Abstract
Objective
To determine if proprioceptive impairments exist in patients with low back pain (LBP). We hypothesized that patients with LBP would exhibit larger trunk proprioception errors than healthy controls.
Design
Case-control study.
Setting
University laboratory.
Participants
24 patients with non-specific LBP and 24 age-matched healthy controls.
Interventions
Not applicable.
Main Outcome Measures
We measured trunk proprioception in all 3 anatomical planes using motion perception threshold, active repositioning, and passive repositioning tests.
Results
LBP patients had significantly greater motion perception threshold than controls (P<0.001)(1.3±0.9 vs. 0.8±0.6 degrees). Furthermore, all subjects had the largest motion perception threshold in the transverse plane (P<0.001) (1.2±0.7 vs. 1.0±0.8 degrees for all other planes averaged). There was no significant difference between LBP and healthy control groups in the repositioning tasks. Errors in active repositioning test were significantly smaller than in passive repositioning test (P=0.032) (1.9±1.2 vs. 2.3±1.4 degrees).
Conclusions
These findings suggest that impairments in proprioception may be detected in patients with LBP when assessed with a motion perception threshold measure.
Keywords: Kinesthesis, Low back pain, Proprioception, Rehabilitation, Spine
“Proprioception is the sense of position and movement of one’s own limbs and body without using vision. There are two submodalities of proprioception: the sense of the stationary position of the limbs (limb-position sense) and the sense of limb movement (kinesthesia) 1” Ability to sense stationary position and movement is an important aspect of executing body motion 2. As one of the sensory modalities, proprioceptive signals are obtained from mechanoreceptors imbedded in ligaments, facet joints, intervertebral discs, and muscles 3–6. Among these sources, dense muscle spindles in paraspinal rotators are thought to be crucial for monitoring the midrange motion of the trunk 7–9. Since ability to monitor trunk motion is necessary to generate movement pattern, any deficit in proprioception would affect the quality of movement.
For chronic LBP patients, differences in motor control such as longer muscle reflex latencies 10–13, poor postural control 11, 14–20, and altered muscle recruitment patterns 21–25 have been documented. These differences in motor control could be, in part, attributed to deficits in proprioception. Proprioception has been studied in LBP using various protocols and the outcomes of these studies vary. Some studies have found proprioceptive impairment associated with LBP 9, 26–31, whereas others have found no impairments 32–36. These conflicting results could stem from diversity in the LBP population 28, 30, 31 and/or different testing methods 34, 37.
The variation in methodologies includes different testing positions (standing 27, 32, 34, sitting 28, or 4 point kneeling 27), and different measurement methods (trunk repositioning task 27, 32–35, or motion perception threshold 29, 36). Within-session repeatability of various measurement methods showed that motion perception threshold had the highest intra-class correlation (ICC = 0.89) compared to active and passive repositioning tests (ICC = 0.61 and ICC = 0.58, respectively) 36. Motion perception threshold represents an individual’s sensitivity to a small change in trunk position. Other tests, such as active repositioning and passive repositioning, rely on memory to recall the initial starting position or other prescribed position. This may increase variability and errors 38, 39.
Furthermore, studies assessed proprioception in different planes of motion 29, 32–34, 36 and measured either trunk rotations 29, 36, or translations28. Thus, despite the large number of studies published on proprioception and LBP, no clear conclusions can be reached about a possible impairment in LBP patients.
The goal of this study was to determine if proprioception impairments are associated with LBP in any of the three anatomical planes (sagittal, coronal, and transverse). We also hypothesized that proprioceptive impairments, should they exist, will be detected with the more sensitive motion perception threshold test, but not with repositioning tests.
