Abstract
Purpose
In the randomized phase III trial, Gynecologic Oncology Group protocol 240, the incorporation of bevacizumab with chemotherapy significantly increased overall survival (OS) in women with advanced cervical cancer. A major objective of GOG-240 was to prospectively analyze previously identified pooled clinical prognostic factors known as the Moore criteria.
Experimental Design
Potential negative factors included black race, performance status 1, pelvic disease, prior cisplatin, and progression-free interval <365 days. Risk categories included low-risk (0-1 factor); intermediate-risk (2-3 factors); high-risk (4-5 factors). Each test of association was conducted at the 5% level of significance. Logistic regression and survival analysis was used to determine whether factors were prognostic or could be used to guide therapy.
Results
For the entire population (n=452), high-risk patients had significantly worse OS (p<0.0001). The hazard ratios of death for treating with topotecan in low-risk, mid-risk, and high-risk subsets are 1.18 (95% CI 0.63-2.24), 1.11 (95% CI 0.82-1.5), and 0.84 (95% CI 0.50-1.42), respectively. The hazard ratios of death for treating with bevacizumab in low-risk, mid-risk, and high-risk subsets are 0.96 (95% CI 0.51-1.83; p=0.9087), 0.673 (95% CI 0.5-0.91; p=0.0094), and 0.536 (95% CI 0.32-0.905; p=0.0196), respectively.
Conclusions
This is the first prospectively validated scoring system in cervical cancer. The Moore criteria have real world clinical applicability. Toxicity concerns may justify omission of bevacizumab in some low-risk patients where survival benefit is small. The benefit to receiving bevacizumab appears to be greatest in the moderate- and high-risk subgroups (5.8 month increase in median OS).
Keywords: cervical cancer, prognostic factors, scoring system, antiangiogenesis therapy
Introduction
Women with recurrent and metastatic cervical cancer constitute a population for whom treatment options have been extremely limited (1). With sophisticated radiotherapy planning and concurrent chemotherapy for radiosensitization and sterilization of occult metastatic tumor foci, central control can be achieved which indirectly eliminates candidacy for pelvic exenteration to clear recurrent tumor (2). In those cases when central failure occurs it is commonly accompanied by distant failure which also abrogates any curative intent of exenteration. Previously, chemotherapy using cisplatin plus paclitaxel in these settings had been palliative, with rapid clinical deterioration, worsened quality of life (QoL), and median overall survival (OS) ranging from 7-12 months (3,4). Importantly, many patients with recurrent disease have been pre-irradiated with limited bone marrow reserves, and may be platinum-resistant as a consequence of prior platinum exposure with radiotherapy and subsequent acquired drug resistance (5,6). In addition, many are medically infirm due to renal failure and malnutrition.
Gynecologic Oncology Group (GOG) 240 was developed to study the non-platinum chemotherapy doublet, topotecan plus paclitaxel, as well as anti-angiogenesis therapy (7). Vascular endothelial growth factor (VEGF) has emerged as an important therapeutic target and the monoclonal anti-VEGF humanized antibody, bevacizumab, was found to be active in GOG-227C, a phase II trial in heavily pretreated patients with recurrent cervical cancer (8). The primary endpoint of GOG-240 was OS.
In February 2013, the National Cancer Institute (NCI) and the GOG issued a Press Release stating that compared to chemotherapy alone, the incorporation of bevacizumab led to significantly improved OS (17m vs 13.3m) and progression-free survival (PFS) (8.2m vs. 5.9m) (9). The integration of bevacizumab also significantly improved response rate (RR) (48% vs. 36%) without a significant deterioration in health-related QoL (9,10). On March 10, 2014, the Cancer Drugs Fund approved bevacizumab for women in England with advanced cervical cancer. Following U.S. FDA approval of bevacizumab for advanced cervical cancer on August 14, 2014, the NCCN has listed both triplet regimens studied (i.e. cisplatin-paclitaxel-bevacizumab and topotecan-paclitaxel-bevacizumab) as Category 1 (11).
