Abstract
Objective
Expanded HIV testing coverage could result in earlier diagnosis of HIV, along with reduced morbidity, mortality, and onward HIV transmission.
Design
Longitudinal analysis of aggregate, population-based surveillance data within New York City (NYC) ZIP codes.
Methods
We examined new HIV diagnoses and recent HIV testing to examine whether changes in recent HIV testing coverage (last 12 months) were associated with changes in late HIV diagnosis rates within NYC ZIP codes during 2003–2010, a period of expansion of HIV testing in NYC.
Results
Overall, recent HIV testing coverage increased from 23% to 31% during 2003–2010, while the rate of late HIV diagnoses decreased from 14.9 per 100,000 to 10.6 per 100,000 population. Within ZIP codes, each 10% absolute increase in recent HIV testing coverage was associated with a 2.5 per 100,000 absolute decrease in the late HIV diagnosis rate. ZIP codes with the largest changes in HIV testing coverage among men were more likely to have the largest (top quartile) declines in late HIV diagnosis rates among men (adjusted odds ratio [aOR]men=4.0; 95%CI=1.5–10.8), as compared with ZIP codes with no or small changes in HIV testing coverage. However, this association was not significant for women (aORwomen=1.4 95% CI=0.50–4.3). Significant geographic disparities in late HIV diagnosis rates persisted in 2009/10.
Conclusions
Increases in recent HIV testing coverage may have reduced late HIV diagnoses among men. Persistent geographic disparities underscore the need for continued expansion of HIV testing to promote earlier HIV diagnosis.
Keywords: late HIV diagnoses, HIV testing coverage, area-level analysis, implementation science, HIV incidence
Introduction
Late HIV diagnoses (AIDS diagnoses within 12 months of initial HIV infection) is one of the seven core indicators in the U.S. National HIV/AIDS Strategy for monitoring HIV programs and services. [1, 2] According to latest national estimates (end of year 2010), 32% of persons initially diagnosed with HIV were diagnosed late. [3] Late HIV diagnoses represents missed opportunities for treatment and prevention and pose significant public health challenges in the United States (US). [4]
Persons diagnosed with HIV in the advanced stages (late HIV diagnoses) are less likely to fully benefit from timely HIV treatment, [5–7] and accrue higher treatment-related costs. [8, 9] They also are more likely to die from HIV-related causes than those who were diagnosed earlier in the course of infection. [10–12] From a population perspective, late HIV diagnoses represent missed opportunities to prevent onward HIV transmission. [13, 14] One study estimated that the transmission rate of HIV among persons not yet diagnosed (and consequently unaware of their HIV status) was approximately four times higher compared to the rate of those aware. [15] Persons unaware of their HIV status are the source of infection for an estimated 49% to 66% of individuals newly infected with HIV. [16]
Expanded HIV testing coverage has been associated with increases in the proportion of persons with HIV linked to care, and subsequently, virally suppressed due to earlier HIV treatment initiation. [17, 18] Over the long term, successful expansion of HIV testing would be expected to result in earlier HIV diagnoses and, consequently, in a reduction in the number and proportion of new HIV diagnoses that are late. [17, 19] In New York City (NYC), there have been multiple recent targeted efforts to expand HIV testing. In the Bronx, following ‘The Bronx Knows’ testing campaign, the proportion of HIV diagnoses concurrent with AIDS (i.e., late HIV diagnoses) declined. [20] However, HIV testing rates improved in other areas across NYC during the same time period as the Bronx Knows campaign. We therefore sought to determine whether expanded HIV testing coverage within neighborhoods in all 5 boroughs of NYC was associated with declines in the rate of late HIV diagnosis during 2003–2010 in those same neighborhoods.
METHODS
Study population
Data on HIV/AIDS diagnoses during 2003–2010, reported through September 30, 2011, came from the NYC HIV Surveillance Registry (the Registry), which is a population-based registry of all persons diagnosed with HIV infection and reported to the NYC DOHMH. [21] Name-based reporting of AIDS diagnoses was mandated by New York State (NYS) law in 1983, followed by HIV reporting in 2000 [22] and laboratory reporting of all positive Western Blots, viral loads, CD4 counts, and nucleotide sequence results in 2005. [23] All incoming electronic laboratory reports are matched to cases in the Registry, and non-matching records are sent for field investigation to confirm case status. [24] Only persons residing in NYC at time of HIV diagnosis were included in the analysis. Additionally, we excluded persons who were undomiciled, living in a shelter, and those with non-residential, unknown or missing ZIP codes (representing approximately 3% of remaining HIV diagnoses).
We examined aggregate data stratified by sex on all HIV diagnoses among NYC residents for 171 residential ZIP codes during 2003–04, 2005–06, 2007–08 and 2009–10.
Measures
Late HIV diagnoses
We used the CDC’s definition of late HIV diagnosis as a CD4 count test result ≤200 cells/μL or an AIDS-defining illness within 12 months of the date of HIV diagnosis. [25] We expressed the proportion of persons diagnosed late as a rate per 100,000 population. We calculated the rate of late HIV diagnoses per 100,000 population across ZIP codes in two-year periods (to improve the stability of rates within ZIP codes with small numbers of events), using Census 2010 as the population denominator to standardize rate calculations. All two-year rates were divided by two (i.e. annualized) to represent rates per year. The outcome variable, change in the rate of late HIV diagnosis during the study period, represents the absolute difference in rates between 2003–04 and 2009–10 within each ZIP code. ZIP codes falling within the top 25 percentile of change in late HIV diagnosis rate (i.e., largest declines in rate) were classified as having “large” declines in the late HIV diagnosis rate. The remaining ZIP codes were classified as having “small to no decline”.
