Abstract
Down syndrome (DS) is a significant risk factor for congenital heart disease (CHD), increasing the incidence 50 times over the general population. However, half of people with DS have a normal heart and thus trisomy 21 is not sufficient to cause CHD by itself. Ts65Dn mice are trisomic for orthologs of >100 Hsa21 genes, and their heart defect frequency is significantly higher than their euploid littermates. Introduction of a null allele of Creld1 into Ts65Dn increases the penetrance of heart defects significantly. However, this increase was not seen when the Creld1 null allele was introduced into Ts1Cje, a mouse that is trisomic for about two thirds of the Hsa21 orthologs that are triplicated in Ts65Dn. Among the 23 genes present in three copies in Ts65Dn but not Ts1Cje, we identified Jam2 as necessary for the increased penetrance of Creld1-mediated septal defects in Ts65Dn. Thus, overexpression of the trisomic gene, Jam2, is a necessary potentiator of the disomic genetic modifier, Creld1. No direct physical interaction between Jam2 and Creld1 was identified by several methods. Regions of Hsa21 containing genes that are risk factors of CHD have been identified, but Jam2 (and its environs) has not been linked to heart formation previously. The complexity of this interaction may be more representative of the clinical situation in people than consideration of simple single-gene models.
Keywords: trisomic potentiator, disomic modifier, congenital heart disease, Down syndrome
CONGENITAL heart disease (CHD) is the most frequent birth defect in human beings, affecting nearly 1% of all newborns (9/1000) (http://www.heart.org/HEARTORG). This frequency is far higher in Down syndrome (DS) where almost half of newborns have CHD (Freeman et al. 2008). Many genes have been implicated as potential modifiers of heart development (Locke et al. 2010; Sailani et al. 2013; Glessner et al. 2014); Online Mendelian Inheritance in Man (http://OMIM.org) lists 11,000 genes or syndromes of which CHD is a feature. We proposed a genetic model in which inheritance of multiple, individually benign genetic variants combine effects to reach a threshold beyond which heart development does not proceed normally (Li et al. 2012). On a euploid background, a large number of modifiers of small risk might be required. In this model, trisomy 21 (ts21) contributes a large fraction of risk. As ts21 is not sufficient to cause CHD by itself, it follows that additional risk factors must be necessary to reach the threshold for disease.
We provided biological support for this genetic model using mice with trisomy for regions orthologous to human chromosome 21 (Hsa21). In particular, the Ts65Dn mouse has been studied in this regard (Moore 2006; Williams et al. 2008; Li et al. 2012). We found a significant increase in septal defects in newborn trisomic mice that also carried a null allele of Creld1, a gene that has been associated with atrioventricular septal defect (AVSD) (Maslen 2004; Li et al. 2012). About 4% of newborn Ts65Dn mice have a septal defect and no defects were seen in Creld1+/− mice, however, a third of Ts65Dn;Creld1+/− mice were affected. A similar observation was made with a null allele of Hey2 in place of Creld1. These individually benign mutations complemented each other in a euploid background: 9.7% of Creld1+/−;Hey2+/− mice have septal defects. It has recently been recognized that the freely-segregating marker chromosome that carries these extra Hsa21 orthologous genes in Ts65Dn also contains a third copy of some genes not conserved with Hsa21 (Duchon et al. 2011; Reinholdt et al. 2011). However, the pattern of septal defects in Ts65Dn is similar to that reported for Dp(16)1Yey mice that carry a direct duplication of all Hsa21 orthologous genes on Mmu16 (Liu et al. 2014), albeit at a lower frequency. Thus this trisomic model is not only useful for uncovering individually benign modifier genes, but appears to be relevant to understanding the genetic basis for the high frequency of CHD in DS. We interrogated additional mouse models with segmental trisomy in an effort to localize genes that might contribute to the increased frequency of CHD.
Materials and Methods
Animal husbandry and genotyping
Mice used in the study were maintained in an American Association for Laboratory Animal Science (AAALAS)-certified clean facility with food and water ad libitum. Dp(16Cbr1-ORF9)1Rhr (Ts1Rhr) mice were maintained on the C57BL/6J background (B6J). Both B6EiC3Sn-Ts(16c-tel)1Cje/DnJ (Ts1Cje) and B6EiC3Sn a/A-Ts(1716)65Dn (Ts65Dn) were obtained from the Jackson Laboratory and maintained as a B6xC3H/HeJ advanced intercross. Dr. Akihiko Okuda of the Saitama Medical University in Japan kindly provided mice carrying a null allele of Jam2 (Sakaguchi et al. 2006) on the C57Bl/B6N background through the Large Animal Resources and Genetic Engineering resource (http://www.cdb.riken.jp/arg/mutant%20mice%20list.html; Material Accession number CDB0413K). All procedures were approved by the Institutional Animal Care and Use Committee.
