Abstract
Objective: To compare phase- and task-dependent H-reflex modulation in standing and walking in 2 spinal cord injury (SCI) groups with and without a walker.
Methods: Fourteen subjects with American Spinal Injury Association Impairment Scale D SCI (40±10 years) participated. Tibial nerve was stimulated to evoke 15 H-reflexes (at M-wave 7%–13% of maximum-M).
Results: H-reflex was greater in the walker group during stance (but not standing/swing).
Conclusion: Differences in H-reflex modulation between groups walking with and without a walker may be explained by sensory mechanism that enhances central excitation, difference in motor activation levels between groups, and other complex mechanisms that influence balance or stability.
Keywords: soleus H-reflex, spinal cord injury, standing, walker, walking
More than 50% of ambulatory individuals with spinal cord injury (SCI) use devices to assist with walking.1 The impact of assistive devices on neuronal excitability, although tested in healthy controls,2 remains unclear after SCI. Reflex impairments have been previously reported after SCI in standing and walking.3 Modulation of soleus H-reflexes is highly task specific; there is smaller reflex size during standing compared to the semi-reclined position.4 Similarly, the reflex size is significantly smaller (or absent) during the swing phase compared to the stance phase of walking in non-injured individuals.4 Although the H-reflex modulation is impaired after SCI, it is not clear what specific aspects of standing and walking such as afferent activation and loading during use of assistive devices most directly affect reflex modulation.
Traditionally, some form of walking assistance such as a walker is prescribed to assist SCI-impaired standing and locomotion.5 Although many individuals with SCI use a variety of assistive devices, a walker with 4 contact points affords greater stability.6 Using a walker engages bilateral upper limbs in weight bearing and increases the muscular effort in the arms6 compared to walking with canes or no assistive devices. Although the level of arm weight bearing is difficult to monitor, individuals using walkers may need to use intense arm muscle activity to assist with walking. Soleus H-reflex facilitation is seen during intense arm muscle activity, also known as the Jendrássik effect.7,8 With already hyperexcitable reflexes after SCI, it is not clear whether the assistive device use and the associated arm loading create conditions that further increase or decrease the spinal excitability. A previous study revealed that a phase-dependent modulation of H-reflex excitability decreased in proportion to the use of walking assistive device.4
Because walkers are routinely prescribed to assist with locomotion in the SCI population, it is important to understand the neurophysiological impact of walker use. The first step in developing a deeper understanding is to assess the reflexes of individuals with SCI walking with and without a walker. The purpose of this exploratory study was to understand whether the task- and phase-dependent modulation of spinal reflex excitability was different between the 2 groups of participants with SCI who habitually walked with a walker compared with those who walked habitually without a walker.
