Abstract
Background: The restoration of walking ability in the spinal cord injury (SCI) population is an increasingly important goal in physical therapy. Locomotor training (LT) is often implemented with the aim to restore ambulation. At this point, there are no guidelines for LT in the pediatric SCI population.
Objectives: The aim of this review is to further narrow the effects of LT to the pediatric SCI population and develop recommendations for pediatric LT.
Methods: A thorough search was performed using the following databases: Scopus, CINAHL, PubMed, and Ovid. Studies were selected based on the following inclusion criteria: pediatric SCI population, articles published within last 10 years, human subjects, and LT. Studies looking at other neurological disorders and subjects who were not previously ambulatory were excluded. Five students and one Faculty Research Advisor from the university's Doctor of Physical Therapy Program evaluated the inclusion criteria, conducted a risk of bias assessment using the Downs and Black checklist, and extracted the results.
Results: Six studies were selected for this review. They showed gains in distance, gait speed, walking independence, and participation. There were variations in results when comparing gains in injury level based on the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).
Conclusions: Currently there is insufficient evidence to determine the best clinical practice guidelines for rehabilitation using LT within the pediatric SCI population.
Keywords: children, gait training, LT, pediatric, SCI, treadmill
Background
Currently, there is very little evidence to support the natural progression of recovery in children with spinal cord injury (SCI), making the restoration of walking ability an increasingly important goal in physical therapy.1 It is estimated that 3% to 5% of all SCI cases occur in the pediatric population, resulting in approximately 2 per 100,000 SCI cases in the United States.2 SCIs in children can lead to significant limitations in function. These children often experience primary impairments such as motor and sensory limitations, which in turn affect their participation in activities of daily living (ADLs) and community involvement. Additionally, children with SCI are at an increased risk for secondary complications such as pressure ulcers, scoliosis, pain, bowel and bladder dysfunction, respiratory insufficiency, and immobilization hypercalcemia.1
Locomotor training (LT) is an activity-based rehabilitation strategy that aims to restore both walking and postural control after SCI.3,4 The goal of activity-based therapies is to focus on recovery and minimize compensatory strategies such as the use of assistive devices, as these strategies may interfere with neuroplasticity and neural recovery.5
Neuroplasticity is the capacity of neurons in the central nervous system (CNS) to change their structure and function in response to development, learning, or injury.6,7 After an SCI, neuroplastic changes occur in the brain and spinal cord either spontaneously or dependently based on activity.6,7 Spontaneous plasticity can include, but is not limited to, new axonal sprouting projecting to and from the brain, creation of new synapses, synaptic remodeling, or the unmasking of dormant pathways.6,7 Activity-dependent neuroplasticity occurs secondary to appropriate afferent input from a repetitive task.6,8 Therefore, it is generally thought that neuroplasticity in the CNS is a main contributor to training-induced recovery.9
Locomotor training provides sensory input to the damaged nervous system through the remaining connections within the spinal cord to facilitate activity-dependent neuroplasticity. This means that impaired motor patterns, like the ones used during walking, are believed to be improved via functional training.10 To induce such changes in impaired motor patterns, the appropriate afferent sensory information must be provided by the locomotor system.5,10
Several different types of LT exist, including body weight supported treadmill training (BWSTT) and robotic LT. These devices are utilized with the goal of providing appropriate afferent information for the desired motor pattern by decreasing the postural requirements and level of physical assistance needed.5,10–12 BWSTT uses a treadmill and a harness to provide body weight support (BWS), while physical therapists are positioned to assist and facilitate a reciprocal gait pattern at each leg.13 Robotic LT devices, such as the Lokomat, facilitate reciprocal stepping movement of the legs through a computer-controlled, exoskeleton device overtop a treadmill.12
The literature is inconclusive about whether or not treadmill training is superior to physical therapy strategies that emphasize walking overground.14 However, many LT approaches combine overground training with a form of treadmill training, because evidence supports that repetitive, task-oriented training is crucial for the promotion of neuroplasticity. It is thought that this approach promotes carry over as well as short- and long-term cortical reorganization.14
Previous systematic reviews have looked at the effectiveness of LT on a variety of pediatric neurological conditions. The aim of this review is to further narrow this to the effects of LT on pediatric SCI and develop recommendations for pediatric LT guidelines. With the implementation of LT into a pediatric rehabilitation program following an SCI, it is thought that an increase in functional outcomes and quality of life may result.
Methods
Search and study selection
A thorough search was performed in July 2015 using the following databases: Scopus, CINAHL, PubMed, and Ovid. Key words used during the search were “children”, “pediatric”, “LT”, “gait training”, “treadmill”, and “SCI”. The article searches were restricted and were limited to human studies, written in the English language, and published within the last 10 years. Abstracts, poster presentations, and book chapters were also excluded as possible matches.
A total of 61 publications were compiled from the initial search. Upon the removal of “exact duplicates,” 37 publications were removed, bringing the remaining total to 24 articles.
Each member screened all titles and abstracts, eliminating 13 publications based on the predetermined inclusion criteria. Our inclusion criteria included pediatric populations, SCI, and forms of LT. This resulted in 11 articles, and full-text assessment was performed at this time. Four more studies were eliminated for the following reasons: LT not included in study, unable to discriminate pediatric SCI participants' data from adult participants in 2 studies, and an adult-only study population. An additional publication was obtained and added following the review of references from the recently assessed articles.
The Downs and Black checklist was used to grade the remaining 8 publications during a second full-text assessment, in which 3 students and the faculty research advisor were responsible for grading each research article. Discrepancies between article scores were determined through discussion and resolved via research group consensus. An additional 2 publications were excluded secondary to being beyond the scope of this review's clinical research question or failing to report data specific to the population in question. These exclusions resulted in 6 final articles. Refer to Figure 1 for a detailed description of the search strategy.