METHODS
Subjects
Twenty four subjects with LBP (11 males and 13 females) and 24 healthy controls without a history of LBP (14 males and 10 females) volunteered for the study. Of the 24 LBP subjects, 9 subjects (5 males and 4 females) were tested after wearing a lumbar orthosis for 3 weeks. Because there was no statistical difference in proprioception between these two LBP groups (active repositioning: p=0.134, passive repositioning: p=0.416, motion perception threshold: p=0.38), they were combined as one group for the comparison with the controls. The anthropometric data for LBP and control groups are presented in Table 1. The LBP group consisted of individuals with pain persisting longer than 3 months and with no history of spinal surgery. Potential subjects were excluded if they have nerve symptoms below the knee. Eleven subjects used pain medicine when needed, and 3 used the pain medicine daily. Also, 2 subjects used muscle relaxants daily. The majority of subjects received some form of therapy in the past, including chiropractic, exercise, massage, and ultrasound. Before starting the experiment, all subjects read and signed an informed consent form approved by the Institutional Review Board.
Table 1.
Anthropometric data for each subject group. Data represents means ± SD.
| Control (n=24) | LBP (n=24) | ||
|---|---|---|---|
|
| |||
| Age [yrs] | 42.4 ± 9.0 | 42.6 ± 13.7 | |
| Height [m] | 1.71 ± 0.08 | 1.69 ± 0.1 | |
| Weight [kg] | 73.0 ± 14.8 | 71.3 ± 12.8 | |
| Visual Analog Scale [out of 10] | NA | 4 ± 2.6 | |
| Modified Oswestry disability index 51 [out of 10] | NA | 1.9 ± 1.5 | |
| Number of years since the first episode | NA | 11.6 ± 7.2 | |
| Days with back pain in the past 30 days [number of patients] | 1 to 5 | NA | 1 |
| 6 to 10 | NA | 4 | |
| 11 to 20 | NA | 8 | |
| 20 or more | NA | 11 | |
Protocol
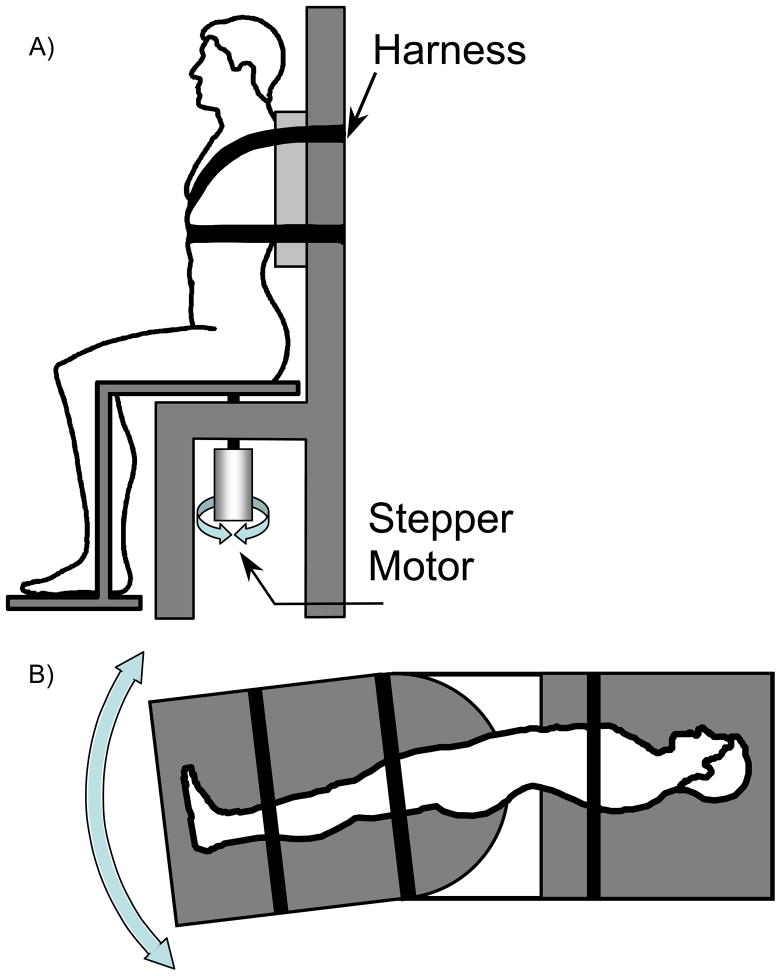
Subjects were tested in seated, supine, and side lying positions, which corresponded to transverse, frontal, and sagittal planes, respectively. The order of testing positions was randomized. The apparatus was modified from Cholewicki et al. 40 to provide rotations in all 3 anatomical planes while immobilizing the upper body (Figure 1). The resolution of the apparatus was less than 0.01 degrees and the accuracy of calibration curve was 0.35 degrees. Subjects were positioned such that the axis of rotation passed through the L4/L5 spinal level. Subjects’ upper bodies were fixed to the apparatus with straps, so that only their lower bodies rotated. Subjects were instructed to keep their arms crossed above their waist and eyes closed to minimize tactile and visual cues. The motor noise from the apparatus was masked by background music.