Genomic DNA was extracted from tail tips and used for genotyping by PCR. Ts1Cje mice were identified using the following primers:
CITE 19UP – CTCGCCAAAGGAATGCAAGGTCTGT,
CITE 324L – CCCTTGTTGAATACGCTTGAGGAGA,
GRIK1 F2 – CCCCTTAGCATAACGACCAG, and
GRIK1 R2 – GGAACGAGACAGACACTGAG.
Ts1Rhr and Ts65Dn PCR typing was performed as described (Duchon et al. 2011; Reinholdt et al. 2011). Genotyping of Creld1 and Jam2 knockout mice was performed by PCR as described (Li et al. 2012; Sakaguchi et al. 2006).
Histology
The progeny of various crosses were collected within hours of birth and processed, embedded, sectioned, and stained as described (Li et al. 2012). Heart morphology for each animal was analyzed with a dissecting stereomicroscope by at least two individuals blinded to genotypes. Photos were taken using a Nikon Digital Sight system (Japan).
Quantitative PCR analysis of Jam2 gene expression
Hearts of 4-week-old mice with different genotypes were dissected and homogenized. Total RNA was extracted using TRIzol (Life Technologies Corporation, Carlsbad, CA). Complementary DNA (cDNA) synthesis was carried out with the AMV Reverse Transcriptase First-strand cDNA Synthesis Kit (Life Sciences, Cat.#LSK1200, Petersburg, FL) using 8 µg of total RNA as template. PCR was carried out using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA). Fluorescent (FAM)-labeled Jam2 (Applied Biosystems) was normalized to a VIC-labeled internal control, β-actin. All comparisons refer to the wild type (WT).
In vitro transcription of messenger RNA
Plasmids were transcribed in vitro using the mMESSAGE mMACHINE SP6 kit (Ambion, Austin, TX). Plasmids were linearized, then purified by precipitation. Transcribed sequence reactions were treated with DNase I, and messenger RNA (mRNA) was purified with lithium chloride. mRNA quality and quantity were confirmed by formaldehyde agarose gel and the NanoDrop8000 (Thermo Fisher Scientific, Waltham, MA).
Zebrafish maintenance and injections
Tubingen Zebrafish were raised in the Zebrafish Core center at the Institute for Genetic Medicine (Johns Hopkins University) under protocol #FI12M263 as described (Westerfield 1993). Zebrafish were maintained at 28°. Males and females were placed together in the morning and embryos were collected 30 min later. One hundred embryos were then injected at the 1–4 cell blastula stage with JAM2 mRNA at 50 pg and 100 pg using a Zeiss Stemi 2000 microscope and PV820 Pneumatic picopump injector. Injected embryos were phenotyped at 24–96 hr postfertilization (hpf) using a Nikon SMZ1500 microscope and imaged with NIS Elements Imaging Software. After imaging, embryos were fixed in 4% paraformaldehyde and transferred to 100% methanol at −20°.
Morpholino rescue
A previously validated translation-inhibiting antisense morpholino (MO) was designed against zebrafish Jam2a (Powell and Wright 2011). One hundred embryos were injected with 2 ng MO, 100 embryos were injected with 100 pg of mRNA, and 100 embryos were injected with both 2 ng MO and 100 pg mRNA; 100 uninjected embryos were used as a control. Embryos were examined at 24 hpf.
Co-immunoprecipitation
Unless otherwise noted reagents were from Thermo Fisher Scientific. α-FLAG antibodies and affinity gel were from Sigma Chemical (St. Louis, MO). Protease inhibitor (PI) and Protein A agarose were from Roche.
Plasmids containing most of the human genes of interest were moved to pcDNA3.1/nV5-DEST with LR clonase. The stop codon was removed from FAM126A, ARHGAP29, and those genes encoding an N-terminal signal sequence, and the sequences moved to pEF-DEST51 by PCR cloning to add a C-terminal V5 tag. Human CRELD1- and CRELD1-R329C-FLAG C-terminal constructs were provided by Cheryl L. Maslen. The CRELD1-E414K construct was produced by site-directed mutagenesis of the WT CRELD1 construct using QuikChangeII XL site-directed mutagenesis kit (Agilent).
