Abstract
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, contact Wolters Kluwer customer service at 866-397-3433. The April 2016 monograph topics are von Willebrand factor (recombinant), daratumumab, elotuzumab, uridine triacetate, and ixazomib. The MUE is on lesinurad.
INDICATIONS
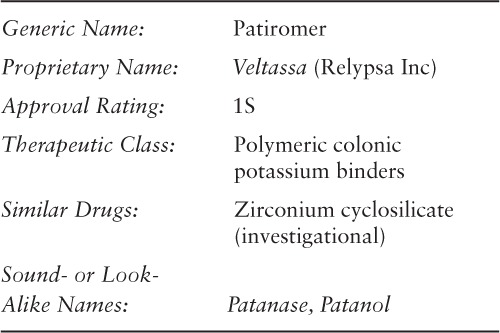
Patiromer is approved by the US Food and Drug Administration (FDA) for the treatment of hyperkalemia.1 Patiromer should not be used for the emergency treatment of life-threatening hyperkalemia because it has a delayed onset of action.1
Hyperkalemia is a potentially life-threatening metabolic condition caused by an inability of the kidneys to excrete potassium, impaired uptake of potassium into cells, increased potassium intake, or a combination of these factors. Contributing causes can include severe heart failure, defects in renal tubular mechanisms, poor renal perfusion, or medications (eg, angiotensin-converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARBs], aldosterone antagonists, potassium-sparing diuretics). Hyperkalemia is defined as an elevated serum potassium level above 5 mmol/L and is typically classified by severity (mild = potassium level 5 to 6 mmol/L; moderate = potassium level 6.1 to 6.9 mmol/L; and severe = potassium level 7 mmol/L and higher; or electrocardiogram [ECG] changes or symptoms occurring at any level). The symptoms of hyperkalemia are nonspecific and can include muscle weakness, flaccid paralysis, nausea, palpitations, arrhythmias, or paresthesias.2–6
Treatment of hyperkalemia can include administration of intravenous (IV) calcium salts, thiazide or loop diuretics, hemodialysis, and nonabsorbable potassium-binding agents (eg, sodium polystyrene sulfonate).2–4,6,7 Nonabsorbable potassium-binding agents have been used for decades to lower serum potassium levels, especially in hospitalized patients. Sodium or calcium polystyrene sulfonate is a nonselective cation exchange resin; the resin releases the sodium or calcium and then binds the potassium, but it may also bind other cations (eg, magnesium, aluminum). The nonabsorbable resin complex (cation plus resin) is then eliminated with bowel movements. Sorbitol is commonly added to the polystyrene sulfonate resin prior to administration to decrease the risk of fecal impaction and to speed up removal of the potassium-resin complex from the body.2–4 However, some patients may develop intestinal necrosis when sorbitol is added to the polystyrene sulfonate formulation.4,8 Patiromer is a nonabsorbable polymer that also binds potassium in the gastrointestinal (GI) tract.2,9,10 Asymptomatic hyperkalemia caused by renin-angiotensin-aldosterone system (RAAS) inhibitors is generally managed by adjusting patiromer doses, following a low-potassium diet, reducing or removing salt substitutes that contain potassium, and discontinuing drugs that impair the excretion of potassium in the kidney (eg, nonsteroidal anti-inflammatory drugs, beta-blockers, cyclosporine, tacrolimus, ketoconazole, potassium-sparing diuretics). Treatment with thiazide or loop diuretics is recommended, especially in patients with renal impairment. In patients with chronic kidney disease (CKD) and metabolic acidosis, sodium bicarbonate administration may be useful in decreasing the serum potassium concentration. Long-term use of polystyrene sulfonate is not recommended because it is generally not tolerated and, if mixed with sorbitol, may increase the risk of mucosal injury of the GI tract.7,11
CLINICAL PHARMACOLOGY
Active secretion of potassium occurs in the proximal and distal portions of the colon. Potassium is pumped from the serum into the colonic epithelial cells via basolateral Na+/K+ and Na+/K+/Cl− pumps. From the colonic epithelial cells, potassium enters into the lumen via apical BK channels. Passive secretion through tight junctions between epithelial cells also occurs, which is the major component of colonic potassium secretion. It is driven by lumen-negative transepithelial voltage, which is approximately 15 to 25 mV. This passive secretion is highest in the distal colon.12
Patiromer (patiromer sorbitex calcium) is a non-absorbed, potassium-binding, anionic polymer consisting of patiromer, potassium-binding polymer, and a calcium-sorbitol counterion. The fluoroacrylate monomers of the molecule are responsible for polymerization, and the carboxylate groups that are bound to the calcium will bind potassium.1,2