Figure 1.
The apparatus for assessing proprioception in A) axial rotation, B) flexion and extension. For lateral bending, the same set up as flexion and extension was used, but the subjects were lying in a supine position.
Motion perception threshold, passive repositioning, and active repositioning were measured with protocols similar to previous studies 36, 40, 41. For motion perception threshold, the stepper motor rotated the lower body at 0.1 degrees/sec away from the neutral position. The subjects were instructed to press a handheld button once they perceived a change in position, and report the direction of motion. The trials were recorded if subject reported the direction correctly. For passive repositioning, the stepper motor moved the subjects’ lower body 15 degrees away from neutral at 2.2 degrees/s. Once 15 degrees was reached, the motor briefly paused and then started to return back towards the neutral position at 1.0 degrees/s. Subjects pressed the button when they perceived that they were back to the neutral position. active repositioning was similar to passive repositioning except that once the motor reached 15 degrees, the clutch was disengaged and the subjects actively repositioned their lower bodies to the perceived neutral position. After reaching their perceived neutral position, subjects pressed the button and the angle was recorded. Subjects were given 2 practice trials in each plane of motion prior to data collection. In lateral and axial planes of motion, we preformed 4 trials in each direction. The data from left and right directions were combined. We increased the number of trials in the flexion and extension directions to five because they could not be combined given that different anatomical structures would be involved.
Analysis
The motion perception threshold was expressed as an absolute value in degrees. For each repositioning trial, an absolute error, referred to as “error” in this paper, was computed as the absolute difference between the subjects’ perceived neutral position and the starting position. We chose absolute error, instead of constant and variable error, as a more general measure of performance. For all tests, the two worst trials were rejected before averaging the values in flexion, extension, lateral bending and axial rotation 36. Data were tested for their normality using an Anderson-Darling test. Box-Cox transformations were applied if data were not normally distributed. For the motion perception threshold test, 2-factor (group and planes), repeated-measures analysis of variance with Tukey’s post hoc test was used (α = 0.05). For repositioning tasks, 3-factor (group, mode (i.e. active or passive), and plane) analysis of variance was used to investigate differences in errors. All statistical analyses were performed with Minitab 13.1a.
RESULTS
Demographic statistics indicated that there was no difference in age (P=0.931), height (P=0.782), or weight (P=0.772) between LBP patients and healthy controls.