GripTite 293 MSR Cells were cotransfected with a V5-tagged gene of interest and FLAG-tagged CRELD1 using Lipofectamine LTX and Plus reagent. After 48 hr, cells were washed with PBS, triturated from the plates in PBS, and pellets were frozen at −80° until use.
Cells were lysed using immunoprecipitation (IP) lysis buffer with PI, precleared with Protein A agarose and incubated with either α-FLAG- (30 μl) or α-V5-affinity gel (20 μl) for 2 hr at 4°. Eluted protein complexes were separated on denaturing NuPAGE gels and transferred to PVDF membranes. For Western blots of IPs using α-FLAG beads, coprecipitated V5-tagged proteins were detected with α-V5-HRP antibody or α-V5 and Clean Blot IP Detection Reagent (HRP). CRELD1-FLAG was detected with α-FLAG M2-AP and Lumi-Phos Western Blotting Reagent. For IPs using α-V5 beads, coprecipitating CRELD1 was detected with either rabbit α-FLAG and α-rabbit-HRP (Cell Signaling) or α-FLAG M2-AP. V5 proteins were detected with α-V5-HRP.
Protein microarray and data analysis
FLAG-tagged human CRELD1 cDNA with the two transmembrane domains removed (ΔCRELD1) (Rupp et al., 2002) was expressed in GripTite 293 cells. The secreted ΔCRELD1 was purified by anti-FLAG M2 affinity gel (Sigma Chemical). The protein was incubated with 17,000 GST-tagged human proteins that were recovered from yeast, and arrayed in duplicate on microscope slides (Jeong et al. 2012). The microarrays were processed with α-FLAG or α-GST as described (Newman et al. 2013). The signal intensity (SI) of each spot is defined as the odds ratio of median values of the foreground and background signals, where a value of one indicates that the query protein did not bind to the substrate protein on the chip. Within-chip normalization was performed and the SI of all spots approximated a normal distribution. A spot was defined as positive if its SI was larger than mean ± 5 std. deviations.
Statistical analysis
Genotype ratios for the crosses produced in this study, the prevalence of heart defects in different mouse genotypes, and the penetrance of heart edema in zebrafish embryos after injection with JAM2 mRNA and/or MO were compared by Fisher’s exact test using GraphPad Prism version 5. The relative quantification of gene expression from different genotypes was compared by Mann–Whitney test. All tests were two-tailed and P-values of P < 0.05 were considered significant.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article and Supplemental Material.
Results
Reduced Creld1 expression increases septal defect frequency in trisomic mice
We showed previously that reduced expression of Creld1 acts in concert with trisomy in Ts65Dn to increase the occurrence of heart defects in Ts65Dn;Creld1+/− mice (Li et al. 2012). To further localize the trisomic genes contributing to CHD, Creld1+/− mice were crossed to Ts1Cje, a mouse model that is trisomic for about 80% of the Mmu16 genes triplicated in Ts65Dn (Figure 1A) (Das et al. 2013). Progeny were killed within hours of birth and evaluated histologically (Figure 2). The genotype ratio of the offspring from this cross was not significantly different from the expected frequency (Supplemental Material, Table S1). The baseline frequency of septal defects was higher in Ts1Cje (5 out of 29) than in Ts65Dn (2 out of 58) (Table 1) (P = 0.04). However, in contrast to the situation in Ts65Dn;Creld1+/− mice, there was no increase in septal defects in Ts1Cje;Creld1+/− mice. We observed defects in 17% of Ts1Cje mice and 13% in Ts1Cje carrying a null allele of Creld1 (P = 0.70).
Figure 1.
Down syndrome mouse models used in this study. (A) Sizes and gene numbers of the three trisomic mouse models used in this study. (B). Fourteen Hsa21-orthologous genes that are expressed in the developing heart which are localized on Ts65Dn but not on Ts1Cje.
Figure 2.
Different types of septal defects were observed in mutant and trisomic mice at P0. (A) Normal heart showing intact ventricular septum at P0; (B) muscular VSD; (C) membranous VSD; (D) normal heart showing atrial septum; (E) ostium secundum ASD; (F) ASD from E at higher magnification. For the incidence of defects in various models, see Table 1 and Table 2. Arrows indicate communication between the chambers. RV: right ventricle; LV: left ventricle; RA: right atrium; LA: left atrium; Bars: A–E, 400 µm; F, 150 µm.
