Abstract
Autism Spectrum Disorder (ASD) is a heterogeneous neurodevelopmental disorder with core symptoms of atypical social interactions and repetitive behaviors. It has also been reported that individuals with ASD have difficulty with multisensory integration, and this may disrupt higher-order cognitive abilities such as learning and social communication. Impairments in the integration of sensory information could in turn reflect diminished cross-modal white matter connectivity. Moreover, the genetic contribution in ASD appears to be strong, with heritability estimates as high as 90%. However, no single gene has been identified, and over 1,000 risk genes have been reported. One of these genes -- contactin-associated-like-protein 2 (CNTNAP2) -- was first associated with Specific Language Impairment, and more recently has been linked to ASD. CNTNAP2 encodes a cell adhesion protein regulating synaptic signal transmission. To better understand the behavioral and biological underlying mechanisms of ASD, a transgenic mouse model was created with a genetic knockout (KO) of the rodent homolog Cntnap2. Initial studies on this mouse revealed poor social interactions, behavioral perseveration, and reduced vocalizations -- all strongly resembling human ASD symptoms. Cntnap2 KO mice also show abnormalities in myelin formation, consistent with a hypo-connectivity model of ASD. The current study was designed to further assess the behavioral phenotype of this mouse model, with a focus on learning and memory. Cntnap2 KO and wild-type mice were tested on a 4/8 radial arm water maze for 14 consecutive days. Error scores (total, working memory, reference memory, initial and repeated reference memory), latency and average turn angle were independently assessed using a 2 × 14 repeated measures ANOVA. Results showed that Cntnap2 KO mice exhibited significant deficits in working and reference memory during the acquisition period of the task. During the retention period (i.e., after asymptote in errors), Cntnap2 KO mice performed comparably to wild-type mice. These findings suggest that CNTNAP2 may influence the development of neural systems important to learning and cross-modal integration, and that disruption of this function could be associated with delayed learning in ASD.
Keywords: Cntnap2, Autism Spectrum Disorder (ASD), Working memory, Spatial memory, Water maze, Neurodevelopment, Genetics, Mouse models
BACKGROUND
Autism Spectrum Disorder (ASD) is a set of neurodevelopmental disorders characterized by a complex behavioral phenotype, encompassing deficits in both social and cognitive domains. Accepted core symptoms are heterogeneous ranging from atypical social interactions and language impairments to repetitive behaviors. Accordingly, individual cases vary substantially in severity and presentation of symptoms. The current estimated prevalence for ASD in the United States is 1 in 68, and is consistently more prevalent in boys than girls (1 in 42 boys versus 1 in 189 girls) (Baio, 2010; Elsabbagh et al., 2012). To date, causal mechanisms underlying ASD remain poorly understood, but likely include a complex combination of polygenic and environmental risk factors (Moreno-De-Luca, 2013).
Ongoing ASD research has focused on the genetic and neurobiological mechanisms of ASD, based on the notion that characterization of the varied neurogenetic features of ASD could provide insight to the diverse behavioral symptoms. The genetic contribution in ASD appears to be strong; for example, monozygotic twin studies estimate the concordance rates are as high as 70%–90% (Bailey et al., 1995; Steffenburg et al., 1989; Rosenberg et al., 2009). However, the relative proportion of ASD that can be accounted for by either rare or common genetic variation remains to be determined, and no single gene has been identified as a major cause. In fact, over 1,000 risk genes have been reported, pointing to a very complex genetic etiology (De Rubeis & Buxbaum, 2015).
One of the autism susceptibility candidate genes -- contactin-associated-like-protein 2 (CNTNAP2) -- was first linked to Specific Language Impairment, and more recently has been linked to ASD (Alarcón et al., 2008; Arking et al., 2008). CNTNAP2 has also been linked to other complex neurological disorders such as schizophrenia, dyslexia and depression in genome-wide association studies (Vernes et al., 2008; Newbury et al., 2011; Peter et al., 2011; Ji et al., 2013). Thus CNTNAP2 mutations could underlie similar endophenotypes across various disorders. In clinically language-impaired populations, CNTNAP2 variants have been associated with difficulties with non-word repetition -- a measure of working memory that critically underlies language and social cognition (Vernes et al., 2008; Peter et al., 2011). Further studies have highlighted significant association between specific SNPs in CNTNAP2 and language endophenotypes of ASD including age at first word (Alarcon et al., 2008) and age at first phrase (Anney et al., 2012).
