle. The Cochrane Central Register of Controlled Trials database was searched with parotid and fine needle as well as parotid and biopsy. Studies were limited to those published in the English language, on human subjects, and in the last 50 years (January 1, 1964, to November 1, 2014). A manual search of the bibliographies of relevant studies was also performed to identify any additional studies.
Title and abstract review was performed, followed by full-text review. Studies were included if they met the following criteria based on the PICOS design (participants, interventions, comparisons, outcomes, and study):
the study examined adults and/or children presenting with clinically or radiographically identified parotid masses who subsequently underwent parotidectomy;
FNA was performed prior to surgery via palpation or ultrasound guidance;
the cytopathology results from the FNA as well as the histopathology results from the surgical specimen are both reported;
the study denoted true positives/negatives and false positives/negatives associated with FNA in diagnosing benign versus malignant disease; and
the study was a randomized or quasi-randomized controlled trial, nonrandomized prospective trial, or retrospective review.
Studies were excluded if
they did not contain sufficient data to determine the number of true positives/negatives and false positives/negatives;
they reported on salivary gland pathology as a whole, without distinguishing between parotid and other salivary glands; and
they were case reports or abstracts that did not contain sufficient data.
Data Collection
Data were extracted from the included studies by 2 independent reviewers (C.C.L., A.R.J.). The main outcomes of interest were the sensitivity, specificity, and posttest probability of parotid FNA in distinguishing malignant from benign disease. To obtain this information, we collected the number of true-positive, true-negative, false-positive, and false-negative results obtained from parotid FNA, with surgical histopathology as the diagnostic gold standard. A true positive was defined as a case where an FNA diagnosis of malignancy was later confirmed on surgical histopathology. Similarly, a true negative was defined as a case where a benign FNA result was subsequently verified as benign on final surgical histopathology. A false positive was defined as an FNA diagnosis of malignancy with subsequent benign surgical histopathology, while a false negative was defined as an FNA diagnosis of benign disease with malignancy discovered after surgery. Finally, we collected data on the frequency of nondiagnostic samples, defined as insufficient cellular content in the aspirate for analysis, as well as the frequency of indeterminate results, defined as sufficient cellular content without clear determination of a benign or malignant diagnosis.
Last, study design, reporting characteristics, and potential risks of bias were tracked. Specifically, we recorded whether the design of a study was prospective or retrospective, whether consecutive patients were described, whether blinding was employed, whether there was a technical description of the FNA method utilized, and whether FNAs were performed under ultrasound guidance.
Statistical Analysis
The sensitivity and specificity for parotid FNA from each study were calculated with the equations shown in Table 1. We also calculated the diagnostic odds ratio, defined as the odds of the FNA being positive for malignancy if the patient has true malignant disease over the odds of the FNA being positive for malignancy if the patient has true benign disease. Finally, we calculated the positive and negative likelihood ratios (LRs) associated with parotid FNA.
Table 1.
Calculation of Diagnostic Test Characteristics.
| Characteristic | Equation |
|---|---|
| Sensitivity | TP/(TP + FN) |
| Specificity | TN/(TN + FP) |
| Diagnostic odds ratio | (TP/FN)/(FP/TN) |
| Likelihood ratio | |
| Positive | sensitivity/(1 − specificity) |
| Negative | (1 − sensitivity)/specificity |
Abbreviations: FN, false negative; FP, false positive; TN, true negative; TP, true positive.
The I2 statistic was used to evaluate heterogeneity among the included studies. It reflects the degree of variability that is due to more than chance alone. We used the following criteria to interpret the I2 statistic: 0%–40% indicates likely unimportant heterogeneity; 30%–60%, moderate heterogeneity; 50%–90%, significant heterogeneity; 75%–100%, considerable heterogeneity.15 For our pooled estimates, we present the associated I2 statistic along with its 95% confidence interval (CI).
Meta-analyses were performed to yield summary estimates of the above diagnostic characteristics of parotid FNA. This was accomplished through a random effects model with weights based on the DerSimonian and Laird method.16 Calculations were performed in Stata 13.0 (College Station, Texas) and Microsoft Excel (Redmond, Washington). Sensitivity and specificity data were calculated according to standard formulas for dichotomously categorized outcomes.17–19 Nondiagnostic and indeterminate results were excluded from the pooled analysis of sensitivity and specificity, as they were unable to be clearly designated as either benign or malignant and surgical histopathology results specific to these findings were often unreported. The sensitivity and specificity from the individual studies were used to construct a receiver operating characteristic curve via a hierarchical regression model.20 Bayesian nomograms were plotted with pooled LRs to determine of posttest probability of malignancy. The presence of publication bias was assessed by performing a Deeks’ funnel plot asymmetry test. P < .05 was considered significant for the presence of bias.
Subgroup analyses were performed to explore whether certain clinical or study design variations contributed to the heterogeneity. Specifically, we performed separate analyses on prospectively designed studies, those involving consecutive series of patients, those that clearly described the FNA technique used, those that used ultrasound guidance to perform the FNAs, as well as studies that examined general patient populations without any specific clinical characteristics.
Ethics
This study involves a review of published literature with no usage of patient data; therefore, it was exempt from institutional review board and ethics committee approval.
Results
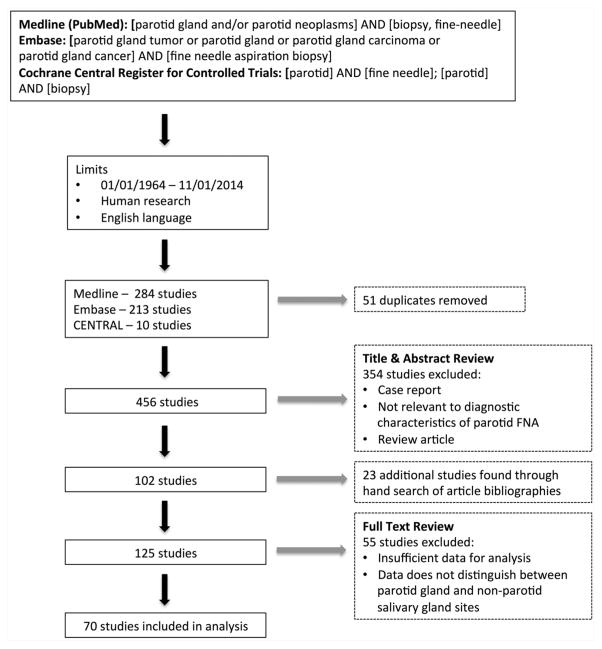
The search strategy yielded a total of 456 studies. After review of titles and abstracts, 354 studies were excluded. A manual bibliography search of relevant studies identified a further 23 studies, leaving 125 studies for full-text review. After studies were excluded that did not contain sufficient data for analysis, 70 studies remained. The search strategy is illustrated in Figure 1.
Figure 1.
Search strategy. FNA, fine-needle aspiration.
Study Characteristics
Tables 2–5 describe the characteristics and individual results of the 70 included studies. Six studies (8%) were prospective in design; the remainder either were retrospective or did not specify. Nineteen studies (27%) used consecutive series of patients, and 36 (51%) reported the FNA technique used. Seven studies examined specific parotid lesion morphologies or pathologies, including Warthin tumors,21–23 metastatic lesions,24 cystic lesions,25 carcinoma ex pleomorphic adenoma,26 and lymphoma of the parotid gland.27 Two studies investigated the diagnostic utility of FNA in the pediatric population28,29; 1 study examined FNAs in patients with symptoms suggestive of malignancy30; and 1 study examined the FNA results of those patients who ultimately received a histologic diagnosis of malignancy.31 Finally, 9 studies included FNAs that were performed under ultrasound guidance.9,32–39
Table 2.