In the motion perception threshold test, the control group perceived smaller trunk displacement as compared to the LBP group (0.8±0.6 vs. 1.3±0.9 degrees, averaged across planes (DF=1, F=45.97, P<0.001)), suggesting that the healthy controls are more perceptive of trunk motion (Figure 2). The plane of motion also had a significant effect on motion perception threshold (DF=3, F=8.04, P<0.001). When averaged across groups, axial rotation had the greatest threshold (1.2±0.9 degrees), followed by extension (1.0±0.7 degrees) and flexion (1.0±0.9 degrees), and then lateral bending (0.9±0.8 degrees). A post hoc analysis revealed that motion perception threshold in axial rotation was significantly greater than the motion perception threshold in other planes of motion (P<0.01). There was no interaction between the effects of group and plane of motion. During the motion perception threshold test, the LBP group reported the wrong direction in 5.4% of trials while the control group reported the wrong direction in 6.3% trials. These error rates were not significantly different between the groups according to the 2 proportion test (P=0.569).
Figure 2.
Motion perception threshold (MPT), and errors in active repositioning (AR) and passive repositioning (PR) tests, averaged across planes of motion. * indicates significant differences (P<0.05).
Errors in the repositioning tests did not differ between LBP and healthy control groups (DF=1, F=0.65, P=0.421) (Figure 2). Generally, active repositioning was significantly more accurate than passive repositioning when averaged across groups and planes of motion (DF=1, F=4.64, P=0.032) (Table 2). There was a significant interaction between the effects of group and plane of motion (DF=3, F=3.0, P=0.031). Post hoc analysis showed errors in extension of the control group were significantly smaller than in flexion (T=−4.07, P=0.001), axial rotation (T=3.05, P=0.048), and lateral bending (T=3.17, P=0.032). However, no such differences existed within the LBP group (Table 2).
Table 2.
Average (means ± SD)motion perception threshold (MPT) (degrees), and average of absolute error (degrees) in active repositioning (AR) and passive repositioning (PR). The LBP patients had significantly greater MPT averaged across planes of motion than healthy controls (P<0.001). There was no significant difference between LBP and healthy control groups in the repositioning errors measured with AR or PR. However, AR had significantly smaller error than PR (P=0.032).
| Flexion | Extension | Axial Rotation | Lateral Bending | Average | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | LBP | Control | LBP | Control | LBP | Control | LBP | ||
| MPT | 0.7 ± 0.4 | 1.3 ± 1.1 | 0.8 ± 0.6 | 1.1 ± 0.7 | 1.1 ± 0.7 | 1.4 ± 0.7 | 0.7 ± 0.5 | 1.2 ± 0.9 | 1.0 ± 0.8 |
| AR | 2.3 ± 1.3 | 2.1 ± 1.1 | 1.6 ± 1.5 | 2.3 ± 2.0 | 1.7 ± 0.9 | 1.8 ± 0.9 | 1.8 ± 0.9 | 2.0 ± 0.8 | 1.9 ± 1.2 |
| PR | 2.7 ± 1.7 | 2.2 ± 1.3 | 1.6 ± 1.4 | 2.2 ± 1.5 | 2.5 ± 1.2 | 2.2 ± 1.4 | 2.5 ± 1.5 | 2.4 ± 1.4 | 2.3 ± 1.4 |
DISCUSSION
Although many studies have investigated proprioception in LBP patients, it is still unclear whether individuals with LBP have impaired proprioception. Most studies tested patients with nonspecific LBP in limited planes of motion; hence, we adapted the protocol from previous studies 36, 40, 41 and tested subjects in all 3 anatomical planes, comparing the LBP group to age-matched healthy controls. Furthermore, our study was designed to investigate position and movement aspects of proprioception, while eliminating inputs from visual and vestibular systems. We hypothesized that if proprioceptive impairments exist in the LBP group, they will more likely be revealed on the more sensitive motion perception threshold test than on the repositioning tests (which rely on memory recall). Our results showed that people with LBP have lower acuity for detecting changes in trunk position during motion perception threshold testing. Repositioning tasks, on the other hand, showed no difference between LBP and control groups. This supports our hypothesis that motion perception threshold is more sensitive for detecting proprioceptive impairments than the repositioning tests (passive repositioning and active repositioning). The values of proprioception measures in the current study were similar to those in previous studies that used motion perception threshold 29, 41, 42 and repositioning tests 40 under similar testing conditions.
