Abstract
Objective
Objective measurements of physical activity (PA), energy expenditure (EE) and energy intake can provide valuable information regarding appropriate strategies for successful sustained weight loss.
Design and methods
We examined total EE by doubly labeled water, resting metabolic rate, PA with activity monitors, and energy intake by the Intake/Balance technique in 116 severely obese undergoing intervention with diet alone (DO) or diet plus PA (D-PA).
Results
Weight loss of 9.6±6.8 kg resulted in decreased EE which was not minimized in the D-PA group. Comparing the highest and lowest quartiles of increase in PA revealed a lower decrease in TDEE (−122±319 vs. −376±305 kcal/d), elimination of the drop in AEE (83±279 vs. −211±284 kcal/d) and greater weight loss (13.0±7.0 vs. 8.1±6.3 kg). Increased PA was associated with greater adherence to energy restriction and maintenance of greater weight loss during months 7–12.
Conclusion
Noncompliance to prescribed PA in the DO and D-PA groups partially masked the effects of PA to increase weight loss and to minimize the reduced EE. Increased PA was also associated with improved adherence to prescribed caloric restriction. A strong recommendation needs to be made to improve interventions that promote PA within the context of behavioral weight loss interventions.
Introduction
A potential confounder in achieving and sustaining weight loss is that energy expenditure decreases with weight loss.1–4 It has been suggested that a decrease in leptin levels is involved in the decrease in RMR with weight loss, but this is not a consistent finding.5–7,14,30 Furthermore, addition of PA to weight loss programs has often, but not always, been shown to minimize the decrease in EE. 8–14
Although severe obesity is increasing more rapidly than moderate obesity,15,16 there are few reports of changes in EE in response to weight loss in this population. The effect of weight loss on EE in severely obese individuals after bariatric surgery has been reported in a few studies.17–19 For example, RMR decreased by 573 kcal/d following bariatric surgery (53 kg weight loss), while TDEE decreased by 860 kcal/d.19
The effect of increased PA on caloric intake is also not clear.20 In a 16 day study in which both intake and physical activity level were accurately assessed, subjects compensated for about 30% of the exercise-induced energy deficit.21 However, we could find no reports of the effect of increased physical activity on intake during long term prescribed caloric deficit when using objective measurement of both PA and EI.
To address these unresolved issues, we examined the effects of weight loss through diet alone or diet plus PA on changes in body composition, EE and EI in severely obese individuals. As previously reported, this intervention resulted in a 10% weight loss in the D-PA group, and 8.4% in the DO group at 12 months.22 We now focus on changes in physical activity, EE and EI during intervention in the D-PA and DO groups. In addition, we examined these relationships by quartiles of objectively assessed changes in PA regardless of intervention assignment.
Methods and Procedures
Design
A detailed description of participants and interventions has been reported.22 The study was approved by the human ethics committee of the University of Pittsburgh. Subjects were 47.8±6.4 y, 119.0±17.5 kg, with an average BMI of 43.6±5.4 kg/m2. Most subjects were female (87%), and 35% were African American. We focus primarily on the first 6 months of intervention, for which we have a complete dataset.
The intervention was a lifestyle intervention consisting of prescribed caloric restriction (CR), with one group randomized to increased PA for the entire 12 months (D-PA), while the other group had the increased PA delayed until the second 6 months, so that the first 6 months were diet only (DO).22 Prescribed intake was 1200–1500 kcal/d for those weighing <90.7kg, 1500–1800 kcal/d for those weight ≥ 90.7 but <113.4 kg, and 1800–2100 kcal/d for those weighing > 113.4 kg. For comparison purposes, prescribed energy intake was taken as the midpoint of the prescribed intake range. Moderate-intensity PA, similar in intensity to brisk walking, was prescribed and progressed to 60 minutes, 5 days per week.
Body weight, height and waist circumference were measured using standard protocols. Body composition was determined by dual energy X-ray absorptiometry (DXA; Prodigy, GE Healthcare, Madison, WI, USA) or by air displacement plethysmography (BodPod, COSMED, Chicago, IL, USA) in 24 subjects (both pre- and post-intervention) who exceeded the weight capacity of the DXA. The BodPod has been shown to be relatively accurate for assessing body composition and changes in body composition compared with DXA.23 Abdominal visceral adipose tissue and hepatic fat content were quantified at baseline and at 6 months using computed tomography as previously described.24 Serum leptin levels were assessed by ELISA (Human leptin ELISA kit, RnD Systems).
Total daily energy expenditure
TDEE was assessed at baseline and at 6 months using DLW as previously described.25 The rate of CO2 production was calculated 26, and total EE was calculated by multiplying by the energy equivalent of at an RQ of 0.86.
Resting Metabolic Rate
RMR was measured at baseline, 6 and 12 months by indirect calorimetry (ParvoMedics Inc., TrueOne® 2400 Metabolic Measurement System with Canopy, Sandy, Utah).25
Physical Activity
Multi-sensor physical activity monitors (Sensewear Pro3, BodyMedia, Pittsburgh, PA) were worn during the DLW periods and at 12 months. Parameters examined (SenseWear Pro armband software version 5.1) include time spent in moderate to vigorous physical activity (MVPA; >3 METS), in vigorous activity (6–9 METS), in sedentary (0–3 METS; therefore includes light activity and sedentary time) activity, hours of sleep, and steps/day. Only days in which monitors were worn > 85% of the day (97±2%) were included in the analyses (7.9±1.8 days). These devices have been shown to provide accurate for estimation of a variety of activities.27–30 In addition, we have previously observed lower minutes of moderate to very vigorous activity, greater steps/d, and greater time spent in sedentary behavior in the severely obese, which corresponded to a lower AEE.25
Energy expended in PA (AEE) was calculated by subtracting RMR and the thermic effect of food (10%) from TDEE.
