Abstract
Background
American Society of Clinical Oncology (ASCO) guidelines recommend only office visits and mammograms as the primary modalities for patient surveillance after treatment for breast carcinoma. We aimed to quantify differences in posttreatment surveillance among medical oncologists, radiation oncologists, and surgeons.
Methods
We emailed a survey to the 3245 ASCO members who identified themselves as having breast cancer as a major focus of their practice. Questions assessed frequency of use of 12 specific surveillance modalities for five posttreatment years.
Results
Of 1012 total responses, 846 were evaluable: 5% from radiation oncologists, 70% from medical oncologists and 10% from surgeons; 15% were unspecified. Marked variation in surveillance practices were noted within each specialty and among specialties.
Conclusion
There are notable variations in surveillance intensity. This suggests overuse and/or underuse and/or misuse of scarce medical resources.
Keywords: Breast Cancer, Surveillance, Follow-up, Surgeon, Medical oncologist, Radiation oncologist
Introduction
Breast carcinoma is the most commonly diagnosed cancer in the USA except for nonmelanoma skin cancer. It is a leading cause of cancer-related death in women worldwide. It is estimated that over 1.1 million men and women were diagnosed with breast cancer and that over 400,000 women died from it worldwide in 2002.1 In the United States, the relative five-year survival rate for breast cancer was 89% for the 1996–2004 period.2 There are more than two million breast cancer survivors in the US at present and posttreatment surveillance is warranted for essentially all of them.3 A steady increase in the number of breast cancer survivors is projected. This will increase costs and place a significant burden on those responsible for posttreatment surveillance.3
In wealthy countries, care of patients with potentially curable breast cancer is fairly well standardized. Two large, well-designed randomized controlled trials regarding posttreatment breast cancer surveillance have been published.4,5 In both trials, surveillance with annual mammograms and regular clinical visits alone was compared to an intensive surveillance strategy with additional tests such as chest x-rays, bone scans and liver ultrasonography. Both trials showed no significant difference in five-year mortality rates between the less intensive and more intensive surveillance groups. Since the available evidence shows that additional surveillance tests do not improve outcomes, American Society of Clinical Oncology (ASCO) guidelines currently recommend only office visits and mammograms for surveillance.6 Similar recommendations have been published by other authoritative sources.7,8,9
The primary purpose of our study was to measure the intensity of patient surveillance strategies used by expert clinicians after potentially curative treatment for breast carcinoma. We have previously documented marked variation in surveillance strategies among ASCO members who have breast cancer treatment as a primary clinical focus.10 In addition, these experts often recommend diagnostic tests not recommended by ASCO for surveillance of asymptomatic patients with no worrisome findings on physical examination after curative- intent therapy for breast carcinoma. We also sought to quantify a likely source of variation in posttreatment surveillance intensity, namely the variation among medical oncologists, radiation oncologists and surgical oncologists.
Methods
We designed a survey instrument with four vignettes depicting idealized generally healthy women with breast cancer of differing prognoses. In each vignette, the patient described had received curative-intent initial treatment but had a different American Joint Commission on Cancer stage, burden of disease, and/or biomarker profile: Stage 0 (TisN0M0), estrogen receptor (ER) positive, progesterone receptor (PR) positive ductal carcinoma in situ; Stage IIA (T2N0M0), ER positive, PR positive, HER2/neu nonamplified invasive ductal cancer; Stage IIA (T1N1M0), ER negative, PR negative, HER2/neu nonamplified invasive ductal cancer; and Stage IIIA (T3N2M0), ER positive, PR positive, HER2/neu amplified invasive ductal cancer.
