Abstract
Background: Obese patients experience a higher risk of venous thromboembolism (VTE) than their nonobese counterparts, which may warrant a more aggressive approach to thromboprophylaxis in this population.
Objective: The purpose of this study was to compare rates of nosocomial VTE in obese patients treated with high-dose versus conventional-dose subcutaneous unfractionated heparin sodium (UFH) for thromboprophylaxis.
Methods: A retrospective, single-center, cohort study was conducted to evaluate obese, adult, hospitalized patients admitted between April 2011 and April 2014 who received heparin 5,000 or 7,500 units subcutaneously every 8 hours for thromboprophylaxis. The primary outcome assessed the rate of nosocomial VTE in obese patients treated with high-dose heparin (7,500 units subcutaneously q 8 h) versus conventional-dose heparin (5,000 units subcutaneously q 8 h). Additionally, a secondary outcome assessed safety by quantifying bleeding events.
Results: Nosocomial VTE occurred in 2/196 (1.02%) patients who received high-dose heparin thromboprophylaxis and in 5/2,182 (0.23%) patients who received conventional-dose heparin (p = .05). Bleeding occurred in 0/196 (0%) patients in the high-dose heparin group and in 2/2,182 (0.09%) patients in the conventional-dose heparin group (p = .67). All bleeding events were minor.
Conclusions: This study failed to demonstrate a statistically significant reduction in the rate of nosocomial VTE in obese patients who received high-dose heparin thromboprophylaxis. Despite receiving a higher heparin dose, no increased risk of bleeding was observed in the high-dose group. Further investigation is needed to identify the optimal heparin dose for thromboprophylaxis in obese patients.
Keywords: obese, thromboprophylaxis, unfractionated heparin, venous thromboembolism
Venous thromboembolism (VTE), a term incorporating both deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the most common and preventable causes of death in the United States it accounts for 200,000 deaths annually.1 Hospitalization increases the risk of VTE 8-fold, with 50% to 75% of events occurring while patients are on a medical service.2 Additional risk factors for VTE include active malignancy, previous VTE, recent trauma or surgery, immobility, pregnancy, hereditary or acquired coagulopathies, increasing age, heart or respiratory failure, acute myocardial infarction (MI) or stroke, acute infection, ongoing hormonal treatment, and obesity.3,4
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or greater, has increased in prevalence over the last several decades; currently more than 35% of the United States population is obese.5 Several studies have shown that obese patients are at 2 to 3 times greater risk for VTE.6–8 In the Nurses' Health Study, a large prospective cohort study, there was a linear increase in the risk of idiopathic PE and increasing BMI, with an 8% increase in risk for every 1 kg/m2 increase in BMI.9
Thromboprophylaxis is recommended with unfractionated heparin sodium (UFH) or another anticoagulant for the prevention of VTE in acutely ill hospitalized medical patients.2 However, the most recently published guidelines from the American College of Chest Physicians do not provide a specific recommendation regarding the dosing of heparin for the prevention of VTE in obese patients. Considering that both hospitalization and obesity increase patient risk for VTE, emphasis should be placed on providing adequate thromboprophylaxis in obese patients while minimizing bleeding complications.
Patients being treated for acute VTE using a weight-based intravenous (IV) heparin dosing nomogram have been shown to reach therapeutic anticoagulant levels more quickly and have a significantly lower rate of recurrent thromboembolic events with no significant difference in major or minor bleeding compared with patients using a fixed-dose IV heparin dosing nomogram.10,11 Since obese patients require higher doses of IV heparin to meet therapeutic endpoints in the treatment of acute VTE, it is reasonable to postulate that the same may be true when using subcutaneous heparin for thromboprophylaxis.
