Abstract
Previous research associates smaller hippocampal volume with posttraumatic stress disorder (PTSD). It is unclear, however, whether treatment affects hippocampal volume or vice versa. Seventy-six subjects, 40 PTSD patients and 36 matched trauma-exposed healthy resilient controls, underwent clinical assessments and magnetic resonance imaging (MRI) at baseline, and 10 weeks later, during which PTSD patients completed ten weeks of Prolonged Exposure (PE) treatment. The resilient controls and treatment responders (n=23) had greater baseline hippocampal volume than treatment non-responders (n=17) (p=0.012 and p=0.050, respectively), perhaps due to more robust fear-extinction capacity in both the initial phase after exposure to trauma and during treatment.
Keywords: PTSD, Hippocampus, Prolonged Exposure Treatment, Resilience, Treatment Response, MRI
1. Introduction
Previous studies suggest smaller hippocampal volume is a heritable vulnerability for posttraumatic stress disorder (PTSD) (Gilbertson et al., 2002). Hippocampal volume has not evinced change over the course of PTSD post onset (Bonne et al., 2001), suggesting a trait-like quality. However, current research has not clarified whether treatment affects hippocampal volume (Apfel et al., 2011), or whether hippocampal volume influences treatment response (van Rooij et al., 2015).
Although hippocampal volume increases following psychopharmacological treatment (Vermetten et al., 2003), whether psychotherapy affects hippocampal volume is unclear. Whereas previous research indicated increased hippocampal volume following cognitive behavioral therapy (N=39) (Levy-Gigi et al., 2013) and Eye Movement and Desentization and Reprocessing (EMDR) (N=10) (Bossini et al., 2011), other studies failed to find any relationship between hippocampal volume changes and psychotherapy (Lindauer et al., 2005, (N=18); van Rooij et al., 2015, (N=47)).
If hippocampal volume predicts risk of developing PTSD after trauma, it may also influence treatment response. To examine this possibility, we evaluated patients with PTSD and resilient trauma-exposed healthy controls (TEHCs), matched for trauma type and demographic variables, using magnetic resonance imaging (MRI) at baseline and 10 weeks later, during which interval PTSD patients received Prolonged Exposure (PE), a first line cognitive-behavioral PTSD treatment (Foa et al., 2008). We hypothesized that larger baseline hippocampal volume would positively predict PE treatment response. We also assessed whether PE was associated with changes in hippocampal volume.
2. Methods
Seventy-six participants with adult trauma (PTSD=40, TEHC=36) received assessment by medical examination, the Structured Clinical Interview for DSM-IV Axis I Disorders (First and Gibbon, 2004), Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995), and Hamilton Depression Rating Scale (HAM-D-17). Participants answered the Life Events Checklist (LEC) to assess trauma history and determine duration and number of exposures to potentially traumatic events. PTSD group exclusion criteria included substance/alcohol dependence within the past six months, or abuse within the past two months; psychotropic medication use four weeks prior to participation; HAM-D >24, and CAPS <50. TEHC group exclusion criteria included current or past Axis I disorders and CAPS >19. PTSD participants’ index trauma was to have occurred after age 16. Participants in the PTSD and TEHC groups were matched on demographic variables and trauma type. Treatment response was defined a priori as ≥30% reduction from baseline CAPS score (Brady et al., 2000).
T1-weighted structural images were acquired on a 1.5 T GE Twin Speed MRI Scanner (TR/TE/Flip angle=7.25ms/3ms/7°; 1×1mm in plane × 1.3mm). After inspection for motion artifacts or gross abnormalities, volume values for both left and right hippocampus were obtained using Freesurfer 5.1(http://surfer.nmr.mgh.harvard.edu) standard surface-based reconstruction pipeline (Dale et al., 1999). PTSD participants received 10 weekly PE sessions. The New York State Psychiatric Institute Institutional Review Board approved all procedures, and all participants provided written informed consent.