Study Design and Risk of Bias.a
| Author (Year) | Prospective | Consecutive | Blinding | Description of FNA Technique |
|---|---|---|---|---|
| Akbas et al (2004)34 | ● | ● | ● | ■ |
| Al-Khafaji et al (1998)57 | ● | ■ | ● | ■ |
| Ali et al (2010)80 | □ | ■ | ● | □ |
| Arabi et al (2006)47 | □ | ● | ● | □ |
| Atula et al (1996)41 | □ | ● | ● | ■ |
| Aversa et al (2006)81 | □ | ● | ● | □ |
| Awan et al (2004)59 | □ | ● | ● | ■ |
| Bajaj et al (2005)33 | □ | ● | ● | □ |
| Balakrishnan et al (2005)82 | □ | ■ | ● | ■ |
| Behbehani et al (1990)45 | □ | ● | ● | □ |
| Behzatoglu et al (2004)83 | ● | ● | ● | ■ |
| Berg et al (1986)60 | ● | ● | ● | □ |
| Bigorgne et al (2013)48 | ● | ● | ● | □ |
| Bono et al (1983)84 | ● | ● | ● | □ |
| Califano et al (1992)85 | ● | ● | ● | ■ |
| Carrillo et al (2009)40 | ■ | ■ | ■ | ■ |
| Contucci et al (2003)73 | ● | ● | ● | ■ |
| Costas et al (2000)74 | ● | ● | ● | ■ |
| de Ru et al (2007)37 | ■ | ● | ● | □ |
| Deans et al (1995)86 | □ | ● | ● | □ |
| Deneuve et al (2010)75 | □ | ■ | ● | □ |
| Fakhry et al (2012)49 | □ | ● | ● | ■ |
| Fakhry et al (2014)76 | □ | ● | ● | ■ |
| Fassnacht et al (2013)77 | □ | ■ | ● | ■ |
| Fassnacht et al (2013)21 | □ | ■ | ● | □ |
| Feld et al (1999)38 | □ | ● | ● | ■ |
| Filopolous et al (1998)42 | ● | ■ | ● | ■ |
| Fundakowski et al (2014)53 | □ | ● | ● | □ |
| Gobic et al (2010)36 | □ | ● | ● | ■ |
| Gooden et al (2002)87 | □ | ■ | ● | □ |
| Haldar et al (2015)10 | □ | ● | ● | ■ |
| Henrys et al (2014)32 | □ | ■ | ● | □ |
| Horii et al (1998)24 | ● | ● | ● | ■ |
| Huang et al (2012)9 | □ | ● | ● | ■ |
| Inohara et al (2008)61 | □ | ● | ● | ■ |
| Iqbal et al (2011)62 | ■ | ● | ● | □ |
| Jafari et al (2009)50 | □ | ■ | ● | ■ |
| Javadi et al (2012)78 | ● | ● | ● | □ |
| Jeong et al (2013)46 | □ | ■ | ● | ■ |
| Kamal et al (1997)88 | ● | ● | ● | □ |
| Lee et al (2013)89 | □ | ● | ● | □ |
| Lim et al (2007)63 | □ | ● | ● | □ |
| Lurie et al (2002)90 | ■ | ■ | ● | ■ |
| Malata et al (1997)31 | □ | ● | ● | □ |
Abbreviation: FNA, fine-needle aspiration.
A black square, ■, indicates that the study was prospective, involved consecutive patients or blinding, or included a description of the FNA technique. A white square, □, indicates that the study was not prospective, did not involve consecutive patients or blinding, or did not include a description of the FNA technique. A bullet, ●, indicates that information was not reported.
Table 5.
Results of Individual Studies.a
| Author (Year) | FNAs, n | TP | FP | FN | TN | ND | ID |
|---|---|---|---|---|---|---|---|
| Mathew et al (1997)28 | 6 | 3 | 0 | 1 | 0 | 1 | 1 |
| McGuirt et al (1995)55 | 18 | 6 | 0 | 0 | 8 | 0 | 4 |
| Mohammed et al (2008)91 | 211 | 21 | 6 | 14 | 148 | 22 | ● |
| Nasuti et al (2000)25 | 29 | 1 | 1 | 0 | 21 | 0 | 6 |
| Newberry et al (2014)56 | 13 | 0 | 0 | 2 | 4 | 1 | 6 |
| Nouraei et al (2005)26 | 14 | 4 | 0 | 9 | 0 | 1 | 0 |
| Paris et al (2005)64 | 148 | 25 | 5 | 6 | 97 | 15 | 0 |
| Piccioni et al (2011)39 | 176 | 13 | 1 | 3 | 123 | 36 | 0 |
| Reddy et al (2008)22 | 35 | 0 | 0 | 0 | 30 | 0 | 5 |
| Riley et al (2005)92 | 86 | 29 | 3 | 2 | 52 | ● | 0 |
| Rodriguez et al (1989)79 | 64 | 11 | 1 | 2 | 32 | 18 | 0 |
| Schelkun et al (1991)51 | 10 | 4 | 0 | 0 | 4 | ● | 2 |
| Seethala et al (2005)43 | 220 | 43 | 12 | 9 | 130 | 12 | 14 |
| Shashinder et al (2009)93 | 76 | 16 | 2 | 5 | 53 | 0 | 0 |
| Takashima et al (1999)94 | 26 | 12 | 0 | 2 | 10 | 2 | 0 |
| Tew et al (1997)95 | 195 | 18 | 0 | 2 | 109 | 29 | 37 |
| Tsai et al (2002)96 | 40 | 3 | 1 | 2 | 34 | 0 | 0 |
| Upton et al (2007)30 | 62 | 20 | 2 | 1 | 29 | 10 | 0 |
| van Lierop et al (2007)97 | 112 | 8 | 1 | 3 | 55 | 45 | 0 |
| Veder et al (2010)23 | 133 | 0 | 2 | 0 | 131 | 0 | 0 |
| Weinberger et al (1992)44 | 49 | 9 | 2 | 3 | 30 | 2 | 3 |
| Wyss et al (2013)27 | 21 | 4 | 0 | 0 | 0 | 0 | 17 |
| Yerli et al (2010)35 | 23 | 3 | 0 | 1 | 16 | 3 | 0 |
| Zafar et al (1997)54 | 28 | 8 | 0 | 2 | 17 | 0 | 1 |
| Zbaren et al (2008)65 | 116 | 50 | 5 | 18 | 37 | 6 | 0 |
| Zurrida et al (1993)98 | 246 | 31 | 0 | 14 | 178 | 23 | 0 |
Abbreviations: FN, false negative; FNA, fine-needle aspiration; FP, false positive; ID, indeterminate; ND, nondiagnostic; TN, true negative; TP, true positive.
The number of each category is shown. A bullet, ●, indicates that information was not reported.
Considerable heterogeneity was found among studies, with an I2 statistic of 72.4% (95% CI, 65.5%–79.3%) for sensitivity and 78.6% (95% CI, 73.6%–83.6%) for specificity. With such high heterogeneity, the pooled estimates should be interpreted with caution. Point estimates of heterogeneity remained high throughout the subgroup analyses (Table 6). Two exceptions were the specificity of prospective studies, which had a heterogeneity point estimate of 0%, and the sensitivity of studies utilizing ultrasound guidance, which had a heterogeneity point estimate of 14.7%; however, the I2 statistic associated with both had notably wide 95% CIs (0%–100% and 0%–72.7%, respectively).
Table 6.
Estimates of the Diagnostic Characteristics of Parotid FNA.a
| Sensitivity | I2 Sensitivity | Specificity | I2 Specificity | DOR | LR+ | LR− | |
|---|---|---|---|---|---|---|---|
| All studies | 0.780 (0.733, 0.821) | 72.4 (65.5, 79.3) | 0.977 (0.966, 0.985) | 78.6 (73.6, 83.6) | 153 (94.7, 246) | 34.3 (22.8, 51.7) | 0.225 (0.184, 0.275) |
| Prospective design | 0.882 (0.509, 0.982) | 87.8 (78.5, 97.0) | 0.995 (0.960, 0.999) | 0 (0, 100) | 1422 (89.8, 22,540) | 169 (21.6, 1317) | 0.119 (0.021, 0.678) |
| Consecutive series of patients | 0.745 (0.642, 0.826) | 75.6 (64.5, 86.8) | 0.979 (0.954, 0.991) | 83.1 (76.1, 90.1) | 137 (54.5, 345) | 35.8 (16.2, 79.2) | 0.261 (0.182, 0.374) |
| With technical description | 0.785 (0.724 0.835) | 71.2 (61.3, 81.1) | 0.965 (0.946, 0.977) | 72.9 (63.7, 82.1) | 99.4 (58.3, 169) | 22.2 (14.4, 34.1) | 0.223 (0.172, 0.289) |
| General populationb | 0.787 (0.740, 0.827) | 74.1 (67.5, 80.7) | 0.976 (0.964, 0.984) | 80.1 (75.4, 84.8) | 150 (92.8, 243) | 32.8 (21.8, 49.3) | 0.218 (0.178, 0.268) |
| FNA, ultrasound guidance | 0.848 (0.760, 0.908) | 14.7 (0, 72.7) | 0.980 (0.951, 0.992) | 57.6 (26.2, 89.0) | 272 (81.0, 912) | 42.2 (16.8, 106) | 0.155 (0.095, 0.253) |
Abbreviations: DOR, diagnostic odds ratio; FNA, fine-needle aspiration; LR, likelihood ratio.
In parentheses, 95% confidence interval.
Studies that are not conducted on a specific disease or patient population.
Diagnostic Accuracy
The results of 6784 FNAs were reported in the 70 included studies. Among these, there were 518 nondiagnostic and 385 indeterminate results, constituting 13.3% of FNAs performed. A further 7 studies22–24,26–28,31 were excluded from the numeric pooled analysis, as they contained zero values such that sensitivity or specificity could not be calculated. A meta-analysis was performed on the remaining 63 studies, which contained data describing 5647 FNAs.
