Abstract
Cancer cachexia leads to involuntary weight loss resulting from the atrophy of skeletal muscle and adipose tissues. Now, in metastatic mouse models of cancer, investigators reveal a cross talk pathway between bone and muscle that provides a new understanding of wasting in advanced cancers.
Cachexia is a condition linked to chronic illnesses, and it is most easily diagnosed by a marked loss of body weight. Cachexia is distinct from anorexia or loss of appetite, conditions in which the loss of weight derives predominantly from the utilization of adipose stores, whereas skeletal muscle mass is preserved1. In cachexia, weight loss is derived from a reduction of muscle mass, and it contributes to an overall state of weakness and fatigue that, depending on the illness, can compromise treatment strategies and quality of life.
In cancer, cachexia is generally classified as a loss of more than 5% of a patient’s pre-illness body weight, and it is estimated to occur in more than 80% of people with cancer2,3. The gastrointestinal cancers, including esophageal and pancreatic cancer, are most commonly associated with cachexia, whereas malignancies such as breast and lung cancer are generally associated with cachexia only during late-stage disease4. A study in this issue of Nature Medicine5 provides a new perspective on cachexia by highlighting the importance of muscle dysfunction in people with cancer, rather than the loss of lean tissue and fat mass. Furthermore, the study suggests that this reduction in muscle function occurs in patients with advanced disease, specifically with metastasis localized to bone.
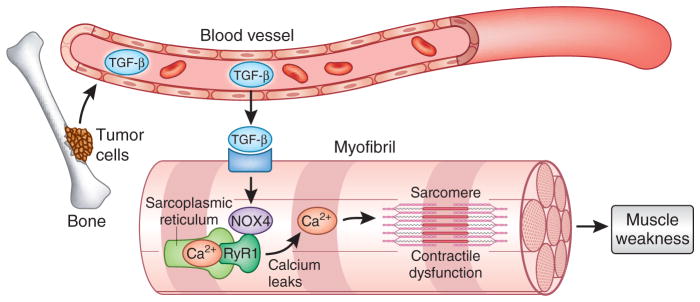
Waning et al.5 studied mouse models of tumor metastasis in which mice received injections of several tumor cell lines, including MDA-MB-231 and MCF-7 (breast), A549 and RWGT2 (lung), PC3 (prostate) and JJN3 (multiple myeloma). In these mice, the researchers were able to demonstrate that tumors that metastasize to bone and induce osteolysis cause the release of transforming growth factor-β (TGF-β) from the bone extracellular matrix into the general circulation (Fig. 1). In contrast to the physiological role of TGF-β that regulates cell growth and differentiation, the authors find that circulating, pathological levels of TGF-β activate a SMAD3 signaling pathway in both proximal and distal skeletal muscles that leads to the oxidation and nitrosylation of the ryanodine receptor5 (RyR1). Under normal conditions, the protein calstabin stabilizes RyR1 (ref. 6). The loss of calstabin from the RyR1 complex, in conjunction with the post-translational modification of RyR1 in advanced cancers with bone metastasis, results in leaky Ca2+ channels in the sarcoplasmic reticulum. This imbalance of Ca2+ homeostasis presumably alters normal binding of Ca2+ to troponin (a protein that is central to muscle contraction) in the sarcomere, leading to an impaired contractile apparatus and eventual muscle weakness. The authors further connected SMAD3 signaling to RyR1 oxidation in the muscle tissue of these mice by showing that TGF-β signaling through SMAD3 transcriptionally induces the NAPDH oxidase 4 (Nox4) gene, whose function in membranes results in the production of reactive oxygen species that oxidize multiple proteins, including RyR1. Thus, the authors showed that tumors that metastasize to bone and release TGF-β lead to lower muscle force production regardless of whether there is a reduction in muscle mass and body weight.
Figure 1.
Bone metastasis leads to skeletal muscle weakness. Metastatic cancer cells induce the breakdown of bone and the release of TGF-β into the circulation. This increase in TGF-β signals myofibers in skeletal muscle to promote NOX4 expression that, in turn, causes post-translational modifications of the ryanodine receptor channel (RyR1) and calcium leaks from the sarcoplasmic reticulum. Altered calcium homeostasis alters the regulation of sarcomere proteins, leading to contractile dysfunction and muscle weakness.