Figure 1.
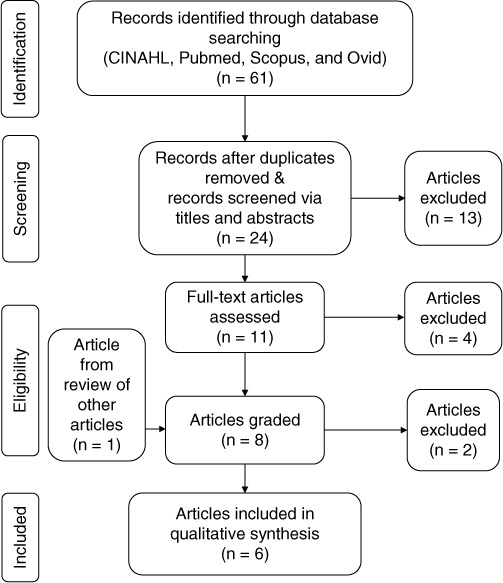
Search strategy flow diagram depicts the search strategy used to include and exclude publications specific to the research question in the systematic review.
Risk of bias assessment
A risk of bias assessment was performed using the Downs and Black checklist, which was developed to assess methodological quality of both randomized and nonrandomized research studies. The checklist was found to have high internal consistency, good test-retest and interrater reliability, and good face and criterion validity.15 Each article was graded with the Downs and Black checklist, and the scores are shown in Table 1. Additionally, researchers extracted injury level, intervention characteristics, outcomes measures, and study conclusions for each individual study (Table 2).
Table 1.
Downs and Black checklist scores
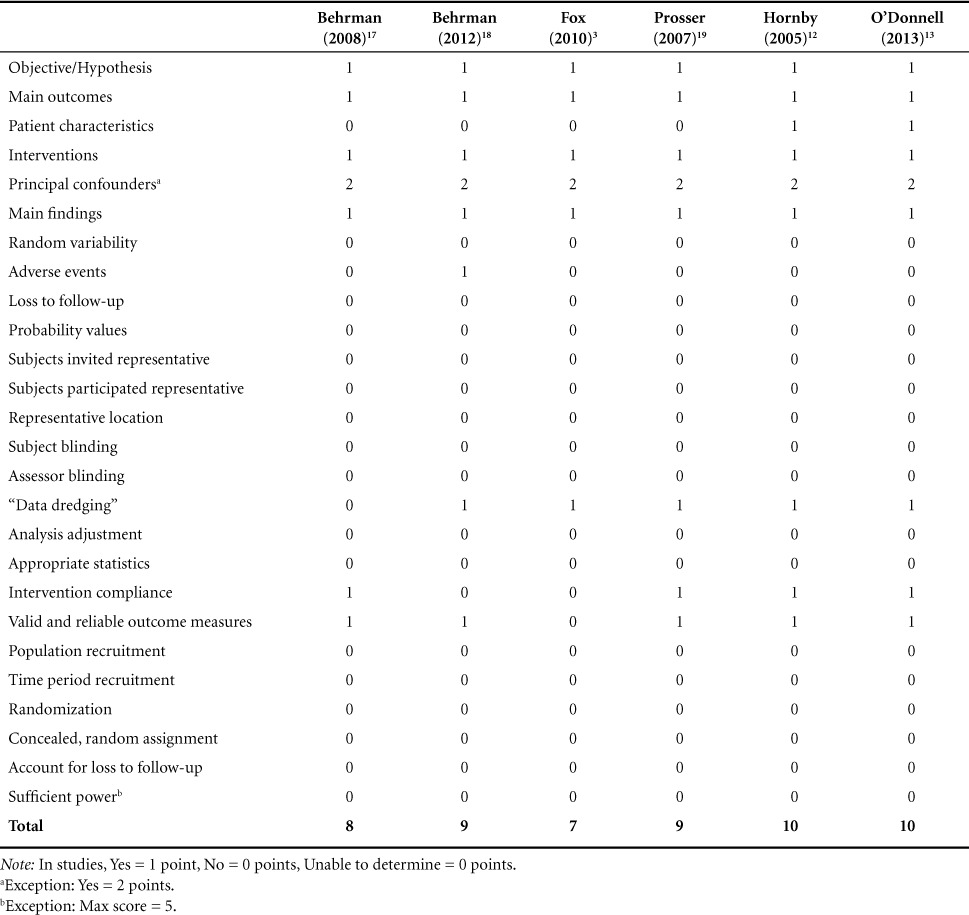
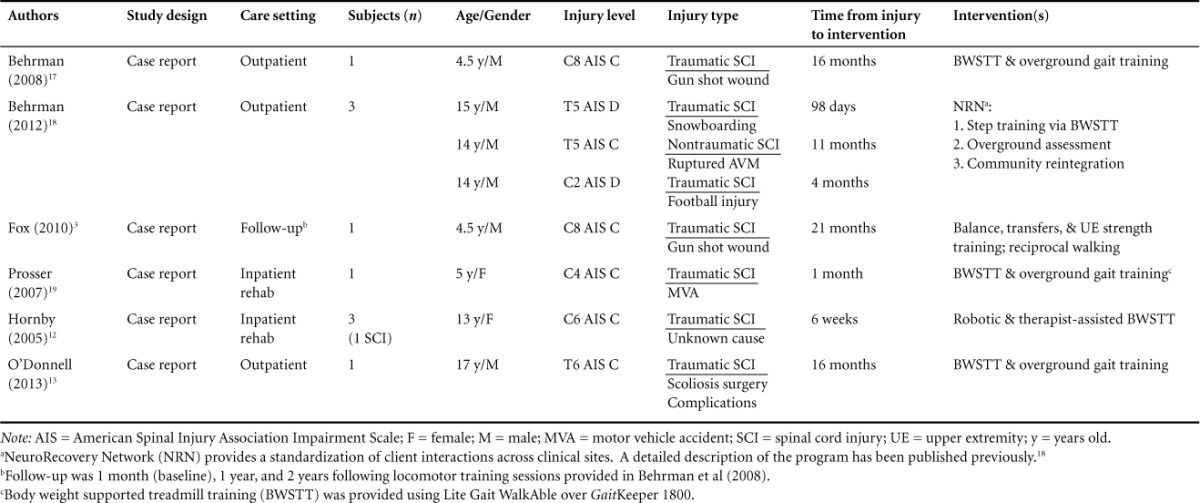
Table 2.