Table 1. Frequency of heart defects on mutant and trisomic genetic backgrounds.
| Phenotype | Genetic background | % of affected | Total no. | Type of septal defect |
|---|---|---|---|---|
| Creld1+/− | B6J/C3Ha | 0 | 18 | Not applicable |
| B6J | 0 | 27 | ||
| Ts1Cje | B6J/C3Hb | 17.2 | 29 | 4 membranous VSDs,c 1 secundum ASDd |
| Ts1Cje;Creld1+/− | B6J/C3Hb | 13 | 31 | 3 membranous VSDs, 1 secundum ASD |
| Ts1Rhr | B6J | 11.1 | 18 | 2 muscular VSDs |
| Ts1Rhr;Creld1+/− | B6J | 8 | 25 | 1 membranous VSD, 1 secundum ASD |
50% B6, 50% C3H.
75% B6, 25% C3H.
VSD, ventricular septal defect.
ASD, atrial septal defect.
Consistent with the idea that a gene that is trisomic in Ts65Dn but not Ts1Cje is required to see the Creld1+/−-influenced increase in heart defects, we detected no interaction between Creld1+/− and trisomy in another model, Ts1Rhr (Olson et al. 2004). These mice are trisomic for 33 of the genes that are triplicated in Ts1Cje and Ts65Dn. The genotype ratio of the offspring from this cross was not significantly different from the expected frequency (Table S2). Septal defects were seen in 8% of Ts1Rhr;Creld1+/− mice, which was not significantly different than the 11.1% frequency in Ts1Rhr itself (P = 0.12) (Table 1). The different outcomes in Ts65Dn compared to both Ts1Cje and Ts1Rhr suggest that a trisomic gene(s) that is necessary (but not necessarily sufficient) for the Creld1 modifier effect on penetrance is localized on the proximal portion of the segment that is triplicated in Ts65Dn (Figure 1B).
Trisomy for Jam2 acts in concert with Creld1
Twenty-three orthologs of Hsa21 genes plus a cluster of KRTAP-related genes that are trisomic in Ts65Dn are not triplicated in Ts1Cje (Starbuck et al. 2014). We identified 14 of these that are expressed in the developing heart (Figure 1B) (http://www.tigem.it/ch21exp/AtlasNewL.html; http://www.genecards.org/; http://www.ncbi.nlm.nih.gov/pubmed). We considered these to be candidates for increased CHD in the presence of decreased Creld1 expression on a trisomic background. Three of these are membrane proteins; Creld1 has been identified as a cell surface protein and more recently has been described in endoplasmic reticulum as well (Rupp et al. 2002; Maslen 2004; Mass et al. 2014). Among the 14 heart-expressed genes, JAM2 is a cell membrane protein with immunoglobulin-like domains that is concentrated at cell-to-cell junctions in heart endothelial cells of both large and small vessels, and it has been implicated in angiogenesis defects in Tc1 mice (Reynolds et al. 2010). Mouse Jam2 was identified in a gene expression-based search for stemness genes in embryonic stem (ES) cells where it was highly expressed in ES cells but quickly down regulated as they began to differentiate (Cunningham et al. 2000). Surprisingly, no phenotype was detected in a thorough study of Jam2−/− mice (Sakaguchi et al. 2006). However, in a screen of Hsa21 gene effects on early embryonic zebrafish development (S. Edie, N. A. Zaghloul, D. K. Klinedinst, J. Lebron, N. Katsanis, R. H. Reeves, in preparation), we found that overexpression of JAM2 causes maldevelopment of the heart.
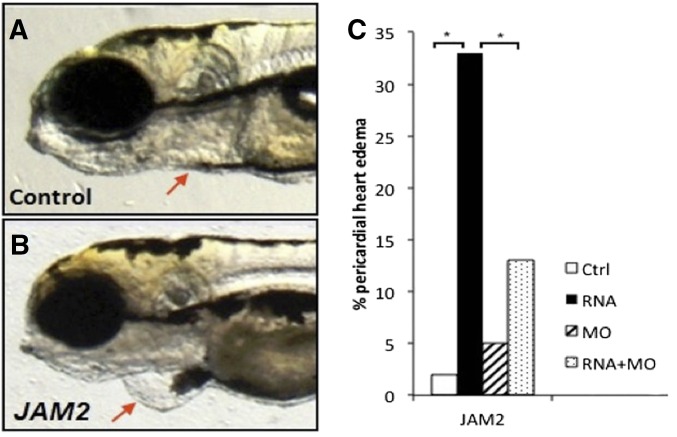
We cloned a human JAM2 ORF into the pCS2 vector, synthesized mRNA and injected zebrafish embryos with JAM2 mRNA. Injected embryos showed a high frequency of pericardial edema and this phenotype was robustly replicated over multiple injections (P < 0.0001, Fisher’s exact test) (Figure 3). The edema phenotype was partially rescued by co-injecting translation-blocking MOs targeted against the zebrafish ortholog, jam2a (P = 0.001), indicating that the effect is due to mRNA expression and not to nonspecific toxicity. Further, the pericardial edema phenotype was not observed when any of >100 other Hsa21 cDNAs was injected in the same paradigm.