Energy Intake
Energy intake was determined by applying the Intake/Balance (I/B) method31 as follows: 1) At baseline, energy requirements were assumed to equal TDEE, while during intervention the TDEE at baseline and month 6 were averaged, assuming a linear change over time. 2) Δ Energy Stores (energy deficit) = ΔFM x 9.3 kcal/g + ΔFFM x 1.1 kcal/g.32 3) EI = EE + Δ Energy stores.
Statistics
Subject characteristics and components of EE were compared using general linear model analysis of variance (GLM: SAS release 9.2 for Windows; SAS Institute Inc, Cary, NC). Data are presented as means±SD unless indicated otherwise. Various parameters were used in analysis of variance models of EE as covariates to adjust for differences in body composition. Change in EE components was analyzed with analysis of variance with and without adjustments. Post hoc tests for differences in group means were accomplished using Fisher’s Least Significant Difference test. Regression analysis was conducted on initial and follow-up EEs. Stepwise variable selection regression was used to develop models to explain the variance in and change in EE. Only significant predictors were able to enter the models. Race was coded as 0 for Caucasian and 1 for African American, and sex coded was as 1 for male and 2 for female. Data were further stratified by quartile of the increase in steps/d during the intervention. Chi-square tests were used to compare proportion of individuals achieving specific caloric restriction levels between groups.
Results
Intervention assignment
As previously reported, significant reductions in fat and FFM were observed after 6 months of intervention (Table 1)22. The group prescribed increased PA lost more weight and body fat. In response to weight loss there was a significant reduction in EE, which was not different by intervention group (Table 1). Prediction equations were developed using baseline data to determine whether the decrease in EE was greater than expected due to loss of weight or FFM and fat mass:
Table 1.
Change in parameters by intervention group at 6 months.*
| Intervention Group* | p | |||
|---|---|---|---|---|
| Parameter, change | DO (n=57) | D-PA (n=61) | Change | Group |
| weight, kg | −8.1 ± 5.9 | −11.0 ± 7.3 | 0.0001 | 0.02 |
| FFM, kg | −2.1 ± 2.7 | −2.4 ± 2.6 | 0.0001 | 0.53 |
| Fat, kg | −5.9 ± 4.5 | −8.6 ± 6.4 | 0.0001 | 0.01 |
| RMR, kcal/d | −114 ± 140 | −164 ± 201 | 0.0001 | 0.13 |
| RMR adj†, kcal/d | −62 ± 20 | −58 ± 20 | 0.005 | 0.87 |
| Leptin, ng/ml | −12.8 ± 19.8 | −12.2 ± 17.2 | 0.0001 | 0.84 |
| TDEE, kcal/d | −167 ± 308 | −209 ± 284 | 0.0001 | 0.45 |
| TDEE1 adj†, kcal/d | −119 ± 38 | −71 ± 37 | 0.002/0.055 | 0.37 |
| TDEE2 adj†, kcal/d | −98 ± 40 | −27 ± 39 | 0.02/0.49 | 0.21 |
| AEE, kcal/d | −36 ± 264 | −22 ± 288 | 0.33/0.55 | 0.78 |
| Steps, #/d | 406 ± 1841 | 1347 ± 2768 | 0.22/0.0001 | 0.05 |
Values are means ± SD. The p values indicate whether the observed change is significantly different from 0 for both intervention groups unless otherwise indicated for DO/D-PA, and whether the observed changes were significantly different by intervention group. Sample size reduced due to missing data for the following measures: TDEE and RMR (−1 for both DO and D-PA); AEE (−1 and −2); and Steps (−4 and −6).
LS Means ± SEM, adjusted using equations developed at baseline.
Decrease in RMR
When comparing RMR to predicted, RMR was still significantly lower after intervention, and the decrease was not minimized in the D-PA group (Table 1). The unadjusted decrease in RMR was correlated with loss of weight (r=0.62, p<0.0001), FFM (r=0.41, p<0.0001) and fat mass (r=0.57, p<0.0001), change in AEE (r=0.37, p<0.0001), and energy deficit (r=0.60, p<0.0001). When body composition changes were included, the first parameter to enter the model was change in body fat (partial R2=0.33) followed by change in FFM (partial R2=0.06), explaining 39% (p<0.0001) of the variance in RMR change, which was no better than the 39% of variation explained by weight loss. When each significant parameter was included in stepwise multiple regression, the first parameter to enter the model was energy deficit (partial R2=0.35) followed by the change in AEE (partial R2=0.12), and change in FFM (partial R2=0.05):
We next examined whether the decline in RMR was related to decreased leptin levels. There was a significant decrease in leptin with weight loss which was not different by intervention assignment (Table 1). The decrease in leptin was correlated with energy deficit (r=0.49, p<0.0001), decrease in body fat (r=0.46, p<0.0001) FFM (r=0.46, p<0.0001), and RMR (r=0.32, p<0.0005). When including change in FFM and fat mass, change in leptin did not enter the model for change in RMR. Furthermore, although change in leptin did enter with change in FFM after eliminating change in body fat, the variance explained was lower than when including change in body fat (R2=0.35 vs. R2=0.51).