In the MDA-MB-231 breast tumor model, the authors also found that the degree of muscle weakness and leaky Ca2+ channels both correlated with the extent of bone metastasis but not with primary tumors. This implies either that the source of TGF-β is enriched in the bone-tumor microenvironment compared to primary tumors, or that the levels and activities of enzymes responsible for the release of TGF-β from the extracellular matrix are themselves elevated at the site of a decaying bone owing to the metastasis. Furthermore, the authors identified a loss of calstabin, and activation of SMAD3 and NOX4 (encoded by Nox4) in muscle biopsies from individuals affected with advanced breast and prostate cancer, which adds to the significance of their findings in the animal models. However, given the limited human sample sizes, these data will need to be repeated with properly powered cohorts in multiple tumor types associated with bone metastasis. These findings will also need to be correlated with circulating levels of TGF-β and proportional SMAD3 activity and NOX4 expression levels in skeletal muscles.
Perhaps most significantly, Waning et al.5 identified several effectors within the TGF-β–NOX4–RyR1 signaling axis that could serve as therapeutic targets to prevent bone metastasis–induced muscle dysfunction. These include the use of chemical inhibitors (SD208 and bisphosphonate) and neutralizing antibodies (ID11) to directly target TGF-β signaling, all of which lowered SMAD3 phosphorylation and prevented leaky RyR1 channels in muscles. In addition, to assess the role of RyR1 in cancer cachexia, investigators used the RyR1 channel stabilizer S107, which has been shown to improve muscle function in muscular dystrophy and aging7,8. S107 functions by maintaining the interaction of calstabin with RyR1 independently of its effect on the post-translational modifications of RyR1. Thus, the closed state of RyR1 is stabilized, preventing Ca2+ leaks and deficits in muscular function. Mice with bone metastasis that were treated with S107 showed improved muscle function without changes to muscle mass or whole body weight. Finally, to ensure that post-translational modifications of RyR1 were responsible for the tumor-induced contractile dysfunction, mice with breast metastases were treated with the Nox4 inhibitor GKT137831, a drug that lowers oxidation and nitrosylation of proteins. GKT137831 prevented the metastasis-associated post-translational changes on muscle proteins, resulting in the stabilization of calstabin. These findings are particularly important, as no pharmacological therapy currently exists for cancer cachexia.
Another important connection made by the authors extends their findings beyond cancer cachexia. Osteoclastic bone resorption and the release of TGF-β also occur in a non-tumor mouse model of Camurati-Engelman disease (CED), a heritable human skeletal disease that is often associated with TGF-β mutations. Waning et al.5, showed that muscles from CED-model mice also have leaky RyR1 channels and contractile force deficits that are similar to those of mice with bone metastasis. However, they did not determine whether muscle weakness in CED-model mice is associated with reductions in muscle mass or changes in body weight that are attributable to the loss of lean and fat mass.
The findings by Waning et al.5 expand our understanding of cancer cachexia beyond the more commonly studied gastrointestinal cancers that are associated with severe weight loss. Activation of the SMAD3–NOX4 signaling axis, leading to muscle dysfunction, would not be predicted in people with gastrointestinal cancers because these cancers metastasize to soft tissue rather than bone9. However, it will be interesting to determine whether a role exists for TGF-β in these patients and in other mouse models without bone metastases. Furthermore, it should be tested whether the typical atrogene signature that accounts for the reduction in muscle mass and weight loss—characterized by elevated expression of genes encoding subunits of the ubiquitin proteasome and autophagy systems—is induced by TGF-β–triggered SMAD3 activation in mice and patients with bone metastasis.
Cachexia is characterized by ongoing loss of skeletal muscle mass that ultimately leads to progressive functional impairment2. The current findings from Waning et al.5 potentially expand on this definition by suggesting that—at least in cancer—cachexia could include people who experience muscle dysfunction without loss of lean muscle or reductions in body weight. It will be intriguing in future clinical studies to assess whether deficits in muscle function correlate with advanced cancers that have progressed to bone metastasis, and more provocatively, whether people with these cancers would benefit from combinatorial therapies that include compounds that target the TGF-β–NOX4–RyR1 axis in order to enhance muscle contractility and performance.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Body JJ. Curr Opin Oncol. 1999;11:255–260. doi: 10.1097/00001622-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, et al. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Hopkinson JB, Wright DN, McDonald JW, Corner JL. J Pain Symptom Manage. 2006;32:322–331. doi: 10.1016/j.jpainsymman.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Dewys WD, et al. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 5.Waning DL, et al. Nat Med. 2015;21:1262–1271. doi: 10.1038/nm.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx SO, Ondrias K, Marks AR. Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger AM, et al. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson DC, et al. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yachida S, Iacobuzio-Donahue CA. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]