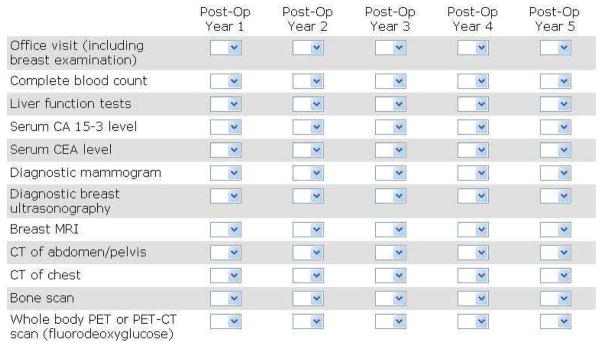
The survey featured questions based on these vignettes designed to quantify the surveillance practices of ASCO members. Using an online web-based technique (surveymonkey.com), the survey was emailed to the 3245 ASCO members who identified themselves as having breast cancer as a major focus of their practice. Each recipient was asked to indicate the number of annual office visits and surveillance tests he/she recommended for his/her own patients during posttreatment years 1 to 5 for each vignette. The list of 12 modalities given on the survey was compiled after a thorough review of the relevant literature and an informed evaluation by local experts indicated that the list was comprehensive (Table 1). Tests performed in the office and tests routinely performed in the hospital outpatient setting were all included. The survey instrument is available on request from margenthale@wudosis.wustl.edu.
Table 1. List of modalities offered on the survey.
This is how the questionnaire appeared on our survey instrument. A drop-down box of numbers was provided for each cell in the matrix to indicate the number of times a particular modality was recommended in a particular posttreatment year for one of the four vignettes in our survey.
On receipt of completed surveys, responses for all four vignettes were entered into a computer program (SAS 9.2 Enhanced Logging Facilities, Cary, NC) for statistical analysis. Mean, standard deviation, median and range of recommended frequency of use were calculated for each surveillance modality in each postoperative year for data from all four vignettes as a group. Repeated-measures analysis of variance (ANOVA) was used to judge whether the practices of medical oncologists, radiation oncologists and surgical oncologists differed significantly. This method of statistical analysis was chosen because it can be used when the same variable has been measured under different conditions on the same subjects.
Results
There were 1012 total responses; 846 were evaluable. There were 5% from radiation oncologists, 70% from medical oncologists, 10% from surgical oncologists, and 15% were unspecified. Marked variation in surveillance intensity was documented when results from all four vignettes were grouped and the composite data on follow-up practice patterns were evaluated.10 For this report we subdivided this dataset according to physician specialty. The most frequently used tests were office visit, mammogram, complete blood count, and liver function tests. The least frequently used tests were chest CT, abdomen CT, bone scan and PET scan.
We first assessed the frequency of recommended use for the two modalities endorsed by ASCO (office visit and mammogram). There was statistically significant variation in the recommended frequency of both surveillance modalities across specialties (Table 2). Although the intensities of surveillance among physicians of the three specialties were statistically significantly different, they were all clinically small. An example of the variation in recommended use of a modality not recommended by ASCO is liver function tests (Table 2). The intensities of recommended use of liver function tests were also statistically significantly different but clinically small. All ten modalities not recommended by ASCO guidelines were utilized by some clinicians in every posttreatment year, sometimes very often.10 To illustrate more fully the variation between and within specialties, we depicted the data as mean ± SD in Table 2 and as median and range in Table 3.
Table 2. Surveillance intensity – all evaluable responses for common surveillance modalities subdivided by physician specialty* (N=846).
These data show the variability in surveillance intensity among the three specialty groups (medical oncologists, radiation oncologists and surgeons) and within each group.