Decrease in TDEE and AEE
Although the decrease in TDEE was not different between groups, the D-PA group lost more weight. Therefore, we examined whether adjusting for change in body weight or composition would reveal a difference. Examining the decrease in TDEE using either Equation revealed a significant decline in the DO group but not in the D-PA group (Table 1). While this indicated that PA minimized the decrease in TDEE, there was no significant difference between intervention groups.
There was no significant change in AEE during intervention in either group (Table 1). However, the activity monitors detected a significant increase in the number of steps/day in the D-PA group (Table 1).
Objective changes in physical activity
Since we anticipated that the addition of PA would minimize the decrease in EE in response to weight loss we examined objectively assessed changes in PA during intervention. Although counseled to maintain PA levels, over a third (38%) of the DO group showed an increase in steps of at least 1000/d and 19% had an increase of at least 2000 steps/d, equivalent to walking an additional mile/d. Furthermore, 40% of the D-PA group showed an increase of less than 500 steps/d, and 27% showed no increase or even a decrease.
Therefore, we examined the response to objectively assessed increase in PA, based on quartiles of increase in steps/day regardless of intervention assignment (Table 2). Although more D-PA participants were in the highest quartile (19 vs. 8), 23 (42%) were in the lowest 2 quartiles. Change in minutes of MVPA increased significantly across quartiles of change in steps. The prescribed PA for the D-PA group (60 minutes/d, 5 d/week) would have resulted in an average increase of 43 minutes of activity/d. Only 22% of the D-PA group achieved a mean increase in activity of 47 minutes/d, although 55% achieved an increase of at least 10 minutes/d. However, 40% of the D-PA had no change or a decrease in MVPA during intervention. A substantial proportion of participants in the DO group increased PA, with 8% achieving the minutes prescribed for the D-PA group, and 37% increasing PA by at least 10 minutes/d.
Table 2.
Change in parameters at 6 months by Quartile of change in number of steps/d.*
| Quartiles, Change in Number of Steps/d | ||||
|---|---|---|---|---|
| Parameter | 1 | 2 | 3 | 4 |
| N (DO/D-PA) | 15/12 | 15/11 | 14/13 | 8/19 |
| Steps #/d* | *−1886 ± 1431a | *87 ± 312b | *1507 ± 508c | *3821 ± 1762d |
| MVPA, min/d | *−47 ± 37a | −4.9 ± 21b | 14 ± 32b | *47 ± 94c |
| Sedentary, min/d | *61.8 ± 45.7a | 11.4 ± 38.1b | −7.7 ± 41.2b | *−52.2 ± 59.7c |
| RMR adjusted, kcal/d | *−81 ± 132 | *−83 ± 120 | −43 ± 161 | *−68 ± 167 |
| TDEE1 adjusted, kcal/d | *−129 ± 362 | *−115 ± 273 | *−156 ± 274 | −1 ± 227 |
| Waist, cm | *−4.6 ± 5.8 | *−7.0 ± 4.7 | *−7.3 ± 7.2 | *−8.6 ± 6.5 |
| Visceral fat, cm2 | −11.1 ± 50.5 | *−24.2 ± 46.3 | *−34.6 ± 49.8 | *−42.6 ± 65.2 |
| Liver fat, liver-spleen HU | 0.06 ± 0.14 | 0.06 ± 0.13 | *0.10 ± 0.16 | *0.14 ± 0.21 |
Values are mean change ± SD between baseline and 6 months. Means with different superscripts are significantly different.
HU = Hounsfield units. Liver fat measures at 6 months were missing due to scheduling issues for 1 subject in the Quartiles 1, 2 and 3 groups.
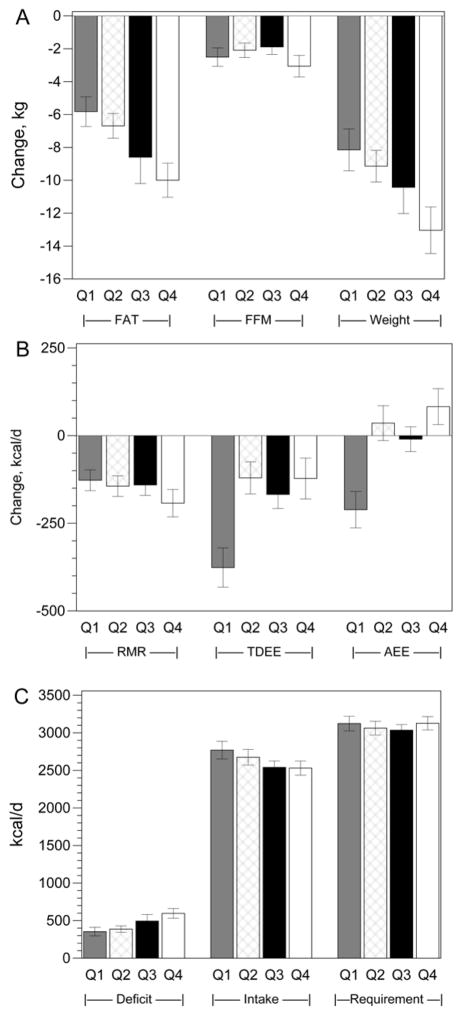
Weight loss and fat loss increased with greater increases in steps/d, but there was no difference in loss of FFM (Figure 1A). Neither the unadjusted (Figure 1B) nor the adjusted (Table 2) decrease in RMR differed by quartiles of change in steps/d. However, the decrease in TDEE was smaller and the decrease in AEE was completely eliminated in the upper 3 quartiles (Figure 1B).
