Climate change is predicted to affect the aerobic capacity available for sustainable performances and fitness in fishes. This study reveals trade-offs between aerobic and anaerobic components of swimming performance and metabolic scope, and highlights the possibility of overestimating sustainable aerobic performances of fishes in relation to climate change.
Keywords: Aerobic metabolic scope, anaerobic metabolism, oxygen- and capacity-limited thermal tolerance (OCLTT), sea bream (Sparus aurata), trade-off, Trinidadian guppy (Poecilia reticulata)
Abstract
Ongoing climate change is predicted to affect the distribution and abundance of aquatic ectotherms owing to increasing constraints on organismal physiology, in particular involving the metabolic scope (MS) available for performance and fitness. The oxygen- and capacity-limited thermal tolerance (OCLTT) hypothesis prescribes MS as an overarching benchmark for fitness-related performance and assumes that any anaerobic contribution within the MS is insignificant. The MS is typically derived from respirometry by subtracting standard metabolic rate from the maximal metabolic rate; however, the methodology rarely accounts for anaerobic metabolism within the MS. Using gilthead sea bream (Sparus aurata) and Trinidadian guppy (Poecilia reticulata), this study tested for trade-offs (i) between aerobic and anaerobic components of locomotor performance; and (ii) between the corresponding components of the MS. Data collection involved measuring oxygen consumption rate at increasing swimming speeds, using the gait transition from steady to unsteady (burst-assisted) swimming to detect the onset of anaerobic metabolism. Results provided evidence of the locomotor performance trade-off, but only in S. aurata. In contrast, both species revealed significant negative correlations between aerobic and anaerobic components of the MS, indicating a trade-off where both components of the MS cannot be optimized simultaneously. Importantly, the fraction of the MS influenced by anaerobic metabolism was on average 24.3 and 26.1% in S. aurata and P. reticulata, respectively. These data highlight the importance of taking anaerobic metabolism into account when assessing effects of environmental variation on the MS, because the fraction where anaerobic metabolism occurs is a poor indicator of sustainable aerobic performance. Our results suggest that without accounting for anaerobic metabolism within the MS, studies involving the OCLTT hypothesis could overestimate the metabolic scope available for sustainable activities and the ability of individuals and species to cope with climate change.
Introduction
Effects of climate change (e.g. increased temperature, ocean acidification and hypoxia) are predicted to have profound effects on the physiology of aquatic ectotherms, including fishes (Pörtner and Peck, 2010; IPCC, 2014; Deutsch et al., 2015). This has raised conservation concerns for the persistence of fish populations and increased the interest in developing predictive models for effects of climate change on different species (Jørgensen et al., 2012; Farrell, 2016). The oxygen- and capacity-limited thermal tolerance (OCLTT) hypothesis uses the metabolic scope (MS) to derive a range of tolerable temperatures (thermal window) of organisms with an optimal temperature for oxygen supply to sustain life, including growth, foraging, migration and reproduction (Pörtner and Farrell, 2008; Pörtner and Peck, 2010; Holt and Jørgensen, 2015; Motyka et al., 2016; Verberk et al., 2016). The MS is defined as the difference between the maximal metabolic rate (O2max) and standard metabolic rate (O2stand). Importantly, OCLTT is concerned solely with the aerobic component of MS (Pörtner and Farrell, 2008; Eliason et al., 2011; Clark et al., 2013), essentially making the assumption that any anaerobic component is insignificant. Anaerobic metabolism within the MS is important because it depletes substrates (e.g. glycogen), accumulates metabolic waste (e.g. lactate) and results in fatigue (Alexander, 1989; Goolish, 1991a; Sänger and Stoiber, 2001; Hedrick et al., 2015). The fraction of the MS that is influenced by anaerobic metabolism is therefore not available for sustainable activity. Given that the OCLTT hypothesis concerns only aerobic performance (Pörtner, 2010), it is imperative that any anaerobic component of the MS is known and corrected for to ensure accurate predictions. Although previous studies have acknowledged an anaerobic component within MS (Goolish, 1991a; Reidy et al., 2000; Svendsen et al., 2012; Careau et al., 2014; Norin et al., 2014), the component has, to date, received little quantitative attention in relation to OCLTT.
Fish acquire energy to accomplish different types of physiological work, such as biosynthesis (e.g. somatic growth), maintenance (e.g. circulation, respiration and osmoregulation) and generation of external work to allow locomotion (Careau et al., 2014). If all these functional traits were running at maximal rate, the oxygen requirements would exceed the available supply, forcing individual organisms to prioritize their oxygen budget within the finite size of MS (Killen et al., 2007; Guderley and Pörtner, 2010; Holt and Jørgensen, 2015). For example, the metabolic costs of locomotion and digestion exhibit a trade-off in several fish species (Priede, 1985; Jordan and Steffensen, 2007; Altimiras et al., 2008; Li et al., 2010), which has ecological and evolutionary implications in relation to important performance traits (e.g. predator evasion, foraging, growth, migration and reproduction; Reidy et al., 2000; Oufiero and Garland, 2009). In some fish species, however, metabolism associated with digestion may be additive to the metabolism associated with locomotion (Jourdan-Pineau et al., 2010). Performance trade-offs often play important roles in relation to phenotypic variation found between individuals (Oufiero et al., 2011; Seebacher and Walter, 2012; Svendsen et al., 2015) and may take place when two antagonistic traits cannot be optimized simultaneously because of conflicting demands on the same capacity (Priede, 1985; Roff and Fairbairn, 2007; Svendsen et al., 2015), such as the oxygen budget (Farrell, 2007; Altimiras et al., 2008). This implies that excellence in one trait comes at the cost of performance in a different trait (Vanhooydonck et al., 2014; Walker and Caddigan, 2015), which is classically exemplified by the conflicting relationship between sprinters and endurance athletes (Reidy et al., 2000; Van Damme et al., 2002; Marras et al., 2013). To date, evidence of the corresponding locomotory trade-off in fishes remains inconclusive, with some studies finding support (Reidy et al., 2000; Ojanguren and Braña, 2003; Langerhans, 2009; Oufiero et al., 2011; Ellerby and Gerry, 2011; Yan et al., 2012), whereas others have not (Claireaux et al., 2007; Oufiero and Garland, 2009; Seebacher and Walter, 2012; Marras et al., 2013).
In many fish species, locomotor performance is powered by the myotomal musculature, consisting of segmented red and white muscle fibres. Red oxidative muscles are slow contracting, fuelled by aerobic metabolism and power steady, sustainable swimming (Webb, 1993, 1998; Kieffer, 2000; Sänger and Stoiber, 2001). When approaching swimming speeds that exceed the power capacity and contraction speed of the red muscle fibres, a gait transition to unsteady, unsustainable, burst-assisted swimming occurs with the activation of fast white muscle fibres. White fibres are mainly fuelled by anaerobic metabolism, and their activation depletes substrates (e.g. glycogen), accumulates metabolic waste (e.g. lactate) and results in fatigue (Webb, 1993; Kieffer, 2000; Sänger and Stoiber, 2001).
Anaerobic metabolism may occur at submaximal exercise levels (Goolish, 1991a; Svendsen et al., 2010) and before reaching the maximal aerobic metabolic rate (Burgetz et al., 1998; Lee et al., 2003; Hinch et al., 2006; Teulier et al., 2013). Hence, the gait transition to burst-assisted swimming can be used to partition swimming performance and the MS (Peake and Farrell, 2004; Peake, 2008; Marras et al., 2013; Svendsen et al., 2015) into a sustainable aerobic component and an unsustainable component strongly influenced by anaerobic metabolism. While trade-offs related to sustainable (aerobic) and unsustainable (anaerobic) swimming performances have been examined (Ellerby and Gerry, 2011; Yan et al., 2012; Marras et al., 2013), to date no study has tested for trade-offs related to sustainable and unsustainable components of the MS.