CNTNAP2 is located on chromosome 7, and is responsible for encoding a cell adhesion protein regulating synaptic signal transmission (Alarcón et al., 2008).To better understand the behavioral and biological underlying mechanisms of ASD, a transgenic mouse model was created with a genetic knockout (KO) of the rodent homolog Cntnap2 (Poliak et al., 2003). Initial behavioral studies of this mouse revealed poor social interactions, perseveration, and reduced pup vocalizations -- all strongly resembling human ASD symptoms (Peñagarikano et al., 2011; Penagarikano & Geschwind 2012). CNTNAP2’s role in neurodevelopment has been further studied using this mouse model, revealing that Cntnap2 KO mice show abnormalities in myelin formation -- consistent with a hypo-connectivity model of ASD (Poliak et al., 2003). These mice also exhibit abnormal cortical neural synchrony (i.e., enhanced asynchrony), fewer inter-neurons (which are mostly inhibitory), and atypical neuronal migration (Peñagarikano et al., 2011). All of these cellular anomalies can be linked to current biological theories for mechanisms of ASD. More recent studies from our lab revealed that the KO mice exhibit unexpected enhancements in acoustic frequency processing, despite impairments on more complex silent gap detection tasks (Truong et al., 2015). The latter results have been linked with anomalies at the level of the thalamus, and also could also reflect atypical patterns of cortical connectivity.
The current study was designed to further assess the behavioral phenotype of the Cntnap2 KO mouse model, with a focus on putative anomalies in spatial learning and memory. Specifically, impairments in working memory have been noted in individuals with ASD, and these deficits are more pronounced when the task load is high (Barendse et al., 2013). It is also important to note that although most of these working memory impairments in ASD are found in the spatial domain (e.g. Lind et al., 2013), they have also been observed in complex verbal working memory tasks (Schuh & Eigsti, 2012; Steele et al., 2007; Luna et al., 2007; Williams, Goldstein, & Minshew, 2005; Willims et al., 2005). Previous studies investigating the Cntnap2 KO mice, however, found similar learning rates on the Morris Water maze task for KOs versus WT controls. This result suggests a lack of spatial learning and memory impairments (Peñagarikano et al., 2011). However, when presented with a maze reversal task, Cntnap2 KOs did show significant impairments in learning the new platform location (Peñagarikano et al., 2011). These results reinforce the notion that difficulty of task may play a role in inconsistent findings for memory deficits associated with ASD. Our goal was to further assess Cntnap2 KOs spatial memory ability utilizing a more difficult 4/8 arm radial water maze task. This task also allows for the analysis of both reference and working memory, while introducing a higher cognitive load (compared to Morris Water maze, with only one platform). Finally, this task generates a more extended learning curve, allowing us to adequately evaluate performance during acquisition and retention periods separately.
MATERIALS AND METHODS
Subjects
10 Cntnap2 KO mice (B6.129(Cg)-Cntnap2tm1Pele/J; stock number 017482) and 11 wild type (WT) controls (C57BL/6J; stock number 000664) were obtained from The Jackson Laboratory (Bar Harbor, ME)1. 1 Subjects were delivered to the University of Connecticut, Department of Psychology at 7 weeks of age. Upon arrival, subjects were single housed in standard plexiglass laboratory cages (12:12 light/dark cycle) with food and water available ad lib. Only male subjects were used for testing, based on evidence of a higher incidence of ASD and developmental language impairments in males as compared to females (Baio, 2012). Maze testing began when the animals were around 24 weeks of age, and occurred during the subjects’ light cycle. All procedures were performed blind to subject genotype and were conducted in compliance with the National Institutes of Health and approved by the University of Connecticut’s Institutional Animal Care and Use Committee (IACUC).
