The emergence of ‘bath salts’ products containing synthetic cathinones represents a global public health threat. Synthetic cathinones have significant abuse potential, and the effects of high-dose administration can be life-threatening. More preclinical and human research is needed to understand the complex pharmacology and toxicology of these substances.
‘BATH SALTS’ PRODUCTS CONTAIN SYNTHETIC CATHINONES
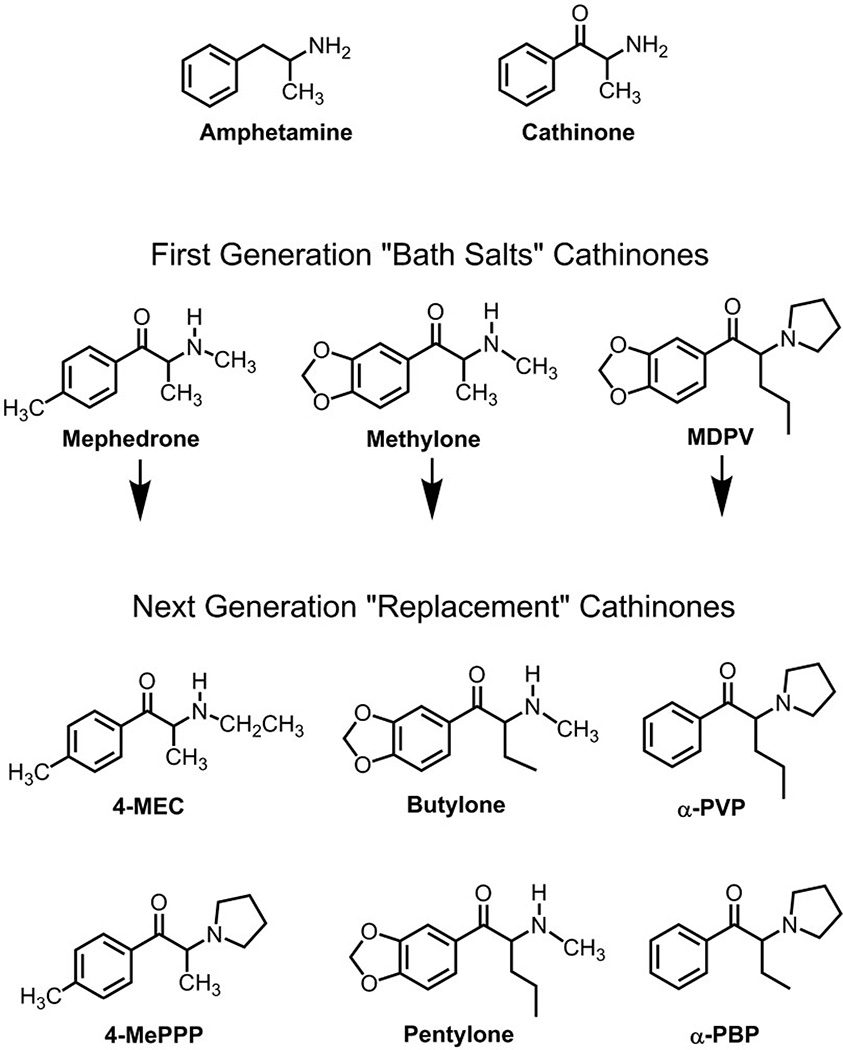
During the past several years, there has been a dramatic rise in the abuse of so-called ‘bath salts’ products that are purchased as alternatives to illicit drugs such as cocaine and 3,4-methylenedioxymethamphetamine [1]. Bath salts are purposely mislabeled and have no use as bath additives; instead, these products contain synthetic analogs of cathinone, an amphetamine-like stimulant found in the khat plant Catha edulis. The marketing of bath salts and related products (e.g. ‘research chemicals’) via the internet has fostered the widespread availability of synthetic cathinones on a global scale. Most bath salts powders are administered intranasally or orally, although some users self-administer by the intravenous route. Clinical evidence indicates that recreational doses of bath salts enhance mood and increase alertness, while high doses or chronic use can lead to serious medical complications, including psychosis, hyperthermia, tachycardia and sometimes death [2,3]. Figure 1 shows three cathinones found commonly in bath salts products: 4-methyl-N-methylcathinone (mephedrone), 3,4-methylenedioxy-N-methylcathinone (methylone) and 3,4-methylenedioxypyrovalerone (MDPV). MDPV is the main substance detected in blood and urine from patients hospitalized for bath salts overdose in the United States, whereas mephedrone is associated more commonly with adverse clinical outcomes in Europe [2,3]. Due to public health risk posed by bath salts, the governments of many countries, including the United States, have passed legislation banning the sale, possession and use of mephedrone, methylone and MDPV [4,5].