Based on existing data on fish swimming performance and metabolism (Svendsen et al., 2013, 2015), the present study examined the OCLTT assumption that the fraction of the MS which is influenced by anaerobic metabolism, is insignificant. At increasing swimming speeds, the gait transition speed (UGT) to burst-assisted swimming was used to partition swimming performance into a sustainable and strictly aerobic component (Usus) and an unsustainable component influenced by anaerobic metabolism (Uunsus). This partitioned MS into the MS associated with sustainable swimming speeds ≤ UGT (sustainable metabolic scope; MSsus) and the MS associated with unsustainable swimming speeds > UGT (unsustainable metabolic scope; MSunsus). Using these data, we tested for trade-offs between the two swimming performance measures (Usus and Uunsus) and between the two measures of the MS (MSsus and MSunsus). We predicted negative correlations between both groups of measures, implying that individuals cannot optimize Usus and Uunsus or MSsus and MSunsus simultaneously.
Materials and methods
Animals
A total of 13 gilthead sea bream (Sparus aurata; unknown sex; mean ± SEM body mass 79.8 ± 2.4 g and length 14.8 ± 0.2 cm) from a fish farm (Ferme Marine de Douhet) in France were maintained in a holding tank (0.7 m3) with seawater (salinity of 30‰) at 10°C. In addition, 18 guppies (Poecilia reticulata; female) (body mass 0.296 ± 0.009 g and length 3.0 ± 0.0 cm) were captured in Trinidad and maintained in freshwater holding tanks (30 l) at 26°C. Fish were acclimated to the laboratory for at least 2 weeks and fed daily on commercial fish food.
Respirometry
Two swimming respirometers (8.4 and 0.17 l) were used to measure oxygen consumption rate (O2; in milligrams of oxygen per kilogram per hour) as a function of swimming speed (U; in centimetres per second) in S. aurata and P. reticulata. Temperature-controlling instruments (TMP-REG; Loligo Systems, Tjele, Denmark) were employed to maintain temperatures at 10 and 26°C (±0.1°C), respectively. Oxygen partial pressure (in kilopascals) inside the respirometers was measured using fibre-optic sensor technology (PreSens, Regensburg, Germany). Intermittent flow respirometry (Forstner, 1983) was applied in accordance with previous studies (Steffensen, 1989; Peixoto et al., 2016). The software AutoResp (Loligo Systems Aps, Tjele, Denmark) was used to collect data and calculate O2 from measurements of oxygen content inside the respirometers (Peixoto et al., 2016).
Experimental protocol
Poecilia reticulata were fasted for 24 h, whereas S. aurata were fasted for 48 h to ensure post-absorptive states and then transferred to the respirometers. Fish were acclimated to the respirometers for 8–12 h (overnight) while swimming at a speed of 0.5 body lengths (BL) s−1 (S. aurata) and 2 BL s−1 (P. reticulata) prior to collection of data. These speeds were the minimal swimming speeds that ensured positive rheotaxis. Critical swimming speed protocols were then used to measure O2 at increasing swimming speeds until fatigue (Brett, 1964; Svendsen et al., 2013, 2015). The time interval at each swimming speed was 30 min for S. aurata and 12 min for P. reticulata, both including 15 s of speed increment to reach each new test speed.
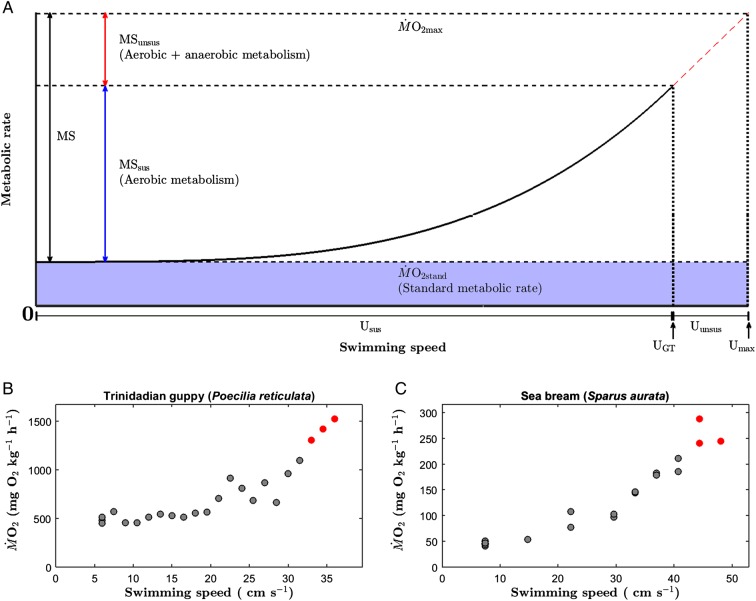
Unlike steady swimming, burst-assisted swimming is partly fuelled by anaerobic metabolism (Peake and Farrell, 2004; Peake, 2008; Marras et al., 2013). The number of bursts correlates positively with excess post-exercise oxygen consumption and therefore anaerobic metabolism (Svendsen et al., 2010), suggesting that the onset of burst-assisted swimming can be used as a reliable indicator of anaerobic metabolism (Svendsen et al., 2015). Hence, in parallel with the measurements of O2, the onset of burst-assisted swimming was recorded. The gait transition speed, UGT, was defined as the highest swimming speed that was supported using only a steady, undulatory locomotory gait (equivalent to USTmax of Peake, 2008). The Usus was defined as the range of sustainable swimming speeds between zero speed and maximal swimming speed maintained by steady swimming (UGT), whereas Uunsus was defined as the range of unsustainable swimming speeds higher than UGT until the maximal swimming speed (Umax; Fig. 1A).
Figure 1:
(A) Conceptual model and (B and C) raw data describing the metabolic rate as a function of swimming speed. (A) Schematic illustration showing the metabolic rate as function of swimming speed, including metabolic scope (MS; (black double-headed arrow) and gait transition speed (UGT) as the highest sustainable swimming speed, equivalent to USTmax of Peake (2008). Using UGT, swimming performance is partitioned into sustainable (ranging from zero speed to UGT) and unsustainable (swimming speeds higher than UGT until Umax) components. The metabolic rate at UGT is used to distinguish between sustainable metabolic scope (MSsus; blue double-headed arrow) and unsustainable metabolic scope (MSunsus; red double-headed arrow). (B and C) Raw data showing oxygen consumption rate (O2; in milligrams of oxygen per kilogram per hour) as a function of swimming speed (in centimetres per second) in an individual Trinidadian guppy (Poecilia reticulata; B) and gilthead sea bream (Sparus aurata; C) used in this study. Data are adapted from Svendsen et al. (2013, 2015). Grey symbols represent O2 when no burst-assisted swimming occurred, whereas red symbols represent O2 when burst-assisted swimming occurred.
Data analyses
Applying data from individual fish, the relationships between U and O2 were described using an exponential equation (Brett, 1964; Fig. 1A). The analyses were limited to steady swimming speeds because the relationship between U and O2 tends to vary at speeds faster than UGT (Schurmann and Steffensen, 1997; Svendsen et al., 2013, 2015; Killen et al., 2015). Extrapolating the exponential equation to zero speed provided an estimate of the standard metabolic rate (O2stand; Brett, 1964; McKenzie et al., 2003; Arnott et al., 2006; Fig. 1A). Maximal metabolic rate (O2max) was defined as the highest O2 measured during the complete swimming protocol (i.e. until fatigue; Binning et al., 2014). The MS was calculated as the difference between O2max and O2stand in individual fish (Fig. 1A).
The metabolic rate corresponding to maximal sustainable swimming speed was defined as the maximal metabolic rate that was maintained aerobically at speeds up to, and including, UGT without the accumulation of anaerobic metabolic products that contribute to performance (U) and negatively impact endurance (Hillman et al., 2014). Hence, swimming performance and MS were partitioned into the following two different components: (i) sustainable components supported by aerobic metabolism alone (Usus and MSsus); and (ii) unsustainable components strongly influenced by anaerobic metabolism (Uunsus and MSunsus; Fig. 1A).
To test for trade-offs in S. aurata and P. reticulata at the intraspecific level, components were correlated using least-squares linear regression (Zar, 2010). Regressions were multivariate, with the unsustainable components (i.e. Uunsus and MSunsus) as the dependent variables and the sustainable component (i.e. Usus and MSsus) and body size (i.e. length and mass) as the independent variables. This was done to test whether predicted correlations were revealed independently of factors related to body size. Multivariate regressions were carried out using stepwise backward elimination. The trade-off related to swimming performance was tested by correlating Usus and Uunsus, whereas the trade-off related to the MS was tested by correlating MSsus and MSunsus. In both cases, a trade-off would be revealed by a significant negative relationship.