Methods
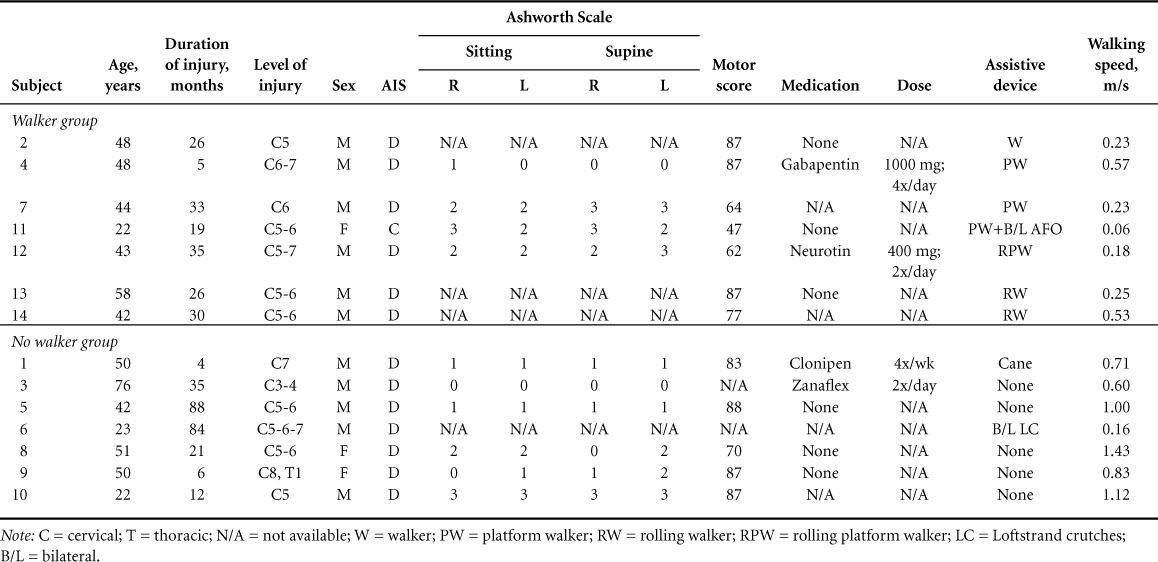
Fourteen participants with a motor incomplete SCI (age, 40±10 years; American Spinal Injury Association Impairment Scale [AIS] level D) participated in the study. Table 1 summarizes the demographic data of participants with SCI. All participants reviewed and signed an informed consent form approved by the institutional review board. Inclusion criteria were age 18 to 65 years; first time SCI diagnosis; SCI etiology ranging from trauma, vascular, or orthopedic injury; cervical or thoracic level injury; AIS categories C or D; medical status approval from participant's physician; no other pre-existing neural degenerative conditions; and the ability to walk independently (with or without an assistive device) for at least 20 feet. The medication-related information was recorded through a participant interview, and the comfortable walking speed was assessed by asking the participants to walk on a 0.63-m-long digitized mat with embedded microswitches. All participants walked 3 times on the mat, and comfortable walking velocity was determined using GAIT MAT IIa software (EQ, Inc, Chalfont, PA). All the assessments were done by an experienced physical therapist.
Table 1.
Demographic data of participants with SCI
The tibial nerve was stimulated in the popliteal fossa (at 5-second intervals) to evoke soleus H-reflexes on the more affected side (based on AIS-Motor score). We adjusted the stimulus intensity to ensure that the M-wave size was within a range of 7% to 13% of the maximum M-wave (Mmax). For detailed data collection methods, refer to the 2010 Phadke et al article.4 Briefly, we plotted the H-reflex recruitment curve to ensure that the H-reflexes were evoked on the rising phase of the recruitment curve. Data acquired at a sampling rate of 10 KHz per channel were stored digitally and analyzed offline using the Data-Pac III software (Run Technologies). The H-reflex amplitude was measured using the raw peak-to-peak amplitude, which was then normalized to the Mmax (tested in a relatively stable standing position).
Testing positions
Standing: We did not provide any specific instructions regarding the use of arms while using assistive devices in any testing position.
Walking: We evoked soleus H-reflexes in the midstance and midswing phases of walking (visually confirmed using foot-switches) at a self-selected pace over ground. Stimuli were presented randomly over the course of walking (eg, every 3–4 steps) to acquire up to 15 responses per phase. We evoked 15 H-reflexes per position in order to collect 10 H-reflexes, required for averaging and for accurate and consistent between-day data.9 The same tester evoked H-reflexes in all the participants and the background EMG 100 ms preceding the H-reflex was recorded for all trials. The soleus H-reflex testing in standing and walking shows an excellent reliability in individuals with SCI.10
Statistical analysis
We formed 2 groups that were comparable in age, chronicity (duration of injury), motor scores, and spasticity: walker (WA) and no-WA (n = 7 in each group (Table 1). We used the Mann-Whitney test to compare groups using IBM SPSS (version 17.0; IBM Corp., Armonk, NY) and XL Toolbox (© 2008-2011 Daniel Kraus, Würzburg, Germany) to create the tables and figures.