Literature to date regarding optimal heparin dosing in obese patients is limited. Currently, there are no published studies directly comparing the rate of nosocomial VTE in obese patients treated with a conventional thromboprophylaxis dose of heparin (5,000 units subcutaneously every 8 hours [q 8 h]), versus an increased dose of heparin (7,500 units subcutaneously q 8 h). However, the results of a retrospective, protocol analysis demonstrated that a median dose of 8,000 units dosed q 12 h (range, 3,000 to 19,000 units q 12 h) was required to achieve goal prophylactic anti-Xa levels in 700 patients who underwent laparoscopic Roux-en-Y gastric bypass surgery.12 There were 3 nonfatal PEs and no DVTs (composite VTE rate of 0.4%), with a major bleeding rate of 1%. When comparing these rates to previously published literature using conventional-dose heparin, thrombosis and bleeding rates were similar to this higher heparin dose. However, there was no comparator conventional-dose group in this study and not all of the patients included in this evaluation were obese; the median patient BMI was 28 kg/m2. Furthermore, mechanical prophylaxis using thromboembolic deterrent hose (TED stockings) and sequential pneumatic compression boots were used in addition to subcutaneous heparin, which may have affected outcomes to a degree.
Wang et al published a retrospective, cohort study in 2014 that analyzed the incidence of VTE in 3,928 morbidly obese mixed medical and surgical patients with a BMI greater than or equal to 40 kg/m2 who received high-dose (heparin 7,500 units q 8 h or enoxaparin 40 mg q 12 h) versus conventional-dose (heparin 5,000 units q 8 h or enoxaparin 40 mg daily) thromboprophylaxis.13 Among patients who received high-dose heparin, there was no difference in the rate of VTE compared with conventional-dose heparin (0.77% vs 1.48%; p = .050). Similarly, the incidence of bleeding in the high-dose heparin group did not differ significantly as compared with conventional-dose heparin group (7.18% vs 8.44%; p = .15). A notable limitation is that the primary and safety endpoints combined all patients who received high-dose thromboprophylaxis into the same treatment arm, regardless of whether they received heparin or enoxaparin, and the number of patients receiving each treatment was not reported.
A more recent retrospective, cohort study evaluated the occurrence of major bleeding and the incidence of confirmed VTE as a secondary endpoint in 398 neurosurgical patients weighing greater than 100 kg who received high-dose (heparin 7,500 units q 8 h) versus traditional-dose (heparin 5,000 units q 8 h) thromboprophylaxis.14 Similar rates of major hemorrhage were observed between treatment arms. The primary outcome of major hemorrhage occurred in 57% in the high-dose group versus 51% in the traditional-dose group (p = .24) at any point during admission and in 14% in the high-dose group versus 11% in the traditional-dose group (p = .33) during any 24-hour period. There was also no difference in the incidence of VTE in patients who received high-dose versus traditional-dose thromboprophylaxis (5.7% vs 9.3%; p = .2). Although this study evaluated neurosurgical patients instead of medically treated patients, the results are similar with no difference in the rate of VTE or bleeding.
PURPOSE/OBJECTIVES
The aim of this study was to compare the rate of nosocomial VTE in obese patients receiving thromboprophylaxis with conventional-dose heparin (5,000 units subcutaneously q 8 h) versus obese patients treated with high-dose heparin (7,500 units subcutaneously q 8 h). Additionally, a secondary outcome assessed safety by quantifying bleeding events in both groups.
METHODS
Design and Sample
This was a retrospective, single-center, cohort study evaluating obese, adult patients admitted to a tertiary care, university-affiliated academic medical center between April 2011 and April 2014. The study was approved by the institutional review board. Obese patients with an order for high-dose or conventional-dose subcutaneous heparin were evaluated for inclusion. Patients had to receive at least 3 consecutive doses of subcutaneous heparin to be included to ensure adequate prophylaxis had been received before the patients were evaluated for the outcomes of interest. Obesity was determined using ICD-9 codes 278, 278.01, 278.03, and V85.3–V85.45 (Figure 1).
Figure 1.