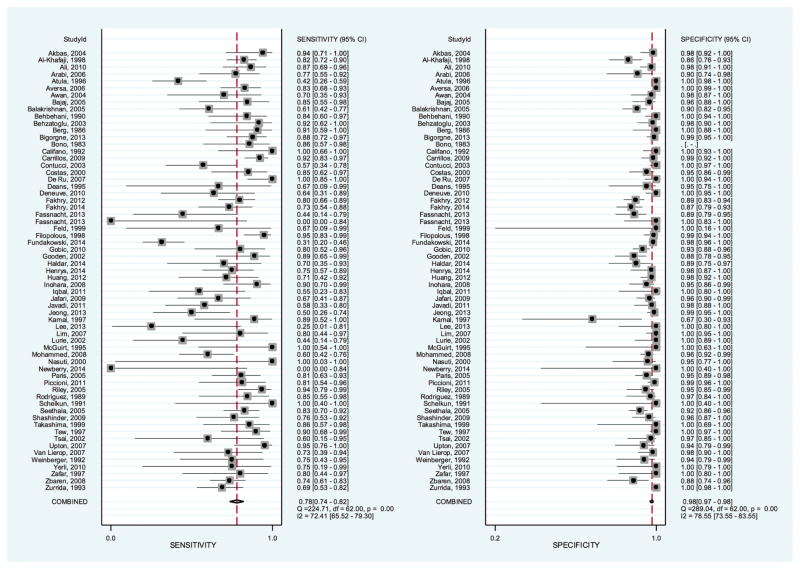
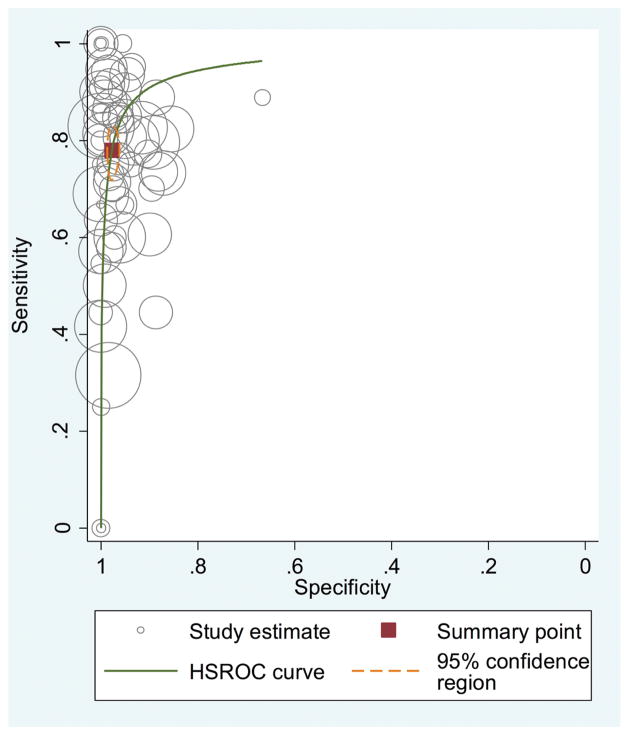
The sensitivity of FNA in distinguishing malignant from benign parotid disease ranged from 0 to 1, and the specificity ranged from 0.670 to 1. The summary estimate for the sensitivity and specificity was 0.780 (95% CI, 0.740–0.820) and 0.980 (95% CI, 0.970–0.980), respectively. Figure 2 presents the forest plot of the sensitivity and specificity values from the individual studies, along with the combined estimate. The diagnostic odds ratio was 153 (95% CI, 94.7–246), while the positive LR was 34.3 (95% CI, 22.8–51.7) and the negative LR was 0.225 (95% CI, 0.184–0.275). Figure 3 shows the receiver operating characteristic curve from all included studies. The area under the curve represents the overall diagnostic accuracy of parotid FNA and was 0.960 (95% CI, 0.940–0.970). If we limited the analysis to only the prospectively conducted studies, the diagnostic accuracy increased to 0.990 (95% CI, 0.980–1.00).
Figure 2.
Summary estimates for the sensitivity and specificity of parotid fine-needle aspiration.
Figure 3.
Receiver operating characteristic curve of fine-needle aspiration in the diagnosis of malignant parotid disease. HSROC, hierarchical summary receiver operating characteristic curve.
Table 6 reports the summary estimates from the subgroup analyses. In the prospective group of studies, the sensitivity and specificity were higher than in the general population of studies: 0.882 (95% CI, 0.509–0.982) and 0.995 (95% CI, 0.960–0.999), respectively. Accordingly, this group was also found to have higher positive and lower negative LRs (169 and 0.119) as compared with the population as a whole and as compared with the other subgroups. The group of studies that performed ultrasound-guided FNAs also had higher sensitivity (0.848; 95% CI, 0.760–0.908) and specificity (0.980; 95% CI, 0.951–0.992) versus those of the general population of studies.
Posttest Probabilities
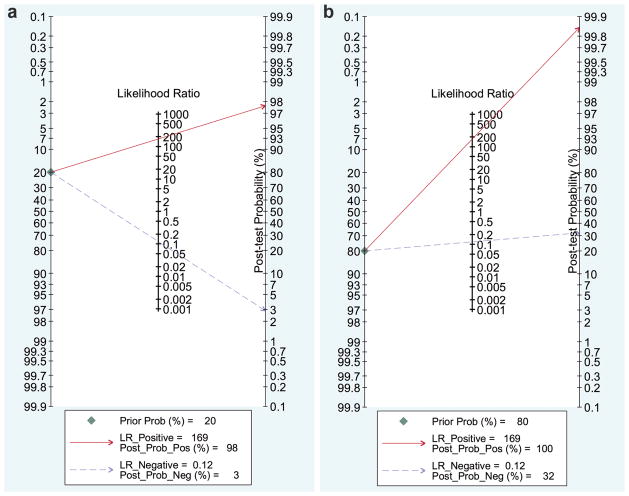
With the LRs from the prospective group of studies, nomograms were constructed for FNA in diagnosing malignant parotid lesions (Figure 4). The posttest probability—or probability that an individual has a malignant lesion given a positive FNA—is dependent not only on the test characteristics (sensitivity and specificity) but also on the pretest prevalence of malignancy in the presenting population. In the setting of a prevalence of 20% (ie, low pretest probability), the posttest probability of malignancy was estimated to be 98%. This posttest probability estimate increased to 100% in the setting of an anticipated 80% prevalence of malignancy (ie, high pretest probability). With negative FNA results in the setting of a 20% pretest probability, the posttest probability of malignancy was 3%. When the pretest probability was estimated at 80%, however, the posttest probability of malignancy given a negative FNA increased to 32%.
Figure 4.
Nomograms for fine-needle aspiration in diagnosing malignant parotid disease in the setting of (a) low and (b) high pretest probabilities.
Nondiagnostic and Indeterminate Results
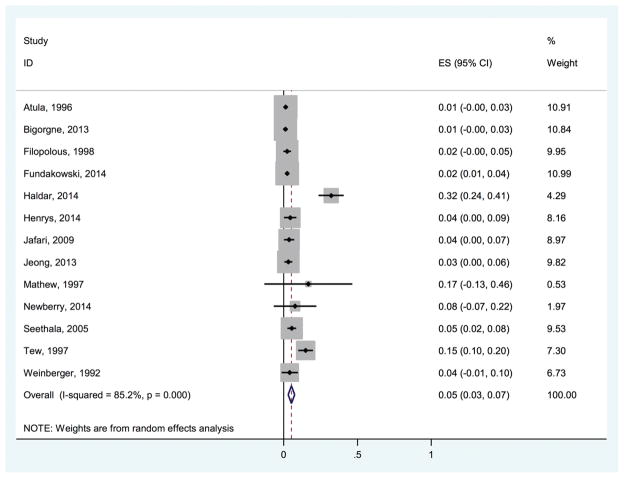
Forty studies reported the frequency of nondiagnostic FNAs. The probability of obtaining a nondiagnostic FNA among these studies ranged from 0.012 to 0.402. Within the studies that reported nondiagnostic FNAs, 13 studies also reported the number of indeterminate results (Figure 5). The pooled estimate of the probability of nondiagnostic samples among studies reporting both nondiagnostic samples and indeterminate results was 0.053 (95% CI, 0.030–0.075). Here again, this pooled result should be interpreted with caution, as the associated I2 statistic was 85.2%. The remaining 27 studies reported nondiagnostic FNAs only. In this group of studies, the pooled estimate of the probability of nondiagnostic samples was 0.140 (95% CI, 0.103–0.176), with an I2 statistic of 90.9%. Among these 27 studies, the lowest number of nondiagnostic samples was reported in those that were prospectively conducted, with a probability of 0.105 (95% CI, 0.000–0.226; I2 = 84.5%).
Figure 5.