Water maze assessment – Visible platform and 4/8 radial water maze
Subjects were initially tested on a visible platform control task (also known as “water escape”) prior to the 4/8 radial water maze task, to evaluate any underlying impairments that might confound further maze testing (i.e., deficits in motivation, swimming, or visual acuity). Subjects were placed in the far end of an oval tub (103 cm × 55.5 cm) filled with room temperature water, and given 45 seconds to swim to a visible escape platform (8.5 cm in diameter; 1 cm above water surface) located at the opposite end of the tub. Latencies to the visual platform were recorded for assessment. None of the subjects displayed any impairments, and there were no observed differences between genotypes on this task. We therefore proceeded to testing on the water version of the 4/8 radial arm maze (adapted from Hyde, Hoplight & Denenberg, 1998).
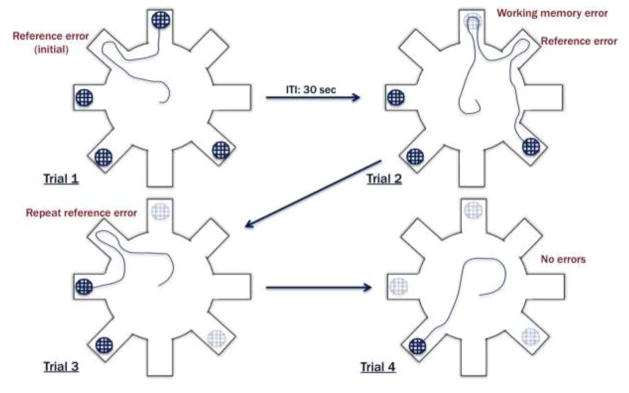
The 4/8 radial arm water maze assesses spatial reference and working memory abilities simultaneously, using a standard 8 arm radial maze with 4 arms containing a submerged goal (escape) platform, and 4 open arms that never contain a platform (Fig. 1). Configuration of goal arms were counterbalanced between subjects, but remained fixed for each subject across all test sessions. Additionally, high contrast extra maze cues were present in the room, and the locations of these remained static for the entire experiment.
Figure 1.
A schematic of the 4/8 radial arm maze and the categorization of memory errors used to evaluate all subjects.
The day prior to testing (Day 1), subjects were given a training session where all arms that would never contain a platform were blocked, forcing the animals to only enter arms containing a platform. Subjects were placed in the middle of the maze and were given 120 seconds to locate a platform. Every subject completed 4 training trials. Each time they found a platform, the recently located platform was removed, and the entrance to that arm was blocked. This ensured that the subject could no longer enter this arm for the remainder of the training session. If the subject failed to find a platform in this time-period, they were guided to the nearest available goal. Once on the platform, subjects remained for 20 seconds and then were removed to their home cage (30 second inter-trial interval; ITI).
Testing began on Day 2 and continued for 14 consecutive days. The testing session followed training procedures, except instead of blocking the goal arm of the most recently located platform, the platform was simply removed during the 30 second ITI. This arm remained open for the remainder of the test session, but contained no escape. Test sessions were recorded using a Sony camera, integrated with the SMART video-tracking program (Panlab, Barcelona, Spain). An arm entry was counted for a subject when all four paws entered an arm. Three types of errors were quantified for analysis: 1) Working memory errors (the number of initial and repeat entries into arms from which a platform had been removed during a testing session on a given day); 2) Initial reference memory errors (the total number of first entries into arms that never contained a goal platform) and; 3) Repeat reference memory incorrect errors (the total number of repeat entries (following the initial entry) into arms that never contained escape platforms). Total errors per test session in each category were tabulated, averaged within Genotype, and used for analysis across days of testing.
Finally, in order to determine whether subjects utilized a spatial or chaining (swimming to successive adjacent arms) strategy to solve the water maze, angles of arm choices were derived and analyzed. Specifically, video tracking data obtained from the SMART system was reviewed, and turn angle entry was calculated to determine the average turn angle utilized across sessions. Lower turn-angle averages (closer to 45°) suggest that subjects preferred adjacent arm choices to solve the maze. Alternatively, higher averages (around 90° and greater) suggest a preference for more spatial strategies to solve the maze.