A major objective in GOG 240 was to prospectively study previously identified pooled prognostic factors known as the Moore criteria (12). If risk stratification were to be validated in the GOG-240 population, two important questions could be asked. First, could risk stratification be used to guide therapy, i.e., to select the optimal chemotherapy backbone? Secondly, does risk stratification identify a cohort that is unsuitable for “standard” therapy due to a low likelihood for response?
Methods
Eligibility Criteria, Study Design, and Treatment
GOG-240 was a phase III randomized trial conducted through the GOG and the Spanish cooperative group, Grupo Español de Investigation en Cancer de Ovario (GEICO) with NCI-supplied bevacizumab (NSC #704865, IND #113912), with central IRB approval and registration (NCT00803062), signed informed consent, and central pathology review (9). Primary endpoints were OS and the frequency and severity of toxicity and secondary endpoints were PFS and RR (9). Prospective validation of the Moore criteria of pooled poor prognostic factors and quality of life were tertiary endpoints. Eligibility required primary Stage IVB or recurrent/persistent cervical carcinoma with measurable disease and GOG performance status 0-1 (9). Using a 2×2 factorial design, participants were randomized to one of four intravenous regimens: paclitaxel (135 mg/m2 over 24 hours or 175 mg/m2 over 3 hours) with cisplatin (50 mg/m2) with or without bevacizumab 15 mg/kg, or paclitaxel 175 mg/m2 over 3 hours on day 1 with topotecan 0.75 mg/m2 over 30 minutes days 1-3 with or without bevacizumab 15 mg/kg (Supplemental Figure SF1A). Cycles were repeated every 21 days until disease progression, unacceptable toxicity, or complete response. Tumor measurements were made using Response Evaluation Criteria in Solid Tumors (RECIST v1) and safety was assessed by the National Cancer Institute's Common Terminology Criteria for Adverse Events. One interim analysis was scheduled at 173 events. A second analysis (271 deaths) occurred 11 months later.
Efficacy
At the first interim analysis, the non-platinum chemotherapy doublet, topotecan-paclitaxel, was reported to be not superior to the cisplatin-paclitaxel chemotherapy backbone (Supplemental Figure SF1B) (9). At the second analysis, the bevacizumab-containing regimens were found to have significantly improved OS, PFS, and RR over chemotherapy alone (Supplemental Figure SF1C) (9).
Pooled Poor Prognostic Factors: Early Development of the Training Set
The Moore criteria were identified by pooling 20 clinical factors from 429 patients enrolled and treated on three prior GOG phase III trials in recurrent/persistent and metastatic cervical cancer (GOG protocols 110, 169, and 179) (12, 13-15). Five factors were prognostic and included performance status >0, pelvic disease, African-American ancestry, disease-free interval <1 year, and prior platinum exposure.
The factors were weighted equally and confirmed to impart a poor prognosis when applied as a training set including low risk (0- 1 factor), mid-risk (2- 3 factors), and high risk (4 or 5 factors). This training set was retrospectively applied to another prior phase III trial in this population (GOG-149) (16).
Pooled poor prognostic factors: Prospective Validation of the Training Set
The Moore criteria were analyzed in the entire study population of GOG-240 to determine whether the risk categories had prognostic significance. Next, each chemotherapy backbone was studied and compared to determine if the risk categories could guide therapy. Finally, the Moore criteria were applied to the subset of patients treated with chemotherapy plus bevacizumab to potentially identify a cohort of patients that were likely not to benefit from anti-angiogenesis therapy, presumably because their risk stratification was so poor it rendered them unsalvageable with contemporary therapy which was defined as chemotherapy (either backbone) plus bevacizumab.