HIV testing
We used estimates of the absolute within-neighborhood change in the percent of having recently tested for HIV in the last 12 months. We used the New York City Community Health Survey (CHS), which is an annual cross-sectional random-digit telephone survey on approximately 10,000 non-institutionalized adults aged 18 years and older administered by the NYC Department of Health and Mental Hygiene (NYC DOHMH) since 2002. The survey is based on a stratified sampling design, which enables enumeration of neighborhood and city-wide estimates. In 2009, the survey started sampling respondents with cell phones and land line phones. [26]
The CHS questions are modeled after the CDC’s Behavioral Risk Factor Surveillance System (BRFSS) and collects self-reported data across a range of socio-demographic, health, and behavioral outcomes such as HIV testing in the past 12 months. The question on HIV testing was first introduced in 2003 and, except for 2004, asked annually through 2010. Data were paired in two-year periods for improved reliability of estimates. Responses from the earliest (2003–2005) and latest (2009–10) survey years at the time of the study were used to generate the exposure variable, recent HIV testing coverage. The average response rate from the CHS between 2003 and 2010 was 36.6% and the cooperation rate (participation among those reached by phone) was 80.6%.
Due to small CHS sample sizes within ZIP codes, we used United Hospital Fund (UHF) neighborhood designations (n=42), which comprise between two and 11 adjacent ZIP codes that represent historical catchment areas of public health care facilities. [27, 28] For each UHF, the absolute age-adjusted percent of persons reporting an HIV test in the past 12 months was calculated for 2003–05 and 2009–10, separately by sex using weights provided by the NYC DOHMH. CHS post-stratification weights are used to adjust for probability of selection while taking into account the respondent’s age, gender, and race. [29] We classified the neighborhood changes in recent HIV testing coverage between 2003/4 and 2009/10 using tertiles: “no/low”, “medium” and “large” increase in recent HIV testing coverage.
HIV incidence
Secular trends (decreases) in HIV incidence might result in a reduction in the rate of late HIV diagnosis simply due to a reduction in new HIV infections (i.e., independently of earlier HIV diagnosis), therefore we examined this as a possible alternate explanation for declines in the rate of late HIV diagnoses. HIV incidence trend data were not available at the ZIP code level, so we used change in rate of non-late HIV diagnoses (i.e., new HIV diagnoses excluding those meeting the above CDC definition of late HIV diagnosis). We calculated the within neighborhood change as the difference between rates of late and new HIV diagnoses per 100,000 during 2003–04 and 2009–10. We classified neighborhood change in our proxy for HIV incidence using tertiles: “no/low”, “medium” and “large” decrease in incidence.
Borough of residence at HIV diagnosis
HIV testing initiatives as well as jurisdictional HIV testing campaigns have taken place in two of the five boroughs (The Bronx beginning in 2008, and Brooklyn beginning in 2010). We therefore examined borough in our multivariate analysis. We selected Queens as the reference group since Queens had not been directly targeted by any DOHMH-led HIV testing initiative as of the end of the study period.
Statistical methods and analysis
Descriptive analysis
We examined the trend and statistical significance of the slopes for rates of new HIV, late HIV, non-late HIV, and AIDS diagnoses, HIV-related mortality, median CD4 count, and HIV testing coverage. We estimated the relative risks for 2009–10 vs. 2003–04 and the relative risk for recent HIV testing coverage for 2009–10 vs. 2003–05. We mapped HIV testing coverage, using tertile categories and late HIV diagnoses using quartile categories, to correspond to the categories used in multivariable analysis. Maps are for the earliest and latest year groups and for changes between these two year groups, by ZIP code. Because HIV testing data from CHS were not available at the ZIP code level, we mapped and examined UHF level testing coverage at the ZIP code level by assigning the same testing coverage for all the ZIP codes within that UHF. Dark blue lines delineate UHFs, while light grey lines are ZIP codes. Maps are based on data for men and women combined.
We calculated the Global Morans’ I to assess spatial autocorrelation [30] for HIV testing coverage and the late HIV diagnosis rate. Moran’s I evaluates whether a pattern observed is clustered, dispersed, or random. A positive Moran’s I indicates that high values (i.e., rates or prevalence) spatially cluster near other high values. A statistically significant value indicates a rejection of the null hypothesis that values are randomly distributed. We calculated Morans’ I for HIV testing at the UHF level using a spatial weights matrix with (k=4) nearest neighbors, and at the ZIP code level for rates of late HIV diagnoses using a spatial weights matrix of (k=10) nearest neighbors. K-nearest neighbor’s specification was chosen because it forces each area-level unit to have the same number of neighbors. We used ArcMap 10.1 [31] to create maps and calculate Moran’s I.