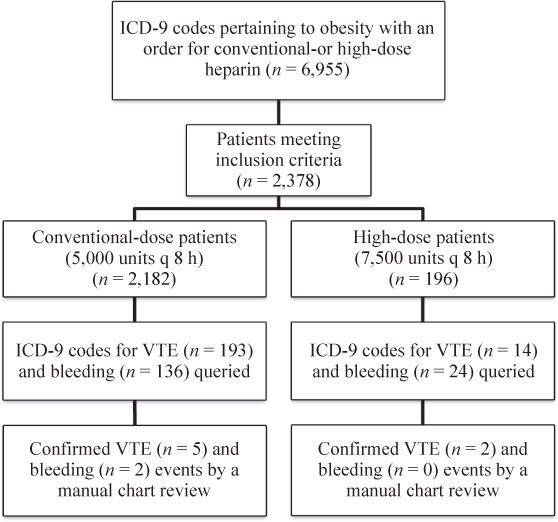
Patient selection scheme. q = every; VTE = venous thromboembolism.
Exclusion criteria were age less than 18 years old, VTE on admission, bleeding on admission, receipt of therapeutic anticoagulation on admission or during hospitalization, receipt of enoxaparin during hospitalization, pregnancy or peripartum, imprisonment, paraplegia, major surgery, inpatient rehabilitation, and history of heparin-induced thrombocytopenia confirmed with a positive Serotonin Release Assay.
The evaluable study population was assessed for the presence of ICD-9 codes pertaining to VTE, bleeding, and hemorrhage to identify potential outcomes. Nosocomial VTE was defined as a VTE acquired during hospitalization without signs and symptoms of VTE on admission or within 30 days of a previous hospitalization. Patients who presented with signs and symptoms of acute VTE on admission who were hospitalized within the previous 30 days and received heparin thromboprophylaxis during that admission were considered to have a nosocomial VTE. Bleeding related to heparin use was defined as bleeding not present at the time of admission, occurring after receipt of at least 24 hours (3 consecutive doses) of subcutaneous heparin. Major bleeding was defined as clinically significant bleeding meeting one of the following criteria: a decrease in hemoglobin of 2 g/dL or more in any 24 hours, transfusion of 2 or more units of blood, or evidence of confirmed bleeding in a critical area such as intracranial hemorrhage or gastrointestinal bleeding.10,14,15 Minor bleeding was defined as any bleeding documented in the medical record and not meeting criteria for major bleeding. To confirm that VTE and bleeding events occurred as a result of hospitalization and while receiving thromboprophylaxis therapy with heparin, a manual chart review for each VTE or bleeding event was performed to determine whether appropriate diagnosis and association could be made. Confirmation of a DVT required evidence of a new DVT on duplex ultrasound, and confirmation of a PE required evidence of a new PE on computed tomography angiography (CTA) or pulmonary ventilation/perfusion scan (V/Q lung scan).
Statistics
An unpaired Student's t test was performed to compare continuous, parametric variables and chi-square test was used to compare categorical variables. A Mann-Whitney U test was performed to compare continuous, nonparametric data. Alpha significance was set at less than .05 for all statistical tests.
RESULTS
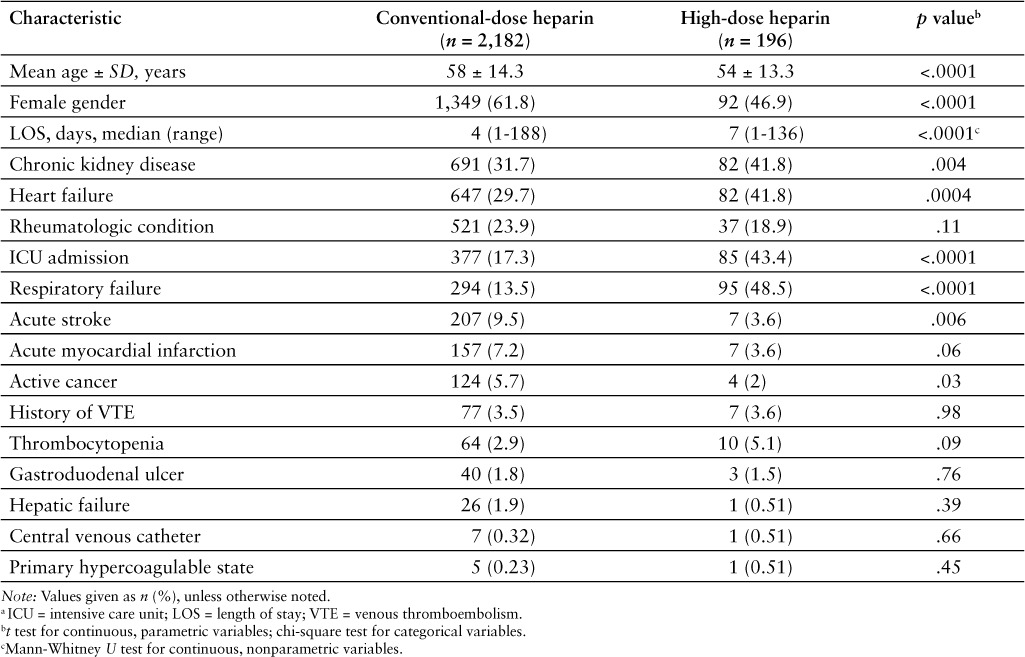