Results
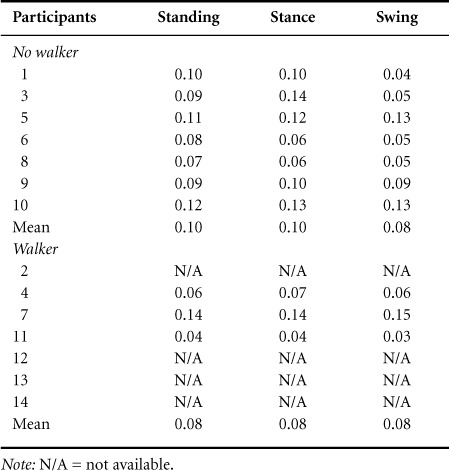
Fourteen participants with diverse ages (range, 22–76 years), injury interval (5–88 months), gender, medication, motors cores, type of walking assistance utilized, and walking speed (0.06–1.43 m/s) participated (see Table 1 for demographics). Age, chronicity, the AIS motor scores, and spasticity were not significantly different between the 2 groups. The mean walking speed of the no-WA group (0.83±0.16 m/s) was significantly faster than the WA group (0.25±0.03 m/s; P < .05). We found, that the H-reflex amplitudes were significantly greater in the WA group during stance phase compared to the no-WA group (P < .05) (see Figure 1). There was no significant difference in the H-reflex amplitudes in the standing (P = .08) and swing phase (P = .30) between the WA and no-WA groups. The background EMG activity (root mean square) did not differ systematically in all testing positions (no statistical analyses were possible due to low sample size) (Table 2).
Figure 1.
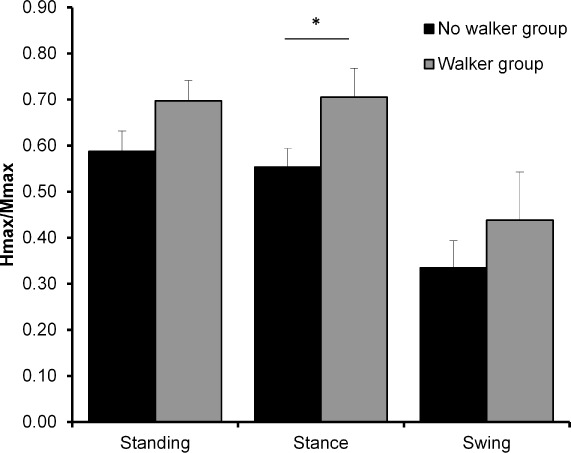
H-reflex amplitude (normalized to Mmax) compared between walker and no-walker groups in three positions (standing, stance, swing). *Statistical differences (P < .05).
Table 2.
Background EMG data
Discussion
We recorded significantly greater stance-phase reflex amplitude in the WA group compared to the no-WA group. The H-reflex amplitude did not differ in swing phase of walking between the 2 groups; however, there was a trend (P = .08) toward a greater reflex amplitude in standing in the WA group. It is possible that walker use alters the stance phase of walking more than the swing phase. The significant between-group differences in walking speed cannot solely explain the differences in reflex excitability, because walking speed alters reflex excitability in healthy controls but not in individuals with SCI.4 Potential mechanisms that may explain the higher soleus H-reflex amplitude in the WA group include unloading of the legs, loading of the arms, increased manual grip pressure, level of impairment, and abnormal muscle activity patterns.
The use of walker may involve some degree of leg unloading, because partial body weight may be borne through the arms. However, a previous report suggests that short-term leg unloading does not change the reflex excitability.11 It is possible that arm loading and manual grip pressure required for squeezing the walker handles activated cutaneous afferents in the hand and wrist known to influence the soleus H-reflex excitability through interlimb neural connections.12 In this study, we did not record the percentage of loading through the arms and legs and were unable to discriminate the effect of arm versus leg unloading on the reflex size. Using a walker also actively restrains the arm swing and increases reflex excitability in the able-bodied controls.13 It is possible that the mechanism underlying the changes seen in the able-bodied controls13 can also help explain the higher reflex excitability in the SCI participant group using a walker in our study. Altered sensory input arising from flexed neck and an upper body posture typically seen with walker use and associated vestibular changes may have also contributed to increase in the reflex excitability.