Figure 1.
Chemical structures of‘bath salts’ cathinones and their next-generation ‘replacement’ analogs. First-generation ‘bath salts’ cathinones are mephedrone, methylone and MDPV, whereas next-generation ‘replacement’ cathinones are those that appeared in the recreational drug market-place after the 2011 emergency scheduling legislation was enacted in the United States. Mephedrone = 4-methyl-N-methylcathinone; methylone = 3,4-methylenedioxy-N-methylcathinone; MDPV = 3,4-methylenedioxypyrovalerone; 4-MEC = 4-methyl-N-ethylcathinone; 4-MePPP = 4-methyl-α-pyrrolidinopropiophenone; butylone = β-keto-N-methylbenzodioxolylbutanamine; pentylone = β-keto-methylbenzodioxolylpentanamine; α-PVP = α-pyrrolidinovalerophenone; α-PBP = α-pyrrolidinobutiophenone
SYNTHETIC CATHINONES TARGET MONOAMINE TRANSPORTERS
Like other stimulant drugs, synthetic cathinones exert their effects by disrupting the function of monoamine transporter proteins expressed on neurons in the central and peripheral nervous systems. Monoamine transporters normally function to translocate neurotransmitter molecules from the extracellular space back into the neuronal cytoplasm. Drugs affecting transporters can be divided into two basic types: (i) amphetamine-like releasing agents and (ii) cocaine-like uptake blockers. Both types of drugs increase the extracellular concentrations of monoamines. Using in-vitro assays, we and others have found that mephedrone and methylone are transporter releasing agents that stimulate efflux of dopamine, norepinephrine and serotonin [6,7]. MDPV is structurally distinct from other cathinones (see Fig. 1), and this drug acts as a potent blocker at transporters for dopamine and norepinephrine [7,8]. In-vivo microdialysis studies in rats show that mephedrone and methylone increase extracellular concentrations of dopamine and serotonin in brain reward pathways, whereas MDPV increases extracellular dopamine without affecting serotonin. The fact that synthetic cathinones stimulate dopamine transmission predicts that bath salts have high abuse liability. Consistent with this notion, studies in rats demonstrate that mephedrone, methylone and MDPV stimulate loco-motor activity and are readily self-administered [6,8–10]. No controlled laboratory-based investigations have been carried out to examine the pharmacology of bath salts constituents in human subjects, and such studies are needed.
LEGISLATIVE BANS CAN HAVE UNINTENDED CONSEQUENCES
A major goal of the legislative ban on synthetic cathinones is to curtail their sale and use, and this strategy might beworking. Since mephedrone, methylone and MDPV were placed into Schedule I control in the United States, reports of bath salts exposures to poison control centers have decreased from 6137 in 2011 to 995 in 2013 [11]. Nevertheless, drug scheduling has a number of unintended consequences. Once synthetic cathinones are rendered illegal, legitimate biomedical research with these substances is prohibited unless investigators have the appropriate licensing and infrastructure to work with scheduled drugs. By definition, Schedule I substances in the United States have no medical value, yet the therapeutic potential of most synthetic cathinones has not been examined, despite the fact that Food and Drug Administration (FDA)-approved cathinone-related medications such as bupropion are widely prescribed [12]. Finally, clandestine chemists have responded to drug scheduling by synthesizing an endless array of new ‘replacement’ cathinones [13,14], and this trend is expected to continue. Figure 1 illustrates examples of replacement cathinones that have appeared in the recreational drug market-place since emergency scheduling of mephedrone, methylone and MDPV in 2011. Importantly, the pharmacology and toxicology of these newer synthetic cathinones are completely unknown. As pointed out by Glennon [15], synthetic cathinones represent a heterogeneous group of compounds, and their biological activity cannot be inferred from existing data but must be determined on a case-by-case basis.