Subjects in both groups had smaller errors in the active repositioning test than in the passive. This is consistent with previous studies in other body segments 38, 39, 43, 44 as well as the trunk 36. It has been suggested that sensory information provided by efferent input to the muscles spindles is greater when the muscle is active, which might improve joint positioning sense 8.
It could be argued that the two passive tests (motion perception threshold and passive repositioning), which represent the proprioceptive sensitivity without significant muscle activation, should produce similar errors. However, the motion perception threshold was significantly smaller than the error in passive repositioning when averaged across groups and planes of motion (Table 2). One reason for this difference might stem from memory recall, since the repositioning tests require the subjects to remember a target position. This introduces memory bias to the repositioning tasks, which varies depending on the time that elapses between the presentation of the target position and the movement execution 39. In contrast, subjects only need to detect a change in position in the motion perception threshold test, which could explain the higher within-session repeatability 36. Therefore, the motion perception threshold test appears more reliable than repositioning tests for assessing proprioceptive deficits.
Although the motion perception threshold test is more reliable than the repositioning tests, the two previous studies that used the motion perception threshold method provided conflicting results. Silfies et al. found no difference in collegiate athletes between those with and without history of LBP 36. However, Taimela had found differences between patients with nonspecific LBP and healthy controls 29, which is similar to the result from this study. One possible explanation for different results is that the ability to detect small motion may be affected more in the older LBP population in the Taimela’s study (average age of 41 years) than the population in the Silfies’ study (average age of 19 years). As part of natural aging process, the neural control of muscles degrades 45, and proprioception is also diminished 46, 47. Thus, the pathology associated with chronic LBP may accentuate the age-related neural changes, resulting in motion perception threshold differences seen only in the older LBP subjects 29 and not the younger athletic population of Silfies et al. 36. It is also possible that the conflicting motion perception threshold results between these two studies are due to the level of proprioceptive training. Since training can improve proprioception 48–50, any proprioception deficit could be masked by the high performance training in collegiate athletes.
O’Sullivan et al. 28 argued that the inconsistencies in proprioception studies are due to the non-homogeneity of subjects. For this study, most LBP subjects had non-specific LBP (1 diagnosis of bulging disc and 1 spondylolysis). When the 2 individuals were excluded, conclusions of this study did not change. Proprioception deficit may exist for subgroups of LBP, such as spinal stenosis 31, herniated disc 30, or patients with lumbar segmental instability with a flexion pattern 28. However, our study indicates that even without such classifications, the impairment in proprioception can be detected if the motion perception threshold test is used.
Although this study showed impaired proprioception in patients with LBP, the current case-control experimental design cannot distinguish between cause and effect. Longitudinal prospective studies are needed to determine if impaired proprioception could be a predisposing factor for LBP. The longitudinal studies can also determine the effects of various treatment modalities on trunk proprioception. Our study did not have sufficient power to correlate the magnitude of motion perception threshold with previous treatment received by the patients. Finally, we did not attempt to sub-classified LBP patients based on any clinical measures. Such sub-classification may reveal more information about the role of proprioception in LBP pathology and help determine prognosis for chronicity.
CONCLUSIONS
While this is yet another study on proprioception in LBP, it contributes some methodological guidelines for future research. For example, with the more sensitive motion perception threshold test, it might be possible to detect proprioceptive differences between various sub-groups of LBP patients and shed more light on the role of such impairments in LBP and its recurrence. Thus, additional studies are still needed until we can fully understand this phenomenon and be able to incorporate such knowledge rationally into the management of LBP.
Acknowledgments
Supported by the National Institutes of Health (grant no. R01 AR051497).
List of Abbreviations
- ICC
intraclass correlation coefficient
- LBP
low back pain
Footnotes
Supplier
Minitab Inc, 1829 Pine Hall Rd, State College, PA 16081.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela S. Lee, Michigan State University Center for Orthopedic Research, Department of Osteopathic Surgical Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, MI.