Multivariate analyses
The multivariate analyses of factors associated with the largest changes in the late HIV diagnosis rate was conducted on 171 ZIP codes between 2003–04 and 2009–10 for each sex. We used generalized estimating equations (GEE) regression with logit link function and an independent correlation structure using robust variance estimation [33] to examine whether changes in HIV testing coverage were associated with “large” declines in the rate of late HIV diagnoses, while accounting the hierarchical nature of the data (ZIP codes nested within UHF neighborhoods). We conducted analyses separately for men and women given known sex differences in HIV testing and late HIV diagnosis. [34] First, we individually assessed the crude association of HIV testing coverage, HIV incidence and NYC borough of residence at HIV diagnoses on the outcome (Model 1). We then estimated the effect of HIV testing coverage adjusting for HIV incidence and borough of residence at HIV diagnosis in stepwise fashion (Models 2 then 3), and then included all variables in one model simultaneously (Model 4). Statistical analyses were conducted in STATA 10.0. [32]
Results
City-wide trends in HIV-related measures
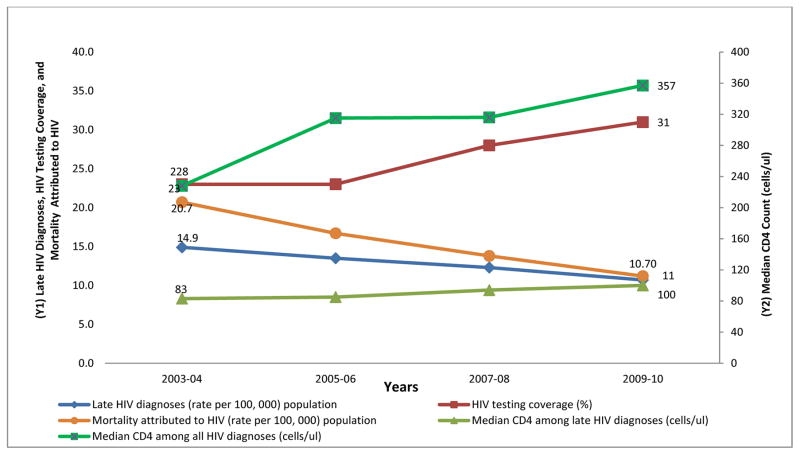
During the seven year period (2003 to 2010), the rate of new HIV diagnoses significantly declined from 49.69 per 100,000 to 39.80 per 100,000 (relative risk [RR] 0.78, 95% CI: 0.76–0.81). The rate of late HIV diagnoses declined from 14.91 to 10.65 per 100,000 corresponding to a RR of 0.70, (95% CI: 0.66–0.74). The rate of non-late HIV diagnoses declined from 34.78 to 29.15 per 100,000 (RR=0.82, 95% CI: 0.79–0.85). The median CD4 count among all persons diagnosed with HIV increased from 228 cells/μL in 2003–04 to 357 cells/μL in 2009–10. The age-adjusted rate of mortality attributed to HIV declined during 2003–04 to 2009–10 by almost half from 20 to 11 per 100, 000 (RR=0.56, 95% CI: 0.52–0.59). Recent HIV testing increased from 23% in 2003–05 to 31% in 2009–10 (Table 1).
Table 1.
Trends in HIV and AIDS outcomes, and HIV Testing Coverage in New York City, 2003 to 2010
| 2003–4 | 2005–6 | 2007–8 | 2009–10 | p-value for trendg | Relative Risk 2009–10 vs. 2003–4 and 95 % CI | |
|---|---|---|---|---|---|---|
|
|
||||||
| New HIV diagnoses | ||||||
| Numbera | 8,124 | 7,661 | 7,381 | 6,508 | 0.78 (0.76; 0.81)§ | |
| Number with CD4 count within 12 months | 4,945 | 5,768 | 5,870 | 5,513 | ||
| Annualized rate per 100,000 population | 49.69 | 46.86 | 45.14 | 39.80 | 0.026 | |
| Late HIV diagnoses within 12 months | ||||||
| Numbera | 2,438 | 2,206 | 2,012 | 1,742 | 0.70 (0.66; 0.74)§ | |
| Number with CD4 count | 2,393 | 2,176 | 1,998 | 1,728 | ||
| Annualized rate per 100,000 population | 14.91 | 13.49 | 12.31 | 10.65 | 0.002 | |
| Men | 10.18 | 9.89 | 8.90 | 7.97 | 0.022 | 0.77 (0.72; 0.83)§ |
| Women | 4.73 | 3.60 | 3.41 | 2.68 | 0.035 | 0.56 (0.50; 0.63)§ |
| Percent due to late HIV diagnoses | 30.01 | 28.80 | 27.26 | 26.77 | ||
| Non-late HIV diagnoses within 12 months | ||||||
| Numbera | 5,686 | 5,455 | 5,369 | 4,766 | 0.82 (0.79; 0.85)§ | |
| Annualized rate per 100,000 population | 34.78 | 33.36 | 32.84 | 29.15 | 0.065 | |
| CD4 count within 12 months | ||||||
| Median CD4 count cells/μL among all HIV diagnosesc | 228.1 | 314.9 | 317 | 357 | 0.024 | |
| Median CD4 count cells/μL among late HIV diagnoses onlyc | 83 | 85 | 94 | 100 | 0.076 | |
| AIDS outcomes | ||||||
| Number of AIDS diagnosesa | 9,733 | 7,981 | 6,880 | 5,510 | 0.55 (0.52; 0.57) | |
| Annualized rate of AIDS diagnoses per 100,000 population | 59.53 | 48.81 | 42.08 | 33.70 | 0.004 | |
| Percent due to late HIV diagnoses | 25.05 | 27.64 | 29.24 | 31.62 | ||
| Number of PLWHAa,b | 96,287 | 102,153 | 106,584 | 110,736 | ||
| Percent of population living with HIV and AIDS | 1.18 | 1.25 | 1.30 | 1.35 | ||
| Mortality attributed to HIVd | ||||||
| Number | 2,959 | 2,501 | 2,083 | 1,681 | ||
| Annualized rate per 100,000 population | 20.07 | 16.78 | 13.83 | 11.22 | 0.001 | 0.56 (0.52; 0.59) |
| HIV testing coverage | ||||||
| Weighted populationf | 1,390,947 | 1,721,272 | 1,928,218 | |||
| Total tested (%) | 23e | 28 | 31 | 0.052 | 1.29 (1.28; 1.29) | |
| Men | 21e | 27 | 29
|
0.061 | ||
| Women | 25e | 29 | 32 | 0.052 | ||
| NYC Population denominators§ | 8,008,278 | 8,175,133 | ||||
As reported by the NYC DOHMH as of September 30, 2011
As of the latest for the two year period
Mean of the Median CD4 Count
Deaths attributed to HIV/AIDS (ICD-10 Codes B20–B24), data are from the DOHMH Bureau of Vital Statistics
Testing coverage assumed same for each two year period (i.e. 2003–04) and (2005–06)
Estimate from NYC Community Health Survey
Regression test for the slope across year
Relative risk are calculated using Census 2000 for first year group and 2010 for last year group. Population denominators estimates are from NYC Department of City Planning redistricting files http://www.nyc.gov/html/dcp/pdf/census/census2010/t_pl_p1_nyc.pdf
The increasing trend in HIV testing coverage corresponded closely with an increasing trend in median CD4 count among all those diagnosed, especially between 2007–08 and 2009–10, and a declining trend in the rates of late HIV diagnosis and HIV-related mortality (Figure 1).