Probability of nondiagnostic fine-needle aspirations among studies reporting both nondiagnostic fine-needle aspiration and indeterminate results. ES, effect size.
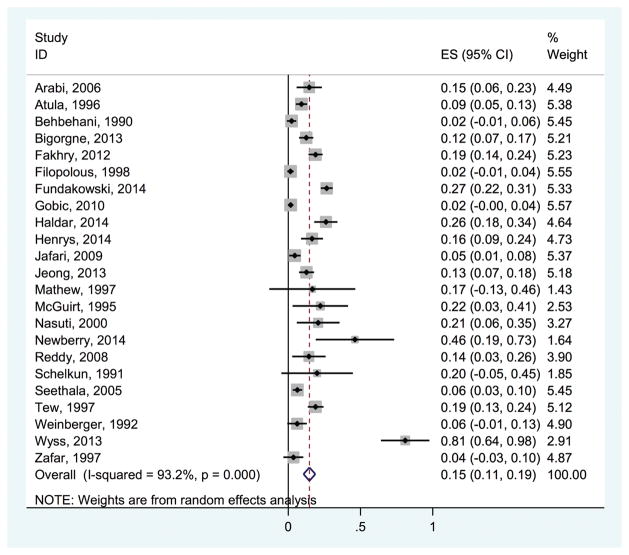
Twenty-three studies reported the frequency of indeterminate results, which ranged from 0.016 to 0.810 (Figure 6). The pooled estimate for the probability of indeterminate results was 0.147 (95% CI, 0.106–0.188), with an I2 statistic of 93.2%.
Figure 6.
Probability of indeterminate fine-needle aspiration results. ES, effect size.
Publication Bias
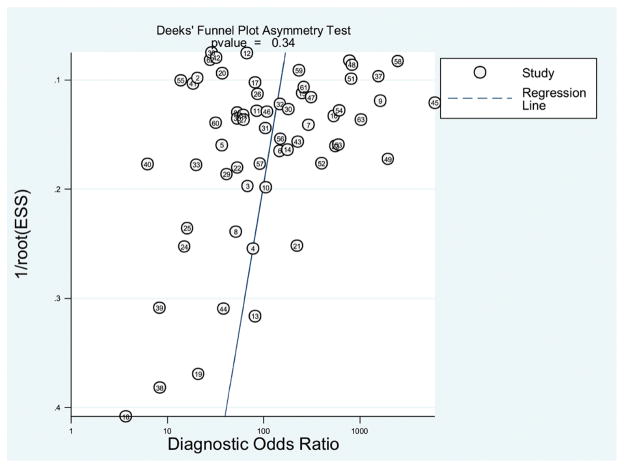
A Deeks’ funnel plot was constructed (Figure 7) and assessed visually for publication bias. The overall symmetrical distribution of study estimates suggests that there is no publication bias. A P value of .341 supports the visual assessment.
Figure 7.
Deeks’ funnel plot for the assessment of publication bias.
Risk of Bias Among Studies
The majority of studies had a retrospective or unspecified study design (91%) and involved nonconsecutive series of patients (72%; Tables 2–5). Only 1 study involved the blinding of cytopathologists and histopathologists, with participants blinded to clinical and imaging data.40
In addition to the significant heterogeneity found in the reported sensitivity and specificity of parotid FNA, there was variability among included studies in how suspicious and malignant results were classified and accounted for in the calculations of the diagnostic test characteristics. In the 23 studies that reported indeterminate data, 5 considered indeterminate results as malignant in their calculations,36,41–44 while 2 considered indeterminate results as benign.45,46 Eight studies removed indeterminate results from their calculations,22,27,47–52 and in 4 studies, it was unclear how indeterminate results were managed.10,32,53,54 Finally, 4 studies presented the raw data and did not calculate sensitivity or specificity.25,28,55,56
Our a priori definition of an indeterminate result included any that were not definitively benign or malignant or non-diagnostic, including results that were “suspicious” for malignancy. In studies where the number of suspicious results and their ultimate diagnoses were clearly stated, they were removed from the counts of true and false positives and negatives. In 12 studies, however, suspicious FNA findings were grouped with those that were clearly malignant, and the numbers associated with each were not explicitly reported.21,33,49,57–65
Discussion
These results suggest that FNA has higher specificity (98%; 95% CI, 97%–98%) than sensitivity (78%; 95% CI, 74%–82%) in differentiating benign from malignant parotid gland lesions, with an overall diagnostic accuracy of 96% (95% CI, 94%–97%). There was significant heterogeneity found among studies (72.4% for sensitivity and 78.6% for specificity). In the prospective cohort of studies, sensitivity and specificity were found to be higher, at 88% and 100%, respectively. As prospectively designed studies represent higher-quality evidence, 88% and 100% may be better reflective of the true diagnostic characteristics of parotid FNA.
An important characteristic of diagnostic tests is that they inform clinicians of the probability that an individual has a disease, conditional on a specific test result. This posttest probability depends on 2 parameters. The first is the LR, which is a function of the sensitivity and specificity of a diagnostic test. The second is the underlying prevalence of the disease in the presenting population—that is, the pretest probability, which is based on clinical characteristics seen in advance of the diagnostic test. For other disease processes, methods to quantify the pretest probability are based on the history and physical examination findings,66 but to date, there is no similar quantification for parotid lesions. Regardless, the related principles apply. A high pretest probability is associated with a higher posttest probability of malignancy in both positive and negative test results. The converse is true for a lower pretest probability. Based on the current analysis, the negative LR of FNA is 0.12. Therefore, the posttest probability of malignancy in a negative FNA result is 3% in the setting of a low pretest probability (20%); however, this rises to 32% when the pretest probability is high (80%). Our analysis determined the positive LR to be 169, which is associated with a 98% to 100% posttest probability of malignancy in a positive FNA, remaining high even over a wide range of pretest probabilities (20%–80%). It is important for clinicians to be cognizant of this when interpreting the results of an FNA, relative to individual patients with specific presentations. For example, a patient presenting with an enlarging parotid mass that is associated with facial nerve paralysis will have a high pretest probability of malignancy. A negative FNA in this case would still be associated with a relatively high posttest probability of malignancy. This is in contrast to a long-standing parotid mass that is asymptomatic, in which case a negative FNA would be more predictive of truly benign disease.
Parotid FNAs can yield a number of intermediate results, and there is variability in the literature in how these intermediate results are handled.67 In data collected through this systematic review, parotid FNA had a reported sensitivity of 0% to 100% and specificity of 67% to 100%. In part, this range can be attributed to the variability among studies in classifying suspicious and indeterminate results. Of the 23 studies that reported indeterminate results, only 15 indicated how these results were classified in the analysis. Five studies classified indeterminate results as malignant, 2 as benign, and 8 studies removed the indeterminate results from the analysis. This variability in intermediate result assignment can significantly alter the estimated sensitivity and specificity of the test. In our analysis, intermediate results were removed to describe the characteristics of parotid FNA in the setting of clearly defined malignant or benign results. However, 12 studies included suspicious results in the malignant category without explicitly stating how many underwent this classification.21,33,49,57–65 As such, the sensitivity and specificity calculations from these studies may include suspicious results that would have otherwise been excluded. This again highlights the variability in interpretation of indeterminate FNA results in the literature.
The underlying reason for the variability in the handling of intermediate results is that there is no universal classification system for the cytologic evaluation and reporting of parotid gland lesions. This is in contrast to, for example, the Bethesda system for reporting thyroid cytopathology.68 Similar to those of the parotid gland, FNAs of the thyroid have been associated with variability in the reporting of intermediate results.69 The Bethesda classification system was established as a solution for this variability, as it clearly defines intermediate cytology findings and reports the risk of malignancy associated with each intermediate category. The French-American-British70 and World Health Organization71 classification systems for hematologic malignancies also offer standard methods for the analysis, reporting, and interpretation of bone marrow aspirates across nonbinary diagnostic categories. In our analysis, individual studies were noted to vary in how indeterminate results were classified. Some studies classified these results as benign, while others classified them as malignant. This inconsistency creates difficulties in interpretation of the data. Furthermore, the lack of agreement on the clinical significance of an indeterminate result impedes proper patient counseling regarding treatment and prognosis. The creation of a classification scheme for parotid gland cytopathology that is analogous to the Bethesda system for thyroid lesions would circumvent the issue of arbitrary classification of intermediate FNA results. A united effort is needed to standardize parotid FNA findings, as this would lead to improved patient care and less heterogeneity in future research, and a multicenter team has undertaken this work (personal communication, Dr Christopher Fundakowski, February 13, 2015).