Patiromer promotes ionization of the polymeric potassium-binding moiety under pH conditions along the colon. The product consists of uniform beads of powder that swell to a minimal degree in water, have low viscosity, and can easily be mixed into beverages.2 Due to its chemical structure, patiromer allows the exchange of monovalent sodium and divalent calcium and magnesium through the length of the GI tract and preferentially binds potassium in the distal colon where potassium concentrations are higher than those of sodium, calcium, and magnesium.9,10 After oral administration, the polymer's calcium ions are exchanged for potassium ions in the colon, which increases fecal potassium excretion, thereby lowering blood potassium levels.2 This causes a reduction in serum potassium under hyperkalemic conditions.9,10
Data from preclinical and clinical studies show that a fixed dose of patiromer has a greater serum potassium–reducing effect when the patient has higher serum potassium values than lower serum potassium values. The amount of potassium excreted in the urine is decreased in an inverse dose-dependent manner in healthy subjects, but patiromer does not produce dose-dependent changes in serum potassium levels. There is no apparent difference between Black and White subjects in the amount of mean fecal potassium excretion.9,10,13,14
PHARMACOKINETICS
COMPARATIVE EFFICACY
Indication: Management of Hyperkalemia
Guidelines
Guideline: Treatment of acute hyperkalemia in adults
Reference: United Kingdom Renal Association, 201415
Comments: Evidence supporting one form of treatment over another is poor. Data regarding IV calcium, sodium bicarbonate, fluids, and diuretics are poor or lacking. Insulin-glucose infusion is the most effective treatment in lowering serum potassium. The use of beta-agonists may be effective in some patients. Potassium-exchange resins are often used, but efficacy in short-term treatment is limited because of slow onset of action.
Studies
Drug: Patiromer vs Placebo
Reference: Pitt B, et al, 2011 (PEARL-HF trial)9
Study Design: Phase 2, randomized, double-blind, placebo-controlled, parallel-group, international, multicenter study
Study Funding: Relypsa Inc
Patients: 120 patients 18 years and older with chronic heart failure were randomized and 105 patients received at least 1 dose of study drug. Eligible patients had a history of chronic heart failure, an indication to initiate spironolactone therapy, a serum potassium concentration of 4.3 to 5.1 mEq/L at screening, and either chronic kidney failure (estimated glomerular filtration rate [GFR] <60 mL/minute) and were receiving 1 or more heart failure therapies (ACE inhibitor, ARB, beta-blocker) or a documented history of hyperkalemia that led to discontinuation of therapy with an aldosterone antagonist, an ACE inhibitor, an ARB, or a beta-blocker within 6 months prior to baseline visit. The average baseline characteristics of the 105 patients were as follows: mean age of 68 years, history of heart failure with a mean duration of just over 4 years, mean left ventricular ejection fraction of 40%, and New York Heart Association (NYHA) class II or III heart failure (most patients). Baseline serum potassium levels were 4.65 and 4.69 mEq/L in the placebo and patiromer groups, respectively. Patients were excluded if they had severe GI disorders, major GI surgery, bowel obstruction, swallowing disorders, significant primary valvular disease, known obstructive or restrictive cardiomyopathy, uncontrolled or unstable arrhythmia, an episode of unstable angina within 3 months of baseline, acute coronary syndrome, transient ischemic attack, QTc segment prolongation greater than 500 ms, recent or anticipated cardiac surgery or intervention, kidney transplant or need for transplantation, need for dialysis during study (currently receiving or anticipated), sustained systolic blood pressure (BP) greater than 170 mm Hg or less than 90 mm Hg, elevated liver enzymes greater than 3 times the upper limit of normal, or any condition with the potential to interfere with study compliance or jeopardize the safety of the patient.