FIGURE 1.
Trends in Late HIV Diagnoses, HIV Testing Coverage, Mortality Attributed to HIV (axis Y1) and Median CD4 Count (axis Y2), NYC, 2003 to 2010
Temporal changes in late HIV diagnosis rates within ZIP codes
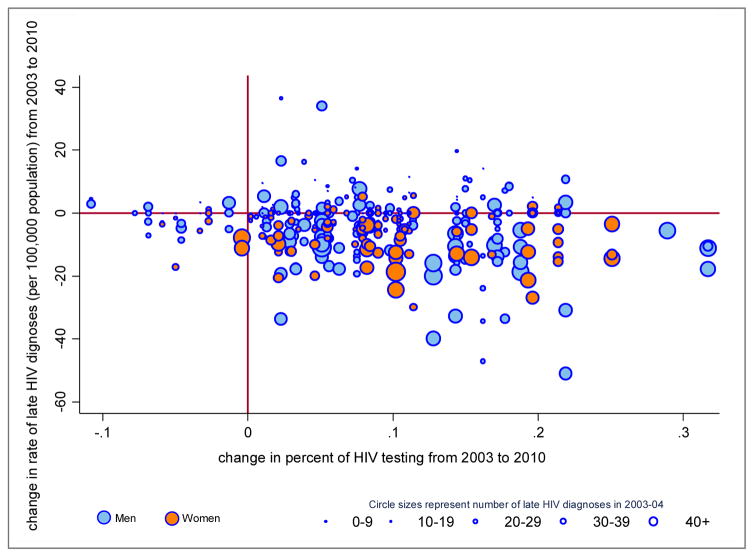
As shown in Figure 2, ZIP codes with larger increases in HIV testing coverage also had larger decreases in the rate of late HIV diagnoses during 2003–2010. The overall crude association showed that each 10% absolute increase in recent HIV testing coverage was associated with a 2.5 per 100,000 absolute decrease in the late HIV diagnosis rate. The sex-specific plots were suggestive of a stronger association for men (blue) than women (orange). Importantly, ZIP codes with larger numbers (>40) of late HIV diagnoses in 2003–4 tended to have both larger improvements in recent HIV testing coverage and larger declines in the rate of late HIV diagnosis.
Figure 2.
Change in late HIV diagnoses rate by change in HIV testing coverage, by sex NYC ZIP codes, 2003–2010
Spatial analysis of temporal changes
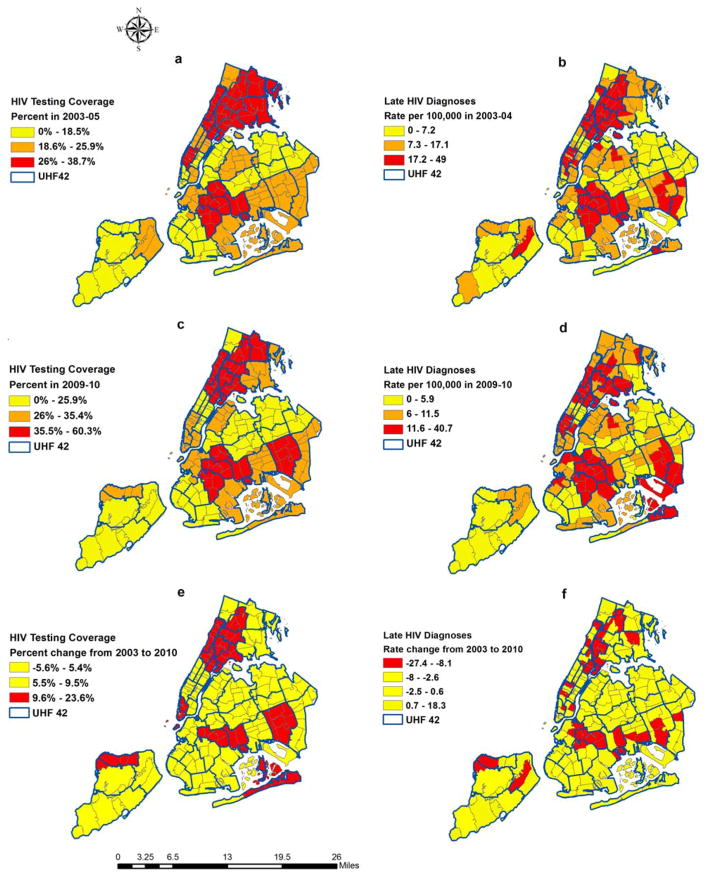
The maps in Figure 3 show that HIV testing coverage was strongly clustered across pockets of areas within the UHF neighborhoods in 2003–05 (Map a), which corresponded to Moran’s I=0.62, z=7.47, p<0.001. The clustering pattern persisted in 2009–10 (Map c), corresponding to a Moran’s I = 0.49, z=6.1, p<0.001. Results from neighborhoods (especially those with increases in HIV testing) (Map e) showed weak evidence of clustering as indicated by the small and non-significant Moran’s I coefficient (Moran’s I=0.12, z=1.74, p=.08).