Analyses compared three groups: treatment responders (n=23), TEHCs (n=36), and non-responders (n=17). Fourteen non-responders dropped out before PE ended (five patients dropped out pre-treatment; four after completing the PE psychoeducational component; and five after the first exposure session). Within the non-responder group, completers and dropouts did not differ on any demographic, clinical, or brain volume variables. Kruskal Wallis tests, t-tests, and one-way analyses of variance (ANOVAs) compared baseline clinical and demographic data. Baseline hippocampal volumes were compared among the responder, non-responder, and TE-HC groups using ANCOVA with post hoc LSD tests; a Group-by-Time repeated-measures ANCOVA compared post-treatment volume change. Both analyses controlled for age, sex, and total brain volume (TBV, included to rule out non-ROI specific trends). All tests were two-tailed with significance α <.05.
3. Results
The treatment responders, non-responders and TEHCs did not differ on demographics or number of traumatic events. In addition, there were no statistically significant baseline differences in total brain volume pre-F(2, 74)=0.070; p>0.25, or post-treatment F(2,50)=0.93; p>0.25. Expected clinical symptom differences and treatment effects appeared pre-(F (2, 74) =337; p<0.001), and post-treatment (F (2, 46) =27.0; p<0.001) (see Table 1).
Table1.
Demographic, Clinical, and MRI Characteristics
| Characteristic | PTSD Treatment Responders (n=23) |
PTSD Non Treatment Responders (n=17) |
Trauma Exposed Healthy Controls (n=36) |
Group Comparison |
|---|---|---|---|---|
| Age, mean (SD), y | 34.4 (8.5) | 37.5 (10.7) | 34.4 (10.8) | F(2, 75) = 0.61; p>.250 |
| Gender, female, % | 78 | 59 | 69 | H(2)=1.73; P > 0.250 |
| Ethnicity | H(2)=0.07; P > 0.250 |
|||
| White, n | 4 | 6 | 11 | |
| African American, n | 3 | 7 | 12 | |
| Hispanic, n | 13 | 4 | 12 | |
| Other, n | 3 | 0 | 1 | |
| Recency of trauma, mean (SD), y |
15.8 (14.4) | 12.4 (9.3) | 11.4 (12.5) | F(2, 75) = 0.87; p>0.250 |
| Age at primary trauma, mean (SD), y |
27.9 (8.8) | 32.1 (12.6) | 25.9 (10.2) | F(2, 75) = 2.09; p=0.130 |
| Total number of Traumatic Events, mean (SD) |
3.7 (3.6) | 3.8 (3.4) | 2.4 (2.4) | H(2)=2.91; P =0.220 |
| Trauma Type | ||||
| Natural Disaster, n | 10 | 4 | 9 | |
| Fire/Explosion, n | 4 | 3 | 2 | |
| Accident, n | 14 | 6 | 12 | |
| Toxic Exposure, n | 2 | 0 | 1 | |
| Physical Assault, n | 8 | 8 | 14 | |
| Assault w/ Weapon, n | 9 | 7 | 6 | |
| Sexual Assault, n | 11 | 7 | 5 | |
| Other Sexual Contact, n | 4 | 3 | 5 | |
| Combat, n | 4 | 2 | 2 | |
| Captivity, n | 5 | 1 | 1 | |
| Illness or Injury, n | 2 | 0 | 4 | |
| Severe Suffering, n | 0 | 2 | 1 | |
| Violent Death, n | 6 | 8 | 3 | |
| Unexpected Death, n | 7 | 5 | 17 | |
| Terrorism, n | 6 | 0 | 5 | |
| Other, n | 8 | 2 | 5 | |
| Baseline CAPS total score, mean (SD) |
81.3 (16.4) | 81.0 (14.5) | 3.2 (3.6) | F (2, 70) = 337; p<0.001 |
| Followup CAPS total score, mean (SD) |
23.0 (20.4) | 68.0 (10.8) | 3.3 (6.1) | F (2, 46) = 27.0; p<0.001 |
| Baseline HAM-D total score, mean (SD |
16.2 (5.7) | 16.5 (5.7) | 2.2 (2.4) | F (2, 70) = 90.1; p=<0.001 |
| Followup HAM-D total score, mean (SD |
8.2 (6.9) | 16.3 (5.7) | 2.9 (3.3) | F (2, 46) = 8.6; p=0.001 |
| Lifetime Alcohol Dependence, n |
2 | 1 | 0 | |
| Right Hippocampus, mean (SD) |
4164.8 (405.8) |
3912.2 (491.2) | 4221.1(443.4) | F (2, 71) =3.26; p=0.04a |
| Left Hippocampus, mean (SD) |
4032.9 (412.5) |
3848.1 (569.8) | 4093.3(339.5) | F (2, 71) =2.84; p=0.06a |
| Total Hippocampal volume, mean (SD) |
8197.7 (792.9) |
7760.2 (1042.1) |
8315.2(758.3) | F (2, 71) =3.42; p=0.038a, η2 = 0.088 |
Abbreviation: TEHC, Trauma Exposed Healthy Controls; CAPS, Clinician Administered PTSD Scale; HAM-D, Hamilton Rating Scale for Depression