USCBs have been studied as an alternative method of obtaining tissue diagnoses from parotid gland tumors. A core needle biopsy obtains more tissue than that of an FNA, thereby improving its yield for histopathology. In a recent meta-analysis examining the diagnostic yield of USCB in salivary gland tumors, the sensitivity was found to be 96% (95% CI, 87%–99%) with a specificity of 100% (95% CI, 84%–100%).72 The disadvantage of USCB is that its performance may require local anesthesia and be associated with a higher incidence of hematoma and facial nerve injury. The rate of hematoma formation and temporary facial nerve weakness following USCB has been reported to be 1.6% and 0.2%, respectively.72 Our analysis suggests that standard FNA has a lower sensitivity versus that of USCB; however, the specificity of the 2 techniques is comparable. The subgroup analysis of studies that performed ultrasound-guided FNAs showed a sensitivity of 85% (95% CI, 76%–91%) and a specificity of 98% (95% CI, 95%–99%). The complication rates associated with FNA appear to be lower in general. In our analysis, 16 studies reported complication data for 1880 patients.* There were 2 cases of hematomas (0.1%), 3 cases of local infection/inflammation (0.16%), 2 cases of pain (0.1%), and no cases of facial nerve injury.
We performed a primary and secondary analysis to investigate the frequency of nondiagnostic aspirates among the included studies. Our primary analysis examined studies that reported nondiagnostic and indeterminate results. In this cohort of studies, the probability of obtaining a nondiagnostic sample was 5.3%. Our secondary analysis examined studies that reported only nondiagnostic samples, and in this group, the probability of obtaining a nondiagnostic sample was 14%. A potential explanation for this difference is that studies that reported only nondiagnostic samples may have included indeterminate results in the nondiagnostic category, thereby overestimating the frequency of nondiagnostic samples. Once again, this highlights the varied nature with which results from parotid FNAs are reported, which contributes to the heterogeneity among studies.
Other sources of heterogeneity are likely both methodological and clinical. First, the FNA technique varied from study to study, including different needle gauges, number of passes, and utilization of ultrasound guidance. These differences in FNA technique may affect sensitivity and specificity, as well as the probability of obtaining nondiagnostic samples and indeterminate results. There may also be clinical variability contributing to the heterogeneity. Among the included studies, the overall prevalence of malignancy ranged from 3.4% to 67%. This may reflect differences in presenting patient populations, as associated with the volume and expertise of the reporting surgical center. Studies conducted at larger or primarily academic centers may involve patients of greater clinical complexity, who may have had previous diagnostic failures at peripheral centers. The varying presence of diagnostically challenging patients among studies may also contribute to the heterogeneity of reported test characteristics. Another limitation of our study is that we included only studies written in the English language. Relevant studies published in other language may thus have been missed.
Conclusion
FNA of the parotid gland has moderate sensitivity and high specificity in differentiating malignant from benign disease. Given the high positive LR, a positive FNA can predict the presence of malignancy with 98% to 100% accuracy, depending on the prevalence of malignancy. However, patients with a negative FNA may still have a tangible posttest probability of malignancy, particularly if the underlying prevalence of disease is high. Physicians should therefore take the pretest probability of malignancy in their patient populations into account when interpreting parotid FNA results. Significant heterogeneity was found among the included studies, particularly in terms of the classification and reporting of intermediate results. An effort to standardize the classification of parotid FNA findings would improve the consistency with which surgeons interpret and approach intermediate results.
Table 3.
Study Design and Risk of Bias.
| Author (Year) | Prospective | Consecutive | Blinding | Description of FNA Technique |
|---|---|---|---|---|
| Mathew et al (1997)28 | ● | ● | ● | ■ |
| McGuirt et al (1995)55 | ● | ■ | ● | □ |
| Mohammed et al (2008)91 | □ | ■ | ● | □ |
| Nasuti et al (2000)25 | □ | ● | ● | ■ |
| Newberry et al (2014)56 | □ | ● | ● | □ |
| Nouraei et al (2005)26 | □ | ● | ● | □ |
| Paris et al (2005)64 | □ | ● | ● | ■ |
| Piccioni et al (2011)39 | □ | ■ | ● | ■ |
| Reddy et al (2008)22 | □ | ● | ● | □ |
| Riley et al (2005)92 | □ | ● | ● | ■ |
| Rodriguez et al (1989)79 | ● | ● | ● | ■ |
| Schelkun et al (1991)51 | ■ | ● | ● | ■ |
| Seethala et al (2005)43 | □ | ● | ● | ■ |
| Shashinder et al (2009)93 | □ | ● | ● | □ |
| Takashima et al (1999)94 | ● | ● | ● | ■ |
| Tew et al (1997)95 | ● | ● | ● | □ |
| Tsai et al (2002)96 | □ | ● | ● | □ |
| Upton et al (2007)30 | □ | ● | ● | □ |
| van Lierop et al (2007)97 | □ | ● | ● | □ |
| Veder et al (2010)23 | ■ | ■ | ● | □ |
| Weinberger et al (1992)44 | □ | ● | ● | ■ |
| Wyss et al (2013)27 | □ | ● | ● | □ |
| Yerli et al (2010)35 | ● | ■ | ● | ■ |
| Zafar et al (1997)54 | ● | ● | ● | ■ |
| Zbaren et al (2008)65 | □ | ● | ● | ■ |
| Zurrida et al (1993)98 | ● | ■ | ● | □ |
Abbreviation: FNA, fine-needle aspiration.
A black square, ■, indicates that the study was prospective, involved consecutive patients or blinding, or included a description of the FNA technique. A white square, □, indicates that the study was not prospective, did not involve consecutive patients or blinding, or did not include a description of the FNA technique. A bullet, ●, indicates that information was not reported.
Table 4.
Results of Individual Studies.a
| Author (Year) | FNAs, n | TP | FP | FN | TN | ND | ID |
|---|---|---|---|---|---|---|---|
| Akbas et al (2004)34 | 82 | 16 | 1 | 1 | 64 | 0 | 0 |
| Al-Khafaji et al (1998)57 | 154 | 61 | 11 | 13 | 65 | 4 | ● |
| Ali et al (2010)80 | 112 | 26 | 2 | 4 | 80 | ● | 0 |
| Arabi et al (2006)47 | 62 | 17 | 3 | 5 | 28 | ● | 9 |
| Atula et al (1996)41 | 219 | 15 | 0 | 21 | 160 | 3 | 20 |
| Aversa et al (2006)81 | 310 | 34 | 0 | 7 | 269 | 0 | 0 |
| Awan et al (2004)59 | 50 | 7 | 1 | 3 | 39 | 0 | 0 |
| Bajaj et al (2005)33 | 69 | 11 | 2 | 2 | 54 | 0 | 0 |
| Balakrishnan et al (2005)82 | 188 | 20 | 10 | 13 | 89 | 56 | 16 |
| Behbehani et al (1990)45 | 85 | 16 | 0 | 3 | 64 | 0 | 2 |
| Behzatoglu et al (2004)83 | 71 | 11 | 1 | 1 | 54 | 4 | ● |
| Berg et al (1986)60 | 42 | 10 | 0 | 1 | 28 | 3 | ● |
| Bigorgne et al (2013)48 | 169 | 29 | 1 | 4 | 112 | 2 | 21 |
| Bono et al (1983)84 | 16 | 12 | 0 | 2 | 0 | 2 | 0 |
| Califano et al (1992)85 | 60 | 9 | 0 | 0 | 51 | 0 | 0 |
| Carrillo et al (2009)40 | 138 | 60 | 1 | 5 | 69 | 3 | 0 |
| Contucci et al (2003)73 | 146 | 12 | 0 | 9 | 118 | 7 | 0 |
| Costas et al (2000)74 | 80 | 17 | 3 | 3 | 57 | 0 | 0 |
| de Ru et al (2007)37 | 82 | 22 | 0 | 0 | 60 | 0 | 0 |
| Deans et al (1995)86 | 25 | 2 | 1 | 1 | 19 | 2 | 0 |
| Deneuve et al (2010)75 | 78 | 7 | 0 | 4 | 67 | 0 | 0 |
| Fakhry et al (2012)49 | 249 | 43 | 16 | 11 | 132 | 0 | 47 |
| Fakhry et al (2014)76 | 138 | 22 | 14 | 8 | 94 | 0 | 0 |
| Fassnacht et al (2013)77 | 125 | 4 | 8 | 5 | 62 | 46 | ● |
| Fassnacht et al (2013)21 | 22 | 0 | 0 | 2 | 20 | 0 | 0 |
| Feld et al (1999)38 | 5 | 2 | 0 | 1 | 2 | 0 | 0 |
| Filopolous et al (1998)42 | 129 | 37 | 1 | 2 | 84 | 3 | 2 |
| Fundakowski et al (2014)53 | 432 | 17 | 4 | 37 | 249 | 10 | 115 |
| Gobic et al (2010)36 | 176 | 12 | 11 | 3 | 147 | 0 | 3 |
| Gooden et al (2002)87 | 144 | 16 | 8 | 2 | 61 | 57 | ● |
| Haldar et al (2015)10 | 115 | 7 | 4 | 3 | 34 | 37 | 30 |
| Henrys et al (2014)32 | 91 | 24 | 1 | 8 | 39 | 4 | 15 |
| Horii et al (1998)24 | 5 | 5 | 0 | 0 | 0 | 0 | 0 |
| Huang et al (2012)9 | 107 | 10 | 2 | 4 | 83 | 8 | ● |
| Inohara et al (2008)61 | 81 | 19 | 3 | 2 | 57 | 0 | 0 |
| Iqbal et al (2011)62 | 30 | 6 | 0 | 5 | 17 | 2 | 0 |
| Jafari et al (2009)50 | 110 | 12 | 3 | 6 | 80 | 4 | 5 |
| Javadi et al (2012)78 | 70 | 11 | 1 | 8 | 45 | 5 | 0 |
| Jeong et al (2013)46 | 158 | 9 | 1 | 9 | 114 | 5 | 20 |
| Kamal et al (1997)88 | 18 | 8 | 3 | 1 | 6 | 0 | 0 |
| Lee et al (2013)89 | 21 | 1 | 0 | 3 | 17 | 0 | 0 |
| Lim et al (2007)63 | 91 | 8 | 0 | 2 | 71 | 10 | 0 |
| Lurie et al (2002)90 | 52 | 4 | 0 | 5 | 32 | 11 | 0 |
| Malata et al (1997)31 | 20 | 14 | 0 | 2 | 0 | 4 | 0 |
Abbreviations: FN, false negative; FNA, fine-needle aspiration; FP, false positive; ID, indeterminate; ND, nondiagnostic; TN, true negative; TP, true positive.