| Modality | Year Postsurgery | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Office visit | |||||
| Medical oncologist | 3.5 ± 1.6 | 3.0 ± 1.2 | 2.5 ± 1.2 | 2.3 ± 1.3 | 2.2 ± 1.4 |
| Radiation oncologist | 3.1 ± 1.4 | 2.7 ± 1.1 | 2.5 ± 1.5 | 2.2 ± 1.5 | 2.1 ± 1.5 |
| Surgical oncologist | 3.0 ± 1.7 | 2.6 ± 1.3 | 2.2 ± 1.2 | 1.9 ± 1.2 | 1.9 ± 1.2 |
| P value | <0.0005 | <0.0005 | <0.02 | <0.002 | <0.02 |
|
| |||||
| Mammogram | |||||
| Medical oncologist | 1.6 ± 1.6 | 1.5 ± 1.8 | 1.4 ± 1.9 | 1.3 ± 1.9 | 1.3 ± 2.0 |
| Radiation oncologist | 1.7 ± 0.9 | 1.4 ± 0.9 | 1.2 ± 1.3 | 1.2 ± 1.3 | 1.2 ± 1.3 |
| Surgical oncologist | 1.4 ± 0.6 | 1.3 ± 0.5 | 1.1 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| P value | 0.399 | 0.336 | 0.195 | 0.090 | 0.098 |
|
| |||||
| Liver function tests | |||||
| Medical oncologist | 2.3 ± 2.1 | 1.9 ± 1.8 | 1.7 ± 1.7 | 1.6 ± 1.7 | 1.5 ± 1.7 |
| Radiation oncologist | 1.4 ± 1.8 | 1.1 ± 1.3 | 1.0 ± 1.3 | 0.9 ± 1.1 | 0.9 ± 1.1 |
| Surgical oncologist | 1.0 ± 1.3 | 0.9 ± 1.2 | 0.8 ± 1.0 | 0.7 ± 0.9 | 0.7 ± 1.0 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
The number in each cell is the number of times a particular modality is recommended in a particular posttreatment year, displayed as mean ± SD. This depiction of the data gives a conservative impression of the variation in practices.
NS = not significant
Table 3. Surveillance intensity – all evaluable responses for three common surveillance modalities subdivided by physician specialty (N=846).
These data also show the variability in surveillance intensity among the three specialty groups (medical oncologists, radiation oncologists and surgeons) and within each group. They also show that some physicians in all three specialty groups recommend every modality during every posttreatment year, often very frequently.
| Modality | Year Postsurgery | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Office visit | |||||
| Medical oncologist | 4 (0 – 12) | 3 (0 – 12) | 2 (0 – 12) | 2 (0 – 12) | 2 (0 – 12) |
| Radiation oncologist | 3 (1 – 12) | 2 (1 – 6) | 2 (1 – 12) | 2 (1 – 12) | 2 (1 – 12) |
| Surgical oncologist | 2 (1 – 12) | 2 (0 – 12) | 2 (1 – 12) | 2 (1 – 12) | 2 (1 – 12) |
|
| |||||
| Mammogram | |||||
| Medical oncologist | 1 (0 – 12) | 1 (0 – 12) | 1 (0 – 12) | 1 (0 – 12) | 1 (0 – 12) |
| Radiation oncologist | 2 (1 – 6) | 1 (0 – 6) | 1 (1 – 12) | 1 (1 – 12) | 1 (1 – 12) |
| Surgical oncologist | 1 (0 – 3) | 1 (0 – 2) | 1 (0 – 2) | 1 (0 – 2) | 1 (0 – 2) |
|
| |||||
| Liver function tests | |||||
| Medical oncologist | 2 (0 – 12) | 2 (0 – 12) | 2 (0 – 12) | 1 (0 – 12) | 1 (0 – 12) |
| Radiation oncologist | 1 (0 – 12) | 1 (0 – 4) | 1 (0 – 4) | 1 (0 – 4) | 1 (0 – 4) |
| Surgical oncologist | 1 (0 – 6) | 0 (0 – 6) | 0 (0 – 4) | 0 (0 – 4) | 0 (0 – 5) |
The number in each cell is displayed as median and range (maximum, minimum). This depiction of the data emphasizes the variation in practices.