Analysis controlled for age, sex, and total brain volume
bAnalysis controlled for age and sex
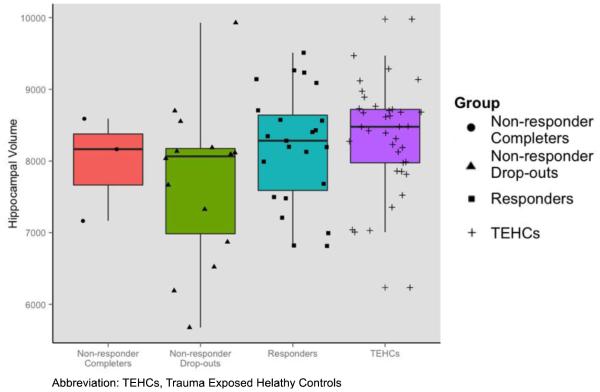
Baseline hippocampal volume showed a significant main effect of group F(2, 71)= 3.42, p=0.038, η2 = 0.088. Post hoc analyses indicated treatment responders had larger hippocampal volumes at trend level than non-responders (p=0.050), and TE-HCs had larger hippocampal volume than non-responders (p=0.012). Treatment responders and TE-HCs did not differ in hippocampal volume (p=0.686) (see Figure 1). Examination of longitudinal effects of PE treatment on hippocampal volume revealed no time × group interaction: F(2, 46)=0.16, p=0.850, η2 =0.007, suggesting that hippocampal volume did not change over the course of the treatment.
Figure 1.
Individual Hippocampal Volumes in Study Groups
4. Discussion
Our study found that PTSD patients responsive to PE treatment and trauma-exposed resilient controls had greater baseline hippocampal volume than treatment non-responders. These findings support evidence that suggests the hippocampus is key to contextual modulation of fear extinction (Ji and Maren, 2007), which is necessary for accurately distinguishing between contextual cues that signal safety and those that signal threat (Shin et al., 2006). Indeed, recent research linked deficits in this process to a smaller hippocampus (Negash et al., 2015). Taken together, our findings indicate that greater hippocampal volume may not only predict better prognosis when facing exposure to a traumatic event, but also better outcome of exposure-based PTSD treatments. This may be explained by the purported mechanism of change in PE, enhancement of safety discrimination during imaginal and in vivo exposure (Foa, 2007). We found no significant association between symptomatic improvement following Prolonged Exposure and change in hippocampal volume, corroborating some previous research (Lindauer et al., 2005; van Rooij et al., 2015).
Several study limitations warrant attention. While the between-group analysis was significant, the post-hoc analysis revealed only a trend level difference between responders and non-responders on baseline hippocampal volume. Additionally, the combination of MRI resolution and version of FreeSurfer used precluded collecting and analyzing hippocampal sub-region data. Other limitations include that we did not assess childhood trauma, overrepresentation (albeit non-significant) of females among PTSD responders, overall number of dropouts and, more specifically, the large number of drop-outs in the non-responder group, raising the possibility that the finding reflects a relationship between smaller hippocampal volume and treatment drop-out. Treatment attrition may reflect a difficulty tolerating exposure treatment (Imel et al., 2013), which may also be tied to similar underlying mechanisms as lack of response. The study deserves replication, and future studies may be advised to examine the issue of attrition vs. lack of response, which we were underpowered to explore. Despite these limitations, we provide new evidence extending current knowledge with regard to the relationship between hippocampal volume and PTSD. Future research might consider investigating whether smaller dorsal hippocampus (specifically, CA1 and CA3) subregions influence treatment response for PTSD, as lesions to these subregions have been previously associated with impaired contextual extinction (Ji and Maren, 2008), a process critical to exposure therapies. We suggest hippocampal volume is a clinically relevant trait in PTSD, potentially critical to the outcome of PE, a first-line PTSD treatment for war veterans and civilians.