The number of each category is shown. A bullet, ●, indicates that information was not reported.
Acknowledgments
We acknowledge and thank Drs Rema Rao and Patrick Royston for their topic expertise. We also thank Dr Christopher Fundakowski for making his primary data available for analysis. J.J.S. thanks Thomas Y. Lin for support during the preparation of this manuscript.
Footnotes
Author Contributions
C. Carrie Liu, systematic search of the literature, data collection, draft of manuscript, edit and approval of final manuscript; Ashok R. Jethwa, systematic search of the literature, data collection, edit and approval of final manuscript; Samir S. Khariwala, interpretation of data, edit and approval of final manuscript; Jonas Johnson, interpretation of data, edit and approval of final manuscript; Jennifer J. Shin, study conception and design, data analysis, edit and approval of final manuscript.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
This article was accepted for presentation at the 2015 AAO-HNSF Annual Meeting &OTO EXPO; September 27–30, 2015; Dallas, Texas.
Disclosures
Competing interests: Jennifer J. Shin, receives textbook royalties from Evidence-Based Otolaryngology (Shin JJ, Randolph GW, editors; Springer, 2008) and Otolaryngology Prep and Practice (Shin JJ, Cunningham MJ, editors; Plural Publishing, 2013). She is a recipient of a Harvard Medical School Shore Foundation/Center for Faculty Development Grant and a Creating Healthcare Excellence through Education and Research Award.
Sponsorships: None.
Funding source: None.
References
- 1.Day TA, Deveikis J, Gillespie MB, et al. Salivary gland neoplasms. Curr Treat Options Oncol. 2004;5:11–26. doi: 10.1007/s11864-004-0002-x. [DOI] [PubMed] [Google Scholar]
- 2.Sungur N, Akan IM, Ulusoy MG, Ozdemir R, Kilinc H, Ortak T. Clinicopathological evaluation of parotid gland tumors: a retrospective study. J Craniofac Surg. 2002;13:26–30. doi: 10.1097/00001665-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lin CC, Tsai MH, Huang CC, Hua CH, Tseng HC, Huang ST. Parotid tumors: a 10-year experience. Am J Otolaryngol. 2008;29:94–100. doi: 10.1016/j.amjoto.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Witt RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope. 2002;112:2141–2154. doi: 10.1097/00005537-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol. 2011;136:45–59. doi: 10.1309/AJCPOIE0CZNAT6SQ. [DOI] [PubMed] [Google Scholar]
- 6.McGuirt WF, McCabe BF. Significance of node biopsy before definitive treatment of cervical metastatic carcinoma. Laryngoscope. 1978;88:594–597. doi: 10.1002/lary.1978.88.4.594. [DOI] [PubMed] [Google Scholar]
- 7.Breeze J, Andi A, Williams MD, Howlett DC. The use of fine needle core biopsy under ultrasound guidance in the diagnosis of a parotid mass. Br J Oral Maxillofac Surg. 2009;47:78–79. doi: 10.1016/j.bjoms.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Tighe D, Haldar S, Mandalia U, et al. Diagnostic investigation of parotid neoplasms: a 14 year experience of freehand fine needle aspiration cytology and ultrasound guided core needle biopsy. Br J Oral Maxillofac Surg. 2014;52:e66. doi: 10.1016/j.ijom.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Huang YC, Wu CT, Lin G, Chuang WY, Yeow KM, Wan YL. Comparison of ultrasonographically guided fine-needle aspiration and core needle biopsy in the diagnosis of parotid masses. J Clin Ultrasound. 2012;40:189–194. doi: 10.1002/jcu.20873. [DOI] [PubMed] [Google Scholar]
- 10.Haldar S, Mandalia U, Skelton E, et al. Diagnostic investigation of parotid neoplasms: a 16-year experience of freehand fine needle aspiration cytology and ultrasound-guided core needle biopsy. Int J Oral Maxillofac Surg. 2015;44:151–157. doi: 10.1016/j.ijom.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Kesse KW, Manjaly G, Violaris N, Howlett DC. Ultrasound-guided biopsy in the evaluation of focal lesions and diffuse swelling of the parotid gland. Br J Oral Maxillofac Surg. 2002;40:384–388. [PubMed] [Google Scholar]
- 12.Roussel F, Dalion J, Benozio M. The risk of tumoral seeding in needle biopsies. Acta Cytol. 1989;33:936–939. [PubMed] [Google Scholar]
- 13.Roussel F, Nouvet G. Evaluation of large-needle biopsy for the diagnosis of cancer. Acta Cytol. 1995;39:449–452. [PubMed] [Google Scholar]
- 14.Yamaguchi KT, Strong MS, Shapshay SM, Soto E. Seeding of parotid carcinoma along Vim-Silverman needle tract. J Otolaryngol. 1979;8:49–52. [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, editors. Assessment of study quality. [Accessed December 1, 2014];Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. http://handbook.cochrane.org. Published 2011.