Figure 1.
Response to objectively assessed change in physical activity. (Q1–Q4 = lowest to highest quartile of increase in steps/d). A) Change in body composition. fat: Q4 > Q2, Q1; Q3 vs. Q1, p<0.07. Weight loss: Q4 > Q1, Q2. B) Change in energy expenditure. RMR: NS, TDEE: Q4 < Q1, Q2, Q3; AEE: Q4 < Q1, Q2, Q3. C) Energy deficit: Q4 > Q1, Q2. Energy intake: Q4 vs Q1 p<0.08.
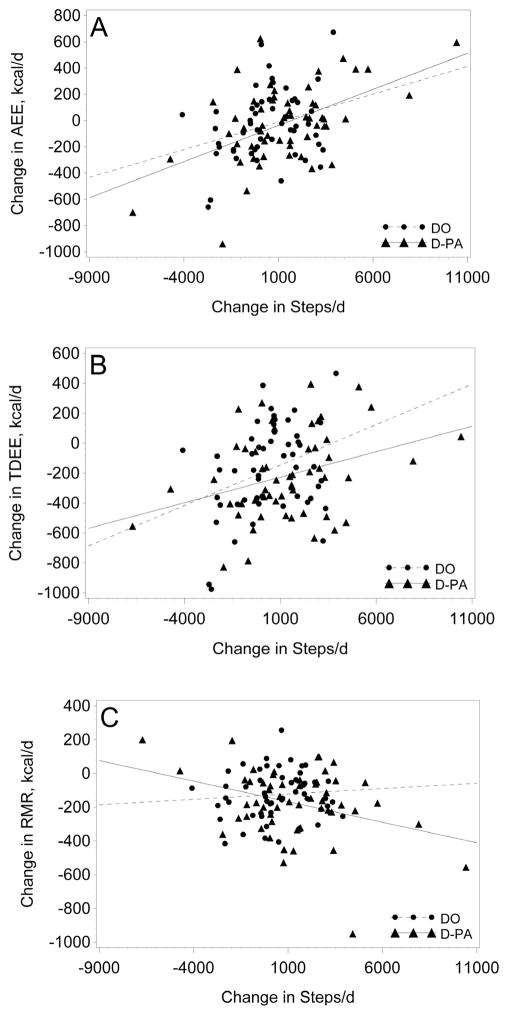
The relationship between change in PA (steps/day) and change in EE components are illustrated in Figure 2. There was a weak to modest relationship (R2 of 0.09 to 0.27) between change in steps/d and change in AEE and TDEE for both intervention groups, with the strongest relationship observed for AEE in the D-PA group (Figure 1A, R2=0.27, p<0.0001). There was no indication that PA minimized the decrease in RMR (Figure 2C). In fact, there was an inverse relationship between change in steps/d and decrease in RMR in the D-PA group.
Figure 2.
Relation between change in energy expenditure in response to intervention by change in steps/d. A) DO (R2=0.09, p<0.04): AEE = 0.042 x Change in Steps −52; D-PA : (R2=0.27, p<0.0001): AEE = 0.055 x Change in Steps −92, B) DO (R2=0.11, p<0.02): TDEE = 0.054 x Change in Steps −200; D-PA : (R2=0.11, p<0.02); TDEE = 0.034 x Change in Steps −261, C) DO (p=0.55): D-PA: (R2=0.11, p<0.02); RMR = −0.0024 x Change in Steps −143.
Relation between PA and energy intake
We next examined the effect of PA on measured EI. Although participants in both intervention groups consumed significantly lower EI than requirements, they consumed approximately 48% more than prescribed. However, 71% of the D-PA group achieved at least 10% CR, and 31% achieved at least 20% CR. Fewer participants in the DO group achieved at least 10% CR (59%), while a similar proportion reached 20% CR (29%). The average energy deficit (−503±367 vs. −356±259 kcal/d) and % CR during intervention (18.6±11.6% vs 14.0±11.7%) were significantly greater in the D-PA group.
To further explore this relationship, we examined EI by quartiles of change in steps/d. There appeared to be a trend for decreasing EI with increasing steps/d, but there were no significant differences (Figure 1C; Q4 vs Q1, 2530±462 vs 2770±576 kcal/d; p<0.08). However, increase in steps/d was associated with a lower intake during intervention (r=-0.19, p<0.05). In addition, a higher increase in steps/d was associated with greater adherence to the energy deficit based on prescribed EI and measured energy requirements (r=0.33, p<0.001).
Quartiles of weight loss
When examining response by quartile of weight loss, the decrease in EE was greatest in those losing the most weight (Table 3). While TDEE and RMR decreased more with greater weight loss, increased PA was also greatest in those who lost the most weight (Table 3). Those who lost the most weight had a lower EI and higher energy deficit. When modeling weight loss, both EI (partial r2=0.25; p<0.0001) and change in steps/d (partial r2=0.055; p<0.005) entered the model. When %CR, which adjusts EI for energy requirements (partial r2=0.28; p<0.0001), and initial weight (partial r2=0.24; p<0.0001) were included, change in steps/d still entered the model (partial r2=0.033; p<0.0001). Therefore, both PA and EI were important determinants of weight loss during intervention.
Table 3.