Discussion
The first aim of this study was to evaluate variation in the frequency of recommended use of currently available posttreatment surveillance modalities by physicians from different specialties. The second aim was to assess adherence to current ASCO guidelines. Our survey results document variation in recommended surveillance intensity among radiation oncologists, medical oncologists and surgeons. We believe this is the first report addressing this potential source of variation. We found marked variation in the use of surveillance methods within each of these specialties as well (Table 3). For patients treated with curative intent, many surveillance tests not recommended by ASCO guidelines are also routinely utilized by clinical experts, sometimes quite frequently, as previously reported.10
We recognize that questionnaires such as the one used in this study have limitations. Electronically distributing this survey to a relevant subset of ASCO members provided a rapid, cost-effective way to reach respondents and facilitated processing of the resulting data. However, of the 3245 members emailed, only 1012 members responded (31% response rate). This is a relatively low response rate for such surveys,11 suggesting that there was probably some nonresponse error. For example, only ASCO members who indicated that breast cancer was a major focus of their practice were surveyed. It is possible that the results presented here may not be representative of the entire ASCO membership. Other ASCO physicians and non-ASCO physicians who provide care to patients with breast cancer are not represented in this study. There are several other described sources of error in conventional surveys such as poor construct validity, coverage error, measurement error, sampling error and specification error.12,13 We believe these are not likely to be as important in this report as nonresponse error. 14 Calculation of confidence interval widths (margins of error) was felt to be inappropriate for our survey.13,14 There are other potential problems unique to electronic surveys. For example, they can be delivered to the junk or spam folder and never noticed. Recall bias (the risk of inaccuracy when survey respondents are asked to quantify their own practices) might well have occurred in our study. The actual practice of patient surveillance reported by ASCO members, the large majority of whom reside in wealthy countries, certainly differs from that of physicians residing in middle-income and low-income countries. Similarly, some ASCO members who practice in wealthy countries serve primarily low-income patients, those who are likely to lack insurance coverage, or otherwise differ from the usual clinical patient population of ASCO members. Their surveillance intensity is very likely to be limited. Regardless of these limitations, this survey provides valuable information. There are no other reports on this topic in the literature, as far as we are aware.
Our data clearly indicate that variation among specialties exists in postsurveillance treatment of breast carcinoma. Similar variation among specialty groups has been reported for management of sentinel lymph node micrometastases in breast cancer patients.15 Our results also suggest that overuse and underuse of surveillance modalities is prevalent. Improved medical education is needed to inform clinicians and trainees about the existing evidence from randomized controlled trials regarding optimal posttreatment surveillance intensity. It is also important that clinicians be aware that unnecessary testing should be avoided in order to prevent unwarranted risks, inconvenience and costs. Winchester notes that frequent organized, multidisciplinary breast cancer meetings tend to promote knowledge of, and adherence to, evidence-based and/or consensus-based standards. Such meetings also typically feature regular self-evaluation studies to ensure compliance with accepted standards.16 Variation in practice apparently unexplained by other factors is usually viewed as evidence of overuse, underuse, and/or misuse of scarce resources,17 so implementing and maintaining an evidence-based standardized surveillance regimen as recommended by ASCO guidelines and promoted at organized multidisciplinary breast cancer meetings could significantly decrease unwarranted variation. This should improve quality of life and prolong survival for patients at acceptable cost. Further efforts should aim to identify the sources of variation observed in this study and provide strategies to eliminate them. This should improve adherence to ASCO, European Society for Medical Oncology and other evidence-based guidelines.
