Abstract
Acute pancreatitis can result in retroperitoneal fat necrosis, typically occurring in the peripancreatic region, with extension into the transverse mesocolon, omentum and mesenteric root. When evaluated with contrast enhanced computed tomography (CECT), acute peripancreatic post necrotic collections typically become lower in attenuation over time, and often appear as homogeneous fluid collections. Saponification as a complication of fat necrosis in patients with acute pancreatitis is a well recognized clinical entity. While retroperitonal fat necrosis is commonly seen on CECT, saponification is not a prominent imaging feature. We present a case of acute pancreatitis complicated by extensive saponification of fat throughout the retroperitoneum and peritoneal lining, mimicking carcinomatosis.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging
Introduction
Acute pancreatitis can result in fat necrosis, typically occurring in the peripancreatic retroperitoneum, omentum and mesenteric root. Saponification may be associated with fat necrosis both in the retroperitoneum and in distant subcutaneous, periarticular, or marrow fat. We present a case of extensive post pancreatitis fat necrosis occurring diffusely throughout the abdomen and resulting in multiple enhancing peritoneal, omental and retroperitoneal masses. To our knowledge this particular radiographic appearance has not previously been described in the literature.
Case Report
A 25-year-old woman with pre-eclampsia underwent a C-section and two days later developed severe acute pancreatitis at an outside hospital. Her clinical course was complicated by renal failure and required an intensive care unit stay. Because of persistent abdominal pain, fevers (despite broad spectrum antibiotic therapy), and failure to thrive she was transferred to our institution approximately one week after initial presentation. At the time of transfer, laboratory evaluation revealed amylase 284, lipase 136, calcium 9.5, hematocrit 28, white blood cell count 16,000. Contrast enhanced abdominal CT (CECT) demonstrated findings consistent with evolving acute pancreatitis including intra- and retroperitoneal fluid, but no defined, drainable fluid collection or glandular necrosis (Figure 1). Infection of the peritoneal and retroperitoneal fluid was excluded and the patient was discharged, only to return with worsening abdominal pain and fullness. Repeat CECT obtained as an outpatient three months later showed evolution of fluid density collections into scattered solid enhancing peritoneal nodules, mesenteric implants, and bulky confluent soft tissue encasement of vessels in the mesenteric root (Figure 2, Figure 3).
Figure 1.
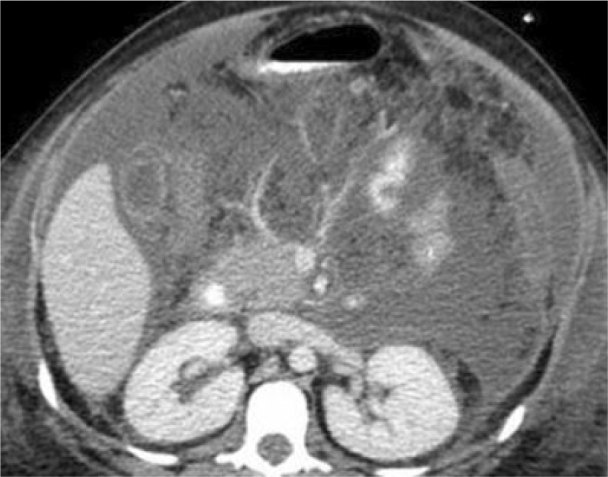
25-year-old woman with presenting with acute pancreatitis. Contrast enhanced CT during initial clinical presentation. Portal venous phase 5 mm axial MDCT image through the inferior pancreatic head level demonstrates retroperitoneal acute peripancreatic fluid extending throughout the mesenteric root and left anterior pararenal spaces. Pancreatic ascites is also present. No pancreatic glandular necrosis is evident.
Figure 2.
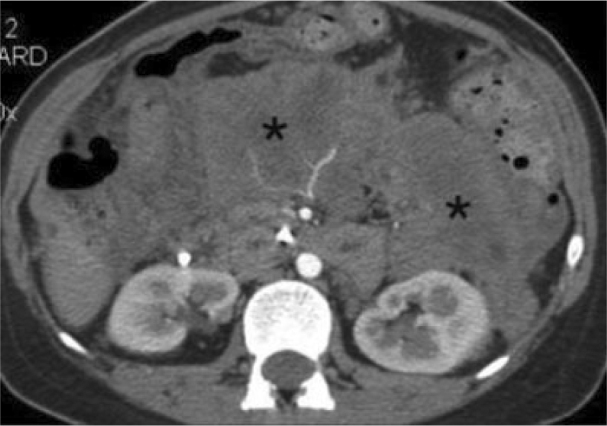
25-year-old woman presenting with acute pancreatitis. Three months later, pancreatic parenchymal phase 2.5 mm MDCT image immediately inferior to the pancreatic head shows that the retroperitoneal peripancreatic acute fluid has evolved in to predominately solid, diffusely enhancing soft tissue density (*).