Jacek Cholewicki, Michigan State University Center for Orthopedic Research, Department of Osteopathic Surgical Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, MI.
N. Peter Reeves, Michigan State University Center for Orthopedic Research, Department of Osteopathic Surgical Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, MI.
Bohdanna T. Zazulak, Department of Orthopaedics and Rehabilitation, Yale-New Haven Hospital/Yale University School of Medicine, New Haven, CT.
Lawrence W. Mysliwiec, Michigan State University Center for Orthopedic Research, Department of Osteopathic Surgical Specialties, College of Osteopathic Medicine, Michigan State University, East Lansing, MI.
References
- 1.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4. New York: McGraw-Hill, Health Professions Division; 2000. p. 443. [Google Scholar]
- 2.Reeves NP, Narendra KS, Cholewicki J. Spine stability: the six blind men and the elephant. Clin Biomech. 2007;22:266–74. doi: 10.1016/j.clinbiomech.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandevia SC, Refshauge KM, Collins DF. Proprioception: peripheral inputs and perceptual interactions. Adv Exp Med Biol. 2002;508:61–8. doi: 10.1007/978-1-4615-0713-0_8. [DOI] [PubMed] [Google Scholar]
- 4.Holm S, Indahl A, Solomonow M. Sensorimotor control of the spine. J Electromyogr Kinesiol. 2002;12:219–34. doi: 10.1016/s1050-6411(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 5.Sjölander P, Johansson H, Djupsjöbacka M. Spinal and supraspinal effects of activity in ligament afferents. J Electromyogr Kinesiol. 2002;12:167–76. doi: 10.1016/s1050-6411(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 6.Solomonow M. Sensory - motor control of ligaments and associated neuromuscular disorders. J Electromyogr Kinesiol. 2006;16:549–67. doi: 10.1016/j.jelekin.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Nitz AJ, Peck D. Comparison of muscle spindle concentrations in large and small human epaxial muscles acting in parallel combinations. Am Surg. 1986;52:273–7. [PubMed] [Google Scholar]
- 8.Gandevia SC, McCloskey DI, Burke D. Kinaesthetic signals and muscle contraction. Trends Neurosci. 1992;15:62–5. doi: 10.1016/0166-2236(92)90028-7. [DOI] [PubMed] [Google Scholar]
- 9.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–94. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25:947–54. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26:724–30. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Reeves NP, Cholewicki J, Milner TE. Muscle reflex classification of low-back pain. J Electromyogr Kinesiol. 2005;15:53–60. doi: 10.1016/j.jelekin.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Cholewicki J, Silfies SP, Shah RA, Greene HS, Reeves NP, Alvi K, et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine. 2005;30:2614–20. doi: 10.1097/01.brs.0000188273.27463.bc. [DOI] [PubMed] [Google Scholar]
- 14.Luoto S, Aalto H, Taimela S, Hurri H, Pyykko I, Alaranta H. One-footed and externally disturbed two-footed postural control in patients with chronic low back pain and healthy control subjects. A controlled study with follow-up. Spine. 1998;23:2081–9. doi: 10.1097/00007632-199810010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Takala E-P, Korhonen I, Viikari-Juntura E. Postural sway and stepping response among working population: Reproducibility, long-term stability, and associations with symptoms of the low back. Clin Biomech. 1997;12:429–37. doi: 10.1016/s0268-0033(97)00033-8. [DOI] [PubMed] [Google Scholar]