Figure 3.
HIV Testing Coverage (a, c, e) and Late HIV Diagnoses (b, d, f), New York City, 2003 to 2010
Rates of late HIV diagnoses showed strong evidence of spatial clustering across ZIP codes in 2003–05 (Map b), with Moran’s I=0.58, z=12.8, p<0.001, and in 2009–10 (Map d), with Moran’s I=0.53, z=11.8, p<0.001. Neighborhoods with the largest changes (declines) in rates of late HIV diagnoses (Map f) were geographically clustered (Moran’s I=0.18, z= 4.17, p<0.001) as indicated by the red shaded regions.
Multivariate analysis of change in HIV testing coverage and change in late HIV diagnosis rates
ZIP codes with the largest changes in HIV testing coverage among men (mean change [MC] =+18%) had three times higher odds of having the largest decline (top quartile) declines in late HIV diagnosis rates among men ([MC] = −17 per 100,000) as compared with ZIP codes with no or small changes in HIV testing coverage ([MC] = +0.09%) (Model 1, Crude odds ratio [OR] =3.1, 95% CI=1.3, 7.0). The association persisted after adjusting for changes in the rate of non-late HIV diagnoses (Model 2, adjusted odds ratio [aOR] =3.2, 95% CI=1.3, 7.4) and borough of residence at HIV diagnosis (Model 3, aOR=4.1, 95% CI=1.7, 10.1) and (Model 4, aOR=3.9, 95% CI=1.6, 09.6).
ZIP codes with the largest changes in HIV testing coverage among women ([MC]=+15.6%) did not have a significantly higher odds of having the largest decline the largest declines (top quartile) in late HIV diagnosis rates among women ([MC] = −14 per 100,000) as compared with ZIP codes with no or small changes in HIV testing coverage ([MC] = +0.05%) in crude (Model 1) or adjusted analyses (Models 3 and 4).
Discussion
Our study found that, during 2003–2010, larger increases in the proportion of persons tested for HIV during the last 12 months were associated with statistically significant declines in the rate of late HIV diagnoses within NYC ZIP codes among men but not women. Among men, the association held after adjusting for changes in non-late HIV diagnoses (a proxy for HIV incidence), and NYC borough of residence at HIV diagnosis. Among women, the relatively higher levels of both HIV testing coverage and lower levels of late HIV diagnoses at the start of the study period may explain the weaker and non-significant association between within-neighborhood changes in HIV testing and changes in late HIV diagnosis rates. Alternatively, HIV testing campaigns may have been able to reach persons with undiagnosed HIV among men more easily than women.
The higher proportion of HIV testing among women compared with men may be partly explained by a greater propensity among women to seek health care and/or voluntary HIV testing, and the fact that women in New York have received universal HIV screening during pregnancy since the 1990’s. National estimates show that 62% of women were screened during pregnancy for HIV in 2002. [35] In 2009–10, CHS data indicated that among NYC women, 61% reported ever testing for HIV compared with 57% among men. Thus, there may have been a floor effect of testing on the late diagnosis rate among women, as the rate of late diagnosis among women in 2003 (4.73 per 100,000) was already lower than what it would become for men in 2009/10 (7.97 per 100,000) (Table 1).
Bronx neighborhoods experienced the largest overall decline in the rates of late HIV diagnoses and largest increase in HIV testing coverage, followed by Manhattan and Brooklyn. The findings within Bronx were expected given the reported impact of the DOHMH’s “The Bronx Knows” borough-wide HIV testing initiative. [36, 37] However, the findings of decreasing late HIV diagnosis rates in Manhattan associated with HIV testing could have been due to a spillover effect whereby residents from Manhattan neighborhoods, particularly on the bordering areas with the Bronx, were exposed to “The Bronx Knows” program via social media, other outreach initiatives, and high inter-borough mobility of NYC residents. [38]
NYC experienced a major increase in median CD4 count among persons newly diagnosed with HIV during the study period. We estimated an annual change of 18.4 cells/μL per year among all persons newly diagnosed with HIV (228 to 357 cells/μL over the entire 7 year study period). This is substantially higher than the increase of 1.5 cells/μL per year reported in a meta-analysis study that used longitudinal clinic-based data that spanned 20 years, [39] and also marginally higher than those reported in a study that used population-based data from Washington, DC [40] (6.6 cells/μL per year; 346 to 379 cells/μL over the 5 year period 2005–2009 compared with 8.4 cells/μL per year in our study; (315 to 357 cells/μL over the same 5-year period).