Figure 3.
Effects of JAM2 expression in zebrafish embryos. (A) Control and (B) 100 pg JAM2-injected embryos at 48 hpf showing pericardial edema. (C) MO rescue, JAM2 mRNA alone, jam2 MO alone, or co-injection mRNA + MO each injected into 100 embryos and phenotyped at 24 hpf. * indicates P < 0.01.
Based on these observations, we tested the hypothesis that Jam2 must be trisomic in mice to see the greatly increased penetrance of septal defects that occurs in Ts65Dn;Creld1+/−, but not in Ts1Cje;Creld1+/− mice that are not trisomic for Jam2. Initial experiments showed that the frequency of heart defects in Ts65Dn seen previously on the trisomic B6J.C3H background (Moore 2006; Williams et al. 2008; Li et al. 2012) was attenuated or lost on the B6N.C3H background. Accordingly, B6N.Jam2−/− mice were backcrossed onto a C57BL/6J background for six or more generations. We used qPCR to compare Jam2 mRNA level in hearts of euploid (WT), Ts65Dn, and Jam2−/− mice. We found that Jam2 expression was increased by about 1.5-fold in Ts65Dn compared to the WT, there was a 40% decrease of Jam2 expression in Jam2+/− compared to the WT, and only background signal was detected in Jam2−/− mice (Figure 4).
Figure 4.
Real-time PCR showing the relative RNA expression level of Jam2 in mice with different genotypes. (A) TaqMan assay showed about 1.5-fold increase of Jam2 expression in Ts65Dn mice compared to WT. (B) TaqMan assay showed about 40% decrease of Jam2 expression in Jam2+/− mice compared to WT, only background level of Jam2 mRNA expression can be detected in Jam2−/− mice. Jam2 mRNA was normalized to β-actin mRNA, P-value is indicated (Mann–Whitney U test).
We performed a two generation, three-way cross to subtract one copy of Jam2 from Ts65Dn;Creld1+/− mice by crossing male Jam2+/−;Creld1+/− to Ts65Dn females. The genotype ratio of the offspring from this cross was not significantly different from the expected frequency (Table S3). In contrast to the 18.3% septal defects in Ts65Dn;Creld1+/− mice, only 4.5% (2 out of 44) of Ts65Dn;Creld1+/−;Jam2+/− (triple) mice had septal defects (P = 0.015) (Table 2). The septal defect penetrance in the triple mice was not different from that in Ts65Dn and Ts65Dn;Jam2+/− (3.4% and 3.8%, respectively). The defect seen in the two affected triple mice was membranous ventricular septal defect (VSD), the most frequent septal defect in Ts65Dn, while half of the affected Ts65Dn;Creld1+/− mice had a secundum atrial septal defect (ASD) (seven membranous VSD and eight secundum ASD).
Table 2. Type and frequency of heart defects in Ts65Dn×Creld1+/−;Jam2+/−.
| Type of defect | ||||
|---|---|---|---|---|
| Membranous VSD | Secundum ASD | % of total | Total | |
| Ts65Dn | 2 | 0 | 3.4 | 58a |
| Ts65Dn;Creld1+/− | 8 | 9 | 18.3 | 93 |
| Ts65Dn;Jam2+/− | 1 | 0 | 3.8 | 26 |
| Ts65Dn;Creld1+/−,Jam2+/− | 2 | 0 | 4.5 | 44b |
| Creld1+/− | 0 | 0 | 0 | 45 |
| Creld1+/−;Jam2+/− | 0 | 0 | 0 | 25 |
Ts65Dn vs. Ts65Dn;Creld1+/−: P = 0.01.
Ts65Dn;Creld1+/− vs. Ts65Dn;Creld1+/−,Jam2+/−: P = 0.03.
No septal defects were detected in Creld1+/-;Jam2+/− mice (0 out of 25) nor in Creld1+/- mice (0 out of 45). Since subtraction of the third copy of Jam2 from Ts65Dn;Creld1+/− mice eliminated the interaction that elevates the penetrance of CHD in these mice; our results indicate that Jam2 plays a necessary role in the cross-talk between trisomy and Creld1 in Ts65Dn. However, carrying two vs. three copies of Jam2 by itself did not affect the frequency of septal defects in Ts65Dn.