Statistical Analysis
Five clinical risk factors were examined for prognostic value with clinical outcome variables (OS, PFS, RR). The variables examined were race (African ancestry or not), performance status (1 or 0), measurable disease in the pelvis (yes/ no), prior platinum as a radiation sensitizer (yes/ no), and progression-free interval from diagnosis of disease (≤365 days or >365 days). Associations with response were assessed with logistic models whereas associations with PFS and OS were assessed with Cox Proportional Hazard models (17). Internal validity of the model was assessed by analyzing the total number of negative risk factors as a continuous variable which was found to be highly significant with a hazard of progression 1.204 (95% CI, 1.088-1.332) and a hazard of death of 1.480 (1.313-1.668).
Each factor was assessed as univariate components in separate models and together in joint models. Total risk score was calculated by adding the number of risk factors to derive a risk classifier. The risk class was assessed for both prognostic and predictive value using logistic and Cox models. The predictive value was evaluated using an interaction term with treatment (cisplatin or topotecan; administration or non-administration of bevacizumab) to assess for significance and describe any potential impact.
Results
Using GOG 240 data, the five risk factors were examined separately and in joint models to assess the strength of association with clinical outcome. All of the risk factors appeared to be detrimental as indicated by their odds ratio estimates being less than 1 even when not statistically significant (Table 1).
Table 1. Frequency and Impact on Progression-Free Survival and Overall Survival of Each Prognostic Factor Examined Separately.
| FACTOR | N (%) | Odds Ratio | 95% CI | HR PFS | 95% CI | HR OS | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| African American | N | 392 (86.7) | 0.685 | 0.372-1.260 | 1.091 | 0.808-1.473 | 1.312 | 0.94-1.827 |
| Y | 60 (13.3) | |||||||
| Performance Status | 0 | 263 (58.2) | 0.560 | 0.371-0.845 | 1.330 | 1.069-1.655 | 1.657 | 1.291-2.127 |
| 1 | 189 (41.8) | |||||||
| Pelvic Disease | N | 210 (46.5) | 0.801 | 0.529-1.215 | 0.946 | 0.755-1.186 | 1.068 | 0.824-1.383 |
| Y | 242 (53.5) | |||||||
| Prior CDDP Radiation | N | 115 (25.4) | 0.263 | 0.160-0.432 | 1.801 | 1.375-2.360 | 2.166 | 1.576-2.979 |
| Y | 337 (74.6) | |||||||
| Progression-Free Interval | <12m | 203 (44.9) | 0.599 | 0.395-0.909 | 1.484 | 1.189-1.854 | 1.950 | 1.506-2.525 |
| ≥12m | 249 (55.1) |
Validation of Moore criteria in entire GOG-240 population (arms 1+2+3+4)
The total risk score integrated all of the risk factors into a single statistic, as was done in the original publication (12). Application of the Moore criteria to the entire GOG-240 study population (i.e., arms 1-4 combined) placed the majority of patients in the mid-risk class (n=303, 67%) (Figure 1). Low-risk patients account for 19% (n=84) and high-risk patients comprise 14% (n=65) of the study population. The distribution of OS, PFS, and RR mirror the low-, mid-, and high-risk subgroup stratification in the direction of statistically significant declination for all three endpoints as one moves from low risk to high risk (Figure 1). For example, patients with 0 or 1 high risk factor (i.e., low-risk cohort) experience 21.8 months OS and 57% RR, while those in the high-risk cohort (4 or 5 factors) have significantly worse OS of 8.2 months (p<0.0001) and 18.5% RR (p<0.001).
Figure 1.

Prospective validation of the Moore criteria in the GOG 240 study population (n=452).
Patients treated with the topotecan-paclitaxel backbone (arms 3+4)
Using the Moore criteria, analysis of the 223 patients randomized to the topotecan-paclitaxel backbone assigns the majority of patients to the mid-risk class (n=151). As one moves from low-risk to high-risk classes, OS significantly decreases from 20.1 months to 8.2 months (p=0.017). The effect on PFS, while not statistically significant, also deteriorates with increasing risk stratification (p=0.066).