- 16.Mientjes MI, Frank JS. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech. 1999;14:710–6. doi: 10.1016/s0268-0033(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 17.Byl NN, Sinnott PL. Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine. 1991;16(3):325–30. doi: 10.1097/00007632-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Henry SM, Hitt JR, Jones SL, Bunn JY. Decreased limits of stability in response to postural perturbations in subjects with low back pain. Clin Biomech. 2006;21:881–92. doi: 10.1016/j.clinbiomech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Brumagne S, Cordo P, Verschueren S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett. 2004;366:63–6. doi: 10.1016/j.neulet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 20.della Volpe R, Popa T, Ginanneschi F, Spidalieri R, Mazzocchio R, Rossi A. Changes in coordination of postural control during dynamic stance in chronic low back pain patients. Gait Posture. 2006;24:349–55. doi: 10.1016/j.gaitpost.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 21.van Dieën JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine. 2003;28:834–41. [PubMed] [Google Scholar]
- 22.van Dieën JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–51. doi: 10.1016/s1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 23.Hodges P, van den Hoorn W, Dawson A, Cholewicki J. Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. J Biomech. 2009;42:61–6. doi: 10.1016/j.jbiomech.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80:1005–12. doi: 10.1016/s0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 25.Lariviere C, Gagnon D, Loisel P. The comparison of trunk muscles EMG activation between subjects with and without chronic low back pain during flexion-extension and lateral bending tasks. J Electromyogr Kinesiol. 2000;10:79–91. doi: 10.1016/s1050-6411(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 26.Field E, Abdel-Moty E, Loudon J. The effect of back injury and load on ability to replicate a novel posture. Journal of Back & Musculoskeletal Rehabilitation. 1997;8:199–207. doi: 10.3233/BMR-1997-8304. [DOI] [PubMed] [Google Scholar]
- 27.Gill KP, Callaghan MJ. The measurement of lumbar proprioception in individuals with and without low back pain. Spine. 1998;23:371–7. doi: 10.1097/00007632-199802010-00017. [DOI] [PubMed] [Google Scholar]
- 28.O’Sullivan PB, Burnett A, Floyd AN, Gadsdon K, Logiudice J, Miller D, et al. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28:1074–9. doi: 10.1097/01.BRS.0000061990.56113.6F. [DOI] [PubMed] [Google Scholar]
- 29.Taimela S, Kankaanpaa M, Luoto S. The effect of lumbar fatigue on the ability to sense a change in lumbar position. A controlled study. Spine. 1999;24:1322–7. doi: 10.1097/00007632-199907010-00009. [DOI] [PubMed] [Google Scholar]
- 30.Leinonen V, Kankaanpaa M, Luukkonen M, Kansanen M, Hanninen O, Airaksinen O, et al. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in discherniation-related back pain. Spine. 2003;28:842–8. [PubMed] [Google Scholar]
- 31.Leinonen V, Maatta S, Taimela S, Herno A, Kankaanpaa M, Partanen J, et al. Impaired lumbar movement perception in association with postural stability and motor- and somatosensory-evoked potentials in lumbar spinal stenosis. Spine. 2002;27:975–83. doi: 10.1097/00007632-200205010-00019. [DOI] [PubMed] [Google Scholar]
- 32.Lam SS, Jull G, Treleaven J. Lumbar spine kinesthesia in patients with low back pain. J Orthop Sports Phys Ther. 1999;29:294–9. doi: 10.2519/jospt.1999.29.5.294. [DOI] [PubMed] [Google Scholar]
- 33.Koumantakis GA, Winstanley J, Oldham JA. Thoracolumbar proprioception in individuals with and without low back pain: intratester reliability, clinical applicability, and validity. J Orthop Sports Phys Ther. 2002;32:327–35. doi: 10.2519/jospt.2002.32.7.327. [DOI] [PubMed] [Google Scholar]