Response by Quartiles of Weight Loss
| Weight Loss Quartiles (month 1–6) | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| N (DO/D-PA) | 20/10 | 13/18 | 12/19 | 12/14 |
| Weight change, kg | *−2.1 ± 2.8d | *−7.2 ± 1.0c | *−11.4 ± 1.3b | *−19.1 ± 5.6a |
| Change in Energy Expenditure and Physical Activity | ||||
| TDEE, kcal/d | −9 ± 258c | *−170 ± 301b | *−185 ± 264b | *−408 ± 228a |
| TDEE, adjusted, kcal/d | 42 ± 149b | −88 ± 249ab | *−111 ± 278a | *−228 ± 366a |
| RMR, kcal/d | −42 ± 132c | *−117 ± 137bc | *−133 ± 143b | *−283 ± 207a |
| RMR, adjusted, kcal/d | −16 ± 141b | −29 ± 135b | *−69 ± 139ab | *−134 ± 171a |
| Steps/day | −9 ± 258c | *−170 ± 301b | *−185 ± 264b | *−408 ± 228a |
| Energy Requirements and Intake During Intervention | ||||
| Requirements, kcal/d | 3125 ± 419 | 3029 ± 389 | 3110 ± 499 | 3119 ± 384 |
| Energy Intake, kcal/d | 3013 ± 419c | 2707 ± 423b | 2627 ± 507b | 2250 ± 334a |
| Energy deficit, kcal/d | *−109 ± 114d | *−319 ± 129c | *−483 ± 136b | *−868 ± 319a |
| Calorie Restriction, % | *3.4 ± 6.1d | *13.1 ± 5.1c | *18.1 ± 5.9b | *32.1 ± 7.9a |
Mean change ± SD is significantly different from 0. Means within rows with different superscripts are significantly different.
Second 6 months of intervention
During months 7–12 of intervention, the DO group was instructed to increase PA to that prescribed for the D-PA group, while the D-PA group was to maintain their increased PA. The DO group significantly increased PA while there was no change in the D-PA group (Table 4). A significant decrease in body weight and body fat were observed only in the DO group. Increased weight loss and fat loss were observed with increasing quartiles of change in steps/d. However, there was no further decrease in RMR.
Table 4.
Changes in response to months 7 to 12 of intervention.
| Month 7–12 Change in Parameter | Intervention Group | Quartiles of increase in steps/d | ||||
|---|---|---|---|---|---|---|
| Group (n) | DO (52) | D-PA (49) | 1 (23) | 2 (24) | 3 (23) | 4 (23) |
| Steps, #/d | *767 (1987) | −124 (2479) | *−2412a (1817) | −243b (268) | *896c (508) | *3090d (1230) |
| MVPA, min/d | 9.4 (40.9) | 1.6 (47.4) | *−39.0a (43.8) | 0.5b (27.8) | 11.3b (23.5) | *49.5c (26.8) |
| Sedentary, min/d | −13.9 (54.3) | −4.6 (44.9) | *40.5a (45.6) | −6.7b (35.6) | *−18.4b (39.3) | *−52.6c (27.9) |
| weight, kg | *−2.1 (5.5) | −1.2 (6.6) | 1.1a (4.0) | −2.2ab (4.0) | −1.9ab (6.9) | *−4.4b (6.2) |
| FFM, kg | −0.2 (1.8) | −0.3 (1.8) | −0.2 (1.9) | −0.4 (1.9) | −0.4 (1.5) | −0.1 (1.9) |
| Fat, kg | *−1.6 (4.8) | −0.4 (6.1) | 1.4a (3.5) | −1.1ab (5.6) | −1.3ab (6.6) | *−3.8b (5.5) |
| Waist, cm | *−3.28 (6.65) | *−1.84 (6.27) | −1.45a (4.14) | *−3.22ab (5.73) | −0.92a (6.96) | *−5.96b (7.41) |
| RMR, kcal/d | −18 (128) | −10 (141) | −4 (143) | −4 (112) | 10 (125) | −45 (114) |
Values are means (SD). Means with * indicate significant (p<0.05) change. Means with different superscripts, between intervention group, or between quartiles of increase in steps/d are significantly different.
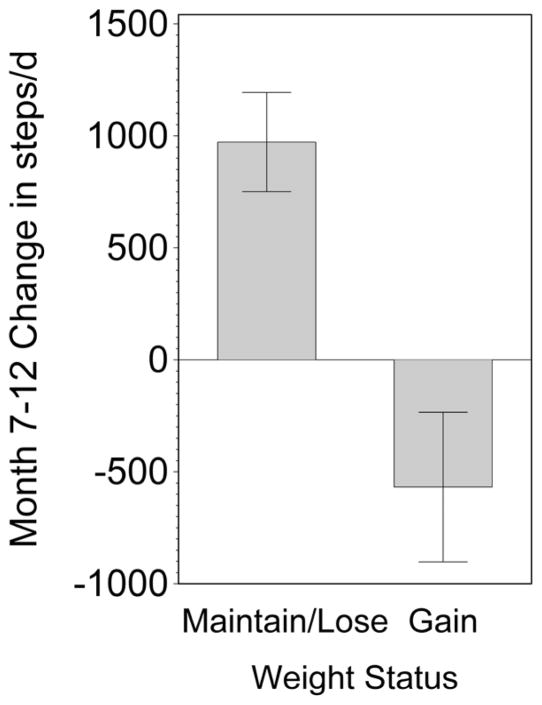
While a majority of participants maintained or continued to lose weight during month 7–12 (n=54; −5.2±5.7 kg), a substantial number gained weight (n=39; +2.9±2.2 kg). There was a significant difference (p<0.001) in change in PA over this period, with those who maintained weight loss showing increased PA while those who gained weight decreased their PA (Figure 3). Minutes of MVPA was unchanged (p=0.22) in those gaining weight but increased (p<0.01) in those maintaining or continuing to lose weight (−8.3±41 vs. 15.5±44 minutes/d; p<0.01). Furthermore, greater increases in steps/d (r = −0.29, p<0.007) and minutes of vigorous activity (r= −0.27, p<0.01) were associated with weight loss.