Patients treated with bevacizumab (arms 2+4)
Analysis of the 227 patients randomized to the regimens administering bevacizumab places the majority (n=152) in the mid-risk class. From low-risk to high-risk subgroups, OS decreases from 22.9 months to 12.1 months, respectively (P=0.0513). The declination of PFS from low-risk to high-risk is not significant (p=0.1417).
Risk stratification of chemotherapy backbones (arms 1+2 vs 3+4)
The Moore criteria and risk assignment were used to determine whether there exists a preferential benefit for patients to be treated with one or the other chemotherapy backbone. Treatment with the topotecan-paclitaxel chemotherapy backbone was not a significant predictor of OS, PFS, or RR. While the Moore criteria themselves are highly prognostic for OS, PFS, and RR, there is no evidence of interaction between the topotecan-paclitaxel backbone and the Moore criteria for OS, PFS, or response (Table 2). The estimates of the hazard ratios of death for treating with topotecan to cisplatin in the low-risk, mid-risk, and high-risk subsets are 1.18 (95% CI 0.63-2.24), 1.11 (95% CI 0.82-1.5), and 0.84 (95% CI 0.50-1.42), respectively, suggesting perhaps a modest (but non-significant) benefit for high-risk patients being treated on the topotecan-paclitaxel backbone. The hazard ratios for PFS were likewise 1.15 (95% CI 0.71-1.85), 1.26 (95% 0.98-1.62), and 1.00 (95% CI 0.60-1.67) in the low, mid, and high risk groups. The odds ratios for responding to therapy in topotecan to cisplatin therapy were 0.43 (95% CI 0.18-1.04) in the low group, 0.67 (95% CI 0.43-1.07) in the mid group, and 1.45 (95% CI 0.41-5.16) in the high risk group. None of the interaction terms were significant.
Table 2. Application of the Prognostic Model to the Chemotherapy Backbones: Cisplatin-Paclitaxel and Topotecan-Paclitaxel.
| Median OS (months) | Median PFS (months) | RR (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low-Risk | Mid-Risk | High-Risk | Low-Riks | Mid-Risk | High-Risk | Low-Risk | Mid-Risk | High-Risk | |
| Cisplatin-Paclitaxel | 26.0 | 16.5 | 8.21 | 10.9 | 7.8 | 5.2 | 66.7 | 48.0 | 15.6 |
| Topotecan-Paclitaxel | 20.0 | 13.8 | 8.25 | 8.5 | 5.7 | 2.8 | 46.0 | 38.0 | 21.0 |
| Hazard Ratio | 1.18 | 1.11 | 0.84 | 1.15 | 1.26 | 1.00 | 0.43 | 0.67 | 1.45 |
| p(Topo+Pac) | 0.6030 | 0.5811 | 0.0601 | ||||||
| p(Moore criteria) | <0.0001 | 0.0072 | 0.0002 | ||||||
| p(Interaction) | 0.6181 | 0.7248 | 0.2988 | ||||||
Risk stratification among women treated with chemotherapy alone vs chemotherapy plus bevacizumab
Eighty-four patients had 0 or 1 risk factor. When these patients are separated by the administration of bevacizumab, the median OS between the groups is very similar (21.8 months in the chemotherapy alone cohort and 22.9 months in the chemotherapy plus bevacizumab cohort, Figure 2A) with a hazard ratio of death estimated at 0.96 (95% CI 0.51-1.83; p=0.9087). Median PFS was also not significantly different among low-risk patients treated with chemotherapy with and without bevacizumab (10.9 vs 8.0 months); (HR 0.85 (95% CI 0.53-1.37).
Figure 2.