In addition to the walker use, there may have been subtle differences in motor control not detected by the AIS motor scores between the 2 groups. Disruption of interlimb coordination and decrease in the complexity of muscle activation patterns during walking with assistive devices14 may be another possible mechanism to explain our findings. Decrease in the complexity and coordination of muscle activation is reflective of impaired motor control. Individuals post-SCI needing assistive devices to ambulate probably experience a greater degree of impaired motor control.15 Thus, it is possible that the individuals with motor incomplete SCI in the walker group were more impaired, resulting in greater reflex excitability.16 Other factors such as balance or trunk control, not tested in this study, may also influence the reflex excitability.
We did not specifically control for the Jendrássik effect (facilitation of soleus by intense arm muscle activity for, for example, squeezing tennis balls8). In the WA group, leaning over the walker for support or squeezing the walker hand grips harder when sensing imbalance may have induced a Jendrássik effect depending on the level of arm muscular force exerted. However, we did not measure the force applied on the walker handles or grip strength in general and are thus unable to discriminate the between-group differences in grip force. It is also possible that Jendrássik effect was greater in the standing and stance phase because of the greater threats to balance in these positions. In the swing phase of walking, participants were likely more stable because the nontested and less-impaired leg was loaded. In contrast, in the stance phase of walking, participants may be less stable as the more-impaired (tested) leg was loaded. Thus, differences in balance requirements may explain the lack of a change in reflex excitability in the swing and standing conditions. It is also possible that the WA group had more impaired reflex excitability unrelated to the walker use and needs to be tested using within-subject design controlling for limb loading and trunk posture in future studies.
Although not statistically different, the WA group was more acute (25 months since injury) compared to the no-WA group (36 months since injury) and may explain the greater reliance on a walker in the WA group. The WA group may have experienced greater balance impairment requiring use of a walker; however, we did not measure balance in this study. A previous study has reported that a greater reflex size in individuals with SCI is associated with a need for greater walking assistance.4 Thus, it is also possible that the WA group experienced higher reflex excitability and hence required use of a walker to maintain balance. We did not specifically examine the effect of walker, and future within-group designs such as those comparing individuals who use a walker and test their reflexes with and without a walker will need to address that. One of the limitations of this study is that we did not control for what the upper extremities were doing; this may have affected our results. Furthermore, one participant used an ankle-foot orthosis, which may also affect modulation of the H-reflex.2
Conclusion
Our exploratory study indicates that walker use may be responsible for a greater reflex amplitude in participants in the walker group compared to those who do not use a walker. It is not clear whether individuals with impaired reflexes need to use a walker or use of a walker results in reflex impairment. Neural plasticity can be a 2-way street, and the direction of the relationship between walker use and reflexes needs to be tested. Future studies will need to test the neurophysiological effect of walker use in individuals who use walkers on a regular basis. Specifically, the Jendrássik effect could be measured with forearm EMG activity to help determine to what extent the Jendrássik maneuver contributes to the effect found in the walker group. Future studies also need to examine the reflex excitability in able-bodied controls using partial upper limb loading.
Acknowledgments
The authors have not conflicts of interest to report.
Financial support: Christopher Reeve Paralysis Foundation grant KA2-000202 (C.K., A.L.B.); National Institutes of Health; the National Center for Medical Rehabilitation Research K-O1 Human Development 01348-01 Award (A.L.B.); Veterans Affairs Rehabilitation Research & Development grant F2182C (A.L.B.), and West Park Foundation (C.P.P.).
Previous publication: This work was presented in an abstract form and as a poster at the Eighth World Congress for Neurorehabilitation; April 2014; Istanbul, Turkey.