Highlights.
PTSD patients responsive to prolonged exposure (PE) treatment and trauma-exposed resilient controls had greater hippocampal volume than treatment non-responders.
These findings support the notion that the hippocampus is key to contextual modulation of fear extinction, which is necessary for accurately distinguishing between contextual cues that signal safety and those that signal threat.
The study found no significant association between symptomatic improvement following PE and change in hippocampal volume.
Acknowledgments
Funding/Support: Preparation of this study was supported by R01 grants MH072833 and MH105355 from the National Institute of Mental Health (Dr. Neria, principal investigator), and the New York State Psychiatric Institute. Dr. Helpman is supported by National Institutes of Health (NIH) grant 5T32MH096724-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration Clinicaltrials.gov Identifier: NCT01576510
Author Contributions: Dr. Neria had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Neria, Papini, Rubin, Mann.
Acquisition, analysis, or interpretation of data: Rubin, Shvil, Papini, Helpman, Markowitz, Mann, Neria.
Drafting of the manuscript: Rubin, Neria, Papini, Helpman, Markowitz, Mann.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Rubin, Papini, Chhetry.
Obtained funding: Neria.
Administrative, technical, or material support: Neria.
Study supervision: Neria, Mann.
Conflict of Interest Disclosures: Dr. Mann receives royalties for commercial use of the C-SSRS from the Research Foundation for Mental Hygiene. No other disclosures were reported.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of sponsoring organizations, agencies, or the U.S. government.
References
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, Neylan TC. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol. Psychiatry. 2011;69(6):541–548. doi: 10.1016/j.biopsych.2010.09.044. doi:10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J. Traum. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. doi:10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. doi:10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis - I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. doi:10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) John Wiley & Sons; New York: 2004. [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. Oxford University Press; New York: 2007. [Google Scholar]
- Foa EB, Keane TM, Friedman MJ, Cohen JA. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. The Guilford Press; New York: 2008. [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. doi:10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imel ZE, Laska K, Jakupcak M, Simpson TL. Meta-analysis of dropout in treatments for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2013;81:394–404. doi: 10.1037/a0031474. doi:10.1037/a0031474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn. Mem. 2008;15:244–251. doi: 10.1101/lm.794808. doi:10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. doi:10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Negash S, Kliot D, Howard DV, Howard JH, Das SR, Yushkevich PA, Pluta JB, Arnold SE, Wolk DA. Relationship of contextual cueing and hippocampal volume in amnestic mild cognitive impairment patients and cognitively normal older adults. J Int Neuropsychol Soc. 2015;21:285–296. doi: 10.1017/S1355617715000223. doi:10.1017/S1355617715000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Gigi E, Szabó C, Kelemen O, Kéri S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biological Psychiatry. 2013;74:793–800. doi: 10.1016/j.biopsych.2013.05.017. doi:10.1016/j.biopsych.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Lindauer RJL, Vlieger E-J, Jalink M, et al. Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychol. Med. 2005;35:1421–1431. doi: 10.1017/S0033291705005246. http://doi.org/10.1017/S0033291705005246. [DOI] [PubMed] [Google Scholar]
- Shin LM. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. doi:10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol. Med. 2015;45:2737–2746. doi: 10.1017/S0033291715000707. doi:10.1017/S0033291715000707. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Southwick SM, Charney DS, Vythilingam M, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. doi:10.1016/S0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossini L, Tavanti M, Calossi S, Polizzotto NR, Vatti G, Marino D, Castrogiovanni PJ. EMDR treatment for posttraumaticstress disorder, with focus on hippocampal volumes: apilotstudy. Neuropsychiatry Clin Neurosci. 2011 doi: 10.1176/jnp.23.2.jnpe1. PMID:21677204. [DOI] [PubMed] [Google Scholar]