The importance of posttreatment surveillance for all types of life-threatening chronic disorders such as cancer is under-appreciated and under-researched. The two large randomized trials of breast cancer surveillance4,5 are nearly two decades old and no longer fully relevant to the practice of breast cancer management. New diagnostic modalities, new treatments for relapse and new methods of primary breast cancer therapy are available. New trials are needed, although they are difficult and expensive to carry out. They should determine whether ASCO guideline-compliant patient care still represents best medical practice. Currently, although the National Institutes of Health and other relevant funding entities consider posttreatment patient surveillance as falling within their realm,18 they do not support comparative trials of low-intensity surveillance (the current evidence-based standard) with an alternative strategy. The public health community has long recognized that “if we want more evidence-based practice, we need more practice-based evidence.”19 Presumably, the community of patients, insurers, advocacy groups, health care professionals, and others will also have to advocate for adoption of enabling language in legislation to change the status quo. This is likely to be difficult. However, unless the evidence base upon which medical practice is founded can be improved, current variation in practices may not improve. Constraints on physician practices by medical management systems is another potential approach but one that has proven to be unpopular with physicians and patients.
Acknowledgments
The authors wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
A portion of this material was presented at the 6th annual Academic Surgical Congress meeting (February 1–3, 2011, Huntington Beach, CA), The 64th Annual Cancer Symposium of the Society of Surgical Oncology (March 2–5, 2011, San Antonio, TX) and the 35th annual meeting of the Association of VA Surgeons (April 10–12, 2011, Irvine, CA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyle P, Levin B. World Cancer Report 2008. Lyon, France: International Agency for Research on Cancer; 2008. p. 412. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Lash TL, Fox MP, Buist DSM, Wei F, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 4.The GIVIO Investigators. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients: A multicenter randomized controlled trial. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- 5.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer: A randomized trial. JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 6.Katcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 Update of the Breast Cancer Follow-Up and Management Guidelines in the Adjuvant Setting. J Clin Oncol. 2008;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 7.Aebi S, Davidson T, Gruber G, Castiglione M. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v9–v14. doi: 10.1093/annonc/mdq159. [DOI] [PubMed] [Google Scholar]
- 8.Bevers TB, Anderson BO, Bonaccio E, et al. Breast Cancer Screening and Diagnosis. JNCCN. 2009;7:1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 9.Rojas MPMP, Telaro E, Moschetti I, et al. Follow-up strategies for women treated for early breast cancer. 1. Oxford, United Kingdom: The Cochrane Library; 2009. Published online 21 Jan 2009. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001768.pub2/abstract. [Google Scholar]
- 10.Margenthaler JA, Allam E, Chen L, et al. Current practice patterns. 2011. Breast cancer patient surveillance after curative-intent primary treatment. JOP in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings SM, Savitz LA, Konrad TR. Reported response rates to mailed physician questionnaires. Health Serv Res. 2001;35:1347–1355. [PMC free article] [PubMed] [Google Scholar]
- 12.de Leeuw ED, Hox JJ, Dillman DA. International Handbook of Survey Methodology. New York, NY: Lawrence Erlbaum Associates; 2008. pp. 16–17. [Google Scholar]
- 13.Dillman DA, Smyth JD, Christian LM. Internet, mail and mixed-mode surveys. 3. Chapter 3. Hoboken, NJ: John Wiley & Sons; 2009. Coverage and Sampling. [Google Scholar]
- 14.Dillman DA. personal communication. Aug 11, 2011.
- 15.Wasif N, Ye X, Giuliano AE. Survey of ASCO members on management of sentinel node micrometastases in breast cancer: Variation in treatment recommendations according to specialty. Ann Surg Oncol. 2009;16:2442–2449. doi: 10.1245/s10434-009-0549-7. [DOI] [PubMed] [Google Scholar]
- 16.Winchester D. Post-treatment surveillance of breast cancer patients in an organized multidisciplinary setting. J Surg Oncol. 2011;103:358–361. doi: 10.1002/jso.21713. [DOI] [PubMed] [Google Scholar]
- 17.Chassin MR, Galvin RW the National Roundtable on Health Care Quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 18.NCI Expands Office of Cancer Survivorship Website. < http://www.cancer.gov/newscenter/pressreleases/1999/survivorship>.
- 19.Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Eval Health Prof. 2006;29:126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]