Statistical Analysis
A univariate ANOVA was conducted to compare latencies as a function of Genotype. Average total, working memory, total reference memory, initial reference memory, and repeated reference memory errors, and average turn angle, were independently assessed using a 2 × 14 repeated measures ANOVA. Genotype (2 levels: WT and Cntnap2 KO) as the between measure, and Days (14 levels) served as the within measure. A one-way ANOVA was also conducted to assess significance between genotypes of each day of testing for all error types. Some analyses also were performed as a function of test periods, as defined by Acquisition (days 1–7) and Retention (days 8–14) portions of the learning curve.
RESULTS
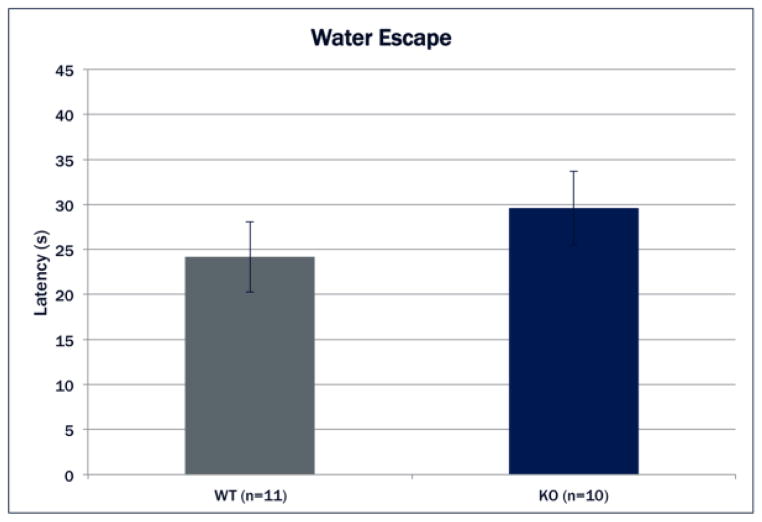
Water Escape
A univariate ANOVA found no main effect of Genotype [F(1,19)=.915, N.S.]. Thus no subjects showed any impairment that might confound a swim-task, and all 10 Cntnap2 KO and 11 WT mice advanced to the testing sessions (Fig. 2).
Figure 2.
Mean latency to platform in the water escape task (+SEM). There were no significant differences between Genotypes on latency to platform indicating no underlying motor or visual impairments.
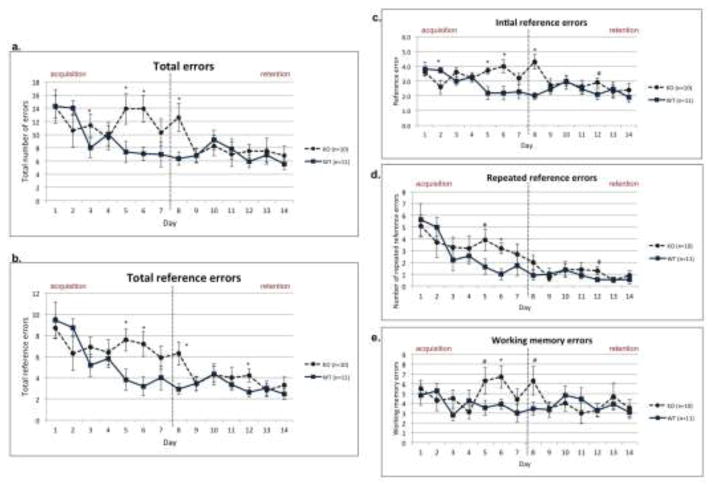
Total errors
The 4/8 radial arm water maze was used to simultaneously measure spatial working and reference memory performance. Analysis of the average number of total errors (working memory, initial reference, and repeated reference memory errors) revealed a significant difference between WT and Cntnap2 KO groups [F(1,19)=4.791, p<0.05] via repeated measures ANOVA, with Cntnap2 KOs making significantly more errors than WTs. A main effect of Day [F(13,247)=4.036, p<.001] was also observed, confirming that both groups reduced errors across days (i.e., showed learning). Within test session an analysis of total errors across days revealed a Day × Genotype interaction [F(13,247)=1.886, p<0.05], with Cntnap2 KOs making significantly more errors during the Acquisition period of testing (days 1–7 of testing) [F(1,19)=5.332, p<.05], but performing comparably to WTs during the Retention period (days 8–14 of testing) [F(1,19)=1.846, N.S.] (Fig. 3a). A one-way ANOVA was conducted to look at differences between genotypes at each day [Day 1: F(1,19)=.001, p>0.05; Day 2: F(1,19)=1.620, p>0.05; Day 3: F(1,19)=4.374, p<0.05; Day 4: F(1,19)=.056, p>0.05; Day 5: F(1,19)=5.498, p<0.05; Day 6: F(1,19)=9.537, p<0.05; Day 7: F(1,19)=1.372, p>0.05; Day 8: F(1,19)=7.374, p<0.05; Day 9: F(1,19)=.003, p>0.05; Day 10: F(1,19)=.177, p>0.05; Day 11: F(1,19)=.118, p>0.05; Day 12: F(1,19)=1.276, p>0.05; Day 13: F(1,19)=.074, p>0.05; Day 14: F(1,19)=.588, p>0.05].