In the most recent time period in our analysis (2009–10), we found significant geographic disparities at the neighborhood level (ZIP code) in the rates of late HIV diagnoses. The highest rates were in East Flatbush within Central Brooklyn and in the South Bronx. These areas are characterized by relatively high levels of socioeconomic disadvantage and a high proportion of racial/ethnic minorities. Our findings correspond to findings from a recent study that showed low socioeconomic status, high proportion of racial/ethnic minorities, high prevalence and incidence of HIV tend to geographically cluster within the same neighborhoods. [41]
Our study has some limitations. First, there might have been factors other than improvements in HIV testing coverage that could partially explain the declines in late HIV diagnoses rates. General declines in HIV incidence could result in fewer people being diagnosed overall as well as late, independently of increasing HIV testing coverage. While our multivariate analyses attempted to control for this, the proxy measure we used for HIV incidence (non-late HIV diagnoses) is imperfect since some persons who were non-late could have been living with HIV for several years without progressing to CD4<200. Reliable population-based data HIV incidence, however, are not available at the ZIP code level in NYC.
Second, there could have been reporting bias of HIV testing behaviors. We used a self-reported measure from a household survey, which included only residents with landline phones through 2009. From 2009 on, the CHS started sampling residents with both landline and cell phones. However, initial evaluations of prevalence estimates of health and behavioral outcomes, showed that the HIV testing prevalence was not markedly different between cellphone and landline respondents with who were primarily landline users vs. those who were primarily cell phone users. [51]
Third, our unit of analysis, ZIP codes, may not accurately represent or differentiate neighborhoods, however, ZIP codes were the smallest unit of aggregation we had HIV surveillance data. Related to use of ZIP codes, we did not have sufficiently precise data on HIV testing coverage at the ZIP code level to match with HIV surveillance data, resulting in less granularity and possibly “scale effects” whereby variance in association between HIV testing and late HIV diagnoses may be different at other levels of aggregation. [53]
Lastly, our findings may not apply to newly diagnosed persons who were homeless or undomiciled or living in a shelter or where residence of ZIP code was unknown.
Our study has several strengths. First, due to the population-based nature of the data sources used in our analysis, our results are generalizeable to all NYC neighborhoods. Second, we were able to examine and quantify the association of changes of HIV testing coverage with changes in late HIV diagnoses longitudinally within ZIP codes throughout NYC, facilitating an ecological assessment of the potential effects that may have resulted from varying degrees of changes in HIV testing coverage.
Conclusion
Our findings suggest that expanded HIV testing has resulted in a reduction in the rate of late HIV diagnoses among men. Given that geographic disparities in the rates of late HIV diagnosis persisted in 2009/10, our findings underscore the need for ongoing expansion of HIV testing because of its potential to reach those areas and populations in which recent HIV testing coverage remains low and the rates of late HIV diagnosis remain high.
Future research should examine whether other behavioral and socioeconomic area-level factors, such as income inequality, social capital, and racial residential segregation are associated with late HIV diagnoses, as well as the impact that disparities in late HIV diagnoses have on other outcomes such as mortality attributed to HIV.
Table 2.
Multivariable Analysis of Factors Associated with the Largest ZIP Code-level Declines in Rates of Late HIV Diagnoses, New York City, 2003 to 2010
| Number of neighborhoods (ZIP codes) | Model 1 Crude OR (95 % CI) | Model 2 aOR (95 % CI) | Model 3 aOR (95 % CI) | Model 4 aOR (95 % CI) | |
|---|---|---|---|---|---|
| Males | |||||
|
|
|||||
| Change╪ in HIV testing coverage | |||||
| No/Low, (0.09 %) | 63 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium, (8.6 %) | 53 | 2.00 (0.85, 4.71) | 2.34 (0.93, 5.90) | 2.22 (0.92, 5.33) | 2.14 (0.86, 5.36) |
| Large, (18.0 %) | 55 | 3.05 (1.34, 6.97)** | 3.22 (1.31, 7.89)* | 3.16 (1.35, 7.40)** | 3.88 (1.57, 9.62)** |
| Change╪ in non-late HIV diagnoses | |||||
| No/Low, (−20 per 100,000 population) | 57 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |
| Medium, (−2.7 per 100,000 population) | 58 | 0.37 (0.16, 0.87)* | 0.34 (0.14, 0.82)* | 0.45 (0.18, 1.15) | |
| Large, (7.1 per 100,000 population) | 56 | 0.81 (0.37, 1.76) | 0.71 (0.32, 1.57) | 1.03 (0.43, 2.47) | |
| Borough of residence at HIV diagnosis | |||||
| Queens | 57 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |
| Bronx | 39 | 5.09 (1.80, 14.4)** | 6.63 (2.18, 20.14)** | 6.62 (2.12,20.60)** | |
| Brooklyn | 38 | 1.62 (0.60, 4.37) | 2.13 (0.75, 6.04) | 2.08 (0.73, 5.96) | |
| Manhattan | 25 | 3.08 (1.49, 9.69)** | 1.94 (1.83, 13.35)** | 4.37 (1.57,12.11)** | |
| Staten Island | 12 | 0.94 (0.18, 4.96) | 1.10 (0.20, 6.12) | 1.05 (0.19, 05.98) | |
| Females | |||||
|
|
|||||
| Change╪ in HIV testing coverage | |||||
| No/Low, (0.05 %) | 63 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Medium, (8.0 %) | 51 | 1.46 (0.56, 3.77) | 1.27 (0.47, 3.45) | 1.03 (0.37, 2.88) | 0.97 (0.32, 2.92) |
| Large, (15.6 %) | 57 | 1.72 (0.70, 4.27) | 1.46 (0.57, 3.79) | 1.61 (0.60, 4.34) | 1.52 (0.54, 4.31) |