Patient characteristics
Results
There were a total of 7 participants, ranging in age from 4.5 to 17 years, with 1 participant followed twice to assess the long-term effects associated with the initial LT received in the Behrman et al16 study. Table 2 presents a detailed depiction of participant characteristics.
Study characteristics
All publications were case studies, implementing pre- and posttest outcome measurements. Four patients received treatment in an outpatient setting, while 2 patients were treated within an inpatient setting.
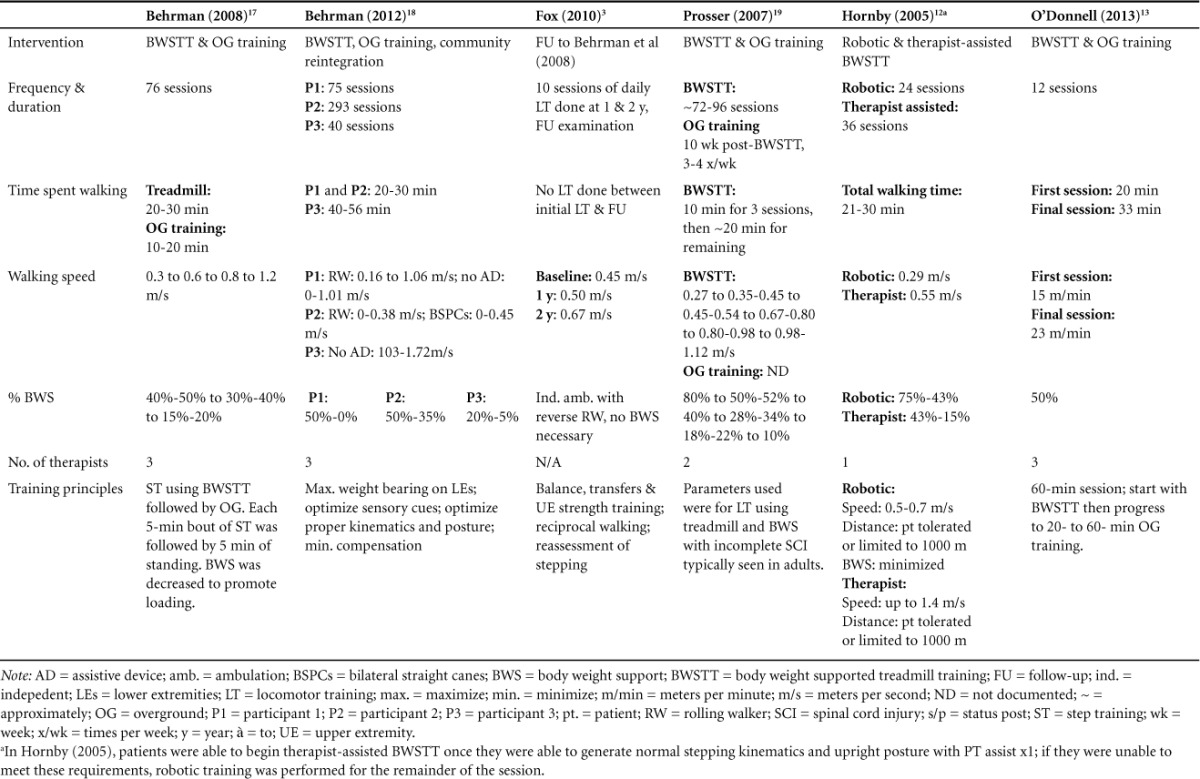
Although the implementation of LT varied to an extent across studies, the primary interventions were all individually based, in which treatment progression was dependent upon the rate of improvement of the participant. Specifics of the type of LT used, progression of treatment approaches, and training principles guiding intervention advancement can be found in Table 3.
Table 3.