Figure 3.
(A) Total number of errors in the 4/8 arm radial water maze task over 14 days of testing (+SEM). Analysis of the average number of total errors (working memory, initial reference, and repeated reference memory errors) revealed a significant difference between WT and Cntnap2 KO groups, with Cntnap2 KOs making more errors. (B) Total number of reference memory errors (+SEM) in the 4/8 arm radial water maze task over 14 days of testing. Analyses revealed that Cntnap2 KOs did make significantly more errors than WT subjects. (C) Total number of initial reference memory errors (+SEM) in the 4/8 arm radial water maze task over 14 days of testing. Cntnap2 KOs made significantly more initial reference memory errors. (D) Total number of repeated reference memory errors (+SEM) in the 4/8 arm radial water maze task over 14 days of testing. Cntnap2 KOs made more repeated reference memory errors across the 14 days of testing but overall performance was comparable between KOs and WTs. (E) Total number of working memory errors (+SEM) in the 4/8 arm radial water maze task over 14 days of testing. Cntnap2 KOs made significantly more working memory errors specifically during the Acquisition period. * p<.05, # p<.10.
Reference Memory
We examined the group differences for four different performance error types including working memory, initial reference memory, repeated reference memory, and total repeated reference memories (METHODS, Fig. 1). A repeated measures ANOVA on total reference memory errors (across Days) revealed that Cntnap2 KOs did in fact make significantly more errors than WT subjects [F(1,19)=4.514, p<0.05]. As seen with total errors, there was also a Day x Genotype interaction [F(1,19)=4.514, p<.05], wherein the Cntnap2 KOs made significantly more errors during the Acquisition period [F(1,19) = 3.305, p<0.05], but performed comparably to the WTs during the Retention period [F(1,19)=2.902, p>0.05] (Fig. 3b). A one-way ANOVA was conducted to look at differences between genotypes at each day [Day 1: F(1,19)=.139, p>0.05; Day 2: F(1,19)=1.878, p>0.05; Day 3: F(1,19)=1.451, p>0.05; Day 4: F(1,19)=.160, p>0.05; Day 5: F(1,19)=6.713, p<0.05; Day 6: F(1,19)=7.680, p<0.05; Day 7: F(1,19)=1.421, p>0.05; Day 8: F(1,19)=8.352, p<0.05; Day 9: F(1,19)=.003, p >0.05; Day 10: F(1,19)=.003, p>0.05; Day 11: F(1,19)=.318, p>0.05; Day 12: F(1,19)=4.494, p<0.05; Day 13: F(1,19)=.048, p>0.05; Day 14: F(1,19)=.880, p>0.05].
Further analysis of reference memory error type also revealed that Cntnap2 KOs made significantly more initial reference memory errors [F(1,19)=5.522, p<.05] (Fig. 3c). A one-way ANOVA revealed the following statistics at each day [Day 1: F(1,19)=.158, p>0.05; Day 2: F(1,19)=5.600, p<0.05; Day 3: F(1,19)=1.947, p >0.05; Day 4: F(1,19)=.033, p>0.05; Day 5: F(1,19)=8.913, p<0.05; Day 6: F(1,19)=7.902, p<0.05; Day 7: F(1,19)=2.533, p>0.05; Day 8: F(1,19) = 18.736, p<0.05; Day 9: F(1,19)=.195, p>0.05; Day 10: F(1,19)=.032, p >0.05; Day 11: F(1,19)=.068, p >0.05; Day 12: F(1,19)=2.987, p>0.05; Day 13: F(1,19)=.051, p>0.05; Day 14: F(1,19)=.766, p>0.05].