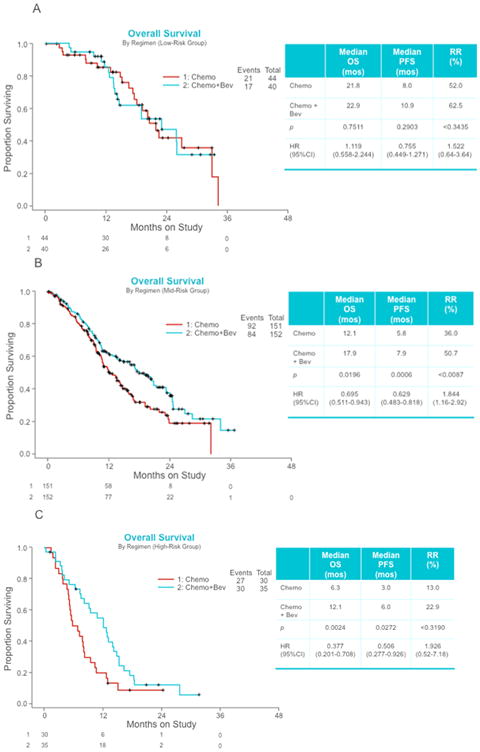
Kaplan Meier overall survival curves following risk stratification according to the Moore criteria among women with advanced cervical cancer treated with chemotherapy with and without bevacizumab. Panel A: Low-risk class (0-1 poor prognostic factor). Panel B: Mid-risk class (2-3 poor prognostic factors). Panel C: High-risk class (4-5 poor prognostic factors).
The mid-risk group (2 or 3 factors) contained 303 patients. As expected, the median OS declined when compared with the low-risk cohort, but in this subgroup the difference in median OS (12.1 months in the chemotherapy alone cohort and 17.9 months in the chemotherapy plus bevacizumab cohort) is highly significant with an estimated hazard of death of 0.673 (95% CI 0.5-0.91; p=0.0094) (Figure 2B). The estimated hazard ratio for the PFS endpoint was 0.694 (95% CI 0.54-0.89; p=0.0047).
Among the 65 patients that had 4 or 5 risk factors, the Moore criteria and high-risk stratification class was highly prognostic between chemotherapy cohorts who did not receive and did receive bevacizumab. The bevacizumab group had better survival with an estimated hazard ratio of death of 0.536 (95% CI 0.32-0.905; p=0.0196) (Figure 2C) and an estimated hazard ratio of the PFS endpoint of 0.506 (95% CI 0.277-0.926; p=0.0272).
The odds ratio of response for those treated with bevacizumab to those who were not in the low, mid, and high risk class patients were 1.52 (95% CI 0.64-3.64), 1.84 (95% CI 1.16-2.92), and 1.93 (95% CI 0.52-7.18), respectively.
Discussion
The original impetus to study poor prognostic markers in advanced cervical cancer was to identify patients a priori who were unlikely to respond to conventional cytotoxic therapy in an effort to avoid administration of futile treatment (18,19). The prognostic model for tumor response was based on five similarly weighted factors that did not interact, allowing for an index based on the total number of risk factors to be derived (13-15).
The Moore criteria were identified in the platinum or cytotoxic era when antiangiogenic agents were not yet employed in randomized clinical trials for cervical cancer patients (20-22). One of the major conclusions of the original Moore criteria analysis was that because even limited toxicity in the face of non-response to treatment or disease progression is unacceptable, then high-risk patients should be spared the toxicity of ineffective therapy and instead be considered for best supportive care or investigational trials. Before GOG 240 was developed, some suggested using the Moore criteria to pull out high-risk patients from subsequent phase III studies. However, at that time, the Moore criteria had not been prospectively validated and for this reason the scoring system was not used to limit eligibility in GOG 240. In GOG 240 we have demonstrated that, compared to high-risk patients treated with chemotherapy alone, those high-risk patients who received bevacizumab had a significantly lower hazard of death not only within the high-risk group but also compared to those at mid-risk level.