There were 2,378 obese patients included in the evaluable study population, 196 patients in the high-dose group, and 2,182 patients in the conventional-dose group (Table 1). Patients in the high-dose group were younger (mean age, 54 vs 58 years; p < .0001), less likely to be female (46.9% vs 61.8%; p < .001), and had a longer length of stay (median 7 vs 4 days; p < .0001). In addition, high-dose group patients were more likely to have chronic kidney disease (41.8% vs 31.7%; p = .04), be admitted to an intensive care unit during their hospitalization (43.4% vs 17.3%; p < .0001), have heart failure (41.8% vs 29.7%; p = .004), and/or experience respiratory failure (48.5% vs 13.5%; p < .0001). However, high-dose group patients were less likely to have an acute stroke (3.6% vs 9.5%; p = .006).
Table 1.
Patient demographic and clinical variables a
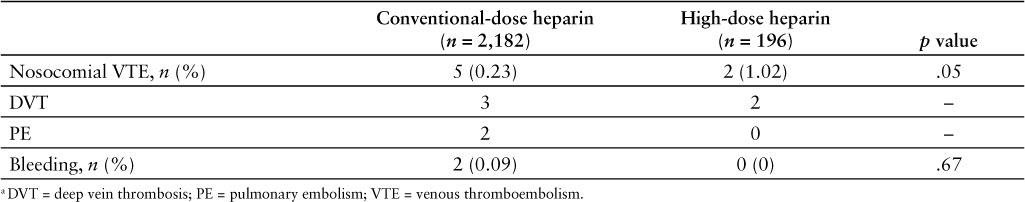
No statistically significant difference was observed in either the primary or secondary endpoints (Table 2). Nosocomial VTE occurred in 2/196 (1.02%) patients who received high-dose heparin thromboprophylaxis and in 5/2,182 (0.23%) patients who received conventional-dose heparin thromboprophylaxis (p = .05). There were 2 DVTs in the high-dose group, and there were 3 DVTs and 2 nonfatal PEs in the conventional-dose group. There were no cases of major bleeding; minor bleeding occurred in 0/196 (0%) patients in the high-dose group and in 2/2,182 (0.09%) patients in the conventional-dose group (p = .67). Observed minor bleeding events included one episode of hematuria and one episode of melenic stool.
Table 2.
Outcomes in obese patients receiving high-dose versus conventional-dose heparin thromboprophylaxis a
DISCUSSION
The results of this study failed to demonstrate a statistically significant reduction in the rate of nosocomial VTE in obese patients treated with high-dose heparin thromboprophylaxis compared with obese patients treated with conventional-dose heparin thromboprophylaxis. There were a higher percentage of VTE events in the high-dose group; however, it appeared that patients who received thromboprophylaxis with high-dose heparin were at greater risk for poorer health outcomes. They had a longer median length of stay with a higher prevalence of known thrombosis risk factors such as heart failure and respiratory failure.3,4 Additionally, patients in the high-dose thromboprophylaxis group had a greater proportion of men with a higher prevalence of chronic kidney disease and ICU admissions, which are known bleeding risk factors.2 Despite the possibility of having a greater baseline bleeding risk, no increased incidence of bleeding was observed with high-dose heparin thromboprophylaxis, suggesting this regimen may be safe without placing obese patients at additional bleeding risk. Although there were statistically fewer acute strokes in the high-dose group, this cannot necessarily be attributed to the higher dose of heparin because data were not collected on whether the finding of acute stroke was present on admission or nosocomial-acquired.