Cntnap2 KOs also made more repeated reference memory errors across the 14 days of testing, but there was no significant main effect of Genotype [F(1,19) = 3.040, N.S] (Fig. 3d). A one-way ANOVA was conducted to look at differences between genotypes at each day [Day 1: F(1,19)=.102, p>0.05; Day 2: F(1,19)=.793, p>0.05; Day 3: F(1,19)=.820, p>0.05; Day 4: F(1,19)=.287, p>0.05; Day 5: F(1,19)=4.131, p<0.10 ; Day 6: F(1,19)=4.935, p<0.05; Day 7: F(1,19)=.635, p>0.05; Day 8: F(1,19)=2.327, p>0.05; Day 9: F(1,19)=.248, p>0.05; Day 10: F(1,19)=.002, p>0.05; Day 11: F(1,19)=.554, p>0.05; Day 12: F(1,19)=3.822, p<0.10; Day 13: F(1,19)=.022, p>0.05; Day 14: F(1,19)=.529, p>0.05].
Working Memory
A repeated measures ANOVA on working memory errors revealed that Cntnap2 KOs made significantly more working memory errors, specifically during the Acquisition period [F(1,19)=4.560, p<.05]. However, they performed comparably to WTs during the Retention period of the task [F(1,19)=.257, p>0.05] (Fig. 3e). Another one-way ANOVA was conducted to look at differences between genotypes at each day [Day 1: F(1,19)=.249, p>0.05; Day 2: F(1,19)=.573, p>0.05; Day 3: F(1,19)=2.556, p>0.05; Day 4: F(1,19)=.782, p>0.05; Day 5: F(1,19)=3.243, p<0.10; Day 6: F(1,19)=5.202, p<0.05; Day 7: F(1,19)=1.003, p>0.05; Day 8: F(1,19)=3.403, p<0.10; Day 9: F(1,19)=.019, p>0.05; Day 10: F(1,19)=.417, p>0.05; Day 11: F(1,19)=.860, p>0.05; Day 12: F(1,19)=.001, p>0.05; Day 13: F(1,19)=.310, p>0.05; Day 14: F(1,19)=.139, p>0.05].
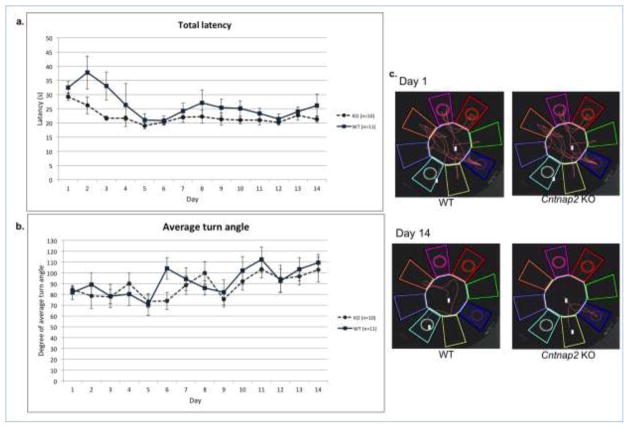
Latency
Total latency across the 4 trials was computed, and a repeated measures ANOVA was performed to analyze Genotype and Day differences (as above). This revealed no significant difference of total latency to the platform during testing sessions, when comparing Cntnap2 KOs and WTs [F(1,19) = 2.842, p>0.05]. There was, however, a main effect of Day, indicating both groups were completing the task more quickly as testing progressed (Fig. 4a).
Figure 4.
(A) Total latency (+SEM) over testing sessions in the 4/8 arm radial water maze task over 14 days of testing. There was no significant difference of total latency to the platform during testing sessions, when comparing Cntnap2 KOs and WTs. (B) Average turn angle (+SEM) over testing sessions in the 4/8 arm radial water maze task over 14 days of testing. There were no significant differences between Genotypes. (C) Examples of swimming tracks from a WT and Cntnap2 KO mouse on testing day 1 and 14.