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol. 2006;59:1331–1332. doi: 10.1016/j.jclinepi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Riley RD, Abrams KR, Sutton AJ, Lambert P, Thompson JR. The Benefits and Limitations of Multivariate Meta-analysis, with Application to Diagnostic and Prognostic Studies. Leicester, UK: University of Leicester; 2005. [Google Scholar]
- 20.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–2884. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 21.Fassnacht W, Schmit S, Weynand B, Marbaix E, Duprez T, Hamoir M. Missed malignancies in patients with assumed Warthin tumour: when should we propose conservative management?. Paper presented at: Annual Meeting of the Royal Belgian Society for Ear, Nose and Throat, Head and Neck Surgery; March 23, 2013; Brussels, Belgium. [Google Scholar]
- 22.Reddy VM, Thangarajah T, Castellanos-Arango F, Panarese A. Conservative management of warthin tumour. J Otolaryngol Head Neck Surg. 2008;37:744–749. [PubMed] [Google Scholar]
- 23.Veder LL, Kerrebijn JD, Smedts FM, den Bakker MA. Diagnostic accuracy of fine-needle aspiration cytology in Warthin tumors. Head Neck. 2010;32:1635–1640. doi: 10.1002/hed.21382. [DOI] [PubMed] [Google Scholar]
- 24.Horii A, Yoshida J, Honjo Y, Mitani K, Takashima S, Kubo T. Pre-operative assessment of metastatic parotid tumors. Auris Nasus Larynx. 1998;25:277–283. doi: 10.1016/s0385-8146(97)10041-4. [DOI] [PubMed] [Google Scholar]
- 25.Nasuti JF, Yu GH, Gupta PK. Fine-needle aspiration of cystic parotid glands lesions: an institutional review of 46 cases with histologic correlation. Cancer. 2000;90:111–116. doi: 10.1002/(sici)1097-0142(20000425)90:2<111::aid-cncr6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Nouraei SA, Hope KL, Kelly CG, McLean NR, Soames JV. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg. 2005;116:1206–1213. doi: 10.1097/01.prs.0000181654.68120.0f. [DOI] [PubMed] [Google Scholar]
- 27.Wyss E, Mueller-Garamvolgyi E, Ghadjar P, Rauch D, Zbaren P, Arnold A. Diagnosis and treatment outcomes for patients with lymphoma of the parotid gland. Laryngoscope. 2013;123:662–669. doi: 10.1002/lary.23750. [DOI] [PubMed] [Google Scholar]
- 28.Mathew S, Ali SZ. Parotid fine-needle aspiration: a cytologic study of pediatric lesions. Diagn Cytopathol. 1997;17:8–13. doi: 10.1002/(sici)1097-0339(199707)17:1<8::aid-dc2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Yoon TM, Lee JK, Lim SC. Clinical utility of fine needle aspiration cytology in pediatric parotid tumors. Int J Ped Otorhinolaryngol. 2013;77:1272–1275. doi: 10.1016/j.ijporl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK. Parotidectomy: ten-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg. 2007;136:788–792. doi: 10.1016/j.otohns.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Malata CM, Camilleri IG, McLean NR, et al. Malignant tumours of the parotid gland: a 12-year review. Br J Plast Surg. 1997;50:600–608. doi: 10.1016/s0007-1226(97)90505-1. [DOI] [PubMed] [Google Scholar]
- 32.Henrys CE, Grigg R. Use of fine-needle aspiration cytology in the diagnosis of parotid neoplasms. Aust N Z J Surg. doi: 10.1111/ans.12939. published online November 26, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj Y, Singh S, Cozens N, Sharp J. Critical clinical appraisal of the role of ultrasound guided fine needle aspiration cytology in the management of parotid tumours. J Laryngol Otol. 2005;119:289–292. doi: 10.1258/0022215054020421. [DOI] [PubMed] [Google Scholar]
- 34.Akbas Y, Tuna EU, Demireller A, Ozcan H, Ekinci C. Ultrasonography guided fine needle aspiration biopsy of parotid gland masses. Kulak Burun Bogaz Ihtis Derg. 2004;13:15–18. [PubMed] [Google Scholar]
- 35.Yerli H, Aydin E, Haberal N, Harman A, Kaskati T, Alibek S. Diagnosing common parotid tumours with magnetic resonance imaging including diffusion-weighted imaging vs fine-needle aspiration cytology: a comparative study. Dentomaxillofac Radiol. 2010;39:349–355. doi: 10.1259/dmfr/15047967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gobic MB, Pedisic D, Bekafigo IS, et al. Fine needle aspiration cytology in the evaluation of parotid gland tumors. Coll Antropol. 2010;34:345–348. [PubMed] [Google Scholar]
- 37.de Ru JA, van Leeuwen MS, van Benthem PP, Velthuis BK, Sie-Go DM, Hordijk GJ. Do magnetic resonance imaging and ultrasound add anything to the preoperative workup of parotid gland tumors? J Oral Maxillofac Surg. 2007;65:945–952. doi: 10.1016/j.joms.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Feld R, Nazarian LN, Needleman L, et al. Clinical impact of sonographically guided biopsy of salivary gland masses and surrounding lymph nodes. Ear Nose Throat J. 1999;78:905, 908–912. [PubMed] [Google Scholar]
- 39.Piccioni LO, Fabiano B, Gemma M, Sarandria D, Bussi M. Fine-needle aspiration cytology in the diagnosis of parotid lesions. Acta Otorhinolaryngol Ital. 2011;31:1–4. [PMC free article] [PubMed] [Google Scholar]
- 40.Carrillo JF, Ramirez R, Flores L, et al. Diagnostic accuracy of fine needle aspiration biopsy in preoperative diagnosis of patients with parotid gland masses. J Surg Oncol. 2009;100:133–138. doi: 10.1002/jso.21317. [DOI] [PubMed] [Google Scholar]
- 41.Atula T, Greenman R, Laippala P, Klemi PJ. Fine-needle aspiration biopsy in the diagnosis of parotid gland lesions: evaluation of 438 biopsies. Diagn Cytopathol. 1996;15:185–190. doi: 10.1002/(SICI)1097-0339(199609)15:3<185::AID-DC2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Filopoulos E, Angeli S, Daskalopoulou D, Kelessis N, Vassilopoulos P. Pre-operative evaluation of parotid tumours by fine needle biopsy. Eur J Surg Oncol. 1998;24:180–183. doi: 10.1016/s0748-7983(98)92895-5. [DOI] [PubMed] [Google Scholar]
- 43.Seethala RR, LiVolsi VA, Baloch ZW. Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck. 2005;27:217–223. doi: 10.1002/hed.20142. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger MS, Rosenberg WW, Meurer WT, Robbins KT. Fine-needle aspiration of parotid gland lesions. Head Neck. 1992;14:483–487. doi: 10.1002/hed.2880140611. [DOI] [PubMed] [Google Scholar]
- 45.Behbehani A, Dashti H, Al-Shahawi M, et al. The value of pre-operative fine-neddle aspiration biopsy in planning the management of parotid gland tumours. Med Princ Pract. 1990;2:27–34. [Google Scholar]
- 46.Jeong WJ, Park SJ, Cha W, Sung MW, Kim KH, Ahn SH. Fine needle aspiration of parotid tumors: diagnostic utility from a clinical perspective. J Oral Maxillofac Surg. 2013;71:1278–1282. doi: 10.1016/j.joms.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Arabi Mianroodi AA, Sigston EA, Vallance NA. Frozen section for parotid surgery: should it become routine? Aust N Z J Surg. 2006;76:736–739. doi: 10.1111/j.1445-2197.2006.03844.x. [DOI] [PubMed] [Google Scholar]
- 48.Bigorgne C, Royer B, Russ G, Bienvenu-Perrard M. Fine-needle aspiration cytology of parotid masses: a 5-year retrospective study of 169 cases. Acta Cytologica. 2013;57:130. [Google Scholar]
- 49.Fakhry N, Antonini F, Michel J, et al. Fine-needle aspiration cytology in the management of parotid masses: evaluation of 249 patients. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:131–135. doi: 10.1016/j.anorl.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Jafari A, Royer B, Lefevre M, Corlieu P, Perie S, St Guily JL. Value of the cytological diagnosis in the treatment of parotid tumors. Otolaryngol Head Neck Surg. 2009;140:381–385. doi: 10.1016/j.otohns.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Schelkun PM, Grundy WG. Fine-needle aspiration biopsy of head and neck lesions. J Oral Maxillofac Surg. 1991;49:262–267. doi: 10.1016/0278-2391(91)90216-9. [DOI] [PubMed] [Google Scholar]
- 52.Tew S, Poole AG, Philips J. Fine needle aspiration biopsy of parotid lesions: comparison with frozen section. Aust N Z J Surg. 1997;67:438–441. doi: 10.1111/j.1445-2197.1997.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 53.Fundakowski C, Castano J, Abouyared M, et al. The role of indeterminate fine-needle biopsy in the diagnosis of parotid malignancy. Laryngoscope. 2014;124:678–681. doi: 10.1002/lary.24341. [DOI] [PubMed] [Google Scholar]
- 54.Zafar A, Shafi M, Hassan SH, Malik S. Fine needle aspiration cytology in parotid lumps. J Pak Med Assoc. 1997;47:188–190. [PubMed] [Google Scholar]
- 55.McGuirt WF, Keyes JW, Jr, Greven KM, Williams IDW, Watson NE, Jr, Cappellari JO. Preoperative identification of benign versus malignant parotid masses: a comparative study including positron emission tomography. Laryngoscope. 1995;105:579–584. doi: 10.1288/00005537-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Newberry TR, Kaufmann CR, Miller FR. Review of accessory parotid gland tumors: pathologic incidence and surgical management. Am J Otolaryngol Head Neck Med Surg. 2014;35:48–52. doi: 10.1016/j.amjoto.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Al-Khafaji BM, Nestok BR, Katz RL. Fine-needle aspiration of 154 parotid masses with histologic correlation: ten-year experience at the University of Texas MD Anderson Cancer Center. Cancer. 1998;84:153–159. [PubMed] [Google Scholar]