Figure 3.
Physical Activity and Weight Loss Maintenance. Significant difference (p<0.001) in change in PA between those who lost or maintained weight loss (n=54; −5.2±5.7 kg) showing increased PA, while those who gained weight (n=39; +2.9±2.2 kg) decreased PA.
Effect of intervention on central and liver fat
Since we had previously shown that waist circumference and liver fat were reduced more in the D-PA group22, we examined the effects of objectively assessed PA (Table 2). Although change in steps/d was inversely associated with change in waist circumference (−0.29; p<0.003) there was no significant difference between quartiles of steps/d. Visceral fat was significantly reduced in the upper 3 quartiles. Liver fat was significantly reduced in only the highest 2 quartiles (p<0.003). Furthermore, regression analysis revealed that even after accounting for the effect of energy deficit (partial R2 = 0.17; p<0.0001) or fat loss (partial R2 = 0.16; p<0.0001), increased physical activity (increased steps/d) was also related to a reduction in liver fat (partial R2 = 0.04; p<0.03, in either the energy deficit or fat loss model).
Discussion
Adherence to prescribed caloric intake
Behavioral interventions resulting in as little as 5% weight loss have been shown to significantly reduce or eliminate disorders associated with obesity.33 The goal of the successful Diabetes Prevention Program (DPP) trial was a 7% weight loss34 and this goal was achieved (7.2% weight loss) and resulted in a significant reduction in development of diabetes.35 This weight loss is normally achieved by prescribing an energy deficit of 500–1000 kcal, with energy requirements calculated from initial body weights. However, actual energy requirements and adherence to prescribed intake are generally not known.
One key aspect of our study was to objectively measure the degree of caloric restriction during intervention. We applied objective measures of energy requirements (longitudinal measures of TDEE by DLW) and intake using the I/B technique during intervention. We show that the intervention induced significant reductions in intake, resulting in a mean energy deficit of 433±327 kcal/d and a weight loss of 9.6 ± 6.8 kg (8.6±5.4% weight loss) over the first 6 months of intervention, which exceeded our 12 month target weight loss of 7%. Based on measured energy requirements during intervention, our prescribed caloric deficit (1419±487 kcal/d) was approximately 3 fold greater than what was achieved, but did result in the target weight loss. However, it may be that a nearly 3 fold greater prescribed deficit is needed to get subjects to achieve the necessary deficit.
Therefore, the adherence in our intervention study (which was similar to the DPP intervention) was typical, and was at least as good as that observed in the DPP study. Our results highlight that although actual energy intake is considerably higher than prescribed, we still achieved the intended weight loss which was similar to other successful weight loss interventions.
Adherence to prescribed physical activity
Our objective PA assessments demonstrated that only 22% of the D-PA group achieved the prescribed increase of 43 minutes of PA per day, while 40% showed no increase, or even a decrease in MVPA. There is a possibility that some of the apparent non-adherence to the PA prescription was a result of a compensatory decline in PA during the remainder of the day or on non-exercise days. 36,37 The other side of PA adherence is that the control group maintains their current level of PA. However, 8% of the control (DO) group increased minutes of MVPA equal to that prescribed for the D-PA group, and 37% increased PA by at least 10 minutes/d. These findings illustrate the problems that non-adherence can introduce when attempting to compare the metabolic effects of the addition of PA in weight loss intervention trials.
Effect of physical activity on intake during intervention
Objective measurements allowed us to examine the relationship between increased PA and intake.38 Energy intake during the intervention was not different between the two intervention groups. However, there was a significant inverse relationship between change in steps/d and EI, as well as a positive relationship between change in steps/d and adherence to prescribed energy deficit during intervention. Therefore, our results indicate that increased PA resulted in better adherence to a lower EI during intervention.
Physical activity and weight loss during intervention
Although we observed greater weight loss in the D-PA compared to DO group, the effect of increased PA on weight and fat loss were even greater in those achieving PA prescription. Therefore, the effect of increased PA to increase body weight loss was partially masked by non-adherence to PA prescription.
Examination of quartiles of weight loss during intervention revealed that although the decrease in EE was greatest in those losing the most weight, the increase in steps/d was also greatest in those losing the most weight. Modeling of the weight loss revealed that both caloric restriction and PA were important determinants of weight loss during intervention.
Relation between weight loss and decrease in energy expenditure
In response to weight loss, EE decreased, confirming previous reports in less obese individuals.2,3,39 There was no minimization of the decrease in EE in the group receiving the prescribed increase in PA, similar to previous reports.9–12 However, when examining participants stratified by objectively measured changes in activity level regardless of intervention assignment, increased PA partially minimized the decrease in TDEE, and totally eliminated the decrease in AEE (Figure 1B).