Average Turn angle
Average turn angle per testing session was recorded and analyzed to assess possible differences in strategies used to complete the task. A repeated measures ANOVA revealed no main effect of Genotype [F(1,19)=.343, p>0.05], but did reveal a significant Day effect [F(13,246)=2.856, p<.05]. Overall, subjects used shorter turn angles during the beginning of testing, but as testing continued, subjects used wider turn angles (indicating more selective arm choices; Fig. 4b).
DISCUSSION
Cntnap2 KO and wild-type mice were tested on a 4/8 radial arm water maze for 14 consecutive days. Results showed that Cntnap2 KO mice exhibited significant deficits in spatial working and reference memory as indicated by higher numbers of errors, specifically during the acquisition period of the task. However, during the retention period (i.e., after an asymptote in errors), Cntnap2 KO mice performed comparably to wild-type mice. These findings indicate that the mutant animals are able to learn, but exhibit delayed learning -- resulting in a different learning curve. It is important to note the differences between Cntnap2 KOs and WTs are particularly robust on days 5 through 8. This likely reflects the floor effect that occurs the first few days of testing, when none of the animals perform substantially better than chance. During day 5–8, however (when WTs begin to learn and improve on the task), Cntnap2 KOs do not appear to learn as quickly as WTs, and continue to exhibit relatively high number of errors during this time period. Importantly, KOs do display some improvement, but not as robust as WTs. This would suggest that once the Cntnap2 KOs begin to learn the platform location they are perseverating on these locations within a testing session. As Cntnap2 KOs are learning the platform locations, they struggling to learn which arms were already visited during the testing session and therefore making more working memory errors. Furthermore, Cntnap2 KO mice and WT mice displayed similar turn angles throughout testing, suggesting they used similar strategies to complete the maze. That is, as testing proceeded, wider turn angles were noted, indicating subjects used more of a spatial strategy and less chaining to find a platform. These findings were likely due to the difficulty of the task used in this study, based on prior findings that failed to show a Cntnap2 deficit when compared to WTs on a simple MWM learning task (Peñagarikano et al., 2011).
Current findings are consistent with deficits in executive learning as demonstrated in ASD (Ozonoff, Pennington & Rogers, 1991; Rosenberg et al., 2009; Hill, 2004). Moreover, our findings may further explain the dyad of core symptoms, given the central role of executive processing in both higher and lower levels of processing. That is, the global connectivity deficiency seen in ASD could contribute to the spatial working memory and learning impairments observed here. This pattern has also been seen in neuroimaging studies with high functioning ASD participants (Barendse et al., 2013; Di Martino et al., 2014). This disconnection may result in problems with sensory integration, and therefore thereby learning. This could explain why Cntnap2 KOs require more experience to effectively learn the maze, as compared to their WT controls.
The impairments observed in the current study also may be explained by the abnormal myelin formation seen in this transgenic mouse model, consistent with the hypo-connectivity theory of the neurobiology of ASD, as well as the spatial learning deficits seen in ASD. Future studies are planned to look into neuroanatomical differences in white matter tracks spanning cortical regions, and correlate these measures to the cognitive differences seen here (using anatomy from these same subjects). Overall, these behavioral findings suggest that CNTNAP2 plays a clear underlying role in the development of neural systems important to learning and cross-modal integration, and disruption of this function could be associated with delayed learning observed in individuals with ASD. Future studies should investigate heterozygous mice performance as well, since this would be relevant due to the presence of heterozygous mutations in the clinical population.
Highlights.
Cntnap2 KOs displayed impairments on a 4/8 radial arm water maze.
Cntnap2 KOs exhibited significant deficits in reference and working memory.
These impairments were specific to the acquisition period of testing.
These findings suggest Cntnap2 KOs displayed delayed learning.
Acknowledgments
This work was supported in part by NSF IGERT Grant 1144399 (J. Magnuson, PI) and by the National Institutes of Health grant P01HD057853 to R.H. Fitch. The authors.