Intervention description
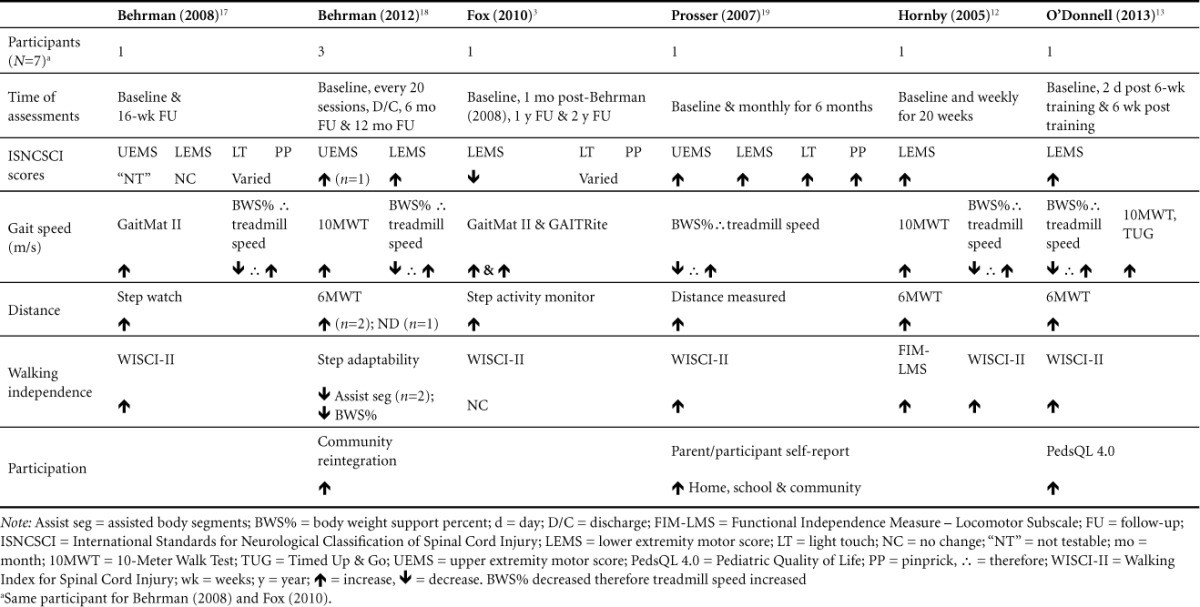
The main outcome variables used to help categorize participants' current level of function across studies3,12,13,16–18 were the following: International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) and the American Spinal Injury Association (ASIA) impairments scale (AIS), gait speed, distance walked, walking independence, and participation. As a classification system, the ISNCSCI and AIS were implemented to determine completeness of injury and grading of the level of neurological injury for all participants. Within this classification system, light touch, pinprick, upper extremity motor score (UEMS), and lower extremity motor score (LEMS) were used as outcome measures to quantify change in neurological function.19 The outcome measures making up the remaining categorizations included Walking Index for Spinal Cord Injury (WISCI II), GaitMat II,20 number of steps, Step Adapt, Berg Balance Scale (BBS), 10-Meter Walk Test (10MWT), 6-Meter Walk Test (6MWT), Community Reintegration interview, GAITRite,21 Gait Pattern, Compensatory Gait Patterns, Step Activity Monitor, Gross Motor Function Measure (GMFM-66), Reciprocal Lower Extremity (LE) Tasks, Pediatric Functional Independence Measure (WeeFIM II), Parent/Patient Participation Self-Report, Timed Up & Go (TUG), Functional Reach Test, and the Pediatric Quality of Life Inventory (PedsQL 4.0). Please refer to Table 4 for outcomes measures and results.
Table 4.
Outcome measures and results by study
Risk of bias within studies
Figure 1 was compiled to help aid in the assessment and analysis of quality among the studies in this systematic review. The Downs and Black grading scale is a 27-question checklist with a maximum score of 32. For all questions except numbers 5 and 27, a grade of 1 is given for a “yes” and a grade of 0 is given to a “no” or “unable to determine.” For question 5's responses, a grade of 2 is given for a “yes,” a grade of 1 is given for “partially,” and a grade of 0 is given for a “no.” For question 27, a total of 5 points could potentially be awarded. Because all the included articles were case studies, the scores were generally low. For example, a maximum score of 10 was noted for Hornby et al12 and O'Donnell et al13 and a minimum score of 7 was noted for Fox et al.3 The mean score after averaging the 6 articles was 8.83.
Behrman et al17 reported 2 adverse events for 2 separate participants. Participant 1 accidentally fractured his ankle while navigating around his room during the time period between his 6-month follow-up and discharge. Also incurring an ankle fracture, participant 2 discontinued participation after 18 sessions for 3 months. These were the only reportable adverse events cited by the reviewed studies.
ISNCSCI classifications
All participants were classified using the ISNCSCI and the AIS.19 The ISNCSCI exam uses light touch through wisps of cotton, and sharp-dull discrimination (pinprick), using a safety pin to assess sensation along 28 dermatomes on each side of the body. The motor examination portion assesses strength bilaterally in the upper (C5-T1) and lower extremities (L2-S1), providing both an UEMS and LEMS. The most caudal and intact level for both sensory and motor scores bilaterally determines the level of the SCI injury. This is known as the neurological level of injury.
Additionally, the AIS portion of the ISNCSCI is used to classify the SCI as either a motor complete or motor incomplete injury. Individuals can fall into the following classifications: motor complete AIS A, sensory incomplete AIS B, and motor incomplete AIS C or AIS D.19 Only one study, by Hornby et al,12 restricted inclusion criteria in which participants had to be classified as an SCI at or above thoracic level 10 (T10) and were motor incomplete (AIS C or D).
At baseline, neurological level of injury and AIS classification included the following3,12,13,16–18: C8 AIS C; T5 AIS D, T5 AIS C, C2 AIS D; C8 AIS C; C4 AIS C; and C6 AIS C. Three of the 7 participants showed improvement in either neurological level and/or AIS classification. Behrman et al17 reported an improvement in participant 2 from T5 AIS C to T8 AIS D and participant 3 from C2 AIS D to C3 AIS D. Hornby et al12 indicated improvement in their participant from C6 AIS C to C6 AIS D. All 6 studies used the ISNCSCI LEMS as an indicator of motor improvement within the bilateral lower extremities. Results showed 6 participants improving LEMS from baseline to follow-up, regardless of change in AIS classification. When first assessed by Behrman et al,16 their participant's LEMS remained unchanged throughout the study. Fox et al3 re-assessed the same participant over the following 2 years, finding a slight decrease in LEMS. When assessing the UEMS, Behrman et al16 were unable to provide a score, indicating the muscles were “not testable” due to triggering of extensor synergy. However, while Behrman et al17 found only a minimal change in UEMS, Prosser et al18 found a sizeable increase in UEMS.