Figure 3.
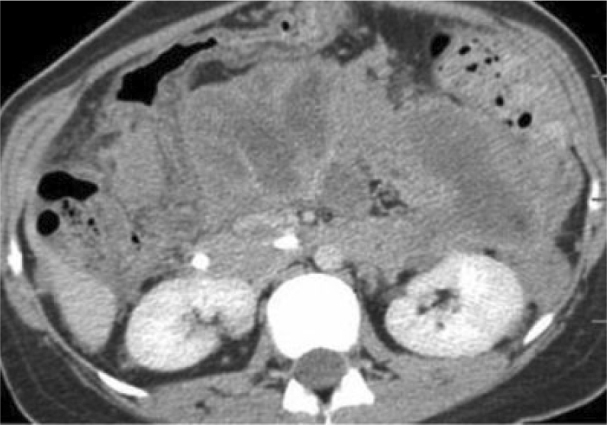
25-year-old woman with presenting with acute pancreatitis. Three months later, portal venous phase demonstrates enhancement of the mass-like evolving collections that appear to encase the mesenteric root vessels.
Though a possible explanation of exuberant granulomatous response secondary to pancreatitis was considered, the finding of mesenteric root soft tissue infiltration with vascular encasement, in addition to the peritoneal and omental nodularity, was concerning for carcinomatosis from an occult epithelial ovarian primary, or primary peritoneal carcinoma.
Because of the unusual appearance, exploratory laparoscopy was performed and demonstrated multiple white, friable peritoneal nodules (Figure 4), as well as extensive adhesions due to implants of similar nodules on the serosal bowel surface. Biopsy of the lesions did not identify a malignant process, but instead was consistent with fat necrosis and fibrosis, with evidence of chronic inflammation. Cultures and gram-stain were negative for infection.
Figure 4.
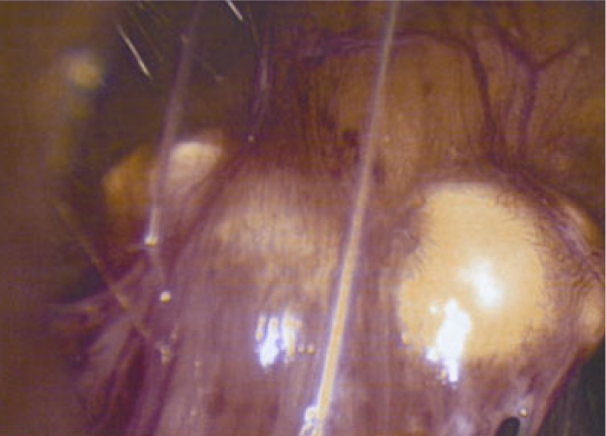
25-year-old woman with presenting with acute pancreatitis. Because of the appearance on imaging, a neoplastic etiology was considered and the patient underwent laparoscopy for tissue sampling. Intraoperative photograph of one of the friable whitish nodules on the peritoneal surface.
The patient continues to require periodic hospitalization for abdominal pain, and supplements her diet with total parenteral nutrition. During a hospitalization six months after the acute pancreatitis episode, the solid enhancing mesenteric root and peritoneal deposits persisted (Figure 5) on CECT.
Figure 5.
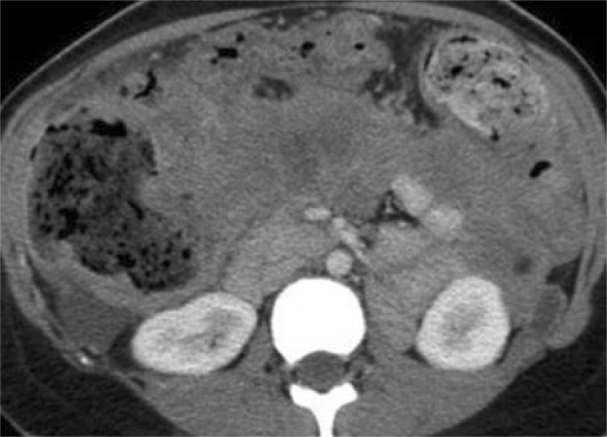
25-year-old woman with presenting with acute pancreatitis. Six months after initial presentation, portal venous phase 5 mm axial MDCT image reveals that the enhancing solid retroperitoneal deposits persist (compare to the ascitic fluid density). The post inflammatory change has an appearance that suggests carcinomatosis rather than the homogeneous, low attenuation more typical for peripancreatic retroperitoneal fat necrosis in patients with evolving acute pancreatitis.