In addition, we calculated the fractions (as percentages) of Umax and MS that were constituted by the unsustainable components (i.e. Uunsus and MSunsus, respectively). The unsustainable fractions (percentages) of Umax and MS were then correlated with the corresponding sustainable components (i.e. Usus and MSsus). The correlations were tested using least-squares linear regressions.
Tests were carried out using the software MATLAB 8.5 (MathWorks, 2015), and results were considered significant at P < 0.05. All values are reported as means ± SEM unless otherwise stated.
Results
Burst-assisted swimming
Burst-assisted swimming was evident as forward movement in the chamber (Fig. 2A), elevated tail beat frequency and amplitude (Fig. 2B) and increased swimming speed (Fig. 2C). Between bursts, fish swam slower than the test speeds (Fig. 2C), leading to backwards movement in the chamber (Fig. 2A) and subsequent bursts until fatigue. Burst-assisted swimming was observed in all fish, except three P. reticulata.
Figure 2:
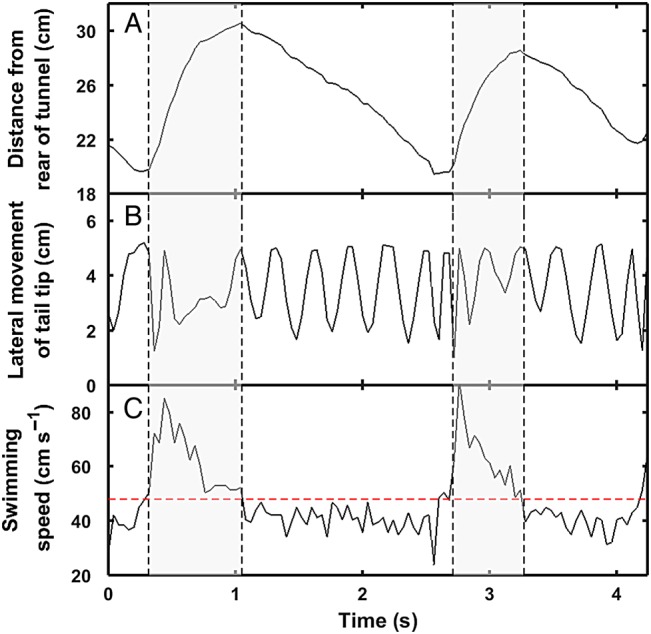
Burst swimming in gilthead sea bream (S. aurata; body length 14.5 cm), with bursts indicated using grey shading. Data were collected in a respirometry chamber [32 cm × 9 cm × 11 cm (length × width × height)], with the water speed adjusted to 48 cm s−1. (A) Longitudinal position of fish snout in the chamber. Bursts are associated with forward movement (grey shading) and followed by backwards movement in the chamber. The y-axis denotes the distance (in centimetres) from the most downstream end of the chamber. (B) Lateral movements of the tail tip as recorded dorsally, with increased tail beat frequency and amplitude providing thrust for each burst. (C) Fish swimming speed in the chamber, with peaks approaching 90 cm s−1 during bursts and speeds below the adjusted water speed (48 cm s−1; indicated by a dashed red line) after bursts. Note that the fish is moving backwards in the chamber (A) when the swimming speed is below 48 cm s−1 (C).
The value of Umax was 46.4 ± 1.5 cm s−1 (range, 37.1–57.0 cm s−1) and 44.0 ± 1.7 cm s−1 (range, 29.5–52.5 cm s−1) in S. aurata and P. reticulata, respectively. Relative to Umax, Uunsus constituted 13.7 ± 1.9% (range, 6.7–27.3%) in S. aurata and 7.1 ± 1.2% (range, 0– 15.6%) in P. reticulata.
Inconsistent correlations between sustainable and unsustainable swimming performances
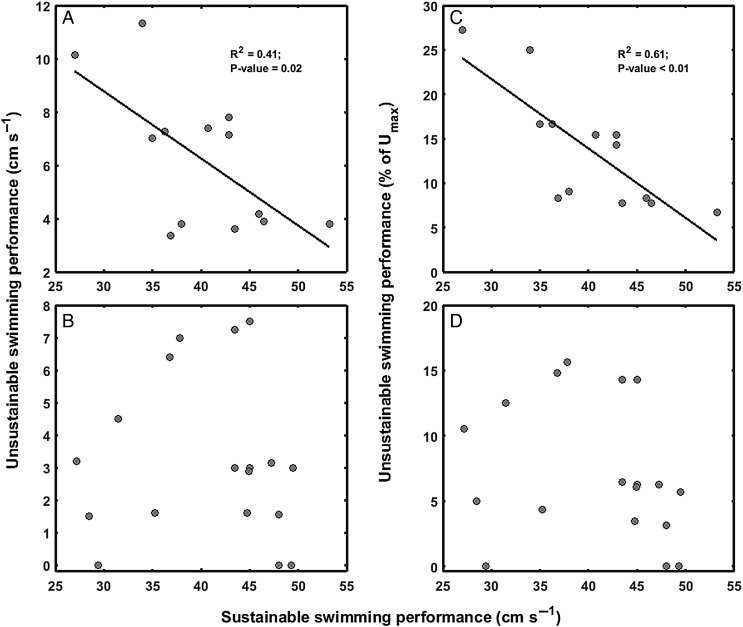
Although a significant negative linear relationship between sustainable swimming performance (i.e. Usus) and unsustainable swimming performance (i.e. Uunsus) was evident in S. aurata (P = 0.02, R2 = 0.41; Fig. 3A), no correlation was found for P. reticulata (P = 0.86, R2 = 0.002; Fig. 3B). For both species, the regression analyses indicated no effects of fish body length. The relationship between Usus and the fraction (percentage) of Umax constituted by Uunsus was significant for S. aurata (P < 0.01, R2 = 0.61; Fig. 3C) but not for P. reticulata (P = 0.33, R2 = 0.06; Fig. 3D). Given that correlations were significant in only one species, these results suggested inconsistent relationships between sustainable and unsustainable swimming performances.
Figure 3:
(A and B) Relationships between sustainable (Usus) and unsustainable (Uunsus) swimming performances (see Fig. 1A for details). (C and D) Relationships between Usus and the fraction (percentage) of Umax constituted by Uunsus. Significant relationships for gilthead sea bream (S. aurata) were found between Usus and Uunsus (A), and between Usus and the fraction (percentage) of Umax constituted by Uunsus (C). (B and D) No significant relationships were found in Trinidadian guppy (P. reticulata).
Consistent negative correlation between sustainable and unsustainable metabolic scopes
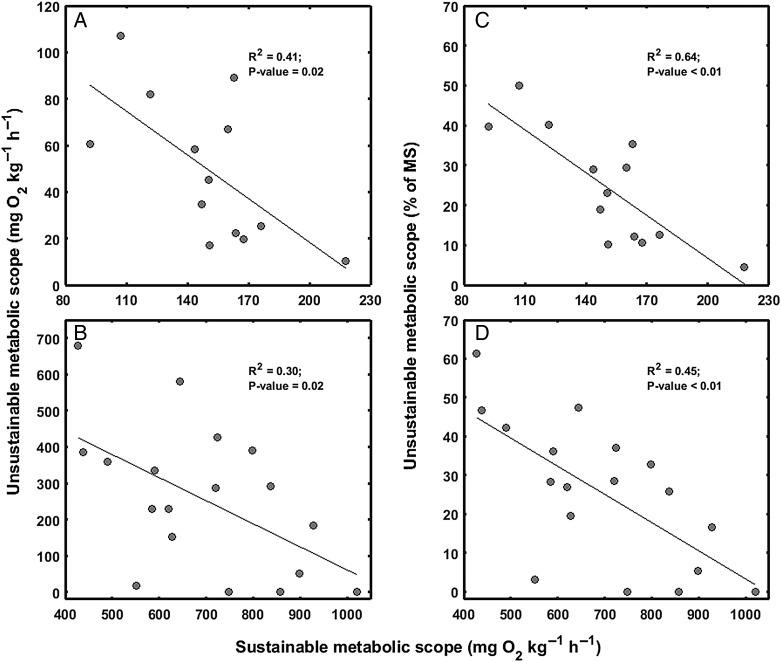
In addition to the trade-off in swimming performance, this study examined the trade-off between MSsus and MSunsus (Figs 1B and C and 4). The value of MSunsus was 49.1 ± 8.6 mg O2 kg−1 h−1 (range, 10.3–107.2 mg O2 kg−1 h−1) and 255.1 ± 47.1 mg O2 kg−1 h−1 (range, 0–677.9 mg O2 kg−1 h−1) in S. aurata and P. reticulata, respectively. Relative to the MS, MSunsus constituted 24.3 ± 3.9% (range, 4.5–50.0%) in S. aurata and 26.1 ± 4.2% (range, 0–61.3%) in P. reticulata. These findings indicated that significant fractions (means, 24.3 and 26.1%) of MS are influenced by anaerobic metabolism and not available for sustainable activities.