No evidence of direct interaction between Jam2 and Creld1 proteins by co-immunoprecipitation
Both Creld1 and Jam2 have been shown to encode membrane proteins (Rupp et al. 2002; Maslen 2004; Sakaguchi et al. 2006). We assessed the possibility that these proteins may interact directly to produce the effects observed in genetic models. Two investigators independently attempted co-immunoprecipitation (co-IP) using both Creld1 and Jam2 as drivers. We could find no evidence for interaction (Figure S2). We then searched for Creld1-interacting proteins that might be possible intermediates for communication between Creld1 and Jam2 using a human protein array.
Proteome microarray using purified human CRELD1 recombinant protein
Purified FLAG-tagged Human CRELD1 without the two transmembrane domains (rhΔCRELD1-FLAG) (Figure 5 and Figure S1) was incubated with protein microarray slides on which about 17,000 yeast-expressed human GST fusion proteins were printed. A negative control (without rhΔCRELD1-FLAG) was included. Signal intensities were determined with a GenePix 4000 scanner. GENEPIX PRO 5.0 software analysis identified about 2000 out of 17,000 proteins that were considered positive using a cut-off of 2 for the signal-to-noise ratio, and the remaining ∼15,000 proteins were considered as negative.
Figure 5.
Expression and purification of ΔCRELD1-FLAG protein. (A) SDS-PAGE and Coomassie blue staining of purified ΔCRELD1-FLAG protein; (B) Western blot to detect the purified protein by anti-FLAG antibody, the first lane is the supernatant of untransfected GripTite 293 cells, the second lane is the supernatant of GripTite 293 cells transfected with pCS2/ΔCRELD1-FLAG.
We prioritized the list of 2000 putative CRELD1-interacting proteins using multiple criteria. First, the proteins were ordered based on intensity of hybridization to the CRELD1 probe peptide. We then assessed groups of 100 proteins using the START program developed by Vanderbilt University (http://bioinfo.vanderbilt.edu/webgestalt/option.php) which is based on Gene Ontology (GO Slim). GO Slim classification includes cellular component, molecular function and biological process. We focused on the cellular component classification because CRELD1 is a membrane protein and we reasoned that true hits would include a large percentage in this category. We found a large number of hits in this category among the top 100 proteins, fewer hits among the 101–200 strongest signal targets, fewer still among the next 100 and so on (Table S4). Based on this we assessed the top 300 proteins using GeneALaCart (A GeneCards Batch Queries Engine; http://gene4.weizmann.ac.il/cgi-bin/BatchQueries/Batch.pl) and made a new target protein list for CRELD1 using the membrane-related and heart expression criteria. We identified 38 such proteins among the top 300 strongest signals (Table S5). JAM2 was not among the top 2000 proteins identified in the protein array.
To verify these interactions, we subcloned these 38 target genes into a mammalian expression vector to produce a V5-tagged protein and performed co-IP experiments with FLAG-tagged full-length human CRELD1. Of the 38 proteins, 10 gave a positive result for association of CRELD1 and the target with α-FLAG antibody on the affinity column. In the inverse experiment, CRELD1-FLAG was pulled down with 9 of the 10 V5-tagged proteins (Table S6). Thus at least 9 of 38 proteins (24%) identified on the large protein array were correctly identified as CRELD1 interactors by this independent measure.
We repeated the co-IPs of the 10 positive proteins with CRELD1 clones that carry the R329C or E414K mutations that have been described in AVSD patients (Robinson et al. 2003; Maslen et al. 2006) with essentially identical results, indicating that mutations in CRELD1 did not affect the interaction with these proteins. We also carried out triple transfections of the V5-tagged target proteins, CRELD1-FLAG and JAM2-myc to determine if JAM2 interacts with CRELD1 indirectly through one of these binding partners. However, Jam2 did not coprecipitate with any of these protein pairs.
Discussion
Our previous demonstration that candidate genetic modifiers predisposing to CHD can be identified in human studies of the genetically-sensitized DS population and validated biologically in the laboratory mouse is expanded here to show a type of genetic relationship not previously described for trisomic gene effects. Variants of Creld1 that are completely benign by themselves are risk factors for CHD that can act additively with other benign modifiers (e.g., Creld1+/− and Hey2+/−) or with trisomy for mouse orthologs of about half of the genes conserved with Hsa21 (Li et al. 2012). Jam2 has no effect on heart development when present at 0, 1, 2, or 3 copies and shows no additive effect with trisomy. However, it must be trisomic and overexpressed to see the increased penetrance of septal defects in mice with only one copy of Creld1.