Three studies measured changes in ISNCSCI sensory scores. Prosser et al18 showed a considerable increase in a 5-year-old participant's scores for both light touch and pinprick from baseline to time of discharge. However, the 4-and-a-half-year-old participant who was evaluated and assessed within 2 studies over a 2-year period3,16 showed variable responses in sensory outcomes from baseline, 1-year follow-up and to the 2-year follow-up.
Gait speed
Five of the 6 studies12,13,16–18 measured change in gait speed from start of LT to end of study assessment using change in walking speed during BWSTT as a measure of functional walking ability. Researchers found an inverse relationship between BWS and gait speed, indicating that as BWS decreased, gait speed increased for all participants.
Behrman et al16 also calculated gait speed using a computerized gait mat (GaitMat II), measuring self-selected and fastest walking speeds, both of which showed increases in velocity. In an attempt to measure long-term functional outcomes associated with LT participation, Fox et al3 conducted a follow-up study 2 years post participation in the Behrman et al16 study. Gait speed measurements obtained from both the GaitMat II and GAITRite showed additional increases in the child's fastest walking speed from baseline over 1 year's time as well as 2 years later.
As an additional assessment for change in gait speed, 5 participants completed a 10MWT at baseline and over periodic follow-up assessments.12,13,17 Four of the 5 participants12,13,17 showed increases in gait speed across assessment intervals. One participant showed improvements from baseline to 2 days post LT on the 10MWT and TUG, which is also an assessment of gait speed, as measured from the time it takes to stand from a chair, walk 3 meters, turnaround, walk back 3 meters, and sit down again.13 When the same participant was reassessed 16 weeks post LT, his 10MWT and TUG speeds had both decreased to being slower than the initial baseline measure.
Distance
All of the studies used distance as an outcome measure; however, different tools were utilized to determine the results.3,12,13,16–18 Two of the studies used step activity monitors: Behrman et al16 used a watch mechanism whereas Fox et al3 wore a device around the ankle. Both studies reported increases in the amount of community ambulation steps. Three studies12,13,17 administered the 6MWT. This outcome measure assesses the distance covered by the participant over a 6-minute period of time. O'Donnell et al13 reported slight improvement after the intervention. Hornby et al12 reported the participant was unable to perform test at baseline, but showed an increase at the conclusion of the training. Behrman et al17 stated that participant 1 and participant 2 showed improvements, but there were no data for participant 3. Prosser et al18 demonstrated increased distance by ambulating 3 to 100 meters each trip independently with a forward rolling walker and left articulated ankle-foot orthoses (AFO) by discharge from inpatient rehabilitations.
Walking independence
Six of the studies chose walking independence as an outcome measure but only 53,12,13,16,18 utilized the WISCI-II. This outcome measure is a 21-item scale with scores ranging from 0 to 20 and assessment of the amount of physical assistance and device(s) required for walking 10 meters following an SCI. In 4 of the studies,12,13,16,18 there was an increase in the walking independence score. Behrman et al16 found an improvement from total dependence (0) to use of rolling walker during ambulation (13). Prosser et al18 reported an improvement from total dependence (0) to use of 2 crutches and a brace during ambulation (12). Hornby et al12 showed improvement from using a walker with no lower extremity bracing (8) to using 2 crutches and no braces with ambulation as primary mode of locomotion (16). Hornby et al12 also performed the Functional Independence Measure (FIM) and showed improvement in the locomotor subscale category for ambulation to a modified independence level. O'Donnell et al13 showed improvement from walking with minimal assistance with a walker (6) to independent ambulation with walker (9). Fox et al3 was the only study that reported no change in score and their subject still needed a reverse rolling walker for independent ambulation. Behrman et al17 demonstrated an increase in walking independence by reporting the decrease in assistance from the therapist and weight percentage in BWS. In both cases 1 and 3, by the end of discharge, no limb assistance was needed from the therapist during ambulation and the percentage of BWS was decreased.
Participation
Only 3 of the 6 studies utilized participation as an outcome measure.13,17,18 Prosser et al18 used parent and patient self-report data collection, describing an increase in ambulation at church, school, and within the community. The patient was able to participate in swimming and ambulate up and down the stairs at home. O'Donnell et al13 selected the PedsQL 4.0 to determine physical, emotional, social, and school functioning. The PedsQL 4.0 is a clinical tool used to assess health-related quality of life in children between the ages of 2 and 8 via physical, social, emotional, and school functioning dimensions. There was a significant improvement in emotional state and sports activity post treatment and again at the 6-week follow-up. Behrman et al17 reported self-reported community integration goals for home and community activities in both the participant and therapist. Participant 1 and participant 3 had a positive transition into the community, while participant 2 experienced challenges returning to school but adjusted well overall.
Discussion
The combined results of the 6 studies suggest that the pediatric SCI population can benefit from receiving LT. As indicated by gait speed, distance, walking independence, and participation, the evidence shows that participants made gains in their ability to ambulate, regardless of change in ISNCSCI classification.
Even though LT in the pediatric population is modeled off the adult population guidelines,11 children are not just small adults and are constantly developing. This continuous development impacts the nervous system where neuroplastic changes are believed to be occurring during LT leading to functional improvements.18,22 The nervous system in a child is not fully developed, so it is essential that the adult guidelines for LT in the SCI population are altered to fit the needs of the pediatric population. With the exception of the NeuroRecovery Network (NRN) implemented by Behrman et al,17 no other LT interventions within this review followed a standardized protocol. The NRN utilizes strict guidelines allowing for the standardization of participants selected based on injury level, current treatment setting, presence of lower extremity movement, and the elimination of spasticity by a certified NRN physician.23 Compensatory strategies are discouraged and the participant is only able to continue in this program if they are making progress, as defined by a preset algorithm. Behrman et al23 demonstrated that the standardized protocol initially developed for adults by the NRN can be applied to children who are 14 and 15 years of age. However, it is unclear whether or not this same protocol will be as effective in children younger in age.