Figure 4:
(A and B) Relationships between sustainable metabolic scope (MSsus) and unsustainable metabolic scope (MSunsus; see Fig. 1A for details). (C and D) Relationships between MSsus and the fraction (%) of MS constituted by MSunsus, Significant negative relationships between MSsus and MSunsus or the fraction (percentage) of MS constituted by MSunsus were found in both gilthead sea bream (S. aurata; A and C) and Trinidadian guppy (P. reticulata; B and D).
In contrast to the swimming performance data (Fig. 3), MS data revealed consistent significant negative correlations between MSsus and MSunsus in both S. aurata (P = 0.02, R2 = 0.41; Fig. 4A) and P. reticulata (P = 0.02, R2 = 0.30; Fig. 4B), indicating a trade-off where individuals exhibiting superior MSsus exhibit inferior MSunsus. The regression analyses indicated no effects of body mass for either species. The relationships between MSsus and the fraction (percentage) of MS constituted by MSunsus were significant for both S. aurata (P < 0.01, R2 = 0.64; Fig. 4C) and P. reticulata (P < 0.01, R2 = 0.45; Fig. 4D).
Discussion
Using two teleost species, this study tested for locomotory performance trade-offs between Usus and Uunsus; however, contrary to our prediction, we found no evidence of a consistent negative correlations. Nevertheless, data indicated significant trade-offs within the MS between MSsus and MSunsus in both species. Earlier studies have acknowledged that anaerobic metabolism may occur within the MS (Goolish, 1991b; Reidy et al., 2000; Svendsen et al., 2012; Norin et al., 2014), but the fraction of the MS influenced by anaerobic metabolism remains largely unknown (Burgetz et al., 1998; Farrell, 2007). The present study is the first to report intraspecific variation in MSunsus in fish, and we found that MSunsus may comprise up to 61% of MS in individual fish. This fraction of MS was associated with burst-assisted swimming and therefore partly fuelled by anaerobic metabolism. On average, MSunsus constituted 24.3 and 26.1% of MS in S. aurata and P. reticulata, respectively, highlighting the importance of anaerobic metabolism within the MS. The fact that a significant fraction of the MS is influenced by anaerobic metabolism, and therefore not available for sustainable activity, could have important implications when MS is used to predict effects of environmental variation, particularly in relation to climate change and OCLTT.
Inconsistent correlations between Usus and Uunsus
The negative correlation between Usus and Uunsus observed in S. aurata indicates a locomotor performance trade-off. Conversely, we found no evidence for the same trade-off in P. reticulata. We recognize the fact that the significant correlation for S. aurata may be coincidental and may not hold true if a future study investigates the same relationship using a substantially increased sample size. Numerous studies have tested the conflicting nature of aerobic and anaerobic swimming performance, with equivocal results (Reidy et al., 2000; Ojanguren and Braña, 2003; Claireaux and Lefrançois, 2007; Oufiero et al., 2011; Seebacher and Walter, 2012; Yan et al., 2012; Marras et al., 2013). Reidy et al. (2000) found a negative relationship between aerobic and anaerobic swimming performance in Atlantic cod (Gadus morhua). This was supported by Oufiero et al. (2011), who presented evidence of a similar trade-off in Trinidadian killifish (Rivulus hartii). In contrast, Marras et al. (2013) did not detect a negative correlation between aerobic and anaerobic swimming performances in European sea bass (Dicentrarchus labrax). Comparing relationships between aerobic and anaerobic swimming performances across studies is difficult, because previous studies have employed a diversity of methods to determine swimming performance in many different species and in disparate environmental conditions (e.g. temperatures).
It has been argued that the spatial separation of red and white muscle fibres in most teleosts (Webb, 1993, 1998; Sänger and Stoiber, 2001; Johnston et al., 2011) is consistent with the absence of a locomotor trade-off because the aerobic and anaerobic muscle structures are recruited separately (Claireaux et al., 2007; Marras et al., 2013). According to this conjecture, optimization of one type of muscle fibre does not come at cost to the other (Marras et al., 2013). However, assuming morphological constraints, a greater proportion of red muscle fibres would presumably occupy the space available for white muscle fibres and thus, suppress anaerobic performance. Consequently, variation in the proportion of white muscle fibres could constitute the mechanistic basis of negative relationships between aerobic and anaerobic swimming performances in individual fish.
Although metabolism is important for swimming performance (Priede, 1985; Binning et al., 2015), physiology is not the sole determinant of swimming performance. Several intraspecific studies have shown that swimming performance may be unaffected by variation in metabolism (Anttila et al., 2014; Svendsen et al., 2015) and strongly affected by morphology (Drucker and Lauder, 2000; Domenici et al., 2008; Langerhans, 2009) and biomechanics (Langerhans, 2009; Shadwick and Goldbogen, 2012; Svendsen et al., 2013). Consequently, intraspecific variation in morphology and biomechanics may mask physiological variation (and trade-offs) and could explain the inconsistent correlations between Usus and Uunsus observed in the present study.
Consistent negative correlations between MSsus and MSunsus
Although this study provides inconsistent evidence for a trade-off related to swimming performance, our data show unambiguous support for a physiological trade-off between MSsus and MSunsus in two teleost species, suggesting that MSsus and MSunsus are two antagonistic traits that cannot be optimized simultaneously.
The mechanistic basis for the apparent trade-offs between MSsus and MSunsus or Usus and Uunsus remain uncertain and poorly described. However, a general prediction is that a functional trade-off exists between sustainable and unsustainable locomotion for fishes using a coupled locomotor system, where the same body parts are used for propulsion in both sustainable and unsustainable locomotion (Yan et al., 2012). The red muscles are oxidative tissues and cannot function without the supply of oxygen in order to yield ATP (Webb, 1998; Seebacher and Walter, 2012). The supply of oxygen to sustain aerobic activity may depend on the ability of the fish to extract oxygen from the water (Priede, 1985; Davison, 1997; Sänger and Stoiber, 2001) and cardiac performance (Farrell, 2007; Eliason et al., 2011; Eliason and Farrell, 2015). In fact, it has been proposed that UGT is limited by the oxygen supply to the heart rather than to the red muscles themselves, implying that fish with superior cardiac performance also exhibit higher UGT (McKenzie and Claireaux, 2010). The present study used the recruitment of white muscles (i.e. gait transition) to partition MS into MSsus and MSunsus, suggesting that MSsus could also be limited by the capacity of the red muscles (e.g. contraction speed; Alexander, 1989; Johnston and Altringham, 1991; Drucker and Lauder, 2000) and not by the oxygen supply alone. The contractile properties of the red muscle fibres may determine a peak power production corresponding to the maximal swimming speed that can be achieved before the white muscles are recruited (i.e. UGT; Drucker and Lauder, 2000). For example, if a fish is constrained by the contraction speed of its red muscles, it may recruit the white muscles at a slow swimming speed, even if the oxygen supply is sufficient. Jayne and Lauder (1994) found that white muscles are recruited before the oxidative capacity of the red muscles is fully exploited, which potentially leaves an aerobic component within the red muscle to be used during unsustainable locomotion (i.e. burst-assisted swimming). Given that red muscles may remove catabolites produced in the white muscles (Wittenberger and Diaciuc, 1965; Johnston and Moon, 1980; Milligan and Girard, 1993; Richards et al., 2002), unexploited aerobic capacity would be available to metabolize anaerobic waste products and elevate MSunsus. Hence, if a fish is using only 70% of the red muscle capacity when the white muscles are recruited, then it would have a substantial aerobic component (30%) within the red muscles to metabolize lactate from unsustainable swimming. Consequently, supporting our findings, anaerobically influenced performance (i.e. Uunsus and MSunsus) would be enhanced at the expense of aerobic performance (i.e. Usus and MSsus) and vice versa.