Discussion
The association of fat necrosis and acute pancreatitis was first described by Fitz [1] and Langerhans [2]. CT findings of acute pancreatitis with resultant retroperitoneal fat necrosis are well known. The distribution is typically peripancreatic, with extension into the mesenteric root, transverse mesocolon, and omentum [3] in severe disease; the appearance is usually homogeneous, sometime slightly heterogeneous, but predominantly low attenuation [4]. We found two case reports that described post pancreatitis intraabdominal enhancing masses consistent with fat necrosis, but none describing the extensive carcinomatosis appearance that developed in our patient. In one report, the authors described a large palpable anterior abdominal mass [5] and in the other, bilateral enhancing renal pseudotumors were noted [6].
The gross appearance of the peritoneal nodules in our patient at laparoscopy was consistent with previously described specimens in the two prior case reports and pathology literature [2]; however, the CECT findings of internal enhancement of soft tissue masses is unique.
Microscopically, fat necrosis is characterized by ghost fat cells that are damaged by pancreatic enzymes [7]. Typically in the peripancreatic region, fat necrosis is characterized by evidence of elevated lipolytic activity, a large quantity of calcium, and an increased concentration of free fatty acids. Importantly, there is generally a noticeable absence of inflammatory infiltration [2]. It may be that in our patient, the inflammation identified microscopically in the laparoscopic biopsy specimens gave rise to the soft tissue enhancement pattern mimicking neoplasm such as primary peritoneal carcinoma [8], and that the soft tissue density in part was contributed to by calcium deposition (saponification). Peripheral fat necrosis involving subcutaneous, periarticular, and marrow fat may also occur during acute pancreatitis episodes and is thought to be due to dissemination of pancreatic lipase through the blood. This clinical feature was not present in our patient.
Footnotes
Published: June 12, 2008
References
- 1.Fitz RH. Acute pancreatitis: a consideration of pancreatic hemorrhage, hemorrhagic, suppurative, and gangrenous pancreatitis, and of disseminated fat necrosis. Med Rec. 1889;35:197. [Google Scholar]
- 2.Lee PC, Howard JM. Fat necrosis. Surg Gynecol Obstet. 1979;148:785–789. [PubMed] [PubMed] [Google Scholar]
- 3.Jeffrey RB, Federle MP, Laing FC. Computed tomography of mesenteric involvement in fulminant pancreatitis. Radiology. 1983;147:185–188. doi: 10.1148/radiology.147.1.6828726. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Morgan DE, Stanley RJ. The Pancreas. In: Lee, Sagel, Stanley, Heiken, editors. Computed Body Tomography with MRI Correlation. Lippincott, Williams and Wilkins; Philadephia, PA: 2006. pp. 1007–1100. [Google Scholar]
- 5.Haynes JW, Brewer WH, Walsh JW. Focal fat necrosis presenting as a palpable abdominal mass: CT evaluation. J Comput Assist Tomogr. 1985;9:568–569. doi: 10.1097/00004728-198505000-00032. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Pedrosa I, Naidich JJ, Rofsky NM, Bosniak MA. Renal pseudotumors due to fat necrosis in acute pancreatitis. J Comput Assist Tomogr. 2001;25:236–238. doi: 10.1097/00004728-200103000-00014. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Keen CE, Buk SJA, Brady K, Levison DA. Fat necrosis presenting as obscure abdominal mass: birefringment saponified fatty acid crystalloids as a clue to diagnosis. J Clin Pathol. 1994;47:1028–1031. doi: 10.1136/jcp.47.11.1028. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cormio G, Di Vagno G, Di Gesù G, Mastroianni M. Primary peritoneal carcinoma: a report of twelve cases and a review of the literature. Gynecol Obstet Invest. 2000;50:203–206. doi: 10.1159/000010311. [PubMed] [DOI] [PubMed] [Google Scholar]