Our genetic data shows that Jam2 is a potentiator of Creld1 in an epistatic interaction leading to maldevelopment of the heart. This does not appear to be based on a direct protein interaction, nor did we identify potential intermediates that connect the two functionally. Indeed, the degree of the effect, while significant, is modest. Cohorts of Ts65Dn trisomic mice that also inherited a null allele of Creld1 saw the incidence of septal defects rise from 4 to 18%, i.e., <20% of offspring were affected.
We have shown that loss of function of Creld1 can act in concert with the trisomic genes in Ts65Dn to create septal heart defects. The most frequent septal defect types we observed in our study are membranous VSD and secundum ASD. The atrioventricular cushions contribute to the formation and perhaps to closure of both the ventricular and atrial septa. CRELD1 is expressed in many human tissues by Northern blot, and it has high expression levels in heart. In situ hybridization using chick embryos showed high expression of CRELD1 in the cardiac atrial muscle and cushion tissue (Rupp et al. 2002), indicating a role for CRELD1 in endocardial cushion formation.
The endocardial cushions arise from a subset of endothelial cells that undergo epithelial-mesenchymal transition (EMT), a process whereby these cells break cell-to-cell adhesions and migrate into the inner heart wall to form endocardial cushions (Brade et al. 2006). In the Creld1 null mouse the endocardial cushions are smaller and hypocellular compared to developmentally matched WT littermates (Redig et al. 2014). Breakage of the cell-cell adhesion between the endothelial cells is an important process during endocardial cushion formation. Jam2 is a cell adhesion molecule that is specifically expressed in endothelial cells (Weber et al. 2007), suggesting that overexpression of the Jam2 gene due to trisomy might slow or inhibit the EMT process by strengthening those cell-cell interactions. As Creld1 is also a membrane protein, it is reasonable to speculate that it may interact with Jam2. However, we did not detect either direct or indirect interaction between Jam2 and Creld1 by co-IP of candidate CRELD1 interacting proteins, nor was Jam2 bound by ΔCRELD1 on a large protein array. If Jam2 is one of the genes responsible for the cross-talk between Ts65Dn and Creld1, it must do so by an indirect mechanism (not direct physical interaction) possibly by affecting endocardial cushion formation through the signaling pathways related to Creld1.
The genetics of CHD are complex, such that only a few highly-penetrant candidate genes have been implicated in human genetic syndromes. The vast majority of CHD is unexplained. If genetic contributions to this anomaly are due to small additive effects of a large pool of individually benign variants, identification of candidate targets for intervention will remain a challenge. The greatly increased frequency of CHD on the sensitized trisomic background will provide an important tool for finding and ameliorating the genetic variation contributing to the most frequent birth defect in human beings.
Acknowledgments
We thank Akihiko Okuda of the Saitama Medical University in Japan for generously providing Jam2−/− mice. This work was supported in part by HL083300 and HD038384 (R.H.R.), American Heart Association grant-in-aid 14GRNT20380202 (C.L.M.), and the American Heart Association fellowship no. 12POST11940039 (H.L.).
Footnotes
Communicating editor: T. R. Magnuson
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.188045/-/DC1
Literature Cited
- Brade T., Manner J., Kuhl M., 2006. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc. Res. 72: 198–209. [DOI] [PubMed] [Google Scholar]
- Cunningham S. A., Arrate M. P., Rodriguez J. M., Bjercke R. J., Vanderslice P., et al. , 2000. A novel protein with homology to the junctional adhesion molecule. Characterization of leukocyte interactions. J. Biol. Chem. 275: 34750–34756. [DOI] [PubMed] [Google Scholar]
- Das I., Park J. M., Shin J. H., Jeon S. K., Lorenzi H., et al. , 2013. Hedgehog agonist therapy corrects structural and cognitive deficits in a Down syndrome mouse model. Sci. Transl. Med. 5: 201ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchon A., Raveau M., Chevalier C., Nalesso V., Sharp A. J., et al. , 2011. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: relevance for modeling Down syndrome. Mamm. Genome 22: 674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S. B., Bean L. H., Allen E. G., Tinker S. W., Locke A. E., et al. , 2008. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet. Med. 10: 173–180. [DOI] [PubMed] [Google Scholar]