Intervention: Patients were randomized 1:1 to the patiromer or placebo group. Patiromer 15 g orally was administered in the morning and evening (for a total daily dose of 30 g), and patients were instructed to mix study drug (supplied as a powder) with water or a low-potassium food prior to administration. In addition, all patients started spironolactone 25 mg/day on day 1, which was increased to 50 mg/day on day 15 if serum potassium was greater than 3.5 to less than or equal to 5.1 mEq/L. If serum potassium was greater than 5.1 to less than or equal to 5.5 mEq/mL, patients remained at the 25 mg/day dose. Patients with serum potassium less than or equal to 3.5 or greater than 5.5 mEq/L were discontinued from the study.
Results
Primary Endpoint(s)
At the end of treatment (day 28), patients receiving patiromer in the modified intention-to-treat (mITT) population had a mean change in serum potassium from baseline of −0.22 mEq/L, compared with a mean change of +0.23 mEq/L in the placebo group. Serum potassium levels were lower in the patiromer group relative to placebo, with a difference between groups of −0.45 mEq/L (p < .001).
Secondary Endpoint(s)
Proportion of patients with serum potassium greater than 5.5 mEq/L at any time during the trial was lower in the patiromer group compared with placebo (7% vs 25%; p = .015).
A higher percentage of patients in the patiromer group compared with placebo was able to increase the dose of spironolactone from 25 mg to 50 mg daily (91% vs 74%; p = .019).
Endpoint(s)
More patients in the patiromer group experienced at least 1 adverse event compared with those in the placebo group (54% vs 31%). The most common adverse events in both groups were due to GI disorders, such as flatulence, diarrhea, constipation, and vomiting (21% vs 6%).
No clinically meaningful treatment-related changes occurred in serum chemistry, hematology, and urinalysis. Serum magnesium values were within normal limits, but a small decrease was observed in both groups (−0.22 vs 0.01 mg/dL for patiromer and placebo, respectively; p < .001); there was no associated increase in the incidence of ventricular arrhythmias.
There were no changes in BP or heart rate from baseline, nor clinically meaningful treatment-related changes in ECG or physical examinations.
Comments: Patients taking patiromer 30 g daily for 28 days experienced a decrease in baseline serum potassium when taking concomitant heart failure medications (an ACE inhibitor, an ARB, or a beta-blocker, in addition to spironolactone). The study was conducted in the United States, Germany, and Eastern Europe. The reduction in serum potassium was observed within 2 days of initiating treatment and persisted throughout the 4-week study. There was a reduced incidence of hyperkalemia and an increase in the proportion of patients in whom the spironolactone dose could be increased. Adverse effects associated with patiromer were mostly GI related and were mild to moderate. Reasons for patient discontinuation included adverse events, protocol noncompliance, serum potassium less than or equal to 3.5 or greater than 5.5 mEq/L, and investigator decision.
Limitations: This study did not take into consideration left ventricular ejection fraction when enrolling patients; most patients had less severe heart failure and did not meet the criteria for initiating spironolactone therapy. The sample size was small and the duration of treatment was relatively short. A larger population size with longer study duration is needed to determine long-term efficacy and safety. In addition, exclusion criteria for “any condition that had the potential to interfere with the study compliance or jeopardize the safety of the patient” were not defined.
Reference: Weir MR, et al, 2015 (OPAL-HK trial)1,10,16–18
Study Design: Phase 3, randomized, international, 2-phase, placebo-controlled study
Study Funding: Relypsa Inc
Patients: In the initial phase, 243 outpatients with persistent hyperkalemia (serum potassium 5.1 to <6.5 mmol/L), CKD (defined as estimated GFR 15 to <60 mL/minute), and receiving a stable dose of an RAAS inhibitor were enrolled. In the initial treatment phase, baseline characteristics of the 243 patients were as follows: mean age was 64.2 years; 58% were male and 98% were White; and 57% of patients had type 2 diabetes, 42% had heart failure, 97% had hypertension, and 25% had a history of myocardial infarction. RAAS inhibitor use was 100% with the following drugs: ACE inhibitor (70%), ARB (38%), dual RAAS therapy (17%), aldosterone antagonist (9%), and renin inhibitor (1%). Patients were excluded if they had any potassium-related ECG changes, severe GI disorders, uncontrolled or unstable arrhythmias or clinically significant ventricular arrhythmias, recent cardiac surgery, kidney or heart transplantation, acute coronary syndrome, transient ischemic attack or stroke within the previous 2 months, confirmed systolic BP greater than or equal to 180 mm Hg or less than 110 mm Hg, diastolic BP of greater than or equal to 110 mm Hg or less than 60 mm Hg, type 1 diabetes, emergency treatment for type 2 diabetes, exacerbation of acute heart failure within 3 months, or NYHA class IV heart failure. Of the 219 patients who completed the initial phase, 107 were eligible and subsequently entered the randomized withdrawal phase of the study. These 107 patients were randomly assigned to continue patiromer (n = 55) or switch to placebo (n = 52), and were required to have an initial treatment-phase baseline serum potassium of at least 5.5 mmol/L, completed the initial phase, a serum potassium reading of 3.8 to 5.1 mmol/L after week 4 of the initial phase, and received patiromer 8.4 to 50.4 g/day.