Due to the inconsistencies between LT interventions and the lack of a standard protocol for the pediatric population, a clinically best guideline for children with SCI cannot be determined at this time. However, to gain insight into which factors may be the most effective for improving ambulation, it is important to note the similarities and the differences that influenced patient progression throughout the interventions. For instance, 5 of the 6 studies focused on segmental control and the ability of the participant to independently maintain proper trunk, pelvis, and lower extremity postural alignment.12,13,16–18 Additionally, as a patient's independence of trunk alignment and limb position increased, BWS decreased, thereby increasing the extent of load bearing through the lower extremities. As BWS decreased and segmental independence increased, gait speed also increased allowing for a more normalized walking speed specific to a more functional gait pattern. Finally, although overground training may or may not have immediately followed BWSTT, every participant progressed to a change in environmental practice at some point in his or her treatment.
Additionally, another variable worth noting is the time from injury to start of treatment and whether these differences had any impact on treatment outcomes. An SCI can be classified as either acute or chronic, meaning it has either been less than 6 months since the time of injury or greater than 6 months. After reviewing the studies, results indicated that 4 of the 7 participants12,17,18 were suffering from an acute SCI at the time LT began, whereas 3 participants13,16,17 did not begin LT until after their injury was chronic. In each study, improvements were noted in LEMS scores as well as treadmill speed and percentage of BWS. Furthermore, Behrman et al16 and Hornby et al12 reported improvements in the WISCI-II for both acute and chronic participants following LT. These findings suggest that while the common belief pertaining to LT is “the sooner the better,” improvements in ambulation can be seen even when initiated in the chronic phase of injury.
Although the ISNCSCI is used both in the adult and pediatric populations, there are limitations to consider when using this classification system in the pediatric population. For instance, children younger than 6 years old are unable to accurately participate in the full examination, as the exams are far too advanced for their cognitive abilities during that developmental time period.24 Furthermore, if the child does not have bowel continence before the injury, it will be difficult to understand the concept of the anal contraction portion of the exam.25 The inability to comprehend deep anal pressure can lead to inconsistencies in the completion of the anorectal assessment as well.26 This raises the question as to whether the ISNCSCI is a reliable classification system for showing change in recovery in the pediatric SCI population.
In addition, previous literature has shown that individuals classified as AIS C and/or D showed the most improvements in functional and participation outcomes after LT followed by overground training and individuals initially classified as an AIS B did not.18 In fact, within 24 hours of initial injury, only 11% of adult patients classified as AIS B, with light touch sensation within sacral dermatomes present, are predicted to become ambulators.27 The studies in this review all used the LEMS scores for indicating functional motor improvement and all individuals were classified as AIS C or D at the initiation of LT. This could be due to some belief that these motor incomplete individuals have better potential for recovery. However, findings by Behrman et al16 suggest that LT may be used to promote walking in nonambulatory individuals even if voluntary lower extremity isolated movement is not present.16 Furthermore, Prosser et al18 reported ambulation in their subject classified as an AIS B 5 days after injury despite previous claims within the literature that these individuals are not predicted to be able to walk post injury. These findings suggest that the ISNCSCI exam and AIS classification may be poor indicators for walking recovery among the pediatric population, especially since there is great variability in scoring seen during test administration with younger children.
Currently, it is believed that preexisting neuronal networks generate movement and are fairly flexible after a nervous system lesion. During LT, therapists aim to work with this flexibility to induce plastic changes and regain locomotor ability.10 In all of the studies we reviewed, subjects were ambulatory prior to their injury; thus, they had already generated stable neuronal networks for locomotion and general movement. Do these flexible neuronal networks still exist in children who were too young to have established locomotor movement patterns at the time of their injury? In a study performed by Heathcock et al,28 researchers aimed to determine the effect of treadmill training after the removal of a spinal tumor in infancy. The subject was nonambulatory prior to LT, but after 20 months of treadmill training, an independent, symmetrical stepping pattern emerged. Although specific neuronal walking pathways had not yet been formed, it was speculated that LT helped to strengthen any spared spinal pathways, teaching the nervous system how to produce alternating steps.28 The notion of step-like movements being present at birth could have explained the improvements noted in this study.10
The principles of neuroplasticity may help explain these comparable gains seen across such varying implementations of treatment protocols. For instance, if patients wanted to walk again, it was important for them to participate in task-specific interventions of ambulation, which included BWSTT as well as BWS overground training to induce neuronal changes specific to gait. BWS, manual facilitation, and tactile cuing all helped to enhance appropriate sensory inputs that not only encouraged correct gait mechanics but also intensified the repetitive nature of the locomotor task itself.4 Behrman et al4 described this change in sensory and load-bearing input as appropriate stimuli necessary to initiate and improve stepping pattern followed by LT through intrinsic neural networks.
Specifically, during locomotion, afferent feedback arises in the muscle receptors, which in turn regulates the step cycle in humans. In order for the swing phase to begin, the leg must be extended, which is signaled by the afferent connections within the hip flexors. When using LT on a treadmill, this same sensory feedback is provided as the belt extends the leg. Similarly, afferent feedback from the receptors in the extensor muscles helps to adjust force production in the ankle extensors.9 As seen in the follow-up study of Fox et al,3 benefits of LT included the generation of reciprocal stepping. By achieving this reciprocal stepping pattern, afferent feedback in turn increases, which then helps to achieve a more stable gait pattern.