REFERENCES
- 1.Poncumhak P, Saengsuwan J, Amatachaya S. Ability of walking without a walking device in patients with spinal cord injury as determined using data from functional tests [published online ahead of print November 7, 2013] J Spinal Cord Med. doi: 10.1179/2045772313Y.0000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair PM, Phadke CP, Behrman AL. Phase dependent modulation of soleus H-reflex in healthy, non-injured individuals while walking with an ankle foot orthosis. Gait Posture. 2014;39(4):1086–1091. doi: 10.1016/j.gaitpost.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Phadke C, Thompson F, Trimble M, Behrman A, Kukulka C. Reliability of soleus H-reflexes in standing and walking post-incomplete spinal cord injury. Int J Neurosci. 2010;120(2):128–136. doi: 10.3109/00207450903337739. [DOI] [PubMed] [Google Scholar]
- 4.Phadke CP, Thompson FJ, Kukulka CG et al. Soleus H-reflex modulation after motor incomplete spinal cord injury: Effects of body position and walking speed. J Spinal Cord Med. 2010;33(4):371–378. doi: 10.1080/10790268.2010.11689715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbeau H, Pepin A, Norman KE, Ladouceur M, Leroux A. Walking after spinal cord injury: Control and recovery. Neuroscientist. 1998;4:14–24. [Google Scholar]
- 6.Bateni H, Maki BE. Assistive devices for balance and mobility:Benefits, demands, and adverse consequences. Arch Phys Med Rehabil. 2005;86(1):134–145. doi: 10.1016/j.apmr.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Delwaide PJ, Toulouse P. Jendrassik maneuver vs controlled contractions conditioning the excitability of soleus monosynaptic reflexes. Arch Phys Med Rehabil. 1980;61(11):505–510. [PubMed] [Google Scholar]
- 8.Tsuruike M, Koceja DM, Yabe K, Shima N. Age comparison of H-reflex modulation with the Jendrassik maneuver and postural complexity. Clin Neurophysiol. 2003;114(5):945–953. doi: 10.1016/s1388-2457(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 9.Mynark RG. Reliability of the soleus H-reflex from supine to standing in young and elderly. Clin Neurophysiol. 2005;116(6):1400–1404. doi: 10.1016/j.clinph.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Phadke CP, Thompson FJ, Trimble MH, Behrman AL, Kukulka CG. Reliability of soleus H-reflexes in standing and walking post-incomplete spinal cord injury. Int J Neuorsci. 2010;120(2):128–136. doi: 10.3109/00207450903337739. [DOI] [PubMed] [Google Scholar]
- 11.Phadke CP, Wu SS, Thompson FJ, Behrman AL. Soleus H-reflex modulation in response to change in percentage of leg loading in standing after incomplete spinal cord injury. Neuroscience Lett. 2006;403(1–2):6–10. doi: 10.1016/j.neulet.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 12.Zehr EP, Frigon A, Hoogenboom N, Collins DF. Facilitation of soleus H-reflex amplitude evoked by cutaneous nerve stimulation at the wrist is not suppressed by rhythmic arm movement. Exp Brain Res. 2004;159(3):382–388. doi: 10.1007/s00221-004-2092-x. [DOI] [PubMed] [Google Scholar]
- 13.Phadke CP, Klimstra M, Zehr EP, Thompson FJ, Behrman AL. Soleus H-reflex modulation during stance phase of walking with altered arm swing patterns. Motor Control. 2010;14(1):116–125. doi: 10.1123/mcj.14.1.116. [DOI] [PubMed] [Google Scholar]
- 14.Hayes HB, Chvatal SA, French MA, Ting LH, Trumbower RD. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury [published online ahead of print February 14, 2014] Clin Neurophysiol. doi: 10.1016/j.clinph.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang SF, Tuel SM, McKay WB, Dimitrijevic MR. Correlation of motor control in the supine position and assistive device used for ambulation in chronic incomplete spinal cord-injured persons. Am J Phys Med Rehabil. 1994;73(4):268–274. doi: 10.1097/00002060-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, Emch GS, Johnson CS, Wrathall JR. Effect of spinal cord injury severity on alterations of the H-reflex. Exp Neurol. 2005;196(2):430–440. doi: 10.1016/j.expneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]