The fate of lactate, however, is not determined by the aerobic capacity of the red muscles alone, and other oxidative tissues, such as the liver, gills and heart (Milligan and Farrell, 1991; Milligan and Girard, 1993; Milligan, 1996; Omlin and Weber, 2013), may also contribute to the metabolism of lactate. Yet, studies have indicated that white muscles have a limited ability to export lactate in rainbow trout (Oncorhynchus mykiss; Weber, 1991; Omlin and Weber, 2013) and suggest that the separation of red and white muscle structures precludes intramuscular lactate shuttles (Weber, 1991; Teulier et al., 2013). If so, the suggestion that limiting contractile properties of the red muscles may leave an aerobic fraction for removal of anaerobic waste products would not underpin the metabolic trade-off observed in the present study.
Assuming that MSsus most directly depends on red muscles, expansion of the aerobic capacity presumably involves increasing the proportion of red muscles and the mitochondrial content of individual fibres (Johnston and Altringham, 1991; Wieser, 1995). Red muscle fibres comprise 10–30 times higher volume densities of mitochondria compared with white muscle fibres (Johnston and Altringham, 1991; Sänger and Stoiber, 2001; Moyes and Genge, 2010), implying that the ratio between the two fibre types could influence the magnitude of MSsus and MSunsus, because aerobic production of ATP takes place in the mitochondria (Johnston and Altringham, 1991; Sänger and Stoiber, 2001). This suggests that a fish with a bigger proportion of red muscle fibres could have more mitochondria per body volume, hence a greater MSsus (Moyes and Genge, 2010). However, it has been argued that the volume density of mitochondria is not always a reliable descriptor of the aerobic potential, owing to differences in mitochondrial cristae density and aerobic enzyme activity (Johnston and Altringham, 1991). Instead, several studies have scaled the content of mitochondrial and glycolytic (i.e. anaerobic) enzymes in relation to body size, and although exceptions exist (Siebenaller et al., 1982; Norton et al., 2000), data have revealed negative scaling relationships between the two types of enzymes (Childress and Somero, 1990; Wieser, 1995; Moyes and Genge, 2010), supporting our findings of a metabolic trade-off between MSsus and MSunsus. Nevertheless, the mechanistic basis of the apparent trade-off between MSsus and MSunsus within the MS needs further attention before firm conclusions can be drawn (Crans et al., 2015).
Fish behaviour in swim tunnels
Since the early work of Brett (1964), swim tunnels have become widely used tools to elucidate patterns of hydrodynamics (Liao, 2007), swimming performance (Schurmann and Steffensen, 1997; Tudorache et al., 2007, 2010) and physiology (Peake and Farrell, 2004; Clark et al., 2013; Svendsen et al., 2013, 2015) of fishes. However, the ecological relevance of swimming performance measures obtained from forced swimming experiments has been questioned because the uniform hydraulic conditions in swim tunnels may be rare in nature (Kemp et al., 2011; Maddock et al., 2013). Instead, spontaneously moving fish perform frequent changes in speed and direction (Tudorache et al., 2009; Steinhausen et al., 2010). Importantly, fish swimming behaviour may be influenced by tunnel dimensions (Tudorache et al., 2007), suggesting that fish swimming behaviour could have been influenced by the tunnel designs used in the present study. It is possible that the correlations between MSsus and MSunsus observed here are partly explained by behavioural responses to the swim tunnels and perhaps not relevant in natural settings. Likewise, it is not known whether intraspecific variation in MSunsus is dependent on the protocol used for data collection. It is possible that the chase protocol to estimate MS (Reidy et al., 2000; Clark et al., 2013; Gräns et al., 2014) would have resulted in less variation between individuals, because this methodology might be less affected by behavioural differences between individuals, including behavioural responses to a swim tunnel. The present study used swimming respirometry because this methodology typically provides the highest measures of O2max (Roche et al., 2013).
Spontaneous use of metabolic scope
How frequently are fish spontaneously using 100% of their MS? Although the answer to this question is largely unknown, previous studies have indicated that this metabolic level may be engaged rarely (Priede, 1985; Lucas et al., 1993; Murchie et al., 2011; Genz et al., 2013; Marras et al., 2013). For example, Lucas et al. (1993) found that the northern pike (Esox lucius) rarely works at the upper limits of metabolism in the wild. Likewise, Murchie et al. (2011) documented that the Bahamas bonefish (Albula vulpes) typically operates at metabolic rates between 40 and 60% of the MS. It is unclear why fish rarely exploit the full extent of their metabolic capacity; however, it is possible that fish refrain from a level of aerobic metabolism that also incurs anaerobic metabolism. The present study found that on average, anaerobic metabolism was present in ∼25% of the MS. Given that the use of anaerobic metabolism is highly inefficient (Johnston and Moon, 1980; Goolish, 1991b), curtails prolonged locomotor performance (Reidy et al., 2000) and is energetically expensive to recover from (Goolish, 1991b; Lucas et al., 1993; Lee et al., 2003; Svendsen et al., 2010), it is likely that fish minimize the use of the MS that includes anaerobic metabolism (Goolish, 1991a; Lucas et al., 1993). This highlights the importance of accounting for MSunsus when the MS is used to estimate the effects of environmental stressors (e.g. temperature and hypoxia) on fish physiology and performance.
Relevance for the oxygen- and capacity-limited thermal tolerance hypothesis and conservation physiology
In the present study, MSunsus occupied between 0 and 61% of the MS in different individuals, suggesting that sustainable metabolic performance may differ substantially between two individuals, even if they exhibit similar MS. Likewise, Lee et al. (2003) indicated that the onset of burst swimming occurred at between 59 and 62% of the critical swimming speed in two salmon species (Oncorhynchus nerka and Oncorhynchus kisutch), leaving ∼40% of the swimming capacity influenced by anaerobic metabolism. Likewise, Burgetz et al. (1998) measured anaerobic metabolism at 70% of the critical swimming speed based on fuel store depletion and accumulation of anaerobic waste. Although the exact contribution of anaerobic metabolism to the total energy consumption (e.g. ATP synthesis rate) remains unknown, the mean percentage (25%) of the MS influenced by anaerobic metabolism observed in the present study still emphasizes the importance of MSunsus when assessing the sustainable component of the MS.
The MS of individual fish is often translated into capacities for fitness-related performances (e.g. growth and locomotion; Guderley and Pörtner, 2010; Eliason et al., 2011; Khan et al., 2014) and related to habitat use (Claireaux and Lefrançois, 2007; del Raye and Weng, 2015) and migratory patterns (Cooke et al., 2013; Eliason et al., 2013) in a number of fish species. Therefore, MS provides an important tool within the field of conservation and climate change management because it acts as a filter between environmental conditions and impacts on population level (Farrell et al., 2008; Jørgensen et al., 2012; Seebacher and Franklin, 2012). For example, the concept of OCLTT has rapidly gained popularity within climate change research of ectotherms, and it hypothesizes that MS, which is considered an aerobic capacity, can be used to predict how local effects of environmental conditions will affect the physiology of fish (Pörtner and Farrell, 2008; Pörtner and Peck, 2010). However, while OCLTT is applied to estimate the physiological persistence of fish, no study measuring MS in relation to the OCLTT hypothesis has, to our knowledge, accounted for the impact of anaerobic metabolism (Cucco et al., 2012; MacMillan et al., 2012; Seth et al., 2013; Norin et al., 2014; Holt and Jørgensen, 2015), although it may be prevalent within the MS as demonstrated by the present study. Therefore, the assumption that the MS, assessed as the difference between O2max and O2stand, is purely aerobic and available for sustainable activities (Reidy et al., 2000; Clark et al., 2013; Farrell, 2016) may be questioned. To improve conservation physiology of fishes, studies of MS in relation to environmental stressors and OCLTT may reveal better predictive value if they take into account that a significant part of MS is influenced by anaerobic metabolism and is unavailable for sustainable performances, as suggested by the present study. For instance, the relationship between temperature and MSsus might differ from the relationship between temperature and MS, although this hypothesis is yet to be to be tested. Comparing normoxic and hypoxic treatments, Dutil et al. (2007) found that MSunsus declines in hypoxia whereas MSsus remains unchanged at least down to 50% air saturation, indicating that MSunsus and MSsus are not necessarily affected equally by environmental variation. The possibility remains that temperature variation affects MSsus and MSunsus differently, warranting further study of aerobic and anaerobic metabolism across environmental temperatures in relation to the OCLTT.