- 34.Descarreaux M, Blouin JS, Teasdale N. Repositioning accuracy and movement parameters in low back pain subjects and healthy control subjects. Eur Spine J. 2005;14(2):185–91. doi: 10.1007/s00586-004-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Åsell M, Sjölander P, Kerschbaumer H, Djupsjöbacka M. Are lumbar repositioning errors larger among patients with chronic low back pain compared with asymptomatic subjects? Arch Phys Med Rehabil. 2006;87:1170–6. doi: 10.1016/j.apmr.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Silfies SP, Cholewicki J, Reeves NP, Greene HS. Lumbar position sense and the risk of low back injuries in college athletes: a prospective cohort study. BMC Musculoskelet Disord. 2007;8:129. doi: 10.1186/1471-2474-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preuss R, Grenier S, McGill S. The effect of test position on lumbar spine position sense. J Orthop Sports Phys Ther. 2003;33:73–8. doi: 10.2519/jospt.2003.33.2.73. [DOI] [PubMed] [Google Scholar]
- 38.Lönn J, Crenshaw AG, Djupsjöbacka M, Pedersen J, Johansson H. Position sense testing: influence of starting position and type of displacement. Arch Phys Med Rehabil. 2000;81:592–7. doi: 10.1016/s0003-9993(00)90040-6. [DOI] [PubMed] [Google Scholar]
- 39.Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. In: Freedman SE, editor. The neurophysiology of spatially oriented behavior. Homewood, IL: The Dorsey Press; 1968. pp. 7–55. [Google Scholar]
- 40.Cholewicki J, Shah KR, McGill KC. The effects of a 3-week use of lumbosacral orthoses on proprioception in the lumbar spine. J Orthop Sports Phys Ther. 2006;36:225–31. doi: 10.2519/jospt.2006.36.4.225. [DOI] [PubMed] [Google Scholar]
- 41.Reeves NP, Cholewicki J, Lee AS, Mysliwiec LW. The effects of stochastic resonance stimulation on spine proprioception and postural control in chronic low back pain patients. Spine. 2009;34:316–21. doi: 10.1097/BRS.0b013e3181971e09. [DOI] [PubMed] [Google Scholar]
- 42.Parkhurst TM, Burnett CN. Injury and proprioception in the lower back. J Orthop Sports Phys Ther. 1994;19:282–95. doi: 10.2519/jospt.1994.19.5.282. [DOI] [PubMed] [Google Scholar]
- 43.Lönn J, Crenshaw AG, Djupsjöbacka M, Johansson H. Reliability of position sense testing assessed with a fully automated system. Clin Physiol. 2000;20:30–7. doi: 10.1046/j.1365-2281.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 44.Pickard CM, Sullivan PE, Allison GT, Singer KP. Is there a difference in hip joint position sense between young and older groups? J Gerontol A Biol Sci Med Sci. 2003;58:631–5. doi: 10.1093/gerona/58.7.m631. [DOI] [PubMed] [Google Scholar]
- 45.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–9. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 46.Petrella RJ, Lattanzio PJ, Nelson MG. Effect of age and activity on knee joint proprioception. Am J Phys Med Rehabil. 1997;76:235–41. doi: 10.1097/00002060-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clin Orthop. 1984:208–118. [PubMed] [Google Scholar]
- 48.Aydin T, Yildiz Y, Yildiz C, Atesalp S, Kalyon TA. Proprioception of the ankle: a comparison between female teenaged gymnasts and controls. Foot Ankle Int. 2002;23:123–9. doi: 10.1177/107110070202300208. [DOI] [PubMed] [Google Scholar]
- 49.Muaidi QI, Nicholson LL, Refshauge KM. Do elite athletes exhibit enhanced proprioceptive acuity, range and strength of knee rotation compared with non-athletes? Scand J Med Sci Sports. 2009;19:103–12. doi: 10.1111/j.1600-0838.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 50.Lephart SM, Giraldo JL, Borsa PA, Fu FH. Knee joint proprioception: a comparison between female intercollegiate gymnasts and controls. Knee Surg Sports Traumatol Arthrosc. 1996;4:121–4. doi: 10.1007/BF01477265. [DOI] [PubMed] [Google Scholar]
- 51.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther. 2001;81:776–88. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]