This study has several strengths. Currently, there are no other published studies directly comparing the rate of nosocomial VTE in medically treated, obese patients using high-versus conventional-dose unfractionated heparin thromboprophylaxis. Furthermore, VTE and bleeding are clinically relevant endpoints that have implications directly applicable to patient management, and this study provides data in an area of clinical practice that has limited evidence. Finally, all VTE and bleeding events were confirmed with a manual chart review in an effort to reduce the likelihood of type I error.
Several limitations from this investigation should be considered. This was a retrospective study that utilized ICD-9 codes to identify and quantify patient outcomes. Inherent to this type of study design, some patients may not have been included in the study secondary to coding errors. Also, minor bleeding events may not have been coded with an ICD-9 code if bleeding severity was considered to be low. Furthermore, since ICD-9 codes were used to identify obese patients, individual patient BMIs were not available for statistical analysis comparing BMIs between the 2 treatment arms.
Another limitation is the small sample size in the high-dose group, which may have been too small and underpowered to conclude that there is no significant difference between treatment arms. Finally, there was significant heterogeneity between baseline characteristics in each treatment group, potentially driving the higher rate of VTE in the high-dose group rather than heparin dose itself.
In conclusion, this study failed to demonstrate a statistically significant reduction in the rate of nosocomial VTE in obese patients who received high-dose heparin thromboprophylaxis. However, there was no increased risk of bleeding observed in the high-dose group despite having received higher doses of heparin. Further investigation is necessary to better guide thromboprophylaxis and identify the optimal subcutaneous heparin dose in the obese population.
ACKNOWLEDGEMENTS
The authors report no conflicts of interest.
REFERENCES
- 1.Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: Controversies and perspectives. Am J Med. 2009;122:1077–1084. doi: 10.1016/j.amjmed.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SR, Lim W, Dunn AS et al. Prevention of VTE in nonsurgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbar S, Noventa F, Rossetto V et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua prediction score. J Thromb Haemost. 2010;8(11):2450–2457. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 6.Allman-Farinelli MA. Obesity and venous thrombosis: A review. Semin Thromb Hemost. 2011;37(8):903–907. doi: 10.1055/s-0031-1297369. [DOI] [PubMed] [Google Scholar]
- 7.Rocha AT, de Vasconcellos AG, da Luz Neto ER et al. Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg. 2006;16(12):1645–1655. doi: 10.1381/096089206779319383. [DOI] [PubMed] [Google Scholar]
- 8.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Kabrhel C, Varraso R, Goldhaber SZ et al. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity. 2009;17(11):2040–2046. doi: 10.1038/oby.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raschke RA, Reilly BM, Guidry JR et al. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119:874–881. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Bernardi E, Piccioli A, Oliboni G et al. Nomograms for the administration of unfractionated heparin in the initial treatment of acute thromboembolism–an overview. J Thromb Haemost. 2000;84(1):22–26. [PubMed] [Google Scholar]
- 12.Shepherd MF, Rosborough TK, Schwartz L. Heparin thromboprophylaxis is gastric bypass surgery. Obes Surg. 2003;13:249–253. doi: 10.1381/096089203764467153. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Milligan PE, Wong CA. Efficacy and safety of high-dose thromboprophylaxis in morbidly obese inpatients. J Thomb Haemost. 2014;111:88–93. doi: 10.1160/TH13-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sameul S, Iluonakhamhe EK, Adair E High dose subcutaneous unfractionated heparin for prevention of venous thromboembolism in overweight neurocritical care patients. J Thromb Thrombolysis. [published online ahead of print March 4, 2015]. [DOI] [PubMed]
- 15.Chesebro JH, Knatterud G, Roberts R et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Circulation. 1987;1:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]