While reviewing the literature regarding LT and SCI in the pediatric population, we found that the Fox et al3 study was the only one investigating the long-term effects of LT. There is very limited knowledge of the long-term, lasting effects of LT as an intervention for aiding in the recovery of SCI in pediatrics. Literature suggests that activity-dependent plastic changes can begin to diminish if the activity is discontinued.6,29 In the Fox et al3 follow-up study to Behrman et al,16 the participant maintained the improvements he made during the initial LT. Yet, when reassessed after only 16 weeks post LT, O'Donnell et al13 found that any gains made by their participant in the 10MWT and TUG following LT had actually decreased below initial baseline measures. To better understand the potentiating effects of LT as a long-term intervention, more studies are needed and a multifactorial approach must be taken when considering the different facets of children's lives affected by SCI.
The International Classification of Functioning, Disability, and Health (ICF) describes the impact that a particular health condition has on overall function.30 One of the domains included in the ICF is participation, which takes function into account with regard to the patient's environmental barriers and facilitators.30 It is important to determine how interventions are impacting a patient's function, specifically with relation to participation in their home, school, and community environments. Only 3 studies assessed participation in this systematic review. The limited reports of participation in this systematic review make it difficult to determine how LT impacted overall function in the participant's home, school, and community environments. The studies that did report on participation used a form of verbal reports from the participant themselves or used the PedsQL 4.0. However, the use of PedsQL 4.0 with the SCI population has been brought into question.31 Therefore, the PedsQL 4.0 might not be the best outcome measure to use with the pediatric SCI population to measure how their overall function is allowing them to participate in school, home, or the community.
Limitations
Throughout the review of the current literature, we found common limitations. There were only 6 studies deemed appropriate for this review, all of which were case studies and none had a control variable. The lack of a controlled condition reduces the ability to confidently report a cause and effect relationship between the changes in outcome measures and LT in patients with SCIs. Of the 6 studies, only 1 followed a standardized protocol,17 indicating the lack of standardized LT interventions within the pediatric SCI population. For example, while implementing interventions, authors selected different intensities, frequencies, session durations, and number of intervention sessions. The number and type of staff used also varied across studies. Additionally, time since injury in relationship to when the intervention was implemented ranged from 1 month19 to 16 months,13,17 suggesting that neurological stability across participants at the start of LT varied throughout as well.
The small, specific, patient population impacts the external validity and generalization of research findings. For example, there was a total of 7 participants, however one was followed long-term within 2 of the 6 articles reviewed. There was also variability within age distribution with 2 participants being 5 years of age and younger and 5 participants between the ages of 13 and 17 years of age.
Across the studies, numerous outcome measures were utilized to provide a means of quantifying potential change in a patient's function. However, there was a lack of consistency as to which measures were selected throughout the studies. After a closer examination of the material, many of the outcome measures had not been tested for reliability within the pediatric spinal cord population. As Hornby et al12 pointed out, there was fair to poor interrater reliability for the FIM mobility subscale for walking and wheelchair use, including a lack of estimated data in the SCI population for the 10MWT, 6MWT, and the TUG. This calls into question the reliability of these assessments in this population.
Conclusion
Currently, studies investigating the benefits of LT in pediatrics with SCI are based on results found within the adult SCI population. Presently, there are no established guidelines specifically for the pediatric population. Although this review showed positive results for gait speed, distance, and participation, further research is needed to determine whether or not prior level of ambulation and time since injury play a role in the ability to regain function following an SCI. Future research designs should utilize controlled research trials to determine a causal relationship between LT and the return of ambulatory function.
In an effort to determine whether LT does induce plastic changes within the nervous system, future studies should use imaging techniques as a way to better assess the changes occurring within the CNS. The following imaging techniques are already being used in an effort to evaluate neurological changes within the CNS: voxel-based morphometry (VBM) for parametric mapping of brain anatomy, voxel-based cortical thickness (VBCT) to assess change in white and gray matter, and diffusion tensor image (DTI) for measures of change in white matter of central pathways.32 The use of imaging under controlled research trial conditions would not only further aid in diagnostic purposes but would also allow for causative relationships to be made regarding which variables have the most beneficial impact within LT interventions. Therefore, due to lack of evidence, additional research gains still need to be made into the investigation as to which LT protocol is most effective in the treatment of pediatric patients with SCIs.
Acknowledgments
No authors declare any sources of conflict of interest. This work was not funded by any organization or agency and was completed as part of a course requirement for students in the doctor of physical therapy program at Thomas Jefferson University. This work was a systematic review of the literature (SRL), therefore no internal review board protocol was needed; however all reporting guidelines consistent with an SRL were included.
Footnotes
*Authors contributed equally to this work.