Intervention: There were 2 phases of this study: a 4-week single-group, single-blind initial treatment phase that was followed by an 8-week placebo-controlled, single-blind, randomized withdrawal phase. In the initial phase, patients were assigned patiromer 4.2 g (potassium level 5.1 to <5.5 mmol/L) or 8.4 g (potassium level 5.5 to <6.5 mmol/L) twice daily for 4 weeks based on their initial potassium level; each dose was administered as an oral suspension in 40 mL of water during breakfast and dinner. During this phase, the patiromer dose was adjusted to reach and maintain a target serum potassium level of 3.8 to 5.1 mmol/L; doses of RAAS inhibitor were not adjusted and were only discontinued if the potassium level was 6.5 mmol/L or greater. In the 8-week treatment withdrawal phase, patients initially treated with patiromer and a RAAS inhibitor were re-randomized to either patiromer or placebo, and prespecified treatment algorithms were developed to manage recurrence of hyperkalemia (ie, increase in the dose of patiromer in the patiromer group or modification of the RAAS inhibitor regimen in the placebo group at the time of first hyperkalemia event).
Results
Primary Endpoint(s)
In the initial phase, the mean change in serum potassium level from baseline to week 4 in the mITT group was −1.01 mmol/L (95% confidence interval [CI], −1.07 to −0.95; p < .001). In patients 65 years and older, the change in serum potassium level was −1.01 mmol/L (95% CI, −1.01 to −0.92; p < .001).
In the treatment withdrawal phase, the median change in serum potassium by week 4 was an increase of 0.72 mmol/L in the placebo group and 0 mmol/L in the patiromer group (between-group difference of 0.72 mmol/L [95% CI, 0.46 to 0.99]; p < .001). In patients 65 years of age and older, the change in serum potassium level was 0.81 mmol/L (95% CI, 0.49 to 1.14; p < .001).
Secondary Endpoint(s)
After week 4 of the initial phase, the proportion of patients with serum potassium levels in the target range of 3.8 to less than 5.1 mmol/L was 76% (95% CI, 70% to 81%), with a mean daily patiromer dose of 12.8 g in patients with mild hyperkalemia and 21.4 g in patients with moderate to severe hyperkalemia. The remaining 24% did not achieve the target dose. Seventy-three percent of patients 65 years and older achieved a serum potassium within the target range.
In the withdrawal phase, the proportion of patients with at least 1 potassium level of 5.5 mmol/L or higher within 8 weeks of withdrawal was 15% (95% CI, 6% to 24%) in the patiromer group and 60% (95% CI, 47% to 74%) in the placebo group (p < .001).
62% of the placebo group and 16% of the patiromer group required an intervention, such as discontinuation of RAAS inhibitor therapy, to manage recurrence of hyperkalemia.
At the end of the withdrawal phase, 94% of patients in the patiromer group and 44% in the placebo group were still receiving RAAS inhibitors.
Endpoint(s)
Post hoc analysis showed that chronic diuretic therapy did not impair the effectiveness of patiromer in patients with CKD.
Adverse events were reported in 47% of patients during the initial phase; mild to moderate constipation was the most common (11%) and the incidence of hypomagnesemia was 3%.