Women with advanced cervical cancer are distinguished by often having been pre-irradiated resulting in diminished marrow reserves and a vasculitis that limits adequate drug distribution and perfusion into irradiated tumor beds. Concurrent chemoradiation leads to acquired drug resistance making cisplatin-based therapies less effective at recurrence. Finally, often poor and lacking access to healthcare, this population is marginalized by society, medically debilitated, malnourished and with diminished renal function due to tumor- and radiation-related hydronephrosis and consequent renal insufficiency/failure. Women with recurrent cervical cancer often do not respond to multiple lines of chemotherapy as do patients with cancer of other types (e.g., ovary, breast). Additional not-yet-developed models that include factors such as income level, nutritional status, and/or renal function may also have clinical utility,
It is not difficult to understand how performance status, short disease-free interval, pelvic disease, and prior cisplatin may impair prognosis. It is less clear how African-American ethnicity worsens outcome. African-Americans may have limited access to care with co-morbidities not reflected in performance status, or they may have biologically worse cervical cancers (23-27). Farley et al reported that in an equal access, unbiased, nonracial environment such as the military, race is not an independent predictor of survival for patients with cervical carcinoma (28). Interestingly, in a GOG ancillary data study in the recurrent cervical cancer population, Plaxe et al found that cisplatin-based chemotherapy was better tolerated by African American women (29).
Angiogenesis confers a poor prognosis in cervical cancer and a molecular cascade involving viral oncoproteins E6 and E7 and their interactions with cellular tumor suppressor gene products p53 and pRb leads to increased hypoxia inducible factor-1α and VEGF production and angiogenesis (30). Through ligand-binding, bevacizumab, sequesters VEGF and inhibits angiogenesis. Although the Moore criteria lack definitive predictive capabilities in chemotherapy guidance (i.e., backbone selection with which to integrate bevacizumab), there is evidence that the risk model can serve as a surrogate for personalized medicine in predicting outcomes among different cohorts treated with bevacizumab.
This risk model is a tool for office practice when counseling patients with advanced disease. Prognostic factors can be compiled and an estimation of RR (and median survival) using anti-angiogenesis therapy can be provided to an individual patient and her family members. Patients with the highest risk stratification (i.e., 4-5 factors) derive the greatest relative benefit from bevacizumab (HR 0.536) compared to those patients who are in the mid- (HR 0.673) or low-risk (HR 0.96) cohorts.
Not all low-risk patients are the same and anticipated toxicity should also be considered when counseling patients. For example, in GOG 240, fistula occurred in 8.6% of patients treated with bevaiczumab, all of whom had been previously irradiated. Additional risk factors for fistula may also include recurrent disease in the irradiated pelvis (with or without distant metastases) and persistent disease following chemoradiation. In a low-risk patient (0-1 factors) treated with chemoradiation prior to recurrence, the Moore criteria can be used to argue against including bevacizumab as the fistula risk is 8.6% with very small survival benefit. We must acknowledge that despite a significantly improved OS and subsequent US FDA regulatory approval, bevacizumab is not curing patients. Development of a fistula may preclude eligibility for participation in a promising immunotherapy clinical trial. When taken in this context, a previously irradiated low-risk patient who is carefully counseled may reasonably choose to not receive bevacizumab.
Performance status is the one Moore factor that is modifiable through medical, nutritional, and possibly spiritual intervention. Eligibility criteria for GOG-240 were more stringent than in preceding trials. Previously, great expense and effort had been invested in patients with very low likelihood of response. Through optimization of medical co-morbidities, correction of malnutrition, improved understanding of renal function with expeditious placement of ureteral stents and/or percutaneous nephrostomies, the GOG-240 population was “healthier” at enrollment than their predecessors in prior trials. To improve survival, we believed that resources (including expensive therapies) are best applied to those who stand the greatest chance of benefiting. The identification of a near-4 month window of improved OS without significant deterioration of QoL suggests that the disease may lend itself to chronicity. Patients deriving benefit (eg., stable disease) but who are intolerable to chemotherapy may have the latter drugs peeled away and continue with bevacizumab monotherapy. Alternatively, those patients responding to anti-angiogenesis therapy may be considered for incorporation of immunotherapy prior to progression. Bevacizumab does not signify the end of advanced cervical cancer, but hopefully represents a small step forward in the treatment of this devastating disease (Figure 3).
Figure 3.