- 58.Aversa S, Ondolo C, Bollito E, Fadda G, Conticello S. Preoperative cytology in the management of parotid neoplasms. Am J Otolaryngol. 2006;27:96–100. doi: 10.1016/j.amjoto.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Awan MS, Ahmad Z. Diagnostic value of fine needle aspiration cytology in parotid tumors. J Pak Med Assoc. 2004;54:617–619. [PubMed] [Google Scholar]
- 60.Berg HM, Jacobs JB, Kaufman D, Reede DL. Correlation of fine needle aspiration biopsy and CT scanning of parotid masses. Laryngoscope. 1986;96:1357–1362. doi: 10.1288/00005537-198612000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Inohara H, Akahani S, Yamamoto Y, et al. The role of fine-needle aspiration cytology and magnetic resonance imaging in the management of parotid mass lesions. Acta Otolaryngol. 2008;128:1152–1158. doi: 10.1080/00016480701827533. [DOI] [PubMed] [Google Scholar]
- 62.Iqbal M, Anwar K, Ullah I, Javed M, Ahmad Khan I, Hussain G. The diagnostic value of fine needle aspiration cytology in masses of the salivary glands. J Postgrad Med Inst. 2011;25:73–77. [Google Scholar]
- 63.Lim CM, They J, Loh KS, Chao SS, Lim LH, Tan LK. Role of fine-needle aspiration cytology in the evaluation of parotid tumours. Aust N Z J Surg. 2007;77:742–744. doi: 10.1111/j.1445-2197.2007.04222.x. [DOI] [PubMed] [Google Scholar]
- 64.Paris J, Facon F, Pascal T, Chrestian MA, Moulin G, Zanaret M. Preoperative diagnostic values of fine-needle cytology and MRI in parotid gland tumors. Eur Arch Otorhinolaryngol. 2005;262:27–31. doi: 10.1007/s00405-003-0730-8. [DOI] [PubMed] [Google Scholar]
- 65.Zbaren P, Guelat D, Loosli H, Stauffer E. Parotid tumors: fine-needle aspiration and/or frozen section. Otolaryngol Head Neck Surg. 2008;139:811–815. doi: 10.1016/j.otohns.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–420. [PubMed] [Google Scholar]
- 67.Shin JJ, Stinnett SS, Randolph GW. Evidence-based medicine in otolaryngology: part 4. Everyday probabilities: nonbinary diagnostic tests. Otolaryngol Head Neck Surg. 2013;149:179–186. doi: 10.1177/0194599813491070. [DOI] [PubMed] [Google Scholar]
- 68.Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 69.Lewis CM, Chang KP, Pitman M, Faquin WC, Randolph GW. Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid. 2009;19:717–723. doi: 10.1089/thy.2008.0425. [DOI] [PubMed] [Google Scholar]
- 70.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 71.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 72.Witt BL, Schmidt RL. Ultrasound-guided core needle biopsy of salivary gland lesions: a systematic review and meta-analysis. Laryngoscope. 2014;124:695–700. doi: 10.1002/lary.24339. [DOI] [PubMed] [Google Scholar]
- 73.Contucci AM, Corina L, Sergi B, Fadda G, Paludetti G. Correlation between fine needle aspiration biopsy and histologic findings in parotid masses: personal experience. Acta Otorhinolaryngol Ital. 2003;23:314–318. [PubMed] [Google Scholar]
- 74.Costas A, Castro P, Martin-Granizo R, Monje F, Marron C, Amigo A. Fine needle aspiration biopsy (FNAB) for lesions of the salivary glands. Br J Oral Surg. 2000;38:539–542. doi: 10.1054/bjom.2000.0465. [DOI] [PubMed] [Google Scholar]
- 75.Deneuve S, Quesnel S, Depondt J, et al. Management of parotid gland surgery in a university teaching hospital. Eur Arch Otorhinolaryngol. 2010;267:601–605. doi: 10.1007/s00405-009-1088-3. [DOI] [PubMed] [Google Scholar]
- 76.Fakhry N, Santini L, Lagier A, Dessi P, Giovanni A. Fine needle aspiration cytology and frozen section in the diagnosis of malignant parotid tumours. Int J Oral Maxillofac Surg. 2014;43:802–805. doi: 10.1016/j.ijom.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Fassnacht W, Schmitz S, Weynand B, Marbaix E, Duprez T, Hamoir M. Pitfalls in preoperative work-up of parotid gland tumours: 10-year series. B-ENT. 2013;9:83–88. [PubMed] [Google Scholar]
- 78.Javadi M, Asghari A, Hassannia F. Value of fine-needle aspiration cytology in the evaluation of parotid tumors. Indian J Otolaryngol Head Neck Surg. 2012;64:257–260. doi: 10.1007/s12070-011-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez HP, Silver CE, Moisa II, Chacho MS. Fine-needle aspiration of parotid tumors. Am J Surg. 1989;158:342–344. doi: 10.1016/0002-9610(89)90130-x. [DOI] [PubMed] [Google Scholar]
- 80.Ali NS, Nawaz A, Rajput S, Ikram M. Parotidectomy: a review of 112 patients treated at a teaching hospital in Pakistan. Asian Pac J Cancer Prev. 2010;11:1111–1113. [PubMed] [Google Scholar]
- 81.Aversa S, Ondolo C, Bollito E, Fadda G, Conticello S. Preoperative cytology in the management of parotid neoplasms. Am J Otolaryngol Head Neck Med Surg. 2006;27:96–100. doi: 10.1016/j.amjoto.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 82.Balakrishnan K, Castling B, McMahon J, et al. Fine needle aspiration cytology in the management of a parotid mass: a two centre retrospective study. Surgeon. 2005;3:67–72. doi: 10.1016/s1479-666x(05)80064-2. [DOI] [PubMed] [Google Scholar]
- 83.Behzatoglu K, Bahadir B, Kaplan HH, Yucel Z, Durak H, Bozkurt ER. Fine needle aspiration biopsy of the parotid gland: diagnostic problems and 2 uncommon cases. Acta Cytologica. 2004;48:149–154. doi: 10.1159/000326308. [DOI] [PubMed] [Google Scholar]
- 84.Bono A, Chiesa F, Sala L, Azzarelli A, Pilotti S, Di Pietro S. Fine-needle aspiration biopsy in parotid masses. Tumori. 1983;69:417–421. doi: 10.1177/030089168306900509. [DOI] [PubMed] [Google Scholar]
- 85.Califano L, Zupi A, Giardino C. Accuracy in the diagnosis of parotid tumours. J Craniomaxillofac Surg. 1992;20:354–359. doi: 10.1016/s1010-5182(05)80365-8. [DOI] [PubMed] [Google Scholar]
- 86.Deans GT, Briggs K, Spence RA. An audit of surgery of the parotid gland. Ann R Coll Surg Engl. 1995;77:188–192. [PMC free article] [PubMed] [Google Scholar]
- 87.Gooden E, Witterick IJ, Hacker D, Rosen IB, Freeman JL. Parotid gland tumours in 255 consecutive patients: Mount Sinai Hospital’s quality assurance review. J Otolaryngol. 2002;31:351–354. doi: 10.2310/7070.2002.34394. [DOI] [PubMed] [Google Scholar]
- 88.Kamal SA, Othman EO. Diagnosis and treatment of parotid tumours. J Laryngol Otol. 1997;111:316–321. doi: 10.1017/s0022215100137211. [DOI] [PubMed] [Google Scholar]
- 89.Lee DH, Yoon TM, Lee JK, Lim SC. Clinical utility of fine needle aspiration cytology in pediatric parotid tumors. Int J Pediatr Otorhinolaryngol. 2013;77:1272–1275. doi: 10.1016/j.ijporl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Lurie M, Misselevitch I, Fradis M. Diagnostic value of fine-needle aspiration from parotid gland lesions. Isr Med Assoc J. 2002;4:681–683. [PubMed] [Google Scholar]
- 91.Mohammed F, Asaria J, Payne RJ, Freeman JL. Retrospective review of 242 consecutive patients treated surgically for parotid gland tumours. J Otolaryngol Head Neck Surg. 2008;37:340–346. [PubMed] [Google Scholar]
- 92.Riley N, Allison R, Stevenson S. Fine-needle aspiration cytology in parotid masses: our experience in Canterbury, New Zealand. Aust N Z J Surg. 2005;75:144–146. doi: 10.1111/j.1445-2197.2005.03331.x. [DOI] [PubMed] [Google Scholar]
- 93.Shashinder S, Tang IP, Velayutham P, et al. A review of parotid tumours and their management: a ten-year-experience. Med J Malaysia. 2009;64:31–33. [PubMed] [Google Scholar]
- 94.Takashima S, Takayama F, Wang Q, Kurozumi M, Sekiyama Y, Sone S. Parotid gland lesions: diagnosis of malignancy with MRI and flow cytometric DNA analysis and cytology in fine-needle aspiration biopsy. Head Neck. 1999;21:43–51. doi: 10.1002/(sici)1097-0347(199901)21:1<43::aid-hed6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 95.Tew S, Poole AG, Philips J. Fine-needle aspiration biopsy of parotid lesions: comparison with frozen section. Aust N Z J Surg. 1997;67:438–441. doi: 10.1111/j.1445-2197.1997.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 96.Tsai SC, Hsu HT. Parotid neoplasms: diagnosis, treatment, and intraparotid facial nerve anatomy. J Laryngol Otol. 2002;116:359–362. doi: 10.1258/0022215021911013. [DOI] [PubMed] [Google Scholar]
- 97.van Lierop AC, Fagan JJ. Parotidectomy in Cape Town: a review of pathology and management. S Afr J Surg. 2007;45:96–98. 100, 102–103. [PubMed] [Google Scholar]
- 98.Zurrida S, Alasio L, Tradati N, Bartoli C, Chiesa F, Pilotti S. Fine-needle aspiration of parotid masses. Cancer. 1993;72:2306–2311. doi: 10.1002/1097-0142(19931015)72:8<2306::aid-cncr2820720804>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]