Funding
This research was supported by a grant (SFRH/BPD/89473/2012) from the Foundation for Science and Technology (FCT) in Portugal to J.C.S., and by the Danish Council for Strategic Research project SUNFISH (sustainable fisheries, climate change and the North Sea ecosystem; grant no. 09-063096). Finally, this study was partly supported by Department of Biology at the University of Copenhagen (grant no. 102-0218/11-5550) and by the Aalborg Zoo Conservation Foundation.
Acknowledgements
We thank Cost Action FA1004 Conservation Physiology of Marine Fishes for support and Paolo Domenici for valuable discussions and encouragement to write this paper. Also, we thank Anne Jensen, Valentina Di Santo and two anonymous reviewers for constructive and helpful comments on the manuscript.
References
- Alexander RM. (1989) Optimization and gaits in the locomotion of vertebrates. Physiol Rev 69: 1199–1227. [DOI] [PubMed] [Google Scholar]
- Altimiras J, Claireaux G, Sandblom E, Farrell AP, McKenzie DJ, Axelsson M (2008) Gastrointestinal blood flow and postprandial metabolism in swimming sea bass Dicentrarchus labrax. Physiol Biochem Zool 81: 663–672. [DOI] [PubMed] [Google Scholar]
- Anttila K, Jørgensen SM, Casselman MT, Timmerhaus G, Farrell AP, Takle H (2014) Association between swimming performance, cardiorespiratory morphometry, and thermal tolerance in Atlantic salmon (Salmo salar L.). Front Mar Sci 1: 76. [Google Scholar]
- Arnott SA, Chiba S, Conover DO (2006) Evolution of intrinsic growth rate: metabolic costs drive trade-offs between growth and swimming performance in Menidia menidia. Evolution 60: 1269–1278. [PubMed] [Google Scholar]
- Binning SA, Roche DG, Fulton CJ (2014) Localised intraspecific variation in the swimming phenotype of a coral reef fish across different wave exposures. Oecologia 174: 623–630. [DOI] [PubMed] [Google Scholar]
- Binning SA, Ros AFH, Nusbaumer D, Roche DG (2015) Physiological plasticity to water flow habitat in the damselfish, Acanthochromis polyacanthus. Linking phenotype to performance. PLoS ONE 10: e0121983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett JR. (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Canada 21: 1183–1226. [Google Scholar]
- Burgetz IJ, Rojas-Vargas A, Hinch SG, Randall DJ (1998) Initial recruitment of anaerobic metabolism during sub-maximal swimming in rainbow trout (Oncorhynchus mykiss). J Exp Biol 201: 2711–2721. [DOI] [PubMed] [Google Scholar]
- Careau V, Killen SS, Metcalfe NB (2014) Adding fuel to the “fire of life”: energy budgets across levels of variation in ectotherms and endotherms. In Martin LB, Ghalambor CK, Woods HA, eds, Integrative Organismal Biology, Ed. 1 John Wiley & Sons, New Jersey. [Google Scholar]
- Childress JJ, Somero GN (1990) Metabolic scaling: new perspective based on scaling of glycolytic enzyme activities. Am Zool 30: 161–173. [Google Scholar]
- Claireaux G, Lefrançois C (2007) Linking environmental variability and fish performance: integration through the concept of scope for activity. Philos Trans R Soc Lond B Biol Sci 362: 2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claireaux G, Handelsman C, Standen E, Nelson JA (2007) Thermal and temporal stability of swimming performance in the European sea bass. Physiol Biochem Zool 80: 186–196. [DOI] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 11: cot001; doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crans KD, Pranckevicius NA, Scott GR (2015) Physiological tradeoffs may underlie the evolution of hypoxia tolerance and exercise performance in sunfish (Centrarchidae). J Exp Biol 218: 3264–3275. [DOI] [PubMed] [Google Scholar]
- Cucco A, Sinerchia M, Lefrançois C, Magni P, Ghezzo M, Umgiesser G, Perilli A, Domenici P (2012) A metabolic scope based model of fish response to environmental changes. Ecol Modell 237–238: 132–141. [Google Scholar]
- Davison W. (1997) The effects of exercise training on teleost fish, a review of recent literature. Comp Biochem Physiol Part A Physiol 117: 67–75. [Google Scholar]
- Del Raye G, Weng KC (2015) An aerobic scope-based habitat suitability index for predicting the effects of multi-dimensional climate change stressors on marine teleosts. Deep Res II 113: 280–290. [Google Scholar]
- Deutsch C, Ferrel A, Seibel B, Pörtner HO, Huey RB (2015) Climate change tightens a metabolic constraint on marine habitats. Science 348: 1132–1135. [DOI] [PubMed] [Google Scholar]
- Domenici P, Turesson H, Brodersen J, Brönmark C (2008) Predator-induced morphology enhances escape locomotion in crucian carp. Proc Biol Sci 275: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker EG, Lauder GV (2000) A hydrodynamic analysis of fish swimming speed: wake structure and locomotor force in slow and fast labriform swimmers. J Exp Biol 203: 2379–2393. [DOI] [PubMed] [Google Scholar]
- Dutil JD, Sylvestre EL, Gamache L, Larocque R, Guderley H (2007) Burst and coast use, swimming performance and metabolism of Atlantic cod Gadus morhua in sub-lethal hypoxic conditions. J Fish Biol 71: 363–375. [Google Scholar]
- Eliason EJ, Farrell AP (2015) Oxygen uptake in Pacific salmon Oncorhynchus spp.: when ecology and physiology meet. J Fish Biol 88: 359–388. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hinch SG, Farrell AP (2013) Cardiorespiratory collapse at high temperature in swimming adult sockeye salmon. Conserv Physiol 1(1): cot008; doi:10.1093/conphys/cot008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby DJ, Gerry SP (2011) Sympatric divergence and performance trade-offs of bluegill ecomorphs. Evol Biol 38: 422–433. [Google Scholar]
- Farrell AP. (2007) Cardiorespiratory performance during prolonged swimming tests with salmonids: a perspective on temperature effects and potential analytical pitfalls. Philos Trans R Soc Lond B Biol Sci 362: 2017–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell AP. (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88: 322–343. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe M, Mathes MT (2008) Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol Biochem Zool 81: 697–709. [DOI] [PubMed] [Google Scholar]
- Forstner H. (1983) An automated multiple-chamber intermittent-flow respirometer. In Gnaiger E, Forstner H, eds. Polarographic Oxygen Sensors, Ed 1 Springer International Publishing, Berlin. [Google Scholar]
- Genz J, Jyde MB, Svendsen JC, Steffensen JF, Ramløv H (2013) Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes. Comp Biochem Physiol A Mol Integr Physiol 165: 54–60. [DOI] [PubMed] [Google Scholar]
- Goolish EM. (1991a) Aerobic and anaerobic scaling in fish. Biol Rev 66: 33–56. [Google Scholar]
- Goolish EM. (1991b) Swimming metabolism of fish: sit-and-wait versus active forager. Physiol Zool 64: 485–501. [Google Scholar]
- Gräns A, Jutfelt F, Sandblom E, Jönsson E, Wiklander K, Seth H, Olsson C, Dupont S, Ortega-Martinez O, Einarsdottir I et al. (2014) Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J Exp Biol 217: 711–717. [DOI] [PubMed] [Google Scholar]
- Guderley H, Pörtner HO (2010) Metabolic power budgeting and adaptive strategies in zoology: examples from scallops and fish. Can J Zool 88: 753–763. [Google Scholar]
- Hedrick MS, Hancock TV, Hillman SS (2015) Metabolism at the max: how vertebrate organisms respond to physical activity. Compr Physiol 5: 1677–1703. [DOI] [PubMed] [Google Scholar]