REFERENCES
- 1.Campbell SK, Palisano RJ, Orlin MN. Physical Therapy for Children. 4th ed. St. Louis, MO: Elsevier Saunders; 2012. [Google Scholar]
- 2.Vitale MG, Goss JM, Matsumoto H, Roye DP., Jr. Epidemiology of pediatric spinal cord injury in the United States: Years 1997 and 2000. J Pediatr Orthop. 2006;26(6):745–749. doi: 10.1097/01.bpo.0000235400.49536.83. [DOI] [PubMed] [Google Scholar]
- 3.Fox EJ, Tester NJ, Phadke CP et al. Ongoing walking recovery 2 years after locomotor training in a child with severe incomplete spinal cord injury. Phys Ther. 2010;90(5):793–802. doi: 10.2522/ptj.20090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: An emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86(10):1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 5.Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: As a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93(9):1588–1597. doi: 10.1016/j.apmr.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Somers MF. Spinal Cord Injury:Functional Rehabilitation. Upper Saddle River, NJ: Prentice Hall; 2001. [Google Scholar]
- 7.Dunlop SA. Activity-dependent plasticity: Implications for recovery after spinal cord injury. Trends Neurosci. 2008;31(8):410–418. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev. 2008;57(1):255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouad K, Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol. 2012;235(1):91–99. doi: 10.1016/j.expneurol.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Wirz M, Colombo G, Dietz V. Long term effects of locomotor training in spinal humans. J Neurol Neurosurg Psychiatry. 2001;71(1):93–96. doi: 10.1136/jnnp.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. J Neurol Phys Ther. 2009;33(1):27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85(1):52–66. [PubMed] [Google Scholar]
- 13.O'Donnell CM, Harvey AR. An outpatient low-intensity locomotor training programme for paediatric chronic incomplete spinal cord injury. Spinal Cord. 2013;51(8):650–651. doi: 10.1038/sc.2013.23. [DOI] [PubMed] [Google Scholar]
- 14.Freivogel S, Mehrholz J, Husak-Sotomayor T, Schmalohr D. Gait training with the newly developed “LokoHelp”-system is feasible for non-ambulatory patients after stroke, spinal cord and brain injury. A feasibility study. Brain Injury. 2008;22(7–8):625–632. doi: 10.1080/02699050801941771. [DOI] [PubMed] [Google Scholar]
- 15.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrman AL, Nair PM, Bowden MG et al. Locomotor training restores walking in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury. Phys Ther. 2008;88(5):580–590. doi: 10.2522/ptj.20070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrman AL, Watson E, Fried G et al. Restorative rehabilitation entails a paradigm shift in pediatric incomplete spinal cord injury in adolescence: An illustrative case series. J Pediatr Rehabil Med. 2012;5(4):245–259. doi: 10.3233/PRM-2012-00225. [DOI] [PubMed] [Google Scholar]
- 18.Prosser LA. Locomotor training within an inpatient rehabilitation program after pediatric incomplete spinal cord injury. Phys Ther. 2007;87(9):1224–1232. doi: 10.2522/ptj.20060252. [DOI] [PubMed] [Google Scholar]
- 19.Kirshblum SC, Burns SP, Biering-Sorensen F et al. International Standards for Neurological Classification of Spinal Cord Injury (revised 2011) J Spinal Cord Med. 2011;34(6):535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair PM, Hornby G, Behrman AL. Minimal detectable change for spatial and temporal measurements of gait after incomplete spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(3):273. doi: 10.1310/sci1803-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dusing SC, Thorpe DE. A normative sample of temporal and spatial gait parameters in children using the GAITRite® electronic walkway. Gait Posture. 2007;25(1):135–139. doi: 10.1016/j.gaitpost.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: An emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86(10):1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 23.Harkema SJ, Schmidt-Read M, Behrman AL, Bratta A, Sisto SA, Edgerton VR. Establishing the neurorecovery network: Multisite rehabilitation centers that provide activity-based therapies and assessments for neurologic disorders. Arch Phys Med Rehabil. 2012;93(9):1498–1507. doi: 10.1016/j.apmr.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Mulcahey MJ, Gaughan JP, Chafetz RS, Vogel LC, Samdani AF, Betz RR. Interrater reliability of the international standards for neurological classification of spinal cord injury in youths with chronic spinal cord injury. Arch Phys Med Rehabil. 2011;92(8):1264–1269. doi: 10.1016/j.apmr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahey MJ, Gaughan J, Betz RR, Johansen KJ. The International Standards for Neurological Classification of Spinal Cord Injury: Reliability of data when applied to children and youths. Spinal Cord. 2007;45(6):452–459. doi: 10.1038/sj.sc.3101987. [DOI] [PubMed] [Google Scholar]
- 26.Vogel L, Samdani A, Chafetz R, Gaughan J, Betz R, Mulcahey MJ. Intra-rater agreement of the anorectal exam and classification of injury severity in children with spinal cord injury. Spinal Cord. 2009;47(9):687–691. doi: 10.1038/sc.2008.180. [DOI] [PubMed] [Google Scholar]
- 27.Crozier KS, Graziani V, Ditunno JF, Jr., Herbison GJ. Spinal cord injury: Prognosis for ambulation based on sensory examination in patients who are initially motor complete. Arch Phys Med Rehabil. 1991;72(2):119–121. [PubMed] [Google Scholar]
- 28.Heathcock JC, Christensen C, Bush K, Butler M, Buehner JJ, Basso DM. Treadmill training after surgical removal of a spinal tumor in infancy. Phys Ther. 2014;94(8):1176–1185. doi: 10.2522/ptj.20130508. [DOI] [PubMed] [Google Scholar]
- 29.Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008;45(2):229–240. doi: 10.1682/JRRD.2007.03.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Towards a Common Language for Functioning, Disability and Health: ICF. World Health Organization; 2002. [Google Scholar]
- 31.Oladeji O, Johnston TE, Smith BT, Mulcahey MJ, Betz RR, Lauer RT. Quality of life in children with spinal cord injury. Pediatr Phys Ther. 2007;19(4):296–300. doi: 10.1097/PEP.0b013e31815a12ef. [DOI] [PubMed] [Google Scholar]
- 32.Freund P, Curt A, Friston K, Thompson A. Tracking changes following spinal cord injury: Insights from neuroimaging. Neuroscientist. 2013;19(2):116–128. doi: 10.1177/1073858412449192. [DOI] [PMC free article] [PubMed] [Google Scholar]