While the addition of PA minimized the decrease in TDEE and AEE, there was no indication of a reduction of the decrease in FFM or RMR. Furthermore, the drop in RMR was greatest in those losing the most weight, even though they were the most active (Table 3). Minimization of the decrease in RMR in response to weight loss has sometimes, but not always been observed.9–12 Results from a recent report of severely obese individuals support our findings that increased PA does not minimize the decrease in RMR in response to weight loss.14 The PA in that study was supervised, consisting of both aerobic and resistance training, with approximately 79% more PA (prescribed 77 min/d vs. 43 min/d in our study). Therefore, even greater aerobic exercise combined with resistance training may not minimize the decrease in RMR with weight loss. These findings are similar to observations over 20 years ago, when it was reported that supervised endurance exercise, weight training or a combination of the two failed to minimize the decrease in FFM or RMR in response to very low calorie diet induced weight loss.8
Relation between change in leptin levels and change in RMR
We examined the relationship between the drop in serum leptin levels and decrease in RMR in response to weight loss. Previous reports had suggested that a decrease in leptin levels may be responsible for the decrease in RMR observed with weight loss.5–7 While we found that the decrease in leptin was related to the decrease in RMR, it was not as strong as the relation between fat loss, and was not independently associated with decrease in RMR after accounting for the decrease in FFM and fat mass. Our finding that fasting leptin levels are not independently associated with the decrease in RMR has been observed by others 14,40. However, assessment of only fasting leptin levels may not capture the true effect of leptin on EE, as 24-h leptin has been shown to be independently related to the metabolic adaptation observed with weight loss.7
Effect of intervention on central and liver fat
We demonstrated that waist circumference and liver fat were reduced with greater increases in PA, and that PA had an independent effect on liver fat beyond that induced by the energy deficit or body fat loss. This finding is similar to a report that showed that increased PA resulted in a reduction in liver triglyceride content without changes in body weight or body fat.33
Physical activity and weight loss/maintenance during months 7–12 of intervention
Although a majority of participants continued to lose weight, 42% gained weight during the second 6 months of intervention. Those who maintained or continued to lose weight increased PA while those who gained weight decreased or maintained level of PA. These results suggest that increasing PA level is important in weight loss maintenance.
In conclusion, although participants consumed more than prescribed, they achieved significant reductions in EI resulting in nearly 10% weight loss. Non-adherence to the PA prescription in both the DO and D-PA groups masked the effect of increased PA to minimize the drop in TDEE and AEE in response to weight loss. This effect was revealed when examining EE by objectively assessed changes in PA. Increased PA was also associated with lower intake, greater adherence to caloric restriction and greater weight loss. In addition, increased PA during the second 6 months of intervention was associated with maintenance of, or increased weight loss. Finally, greater reductions in liver fat were observed with increased PA. Therefore, the addition of PA to a weight loss intervention leads to beneficial effects on both sides of the energy balance equation, resulting in greater weight loss, and potentially to greater weight loss maintenance.
What is already known about this subject?
Effective weight loss interventions generally result in 5–10% weight loss when prescribing an energy deficit of 500–1000 kcal/d.
Physical activity during intervention increases weight loss and may minimize the reduction in energy expenditure observed with weight loss.
The effect of increased physical activity on energy intake during intervention is not known.
What does this study add?
Adherence to prescribed caloric intake (essentially based on DPP) is really quite low, but does result in significant caloric restriction and weight loss. The degree to which participants do not adhere to the prescribed caloric intake during intervention is underappreciated because there are no objective measures of adherence to caloric restriction in most intervention studies. Therefore, our study providing objective assessment of actual caloric intake and relation to prescribed intake is important information for the obesity field.
Noncompliance to maintenance of current PA in the control group (DO) and increased physical activity in the D-PA group partially masked the effects of PA to increase weight loss and to minimize the reduced EE.
Increased PA was associated with lower intake, greater adherence to caloric restriction and greater weight loss. Increased PA during the second 6 months of intervention was associated with maintenance of, or increased weight loss.
Acknowledgments
This study was funded by the Commonwealth of Pennsylvania Department of Health.
The authors’ responsibilities were as follows — JPD, DEK, JMJ and BHG: design of the study; JPD, BHG, JMJ and KCH: collection of data; JPD: analysis of the data, wrote the manuscript, and had primary responsibility for final content; and all authors: interpretation of the data and critical revision of the manuscript.
Abbreviations
- EE
energy expenditure
- TDEE
total daily energy expenditure
- RMR
resting metabolic rate
- AEE
activity energy expenditure
- DLW
doubly labeled water
- FFM
fat free mass
- DXA
dual energy X-Ray absorptiometry
- TBW
total body water
- Q1–Q4
quartile of increase in steps/day
Footnotes
Conflicts of Interest
JMJ conflicts of interest: Scientific Advisory Board for ILSI North America. The other authors report no conflict of interest.
Reprints will not be available.