Comments: The study population included patients from Eastern Europe, the European Union, and the United States. All patients had CKD, persistent hyperkalemia, and were taking a RAAS inhibitor. Evaluation of the primary and secondary endpoints from the withdrawal phase showed that patients taking patiromer experienced a decrease in serum potassium levels and a reduction in recurrence of hyperkalemia. As a result, more patients in the patiromer group were able to continue taking RAAS inhibitors due to better control of serum potassium. Reasons for study discontinuation included elevated potassium levels that met pre-specified withdrawal criteria or potassium levels less than 3.8 mmol/L. Of the 8 patients in the patiromer group who had at least 1 potassium value greater than 5.5 mmol/L through week 8, only 2 met withdrawal criteria. Of patients with at least 1 potassium value greater than 5.1 mmol/L during the withdrawal phase, 91% were taking placebo compared with 43% taking patiromer.
Limitations: Both phases of this study were single-blinded.
Drug: Patiromer
Reference: Relypsa Inc (ClinicalTrials.gov), 2014 (AMETHYST-DN trial)19–24
Study Design: Phase 2, randomized, open-label, dose-ranging, parallel-group, safety/efficacy, international, multicenter study
Study Funding: Relypsa Inc
Patients: 306 patients with hypertension and diabetic neuropathy treated with ACE inhibitors and/or ARBs, with or without spironolactone. Patients were between 30 and 80 years of age at the time of screening, had a diagnosis of type 2 diabetes mellitus and were treated with oral medication or insulin at least 1 year prior to screening, had CKD, received an ACE inhibitor and/or ARB for at least 28 days prior, and had an average systolic BP of 140 to less than 180 mm Hg or average diastolic BP of 90 to less than 110 mm Hg (sitting). Mean age was 66.3 years, 63.2% were male, and 100% were White.
Intervention: The study consisted of a 10-day initial screening, a 4-week run-in period, a treatment initiation phase of 8 weeks, a long-term maintenance phase of 44 additional weeks, and 1 to 4 weeks of follow-up. Patients were assigned to stratum 1 (baseline serum potassium >5 to 5.5 mEq/L) or stratum 2 (baseline serum potassium >5.5 to <6 mEq/L) and randomized to patiromer doses depending on the stratum; if needed (based on patient serum potassium levels), patiromer doses were individually titrated. Within each stratum, patients were randomized to losartan 100 mg daily plus low- or high-dose patiromer, or to their current dose of ACE inhibitor and/or ARB plus spironolactone 25 to 50 mg daily plus low- or high-dose patiromer. The low-dose patiromer group (stratum 1) received a starting dose of 8.4 g/day, 16.8 g/day, or 25.2 g/day orally in 2 divided doses. The high-dose patiromer group (stratum 2) received a starting dose of 16.8 g/day, 25.2 g/day, or 33.6 g/day orally in 2 divided doses.
Results
Primary Endpoint(s)
Least squares mean reductions from baseline in serum potassium level at week 4 or time of first dose titration in patients with mild and moderate hyperkalemia both showed a dose-related response. The change in the mild hyperkalemia group was 0.35 mEq/L (95% CI, 0.22 to 0.48; p < .001) with 4.2 g twice daily, 0.51 mEq/L (95% CI, 0.38 to 0.64; p < .001) with 8.4 g twice daily, and 0.55 mEq/L (95% CI, 0.42 to 0.68; p < .001) with 12.6 g twice daily. The change in the moderate hyperkalemia group was 0.87 mEq/L (95% CI, 0.6 to 1.14; p < .001) with 8.4 g twice daily, 0.97 mEq/L (95% CI, 0.7 to 1.23; p < .001) with 12.6 g twice daily, and 0.92 mEq/L (95% CI, 0.67 to 1.17; p < .001) with 16.8 g twice daily.
Secondary Endpoint(s)
Throughout the 44-week, long-term maintenance period, the mean serum potassium level in stratums 1 and 2 remained in the target range of 3.8 to 5 mEq/L. At week 52, the proportion of patients with serum potassium values in the target range was 83.1% to 92.7% in stratum 1 and 77.4% to 95.1% in stratum 2.
Endpoint(s)
After titration, the mean daily dose for the entire 52-week treatment period was similar to doses at week 4 through 8 (19.4 g/day in stratum 1 and 27.2 g/day in stratum 2).
The most common adverse events with patiromer were worsening of CKD (9.2%), hypomagnesemia (8.6%), worsening of hypertension (7.9%), constipation (6.3%), and diarrhea (5.6%).
There were no cases of severe hypomagnesemia (less than 1 mg/dL); the incidence of mild to moderate hypomagnesemia was less than 10%.