| Change╪ in non-late HIV diagnoses | |||||
| No/Low, (−22 per 100,000 population) | 58 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |
| Medium, (−2.2 per 100,000 population) | 79 | 0.17 (0.07, 0.44)** | 0.18 (0.07, 0.46)** | 0.18 (0.07, 0.48)** | |
| Large, (18 per 100,000 population) | 34 | 0.46 (0.16, 1.23) | 0.47 (0.17, 1.26) | 0.62 (0.21, 1.78) | |
| Borough of residence at HIV diagnoses | |||||
| Queens | 57 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |
| Bronx | 39 | 4.00 (1.21,13.17)* | 3.80 (1.11, 12.94)* | 2.53 (0.68, 09.35) | |
| Brooklyn | 38 | 3.34 (1.11, 09.99)* | 3.72 (1.18, 11.76)* | 3.99 (1.17, 13.59)* | |
| Manhattan | 25 | 2.27 (0.72, 07.16) | 2.32 (0.73, 07.37) | 2.79 (0.37, 15.83) | |
| Staten Island | 12 | 1.70 (0.30, 09.67) | 2.10 (0.35, 12.64) | 2.44 (0.37, 15.83) | |
p<.05,
p<.01,
mean of the continuous distribution within each tertile is reported in (parentheses)
OR= Odds Ratio, aOR= Adjusted Odds Ratio, CI= Confidence Interval
Model 1: Crude association for each variable
Model 2: Change in HIV testing + Change in non-late HIV diagnoses
Model 3: Change in HIV testing + NYC Borough
Model 4: Change in HIV testing + Change in non-late HIV diagnoses + NYC Borough
References
- 1.The White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States. Washington, DC: The White House; 2010. p. 45. [Google Scholar]
- 2.United States Department of Health & Human Services. Secretary Sebelius approves indicators for monitoring HHS-funded HIV services. Washington, DC: United States Department of Health & Human Services; 2013. [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV surveillance report, 2011. Vol. 23. Atlanta, GA: 2013d. table 24. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Vital signs: HIV testing and diagnosis among adults-United States, 2001–2009. Morb Mortal Wkly Rep. 2010a;59:1541–1571. [PubMed] [Google Scholar]
- 5.Sabin CA, Smith CJ, Youle M, Lampe FC, Bell DR, Puradiredja D. Deaths in the era of HAART: Contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. AIDS. 2006;20:67–71. doi: 10.1097/01.aids.0000196178.73174.24. [DOI] [PubMed] [Google Scholar]
- 6.Sabin CA, Smith CJ, Gumley H, Murphy G, Lampe FC, Phillips AN, et al. Late presenters in the era of highly active antiretroviral therapy: Uptake of and responses to antiretroviral therapy. AIDS. 2004;18:2145–2151. doi: 10.1097/00002030-200411050-00006. [DOI] [PubMed] [Google Scholar]
- 7.Price RW, Yiannoutsos CT, Clifford DB, Zaborski L, Tselis A, Sidtis JJ, et al. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS. 1999;13:1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- 8.Fleishman JA, Yehia BR, Moore RD, Gebo KA. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48:1071–1079. doi: 10.1097/MLR.0b013e3181f81c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krentz H, Auld M, Gill M. The high cost of medical care for patients who present late (CD4< 200 cells/μL) with HIV infection. HIV Med. 2004;5:93–98. doi: 10.1111/j.1468-1293.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 10.Losina E, Figueroa P, Duncan J, Divi N, Wolf LL, Hirschhorn LR, et al. HIV morbidity and mortality in Jamaica: Analysis of national surveillance data, 1993–2005. INT J Infect Dis. 2008;12:132–138. doi: 10.1016/j.ijid.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrosbree J, Campbell LJ, Ibrahim F, Hopkins P, Vizcaychipi M, Strachan S, et al. Late HIV diagnosis is a major risk factor for intensive care unit admission in HIV-positive patients: A single centre observational cohort study. BMC Infect Dis. 2013;13:23. doi: 10.1186/1471-2334-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montlahuc C, Guiguet M, Abgrall S, Daneluzzi V. Impact of late presentation on the risk of death among HIV-infected people in France (2003–2009) JAIDS. 2013;64:197–203. doi: 10.1097/QAI.0b013e31829cfbfa. [DOI] [PubMed] [Google Scholar]
- 13.Girardi E, Sabin CA, Antonella d’Arminio Monforte M. Late diagnosis of HIV infection: Epidemiological features, consequences and strategies to encourage earlier testing. JAIDS. 2007;46:S3–S8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Late HIV Testing- 34 States, 1996–2005. Morb Mortal Wkly Rep. 2009;58:661–665. [PubMed] [Google Scholar]
- 15.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 16.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26:893–896. doi: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Results of the expanded HIV testing initiative-25 jurisdictions, United States, 2007–2010. Morb Mortal Wkly Rep. 2011a;60:805–833. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. HIV testing at CDC-funded sites, United States, Puerto Rico, and the U.S. Virgin Isands, 2010. Atlanta, GA: CDC; 2012. [Google Scholar]
- 19.Paltiel AD, Walensky RP, Schackman BR, Seage GR, Mercincavage LM, Weinstein MC, et al. Expanded HIV screening in the United States: Effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 20.Myers JE, Braunstein SL, Shepard CW, Cutler BH, Mantsios AR, Sweeney MM, et al. Assessing the impact of a community-wide HIV testing scale-up initiative in a major urban epidemic. JAIDS. 2012;61:23–31. doi: 10.1097/QAI.0b013e3182632960. [DOI] [PubMed] [Google Scholar]
- 21.Fleming PL, Ward JW, Janssen RS, De Cock KM, Valdiserri RO, Gayle HD, et al. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Morb Mortal Wkly Rep. 1999;48:1–28. [PubMed] [Google Scholar]
- 22.Nash D, Ramaswamy C, Manning S. Implementation of named HIV reporting-New York City, 2001. Morb Mortal Wkly Rep. 2004;52:1248–1252. [PubMed] [Google Scholar]
- 23.State of New York Laws Chapter 308. HIV testing and counseling amendment to New York State public health law article 21. In: Title 10; 2010.