Successive improvement in median overall survival among women with advanced cervical cancer. The phase III experience of the Gynecologic Oncology Group (now, part of NRG Oncology).
This is the first prospectively validated prognostic scoring system in cervical cancer, a disease that is only second to lung and breast in cancer-related mortality worldwide. Advanced cervical cancer is not a disease in which “cure” is an issue. Studies such as this which help to refine treatment selection in a manner that stratifies patients into risk groups for anticipated response to treatment and complications are clinically important. Characterization of gene signatures that confer risk and blood-based predictive protein signatures obtained from women with durable responses to anti-VEGF therapy are needed. Mathematical modeling may be used to combine clinical (i.e., Moore-like) risk factors with molecular ones and through assignation of different weights, the predictive capabilities of the risk model can be refined further.
Supplementary Material
Statement of Translational Relevance.
The phase III international, multi-center trial, GOG 240, demonstrated a significant survival advantage among women treated with chemotherapy plus bevacizumab compared with chemotherapy alone, and directly led to US FDA approval of the two triplet regimens administering bevacizumab. However, anti-angiogenesis therapy in this population may be associated with significant toxicity. Clinical scoring systems may allow for risk stratification. The data presented are a prospective validatation of the Moore clinical prognostic factor scoring system. This system may be used as a clinical instrument to counsel patients regarding their likelihood of response and estimated risk of progression and death by cervical cancer when considering adding anti-angiogenesis therapy to systemic chemotherapy for recurrent/persistent or metastatic cervical cancer. Importantly, based on these data patients at mid- to high-risk may be expected to derive the greatest benefit from the integration of bevacizumab to chemotherapy, while the benefit conferred to low-risk patients appears to be low.
Acknowledgments
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Northwestern Memorial Hospital, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Rush-Presbyterian-St. Luke's Medical Center, Magee Women's Hospital, SUNY Downstate Medical Center, University of Kentucky, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Yale University, University of Wisconsin Hospital, Cancer Trials Support Unit, University of Texas - Galveston, Women and Infants Hospital, The Hospital of Central Connecticut, Georgia Core, Aurora Women's Pavilion of West Allis Memorial Hospital, Grupo Espanol de Investigacion en Cancer de Ovario, University of California, San Francisco-Mt. Zion, St. Joseph's Hospital and Medical Center (Arizona), and Community Clinical Oncology Program.
Funding: This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517) and NRG Oncology Group Grant 1 U10 CA180822.
Footnotes
Financial Disclosure: Drs. K. Tewari, B. Monk and D. Moore disclose serving on an advisory board for Roche/Genentech.
References
- 1.Tewari KS, Monk BJ. Invasive Cervical Cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. 8th. Mosby; 2012. [Google Scholar]
- 2.Monk BJ, Tewari KS, Koh WJ. Multi-modality therapy for locally advanced cervical carcinoma: State of the art and future directions. J Clin Oncol. 2007;25:2952–2965. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 3.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tewari KS, Monk BJ. Gynecologic Oncology Group trials of chemotherapy for metastatic and recurrent cervical carcinoma. Curr Oncol Rep. 2005;7:419–434. doi: 10.1007/s11912-005-0007-z. [DOI] [PubMed] [Google Scholar]
- 5.Tewari KS, Monk BJ. Beyond platinum for metastatic and recurrent carcinoma of the cervix. Onkologie. 2009;32:552–554. doi: 10.1159/000232385. [DOI] [PubMed] [Google Scholar]
- 6.Tewari KS. A critical need for reappraisal of therapeutic options for women with metastatic and recurrent cervical carcinoma: Commentary on Gynecologic Oncology Group protocol 204. Am J Hematol Oncol. 2010;9:31–34. [Google Scholar]
- 7.Tiersten AD, Selleck MJ, Hershman DL, Smith D, Resnik EE, Troxel AB, et al. Phase II study of topotecan and paclitaxel for recurrent, persistent, or metastatic cervical carcinoma. Gynecol Oncol. 2004;92:635–638. doi: 10.1016/j.ygyno.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penson RT, Huang HQ, Wenzel LB, Monk BJ, Stockman S, Long HJ, 3rd, et al. Bevacizumab for advanced cervical cancer: patient reported outcomes of a randomised, phase 3 trial (NRG Oncology – Gynecologic Oncology Group protocol 240) Lancet Oncol. 2015;16:301–311. doi: 10.1016/S1470-2045(15)70004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Cervical Cancer Version 1. 2014 NCCN.org.