References
- 1.Benedict FG, Roth P. Effects of a Prolonged Reduction in Diet on 25 Men: I. Influence on Basal Metabolism and Nitrogen Excretion. Proc Natl Acad Sci U S A. 1918 Jun;4(6):149–152. doi: 10.1073/pnas.4.6.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 3.Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000 May 1;71(5):1138–1146. doi: 10.1093/ajcn/71.5.1138. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay A, Chaput J-P. Adaptive reduction in thermogenesis and resistance to lose fat in obese men. British Journal of Nutrition. 2009;102(04):488–492. doi: 10.1017/S0007114508207245. [DOI] [PubMed] [Google Scholar]
- 5.Doucet E, St Pierre S, Almeras N, Mauriege P, Richard D, Tremblay A. Changes in energy expenditure and substrate oxidation resulting from weight loss in obese men and women: is there an important contribution of leptin? J Clin Endocrinol Metab. 2000 Apr;85(4):1550–1556. doi: 10.1210/jcem.85.4.6500. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002 May;87(5):2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 7.Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011 Sep;96(9):E1512–1516. doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM. Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females. The American Journal of Clinical Nutrition. 1991 Jul 1;54(1):56–61. doi: 10.1093/ajcn/54.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Bryner RW, Ullrich IH, Sauers J, Donley D, Hornsby G, Kolar M, et al. Effects of Resistance vs. Aerobic Training Combined With an 800 Calorie Liquid Diet on Lean Body Mass and Resting Metabolic Rate. J Am Coll Nutr. 1999 Apr 1;18(2):115–121. doi: 10.1080/07315724.1999.10718838. [DOI] [PubMed] [Google Scholar]
- 10.Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997 Apr;65(2):269–277. doi: 10.1037//0022-006x.65.2.269. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JL, Manore MM, Thomas JR. Effects of diet and diet-plus-exercise programs on resting metabolic rate: a meta-analysis. Int J Sport Nutr. 1996 Mar;6(1):41–61. doi: 10.1123/ijsn.6.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. Journal of applied physiology. 1995 Sep;79(3):818–823. doi: 10.1152/jappl.1995.79.3.818. [DOI] [PubMed] [Google Scholar]
- 13.Redman LM, Heilbronn LK, Martin CK, deJonge L, Williamson DA, DeLany JP, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE 2009. 2009;4(2):e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012 Jul;97(7):2489–2496. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003 Oct 13;163(18):2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 16.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007 Jul;121(7):492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coupaye M, Bouillot JL, Coussieu C, Guy-Grand B, Basdevant A, Oppert JM. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obesity surgery. 2005 Jun-Jul;15(6):827–833. doi: 10.1381/0960892054222768. [DOI] [PubMed] [Google Scholar]
- 18.Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Annals of surgery. 2006 Nov;244(5):715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das SK, Roberts SB, McCrory MA, Hsu LKG, Shikora SA, Kehayias JJ, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003 Jul 1;78(1):22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proceedings of the Nutrition Society. 2003;62(03):651–661. doi: 10.1079/PNS2003286. [DOI] [PubMed] [Google Scholar]
- 21.Whybrow S, Hughes DA, Ritz P, Johnstone AM, Horgan GW, King N, et al. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. Br J Nutr. 2008 Nov;100(5):1109–1115. doi: 10.1017/S0007114508968240. [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster BH, DeLany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, et al. Effects of Diet and Physical Activity Interventions on Weight Loss and Cardiometabolic Risk Factors in Severely Obese Adults: A Randomized Trial. JAMA : the journal of the American Medical Association. 2010 Oct 27;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frisard MI, Greenway FL, DeLany JP. Comparison of methods to assess body composition changes during a period of weight loss. Obesity research. 2005 May;13(5):845–854. doi: 10.1038/oby.2005.97. [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999 Apr 1;48(4):839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 25.Delany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. High energy expenditure masks low physical activity in obesity. International journal of obesity (2005) 2013 Oct 23; doi: 10.1038/ijo.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994 Oct;267(4 Pt 1):E585–590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 27.Vyas N, Farringdon J, Andre D, Stivoric J. Machine Learning and Sensor Fusion for Estimating Continuous Energy Expenditure. Ai Mag. 2012 Sum;33(2):55–66. [Google Scholar]
- 28.Jakicic JM, Marcus M, Gallagher KI, Randall C, Thomas E, Goss FL, et al. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Medicine and science in sports and exercise. 2004 May;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 29.Berntsen S, Hageberg R, Aandstad A, Mowinckel P, Anderssen SA, Carlsen K-H, et al. Validity of physical activity monitors in adults participating in free-living activities. British Journal of Sports Medicine. 2010 Jul 1;44(9):657–664. doi: 10.1136/bjsm.2008.048868. [DOI] [PubMed] [Google Scholar]
- 30.Welk GJ, McClain JJ, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity (Silver Spring, Md ) 2007 Apr;15(4):918–928. doi: 10.1038/oby.2007.624. [DOI] [PubMed] [Google Scholar]
- 31.de Jonge L, DeLany JP, Nguyen T, Howard J, Hadley EC, Redman LM, et al. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr. 2007 Jan;85(1):73–79. doi: 10.1093/ajcn/85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. The American Journal of Clinical Nutrition. 2010 Dec 1;92(6):1326–1331. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackburn G. Effect of degree of weight loss on health benefits. Obesity research. 1995 Sep;3(Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 34.The Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002 Dec 1;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol. 1992;263(v):E950–E957. doi: 10.1152/ajpendo.1992.263.5.E950. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Nicklas BJ. Acute impact of moderate-intensity and vigorous-intensity exercise bouts on daily physical activity energy expenditure in postmenopausal women. J Obes. 2011;2011 doi: 10.1155/2011/342431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blundell JE, King NA. Physical activity and regulation of food intake: current evidence. Medicine and science in sports and exercise. 1999 Nov;31(11 Suppl):S573–583. doi: 10.1097/00005768-199911001-00015. [DOI] [PubMed] [Google Scholar]
- 39.Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, et al. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol. 1996;270(3 Pt 2):R496–R504. doi: 10.1152/ajpregu.1996.270.3.R496. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82(11):3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]