Comments: This study was conducted in multiple centers in Eastern Europe. Long-term use of patiromer in stratums 1 and 2 appears to be efficacious and cause minimal adverse effects. Discontinuation of patiromer therapy results in an elevation in serum potassium.
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS
Contraindications
Patiromer is contraindicated in patients with a history of a hypersensitivity reaction to the drug or any of its components (ie, xanthan gum).1
Warnings and Precautions
Patiromer may decrease the GI absorption of other drugs. All other oral medications should be taken at least 6 hours before or after patiromer administration.1
Patiromer therapy should be avoided in patients with GI obstruction, impaction, or motility issues (eg, severe constipation, bowel obstruction, impaction, postoperative bowel motility disorders); patiromer may be ineffective due to poor GI motility and may worsen GI conditions.1 Key exclusion criteria for clinical trials included a history of bowel obstruction, severe GI disorders or major GI surgery, NYHA class IV heart failure, renal artery stenosis, need for dialysis (currently receiving or anticipated), kidney transplantation, and QTc value of greater than 500 ms. Patiromer should be used with caution, if at all, in these populations.9,10
Patiromer does not selectively bind potassium. It also binds magnesium in the colon and may lead to the development of hypomagnesemia. Serum magnesium levels should be monitored in all patients and magnesium supplementation should be considered in those with low serum magnesium levels.1
Chronic toxicity studies show that patiromer is well tolerated, with no target organ toxicity. Genotoxicity and reproductive toxicity studies currently reveal no safety concerns. In addition, the manufacturer received a carcinogenicity waiver and confirmation that peri/postnatal reproductive toxicology studies would not be needed.1,12
Safety and efficacy of patiromer have not been established in pediatric patients.1
ADVERSE REACTIONS
The most common adverse events reported in clinical trials were mild to moderate GI symptoms, including constipation and diarrhea (5% to 11%). Hypomagnesemia was reported (often mild to moderate in severity and occurring in up to 24% of patients). Common adverse events reported in less than 10% of patients included hypertension and worsening of underlying chronic renal failure.9,10,20 The most common adverse reactions reported in the product labeling included constipation (7.2%), hypomagnesemia (5.3%), diarrhea (4.8%), nausea (2.3%), abdominal discomfort (2%), and flatulence (2%).1 Discontinuation of therapy because of adverse events was low: vomiting (0.8%), diarrhea (0.6%), constipation (0.5%), and flatulence (0.5%).1
Laboratory abnormalities included hypokalemia (serum potassium levels <3.5 mEq/L) in 4.7% and hypomagnesemia (serum magnesium levels <1.4 mg/dL) in 9% of patients.1
DRUG INTERACTIONS
Patiromer is not systemically absorbed but does bind to other drugs in the GI tract, which may lead to decreased absorption and reduced clinical effects of other medications. All other oral medications should be taken at least 6 hours before or after patiromer administration.1 Patiromer product labeling includes a boxed warning regarding its impact on other drugs.1 The FDA is now requiring the manufacturer of polystyrene sulfonate to complete a series of similar drug interaction studies to evaluate its impact on the absorption of drugs from the GI tract, even though it has been marketed for more than 50 years.25
RECOMMENDED MONITORING
Serum potassium and magnesium levels should be monitored throughout therapy. Changes in serum magnesium have been reported, although no increases in the incidence of ventricular arrhythmias were noted during clinical trials.9,10
DOSING
The recommended starting dose of patiromer is 8.4 g once daily mixed with water.1 The dose used in a phase 3 study was between 8.4 and 50.4 g/day.10 The dose should then be adjusted based on the serum potassium level and the desired target range. The dose can be up-titrated at 1-week or longer intervals in increments of 8.4 g.1 Pivotal studies used a potassium level of 3.8 to 5 mmol/L as their goal.10 According to the product labeling, the maximum recommended dose is 25.2 g once daily.1 No dosage adjustments are required for patients with renal impairment.1
All other oral medications should be taken at least 6 hours before or after patiromer administration.