- Hillman SS, Drewes RC, Hedrick MS, Hancock TV (2014) Physiological vagility and its relationship to dispersal and neutral genetic heterogeneity in vertebrates. J Exp Biol 217: 3356–3364. [DOI] [PubMed] [Google Scholar]
- Hinch SG, Cooke SJ, Healey MC, Farrell APT (2006) Behavioural physiology of fish migrations: salmon as a model approach. Fish Physiol 24: 239–295. [Google Scholar]
- Holt RE, Jørgensen C (2015) Climate change in fish: effects of respiratory constraints on optimal life history and behaviour. Biol Lett 11: 20141032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change IPCC, Geneva, Switzerland. [Google Scholar]
- Jayne BC, Lauder GV (1994) How swimming fish use slow and fast muscle fibers: implications for models of vertebrate muscle recruitment. J Comp Physiol A 175: 123–131. [DOI] [PubMed] [Google Scholar]
- Johnston IA, Altringham JD (1991) Movement in water: constraints and adaptations. In Hochachka PW, Mommsen TP, eds, Biochemistry and Molecular Biology of Fishes, Ed. 1 Elsevier Science Publishers, Amsterdam. [Google Scholar]
- Johnston IA, Moon TW (1980) Endurance exercise training in the fast and slow muscles of a teleost fish (Pollachius virens). J Comp Physiol B 135: 147–156. [Google Scholar]
- Johnston IA, Bower NI, Macqueen DJ (2011) Growth and the regulation of myotomal muscle mass in teleost fish. J Exp Biol 214: 1617–1628. [DOI] [PubMed] [Google Scholar]
- Jordan AD, Steffensen JF (2007) Effects of ration size and hypoxia on specific dynamic action in the cod. Physiol Biochem Zool 80: 178–185. [DOI] [PubMed] [Google Scholar]
- Jørgensen C, Peck MA, Antognarelli F, Azzurro E, Burrows MT, Cheung WWL, Cucco A, Holt RE, Huebert B, Marras S et al. (2012) Conservation physiology of marine fishes: advancing the predictive capacity of models. Biol Lett 8: 900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan-Pineau H, Dupont-Prinet A, Claireaux G, McKenzie DJ (2010) An investigation of metabolic prioritization in the European sea bass, Dicentrarchus labrax. Physiol Biochem Zool 83: 68–77. [DOI] [PubMed] [Google Scholar]
- Kemp PS, Russon IJ, Vowles S, Lucas MC (2011) The influence of discharge and temperature on the ability of upstream migrant adult river lamprey (Lampetra fluviatilis) to pass experimental overshot and undershot weirs. River Res Appl 27: 488–498. [Google Scholar]
- Khan JR, Pether S, Bruce M, Walker SP, Herbert NA (2014) Optimum temperatures for growth and feed conversion in cultured hapuku (Polyprion oxygeneios)—Is there a link to aerobic metabolic scope and final temperature preference? Aquaculture 430: 107–113. [Google Scholar]
- Kieffer JD. (2000) Limits to exhaustive exercise in fish. Comp Biochem Physiol A Mol Integr Physiol 126: 161–179. [DOI] [PubMed] [Google Scholar]
- Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc Biol Sci 274: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Reid D, Marras S, Domenici P (2015) The interplay between aerobic metabolism and antipredator performance: vigilance is related to recovery rate after exercise. Front Physiol 6: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerhans RB. (2009) Trade-off between steady and unsteady swimming underlies predator-driven divergence in Gambusia affinis. J Evol Biol 22: 1057–1075. [DOI] [PubMed] [Google Scholar]
- Lee CG, Farrell AP, Lotto A, Hinch SG, Healey MC (2003) Excess post-exercise oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon following critical speed swimming. J Exp Biol 206: 3253–3260. [DOI] [PubMed] [Google Scholar]
- Li X-M, Cao Z-D, Peng J-L, Fu S-J (2010) The effect of exercise training on the metabolic interaction between digestion and locomotion in juvenile darkbarbel catfish (Peltebagrus vachelli). Comp Biochem Physiol A Mol Integr Physiol 156: 67–73. [DOI] [PubMed] [Google Scholar]
- Liao JC. (2007) A review of fish swimming mechanics and behaviour in altered flows. Philos Trans R Soc Lond B Biol Sci 362: 1973–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas MC, Johnstone ADF, Priede IG (1993) Use of physiological telemetry as a method of estimating metabolism of fish in the natural environment. Trans Am Fish Soc 122: 822–833. [Google Scholar]
- McKenzie DJ, Claireaux G (2010) The effects of environmental factors on the physiology of aerobic exercise. In Domenici P, Kapoor BG, eds, Fish Eocomotion: An Etho-Ecological Prespective. CRC Press, Boca Raton, pp 296–332. [Google Scholar]
- McKenzie D, Martinez R, Morales A, Acosta J, Morales R, Taylor E, Steffensen J, Estrada M (2003) Effects of growth hormone transgenesis on metabolic rate, exercise performance and hypoxia tolerance in tilapia hybrids. J Fish Biol 63: 398–409. [Google Scholar]
- MacMillan HA, Williams CM, Staples JF, Sinclair BJ (2012) Metabolism and energy supply below the critical thermal minimum of a chill-susceptible insect. J Exp Biol 215: 1366–1372. [DOI] [PubMed] [Google Scholar]
- Maddock I, Harby A, Kemp P, Wood P (2013) Ecohydraulics: an introduction. In Maddock I, Harby A, Kemp P, Wood P, eds, Ecohydraulics: An Integrated Approach, Ed. 1 John Wiley & Sons, New Jersey. [Google Scholar]
- Marras S, Killen SS, Domenici P, Claireaux G, McKenzie DJ (2013) Relationships among traits of aerobic and anaerobic swimming performance in individual European sea bass Dicentrarchus labrax. PLoS ONE 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan CL. (1996) Metabolic recovery from exhaustive exercise in rainbow trout. J Biochem Physiol 113: 51–60. [Google Scholar]
- Milligan CL, Farrell AP (1991) Lactate utlization by an in situ perfused trout heart: effects of workload and blockers of lactate transport. J Exp Biol 373: 357–373. [Google Scholar]
- Milligan CL, Girard S (1993) Lactate metabolism in rainbow trout. J Exp Biol 193: 175–193. [Google Scholar]
- Motyka R, Norin T, Petersen LH, Huggett DB, Gamperl AK (2016) Long-’term hypoxic exposure alters the cardiorespiratory physiology of steelhead trout (Oncorhynchus mykiss), but does not affect their upper thermal tolerance. J Therm Biol. doi:10.1016/j.jtherbio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Moyes CD, Genge CE (2010) Scaling of muscle metabolic enzymes: an historical perspective. Comp Biochem Physiol A Mol Integr Physiol 156: 344–350. [DOI] [PubMed] [Google Scholar]
- Murchie KJ, Cooke SJ, Danylchuk AJ, Suski CD (2011) Estimates of field activity and metabolic rates of bonefish (Albula vulpes) in coastal marine habitats using acoustic tri-axial accelerometer transmitters and intermittent-flow respirometry. J Exp Mar Biol Ecol 396: 147–155. [Google Scholar]
- Norin T, Malte H, Clark TD (2014) Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J Exp Biol 217: 244–251. [DOI] [PubMed] [Google Scholar]
- Norton SF, Eppley ZA, Sidell BD (2000) Allometric scaling of maximal enzyme activities in the axial musculature of striped bass, Morone saxatilis (Walbaum). Physiol Biochem Zool 73: 819–828. [DOI] [PubMed] [Google Scholar]
- Ojanguren A, Braña F (2003) Effects of size and morphology on swimming performance in juvenile brown trout (Salmo trutta L.). Ecol Freshw Fish 12: 241–246. [Google Scholar]