- Glessner J. T., Bick A. G., Ito K., Homsy J. G., Rodriguez-Murillo L., et al. , 2014. Increased frequency of de novo copy number variants in congenital heart disease by integrative analysis of single nucleotide polymorphism array and exome sequence data. Circ. Res. 115: 884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, J. S., L. Jiang, E. Albino, J. Marrero, H. S. Rho et al., 2012 Rapid identification of monospecific monoclonal antibodies using a human proteome microarray. Mol. Cell Proteomics 11: O111.016253. [DOI] [PMC free article] [PubMed]
- Li H., Cherry S., Klinedinst D., DeLeon V., Redig J., et al. , 2012. Genetic modifiers predisposing to congenital heart disease in the sensitized Down syndrome population. Circ Cardiovasc. Genet. 5: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Morishima M., Jiang X., Yu T., Meng K., et al. , 2014. Engineered chromosome-based genetic mapping establishes a 3.7 Mb critical genomic region for Down syndrome-associated heart defects in mice. Hum. Genet. 133: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke A. E., Dooley K. J., Tinker S. W., Cheong S. Y., Feingold E., et al. , 2010. Variation in folate pathway genes contributes to risk of congenital heart defects among individuals with Down syndrome. Genet. Epidemiol. 34: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslen C. L., 2004. Molecular genetics of atrioventricular septal defects. Curr. Opin. Cardiol. 19: 205–210. [DOI] [PubMed] [Google Scholar]
- Maslen C. L., Babcock D., Robinson S. W., Bean L. J., Dooley K. J., et al. , 2006. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome. Am. J. Med. Genet. A. 140: 2501–2505. [DOI] [PubMed] [Google Scholar]
- Mass E., Wachten D., Aschenbrenner A. C., Voelzmann A., Hoch M., 2014. Murine Creld1 controls cardiac development through activation of calcineurin/NFATc1 signaling. Dev. Cell 28: 711–726. [DOI] [PubMed] [Google Scholar]
- Moore C. S., 2006. Postnatal lethality and cardiac anomalies in the Ts65Dn Down syndrome mouse model. Mamm. Genome 17: 1005–1012. [DOI] [PubMed] [Google Scholar]
- Newman R. H., Hu J., Rho H. S., Xie Z., Woodard C., et al. , 2013. Construction of human activity-based phosphorylation networks. Mol. Syst. Biol. 9: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. E., Richtsmeier J. T., Leszl J., Reeves R. H., 2004. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science 306: 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. T., Wright G. J., 2011. Jamb and jamc are essential for vertebrate myocyte fusion. PLoS Biol. 9: e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redig J. K., Fouad G. T., Babcock D., Reshey B., Feingold E., et al. , 2014. Allelic Interaction between and in the Pathogenesis of Cardiac Atrioventricular Septal Defects. AIMS Genet 1: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt L. G., Ding Y., Gilbert G. J., Czechanski A., Solzak J. P., et al. , 2011. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm. Genome 22: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L. E., Watson A. R., Baker M., Jones T. A., D’Amico G., et al. , 2010. Tumour angiogenesis is reduced in the Tc1 mouse model of Down’s syndrome. Nature 465: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. W., Morris C. D., Goldmuntz E., Reller M. D., Jones M. A., et al. , 2003. Missense mutations in CRELD1 are associated with cardiac atrioventricular septal defects. Am. J. Hum. Genet. 72: 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp P. A., Fouad G. T., Egelston C. A., Reifsteck C. A., Olson S. B., et al. , 2002. Identification, genomic organization and mRNA expression of CRELD1, the founding member of a unique family of matricellular proteins. Gene 293: 47–57. [DOI] [PubMed] [Google Scholar]
- Sailani M. R., Makrythanasis P., Valsesia A., Santoni F. A., Deutsch S., et al. , 2013. The complex SNP and CNV genetic architecture of the increased risk of congenital heart defects in Down syndrome. Genome Res. 23: 1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Nishimoto M., Miyagi S., Iwama A., Morita Y., et al. , 2006. Putative “stemness” gene jam-B is not required for maintenance of stem cell state in embryonic, neural, or hematopoietic stem cells. Mol. Cell. Biol. 26: 6557–6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starbuck J. M., Dutka T., Ratliff T. S., Reeves R. H., Richtsmeier J. T., 2014. Overlapping trisomies for human chromosome 21 orthologs produce similar effects on skull and brain morphology of Dp(16)1Yey and Ts65Dn mice. Am. J. Med. Genet. A. 164A: 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Fraemohs L., Dejana E., 2007. The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 7: 467–477. [DOI] [PubMed] [Google Scholar]
- Westerfield M., 1993. The Zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). University of Oregon Press, Eugene, OR. [Google Scholar]
- Williams A. D., Mjaatvedt C. H., Moore C. S., 2008. Characterization of the cardiac phenotype in neonatal Ts65Dn mice. Dev. Dyn. 237: 426–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article and Supplemental Material.