The dry powder should not be ingested alone, but mixed with water. The recommended preparation is 30 mL of water added to an empty glass or cup; empty the entire contents of the packet(s) into the glass or cup, stirring the mixture thoroughly. Then add an additional 60 mL of water, and stir the mixture again. The suspension will appear cloudy. Have the patient drink the mixture immediately. If some powder remains after drinking, more water should be poured into the glass or cup and stirred to attempt to capture the remaining powder; have the patient drink immediately.1 Patiromer can be administered with food, but the food/drug mixture should not be heated (eg, microwaved) or added to heated food or liquids.1
PRODUCT AVAILABILITY
The New Drug Application for patiromer was submitted to the FDA in October 2014 and was approved on October 21, 2015.12,26 It is available in single-use packets containing patiromer 8.4 g, 16.8 g, or 25.2 g in cartons of 4 packets (8.4 g only), and in cartons of 30 packets.1
It is recommended that patiromer be stored in the refrigerator at 2° to 8°C (36° to 46°F). If stored at room temperature (25°C [77°F]), the drug must be used within 3 months of being taken out of the refrigerator.1 Exposure to excessive heat (above 40°C [104°F]) should be avoided.1
DRUG SAFETY/RISK EVALUATION AND MITIGATION STRATEGY (REMS)
No REMS is required for patiromer.26
CONCLUSION
Patiromer is a novel polymeric, nonabsorbed, potassium binder used to treat hyperkalemia. Similar to polystyrene sulfonate, it binds potassium in the GI tract. Patiromer can also bind magnesium but does not require the use of sorbitol. Patiromer has demonstrated the ability to lower serum potassium (starting at day 3) and maintain reduced potassium levels throughout therapy (up to 1 year), even in patients receiving a concomitant ACE inhibitor, an ARB, or an aldosterone antagonist. All patients require monitoring of potassium and magnesium levels throughout therapy. There are no head-to-head comparisons with any of the polystyrene sulfonate resins.
Footnotes
The authors indicate no relationships that could be perceived as a conflict of interest.
REFERENCES
- 1.Veltassa (patiromer) [prescribing information] Redwood City, CA: Relypsa; October 2015. [Google Scholar]
- 2.Tzamaloukas AH, Glew RH. Efficacy and safety of patiromer in prevention and treatment of hyperkalemia. J Sympt Signs. 2013;2(6):475–484. [Google Scholar]
- 3.Nguyen T, Ondrik D, Zhufyak O, To W, He S. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015;28(3):41–45. doi: 10.1097/01.JAA.0000458856.92020.1e. [DOI] [PubMed] [Google Scholar]
- 4.Ingelfinger JR. A new era for the treatment of hyperkalemia? N Engl J Med. 2015;372(3):275–277. doi: 10.1056/NEJMe1414112. [DOI] [PubMed] [Google Scholar]
- 5.Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377–384. doi: 10.1007/s00467-010-1699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Packham DK, Kosiborod M. Potential new agents for the management of hyperkalemia [published online ahead of print July 9 2015] Am J Cardiovasc Drugs. doi: 10.1007/s40256-015-0130-7. [DOI] [PubMed] [Google Scholar]
- 7.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351(6):585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 8.US National Library of Medicine. Carolina Medical Products Company; Sodium polystyrene sulfonate. NDCs 46287-006-01, 46287-006-04, 46287-006-60. DailyMed website. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d48dcf-07bd-4b06-bd6c-7543f1be8357. Updated March 2011. Accessed April 27, 2015. [Google Scholar]
- 9.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–828. doi: 10.1093/eurheartj/ehq502. PEARL-HF Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weir MR, Bakris GL, Bushinsky DA. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/NEJMoa1410853. et al; OPAL-HK Investigators. [DOI] [PubMed] [Google Scholar]
- 11.Esteras R, Perez-Gomez MV, Rodriguez-Osorio L, Ortiz A, Fernandez-Fernadez B. Combination use of medicines from two classes of renin-angiotensin system blocking agents: Risk of hyperkalemia, hypotension, and impaired renal function. Ther Adv Drug Saf. 2015;6(4):166–176. doi: 10.1177/2042098615589905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orwin J. Relypsa analyst briefing. Relypsa website. http://www.relypsa.com/file.cfm/1/docs/Relypsa_2015_Analyst_Day_Presentation.pdf. Published January 2015. Accessed April 27, 2015.