- Omlin T, Weber J-M (2013) Exhausting exercise and tissue-specific expression of monocarboxylate transporters in rainbow trout. Am J Physiol Regul Integr Comp Physiol 304: R1036–R1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oufiero CE, Garland T (2009) Repeatability and correlation of swimming performances and size over varying time-scales in the guppy (Poecilia reticulata). Funct Ecol 23: 969–978. [Google Scholar]
- Oufiero CE, Walsh MR, Reznick DN, Garland T (2011) Swimming performance trade-offs across a gradient in community composition in Trinidadian killifish (Rivulus hartii). Ecology 92: 170–179. [DOI] [PubMed] [Google Scholar]
- Peake SJ. (2008) Gait transition speed as an alternate measure of maximum aerobic capacity in fishes. J Fish Biol 72: 645–655. [Google Scholar]
- Peake SJ, Farrell AP (2004) Locomotory behaviour and post-exercise physiology in relation to swimming speed, gait transition and metabolism in free-swimming smallmouth bass (Micropterus dolomieu). J Exp Biol 207: 1563–1575. [DOI] [PubMed] [Google Scholar]
- Peixoto MJ, Svendsen JC, Malte H, Pereira LF, Carvalho P, Pereira R, Gonçalves JFM, Ozório ROA (2016) Diets supplemented with seaweed affect metabolic rate, innate immune, and antioxidant responses, but not individual growth rate in European seabass (Dicentrarchus labrax). J Appl Phycol 28: 2061–2071. [Google Scholar]
- Priede IG. (1985) Metabolic scope in fishes. In Tytler P, Calow P, eds, Fish Energetics, Ed 1 Croom Helm Ltd, Beckenham, pp 33–64. [Google Scholar]
- Pörtner HO. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. [DOI] [PubMed] [Google Scholar]
- Reidy SP, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203: 347–357. [DOI] [PubMed] [Google Scholar]
- Richards JG, Mercado AJ, Clayton CA, Heigenhauser GJF, Wood CM (2002) Substrate utilization during graded aerobic exercise in rainbow trout. J Exp Biol 205: 2067–2077. [DOI] [PubMed] [Google Scholar]
- Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216: 2103–2110. [DOI] [PubMed] [Google Scholar]
- Roff DA, Fairbairn DJ (2007) The evolution of trade-offs: where are we? J Evol Biol 20: 433–447. [DOI] [PubMed] [Google Scholar]
- Sänger AM, Stoiber W (2001) Muscle fiber diversity and plasticity. Fish Physiol 18: 187–250. [Google Scholar]
- Schurmann H, Steffensen JF (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50: 1166–1180. [Google Scholar]
- Seebacher F, Franklin CE (2012) Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Philos Trans R Soc B Biol Sci 367: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Walter I (2012) Differences in locomotor performance between individuals: importance of parvalbumin, calcium handling and metabolism. J Exp Biol 215: 663–670. [DOI] [PubMed] [Google Scholar]
- Seth H, Gräns A, Sandblom E, Olsson C, Wiklander K, Johnsson JI, Axelsson M (2013) Metabolic scope and interspecific competition in sculpins of Greenland are influenced by increased temperatures due to climate change. PLoS ONE 8: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadwick RE, Goldbogen JA (2012) Muscle function and swimming in sharks. J Fish Biol 80: 1904–1939. [DOI] [PubMed] [Google Scholar]
- Siebenaller JF, Somero GN, Haedrich RL (1982) Biochemical characteristics of macrourid fishes differing in their depth of distribution. Biol Bull 163: 240–249. [Google Scholar]
- Steffensen JF. (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol Biochem 6: 49–59. [DOI] [PubMed] [Google Scholar]
- Steinhausen MF, Fleng Steffensen J, Gerner Andersen N (2010) The effects of swimming pattern on the energy use of gilthead seabream (Sparus aurata L.). Mar Freshw Behav Physiol 43: 227–241. [Google Scholar]
- Svendsen JC, Tudorache C, Jordan AD, Steffensen JF, Aarestrup K, Domenici P (2010) Partition of aerobic and anaerobic swimming costs related to gait transitions in a labriform swimmer. J Exp Biol 213: 2177–2183. [DOI] [PubMed] [Google Scholar]
- Svendsen JC, Steffensen JF, Aarestrup K, Frisk M, Etzerodt A, Jyde M (2012) Excess posthypoxic consumption in rainbow trout (Ocorhynchus mykiss): recovery in normoxia and hypoxia. Can J Zool 90: 1–11. [Google Scholar]
- Svendsen JC, Banet AI, Christensen RHB, Steffensen JF, Aarestrup K (2013) Effects of intraspecific variation in reproductive traits, pectoral fin use and burst swimming on metabolic rates and swimming performance in the Trinidadian guppy (Poecilia reticulata). J Exp Biol 216: 3564–3574. [DOI] [PubMed] [Google Scholar]
- Svendsen JC, Tirsgaard B, Cordero GA, Steffensen JF (2015) Intraspecific variation in aerobic and anaerobic locomotion: gilthead sea bream (Sparus aurata) and Trinidadian guppy (Poecilia reticulata) do not exhibit a trade-off between maximum sustained swimming speed and minimum cost of transport. Front Physiol 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulier L, Omlin T, Weber J-M (2013) Lactate kinetics of rainbow trout during graded exercise: do catheters affect the cost of transport? J Exp Biol 216: 4549–4556. [DOI] [PubMed] [Google Scholar]
- Tudorache C, Viaenen P, Blust R, De Boeck G (2007) Longer flumes increase critical swimming speeds by increasing burst-glide swimming duration in carp Cyprinus carpio, L. J Fish Biol 71: 1630–1638. [Google Scholar]
- Tudorache C, Jordan AD, Svendsen JC, Domenici P, DeBoeck G, Steffensen JF (2009) Pectoral fin beat frequency predicts oxygen consumption during spontaneous activity in a labriform swimming fish (Embiotoca lateralis). Environ Biol Fishes 84: 121–127. [Google Scholar]
- Tudorache C, O’Keefe RA, Benfey TJ (2010) The effect of temperature and ammonia exposure on swimming performance of brook charr (Salvelinus fontinalis). Comp Biochem Physiol A Mol Integr Physiol 156: 523–528. [DOI] [PubMed] [Google Scholar]
- Van Damme R, Wilson RS, Vanhooydonck B, Aerts P (2002) Performance constraints in decathletes. Nature 415: 755–756. [DOI] [PubMed] [Google Scholar]
- Vanhooydonck B, James RS, Tallis J, Aerts P, Tadic Z, Tolley KA, Measey GJ, Herrel A (2014) Is the whole more than the sum of its parts? Evolutionary trade-offs between burst and sustained locomotion in lacertid lizards. Proc Biol Sci 281: 20132677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk WCEP, Overgaard J, Ern R, Bayley M, Wang T, Boardman L, Terblanche JS (2016) Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comp Biochem Physiol A Mol Integr Physiol 192: 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Caddigan SP (2015) Performance trade-offs and individual quality in decathletes. J Exp Biol 218: 3647–3657. [DOI] [PubMed] [Google Scholar]
- Webb PW. (1993) Swimming. In Evans DH, eds, The Physiology of Fishes, Ed 1 CRC Press, Boca Raton, pp 47–73. [Google Scholar]
- Webb PW. (1998) Swimming. In Evans DH, eds, The Physiology of Fishes, Ed 2 CRC Press, Boca Raton, pp 3–24. [Google Scholar]
- Weber BYJ. (1991) Effect of endurance swimming on the lactate kinetics of rainbow trout. J Exp Biol 476: 463–476. [DOI] [PubMed] [Google Scholar]
- Wieser W. (1995) Energetics of fish larvae, the smallest vertebrates. Acta Physiol Scand 154: 279–290. [DOI] [PubMed] [Google Scholar]
- Wittenberger C, Diaciuc IV (1965) Effort metabolism of lateral muscles in carp. J Fish Board Canada 22: 1397–1406. [Google Scholar]
- Yan GJ, He XK, Cao ZD, Fu SJ (2012) The trade-off between steady and unsteady swimming performance in six cyprinids at two temperatures. J Therm Biol 37: 424–431. [Google Scholar]
- Zar JH. (2010) Biostatistical Analysis. Prentice Hall, New Jersey. [Google Scholar]