Footnotes
Jax guarantees “rigorous genetic quality control and mutant gene genotyping programs” for mouse strains with identified molecular mutations (see Terms of Sale).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, … Nelson SF. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. The American Journal of Human Genetics. 2008;82(1):150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, … Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. The American Journal of Human Genetics. 2008;82(1):160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anney R, Klei L, Pinto D, Almeida J, Bacchelli E, Baird G, … Brennan S. Individual common variants exert weak effects on the risk for autism spectrum disorders. Human molecular genetics. 2012;21(21):4781–4792. doi: 10.1093/hmg/dds301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Baio J. Surveillance Summaries. 3. Vol. 61. Centers for Disease Control and Prevention; 2010. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. [PubMed] [Google Scholar]
- Barendse EM, Hendriks MP, Jansen JF, Backes WH, Hofman PA, Thoonen G, … Aldenkamp AP. Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. Journal of neurodevelopmental disorders. 2013;5(1):14. doi: 10.1186/1866-1955-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, Buxbaum JD. Genetics and genomics of autism spectrum disorder: embracing complexity. Human molecular genetics. 2015:ddv273. doi: 10.1093/hmg/ddv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, … Milham MP. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular psychiatry. 2014;19(6):659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, … Fombonne E. Global prevalence of autism and other pervasive developmental disorders. Autism Research. 2012;5(3):160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in cognitive sciences. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain research. 1998;785(2):236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Ji W, Li T, Pan Y, Tao H, Ju K, Wen Z, … He L. CNTNAP2 is significantly associated with schizophrenia and major depression in the Han Chinese population. Psychiatry research. 2013;207(3):225–228. doi: 10.1016/j.psychres.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Lind SE, Williams DM, Raber J, Peel A, Bowler DM. Spatial navigation impairments among intellectually high-functioning adults with autism spectrum disorder: Exploring relations with theory of mind, episodic memory, and episodic future thinking. Journal of abnormal psychology. 2013;122(4):1189. doi: 10.1037/a0034819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca A, Myers SM, Challman TD, Moreno-De-Luca D, Evans DW, Ledbetter DH. Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence. The Lancet Neurology. 2013;12(4):406–414. doi: 10.1016/S1474-4422(13)70011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Paracchini S, Scerri TS, Winchester L, Addis L, Richardson AJ, … Monaco AP. Investigation of dyslexia and SLI risk variants in reading-and language-impaired subjects. Behavior genetics. 2011;41(1):90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. Journal of child Psychology and Psychiatry. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Geschwind D. What does CNTNAP2 reveal about autism spectrum disorder? Trend Mol Med. 2012;18:156–163. doi: 10.1016/j.molmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Raskind WH, Matsushita M, Lisowski M, Vu T, Berninger VW, … Brkanac Z. Replication of CNTNAP2 association with nonword repetition and support for FOXP2 association with timed reading and motor activities in a dyslexia family sample. Journal of Neurodevelopmental Disorders. 2011;3(1):39–49. doi: 10.1007/s11689-010-9065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, … Furley AJ. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. The Journal of cell biology. 2003;162(6):1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Goddard L, Dritschel B, Wisley M, Howlin P. Executive functions in children with autism spectrum disorders. Brain and cognition. 2009;71(3):362–368. doi: 10.1016/j.bandc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Archives of pediatrics & adolescent medicine. 2009;163(10):907–914. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- Schuh JM, Eigsti IM. Working memory, language skills, and autism symptomatology. Behavioral Sciences. 2012;2(4):207–218. doi: 10.3390/bs2040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele SD, Minshew NJ, Luna B, Sweeney JA. Spatial working memory deficits in autism. Journal of autism and developmental disorders. 2007;37(4):605–612. doi: 10.1007/s10803-006-0202-2. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30(3):405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Truong DT, Rendall AR, Castelluccio BC, Eigsti IM, Fitch RH. Auditory processing and morphological anomalies in medial geniculate nucleus of Cntnap2 mutant mice. Behavioral neuroscience. 2015;129(6):731. doi: 10.1037/bne0000096. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, … Fisher SE. A Functional Genetic Link between Distinct Developmental Language Disorders. The New England Journal of Medicine. 2008;359(22):2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. Journal of autism and developmental disorders. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. The profile of memory function in children with autism. Neuropsychology. 2006;20(1):21. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]