- 13.Epstein M, Palmer B, Mayo M Mechanism of action of patiromer: increases fecal K[+] excretion in healthy volunteers independent of race [poster 018] Presented at: 2015 World Congress of Nephrology; March 13–17, 2015; Cape Town, South Africa. Abstract SAT-218. http://www.posters2view.eu/wcn2015/view.php?nu=3287. Accessed April 27, 2015.
- 14.Bushinsky DA, Williams GH, Pitt B Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia [published online ahead of print September 16, 2015] Kidney Int. [DOI] [PMC free article] [PubMed]
- 15.UK Renal Association. Clinical practice guidelines: Treatment of acute hyperkalaemia in adults. Renal Association website. http://www.renal.org/docs/default-source/guidelines-resources/joint-guidelines/treatment-of-acute-hyperkalaemia-in-adults/hyperkalaemia-guideline---march-2014.pdf?sfvrsn=2). Updated March 2014. Accessed April 27, 2015.
- 16.Weir M, Mayo M, Garza D, Stasiv Y, Arthur S, Berman L. Patiromer reduced serum K+ and maintained normokalemia in patients with chronic kidney disease who were hyperkalemic on RAAS inhibitors in the OPAL-HK study [poster 017] Presented at: 2015 World Congress of Nephrology; March 13–17, 2015; Cape Town, South Africa. Abstract SAT-217. http://www.posters2view.eu/wcn2015/view.php?nu=3285. Accessed April 27, 2015.
- 17.Weir MR, Bushinsky DA, Mayo M et al. Patiromer lowers serum K+ and prevents recurrent hyperkalemia in CKD patients ≥ 65 years of age on RAAS inhibitors [abstract] J Am Soc Nephrology. 2015;26 abstract TH-OR035. [Google Scholar]
- 18.Weir MR, Mayo M, Garza D et al. Chronic diuretic therapy does not impair the effectiveness of patiromer in hyperkalemic patients with CKD [abstract] J Am Soc Nephrology. 2015;26 abstract TH-OR035. [Google Scholar]
- 19.Relypsa Inc. RLY5016 in the treatment of hyperkalemia in patients with hypertension and diabetic nephropathy (AMETHYST-DN) ClinicalTrials.gov website. https://www.clinical-trials.gov/ct2/show/record/NCT01371747?term=AMETHYST-DN&rank=1. Updated October 30, 2014. Accessed April 28, 2015. NLM Identifier: NCT01371747.
- 20.Relypsa reports positive to pline results for its 52-week phase 2b trial of patiromer [press release] Redwood, CA: Relapsa Inc; October 17, 2013. http://files.shareholder.com/downloads/AMDA-29YRKX/892209952x0x702040/20E449FE-B842-4C58-AD39-3A5C0B0CA988/RLYP_News_2013_10_17_General_Releases.pdf. Accessed March 18, 2015. [Google Scholar]
- 21.Pitt B, Bushinsky D, Garza D 1-year safety and efficacy of patiromer for hyperkalemia in heart failure patients with chronic kidney disease on renin-angiotensin-aldosterone system inhibitors [abstract] American College of Cardiology Scientific Session 2015; March 14–16, 2015; San Diego, CA. Abstract 1145-184. http://www.abstractsonline.com/pp8/#!/3658/presentation/34899. Accessed March 18, 2015.
- 22.Pitt B, Bakris GL, Bushinsky DA et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17(10):1057–1065. doi: 10.1002/ejhf.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitt B, Dushinsky D, Garza D et al. 1-year safety and efficacy of patiromer for hyperkalemia in heart failure patients with chronic kidney disease on renin-angiotensin-aldosterone system inhibitors [abstract] J Am Coll Cardiol. 2015;65(10S) abstract 855. [Google Scholar]
- 24.Bakris GL, Pitt B, Weir MR. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–161. doi: 10.1001/jama.2015.7446. et al; AMETHYST-DN Investigators. [DOI] [PubMed] [Google Scholar]
- 25.FDA Drug Safety Communication: FDA requires drug interaction studies with potassium-lowering drug Kayexalate (sodium polystyrene sulfonate) US Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm468035.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery. Published October 22, 2015. Accessed October 26, 2015.
- 26.Unger EF. NDA approval letter: Veltassa (patiromer NDA 205739) US Food and Drug Administration website. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/205739Orig1s000ltr.pdf. Published October 21, 2015. Accessed October 26, 2015.