- 24.Torian LV, Wiewel EW, Liu K-L, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;168:1181–1187. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years, United States, 2008. Morb Mortal Wkly Rep. 2008;57:1–8. [PubMed] [Google Scholar]
- 26.New York City Department of Health and Mental Hygiene. The New York City Community Health Survey. New York City, NY: Department of Health and Mental Hygiene; 2013. Survey Data on the Health of New Yorkers. [Google Scholar]
- 27.New York City Department of Health and Mental Hygiene. New York City United Hospital Fund (UHF) neighborhoods and NYC zip code areas. New York City, NY: New York City Department of Health and Mental Hygiene; 2006. [Google Scholar]
- 28.Buchholz N, Resnic S, Konty K. New York City Community Health Survey Atlas, 2010. New York City, NY: The New York City Department of Health and Mental Hygiene; 2012. [Google Scholar]
- 29.New York City Department of Health and Mental Hygiene. Community Health Survey: Public use data. New York City, NY: New York City Department of Health and Mental Hygiene; 2013. [Google Scholar]
- 30.Scott LM, Janikas MV. Spatial statistics in ArcGIS. In: Fischer M, Getis A, editors. Handbook of applied spatial analysis: Software tools, methods, and applications. New York, NY: Springer; 2010. pp. 27–41. [Google Scholar]
- 31.ESRI. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute; 2011. [Google Scholar]
- 32.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 33.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 34.New York City Department of Health and Mental Hygiene. New York City HIV/AIDS Surveillance Slide Sets. New York City, NY: Department of Health and Mental Hygiene; 2013. HIV/AIDS in New York CIty, 2007–2011. [Google Scholar]
- 35.United States Department of Health and Human Services. HIV screening for pregnant women. Rockville, MD: Health Resources and Services Administration; 2012. [Google Scholar]
- 36.New York City Department of Health and Mental Hygiene. Brooklyn knows HIV testing initiative. New York: NYC DOHMH; 2010. [Google Scholar]
- 37.New York City Department of Health and Mental Hygiene. The Bronx Knows HIV testing initiative final report. New York City, NY: Department of Health and Mental Hygiene; 2011. [Google Scholar]
- 38.New York City Department of City Planning. Peripheral travel study. New York, NY: Transportation Division; 2010. [Google Scholar]
- 39.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4 cell count at presentation to HIV care, 1992–2011. Clin Infect Dis. 2013;57:1027–1037. doi: 10.1093/cid/cit421. [DOI] [PubMed] [Google Scholar]
- 40.Castel AD, Greenberg AE, Befus M, Willis S, Samala R, Rocha N, et al. Temporal association between expanded HIV testing and improvements in population-based HIV/AIDS clinical outcomes, District of Columbia. AIDS care. 2013;26:785–789. doi: 10.1080/09540121.2013.855296. [DOI] [PubMed] [Google Scholar]
- 41.Nunn A, Yolken A, Cutler B, Trooskin S, Wilson P, Little S, et al. Geography should not be destiny: Focusing HIV/AIDS implementation research and programs on microepidemics in US neighborhoods. Am J Public Health. 2014;104:775–780. doi: 10.2105/AJPH.2013.301864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.New York City Department of Health and Mental Hygiene. HIV Epidemiology & Fiend Services Surveillance Statistics. New York City, NY: New York CIty Department of Health and Mental Hygiene; 2015. HIV/AIDS annual surveillance statistics. [Google Scholar]
- 43.Schnizlein-Bick CT, Spritzler J, Wilkening CL, Nicholson JK, O’Gorman MR. Evaluation of TruCount absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus-positive adults. Clin Diagn Lab Immunol. 2000;7:336–343. doi: 10.1128/cdli.7.3.336-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaner JSG, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Expanded HAART coverage is associated with decreased population-level HIV-1-RNA and annual new HIV dignoses in British Columbia, Canada. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–335. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dromer F, Mathoulin-Pélissier S, Fontanet A, Ronin O, Dupont B, Lortholary O, et al. Epidemiology of HIV-associated cryptococcosis in France (1985–2001): Comparison of the pre-and post-HAART eras. AIDS. 2004;18:555–562. doi: 10.1097/00002030-200402200-00024. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 48.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 49.Harrison KM, Kajese T, Hall HI, Song R. Risk factor redistribution of the national HIV/AIDS surveillance data: an alternative approach. Public Health Rep. 2008;123:618–627. doi: 10.1177/003335490812300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention into HIV medical care in the United States are moderately high. Improvement in both outcomes will increase the success of a test and treat strategy. AIDS. 2010;24:2665–2678. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 51.Corey C, Eisenhower D, Immerwahr S, Konty K, Norton J, Sanderson M. Epi Research Report. New York, NY: New York City Department of Health and Mental Hygiene; 2010. Including New Yorkers who can only be reached by cell phones in the Community Health Survey: Results from the 2008 cell phone pilot survey. [Google Scholar]
- 52.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System (BRFSS): 2012 summary data quality report. Atlanta, GA: CDC; 2013. [Google Scholar]
- 53.Jelinski DE, Wu J. The modifiable areal unit problem and implications for landscape ecology. Landsc Ecol. 1996;11:129–140. [Google Scholar]