- 12.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:44–49. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactor versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–171. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 14.Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 15.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2006;23:4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Bloss JD, Blessing JA, Behrens BC, Mannel Rs, Rader JS, Sood AK, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2002;20:1832–1837. doi: 10.1200/JCO.2002.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B. 1972;34:187–220. [Google Scholar]
- 18.Tewari KS, Monk BJ. Recent achievements and future developments in advanced and recurrent cervical cancer: Trials of the Gynecologic Oncology Group. Semin Oncol. 2009;36:170–180. doi: 10.1053/j.seminoncol.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr Opin Obstet Gynecol. 2014;26:314–321. doi: 10.1097/GCO.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 20.Tewari KS, Monk BJ. The rationale for the use of non-platinum chemotherapy doublets for metastatic and recurrent cervical carcinoma. Clin Adv Hematol Oncol. 2010;8:108–115. [PubMed] [Google Scholar]
- 21.Tewari KS. Patients with metastatic/recurrent cervical cancer should be treated with cisplatin plus paclitaxel. Expert Panel Clin Ovarian Cancer. 2011;4:90–93. [Google Scholar]
- 22.Moore DH. Chemotherapy for recurrent cervical carcinoma. Curr Opin Oncol. 2006;18:516–519. doi: 10.1097/01.cco.0000239893.21161.51. [DOI] [PubMed] [Google Scholar]
- 23.Somayaji D, Cloyes KG. Cancer Fear and Fatalism: How African-American participants construct the role of research subject in relation to clinical cancer research. Cancer Nurs. 2014;38:133–144. doi: 10.1097/NCC.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 24.Haynes-Maslow L, Godley P, Dimartino L, White B, Odom J, Richmond A, et al. African-American women's perceptions of cancer clinical trials. Cancer Med. 2014;3:1430–1439. doi: 10.1002/cam4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: A report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133:353–361. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brookfield KF, Cheung MC, Lucci J, Fleming LE, Koniaris LG. Disparities in survival among women with invasive cervical cancer: A problem of access of care. Cancer. 2009;115:166–178. doi: 10.1002/cncr.24007. [DOI] [PubMed] [Google Scholar]
- 27.Kang M, Shen XJ, Kim S, Araujo-Perez F, Galanko JA, Martin CE, et al. Somatic gene mutations in African-Americans may predict worse outcomes in colorectal cancer. Cancer Biomark. 2013;13:359–366. doi: 10.3233/CBM-130366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farley JH, Hines JF, Taylor RR, Carlson JW, Parker MF, Kost ER, et al. Equal care ensures equal survival for African-American women with cervical carcinoma. Cancer. 2001;91:869–73. [PubMed] [Google Scholar]
- 29.Plaxe SC, Brooks SE, Tian C, Bloss JD, Moore DH, Long HJ. Influence of race on tolerance of platinum-based chemotherapy and clinical outcomes in women with advanced and recurrent cervical cancer: a pooled analysis of 3 Gynecologic Oncology Group studies. Am J Obstet Gynecol. 2008;199:539e1–6. doi: 10.1016/j.ajog.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Tewari KS, Taylor JA, Liao SY, DiSaia PJ, Burger RA, Monk BJ, et al. Development and assessment of a general theory of cervical carcinogenesis utilizing a severe combined immunodeficiency murine-human xenograft model. Gynecol Oncol. 2000;77:137–148. doi: 10.1006/gyno.2000.5729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