SYNTHETIC CATHINONES PRODUCE DANGEROUS ADVERSE EFFECTS
The emergence of bath salts has placed a significant burden on health-care professionals, especially those providing emergency medical care [1–3]. Most cases of overdose from bath salts are reported to poison control centers and subsequently treated in hospital emergency departments [2,3]. As noted above, symptoms of severe bath salts intoxication include psychosis, hyperthermia and tachycardia, which can be accompanied by combative or violent behaviors; thus, subduing and treating such patients can be a harrowing experience for hospital staff. Perhaps the most dangerous syndrome produced by bath salts is ‘excited delirium’, in which the patient displays extreme agitation, delirium and hyperthermia in conjunction with rhabdomyolysis and ensuing kidney failure [16,17]. Treatment is mostly supportive, with benzodiazepines for agitation and excessive sympathetic stimulation, and aggressive cooling for hyperthermia. At the present time, synthetic cathinones are not detected by routine toxicology screens, so analytical confirmation of bath salts exposure is often impossible. Sophisticated forensic methods for the detection of synthetic cathinones are being reported [18], but such methods are not readily available in most clinical settings. Unfortunately, forensic toxicologists are faced with the prospect of continually developing new analytical methods to keep pace with the appearance of new replacement cathinone analogs.
SUMMARY
To conclude, the emergence of synthetic cathinones as drugs of abuse is a serious problem which negatively impacts global public health. Activation of central dopamine systems by synthetic cathinones portends a substantial risk for addiction, and patients exposed to high doses of these substances can experience life-threatening medical complications. Banning specific synthetic cathinones may be a viable law enforcement strategy, but this approach hinders the critical research that is needed to understand the basic pharmacology and toxicology of cathinone analogs. Given the growing list of new replacement cathinones, it seems likely that health-care professionals, forensic toxicologists and biomedical researchers will continue to face formidable challenges in dealing with this unfolding drug abuse phenomenon.
Acknowledgments
Declaration of interests
M.H.B. is funded by the Intramural Research Program of the National Institute on Drug Abuse, USA.
References
- 1.Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of ‘bath salts’ and ‘legal highs’ (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 3.Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test Anal. 2011;3:454–463. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- 4.Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76:65371–65375. [PubMed] [Google Scholar]
- 5.Drug Enforcement Administration, Department of Justice. Establishment of drug codes for 26 substances. Final rule. Fed Regist. 2013;78:664–666. [PubMed] [Google Scholar]
- 6.Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sci. 2014;97:27–30. doi: 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watterson LR, Watterson E, Olive MF. Abuse liability of novel ‘legal high’ designer stimulants: evidence from animal models. Behav Pharmacol. 2013;24:341–355. doi: 10.1097/FBP.0b013e3283641ec8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Association of Poison Control Centers. [accessed 28 May 2014];Bath Salts. 2014 Available at: http://www.aapcc.org/alerts/bath-salts/ (Archived at http://www.webcitation.org/query?url=http%3A%2F%2Fwww.aapcc.org%2Falerts%2Fbath-salts%2F&date=2014-05-28) [Google Scholar]
- 12.Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectroscopy. J Anal Toxicol. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- 14.Leffler AM, Smith PB, de Armas A, Dorman FL. The analytical investigation of synthetic street drugs containing cathinone analogs. Forensic Sci Int. 2014;234:50–60. doi: 10.1016/j.forsciint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Glennon RA. Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. Adv Pharmacol. 2014;69:581–620. doi: 10.1016/B978-0-12-420118-7.00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive ‘bath salts’ intoxication with methylenedioxypyrovalerone. Am J Med. 2012;125:854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts) Gen Hosp Psychiatry. 2012;34:647–650. doi: 10.1016/j.genhosppsych.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Concheiro M, Anizan S, Ellefsen K, Huestis MA. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal Bioanal Chem. 2013;405:9437–9448. doi: 10.1007/s00216-013-7386-z. [DOI] [PubMed] [Google Scholar]