Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP) infection is increasing in incidence and is associated with increased mortality in liver transplantation (LT) recipients. We performed a retrospective cohort study of all patients transplanted between January 2010 and January 2013 to identify the incidence and risk factors for post-LT CRKP infection and evaluate the impact of this infection on outcomes in a CRKP-endemic area. We studied 304 recipients, of whom 20 (6.6%) developed CRKP and 36 (11.8%) carbapenem-susceptible Klebsiella pneumoniae (CSKP) infections in the year following LT. Among the 20 recipients with post-LT CRKP infection, 8 (40%) were infected in ≥ 2 sites; 13 (65%) had surgical site–intra-abdominal infections; 12 (60%) had pneumonia; and 3 (15%) had a urinary tract infection. There were 6 patients with a CRKP infection before LT, 5 of whom developed a CRKP infection after LT. Significant risk factors for post-LT CRKP infection in multivariate analysis included laboratory Model for End-Stage Liver Disease at LT (odds ratio [OR], 1.07; P = 0.001), hepatocellular carcinoma (OR, 3.19; P = 0.02), Roux-en-Y biliary choledochojejunostomy (OR, 3.15; P = 0.04), and bile leak (OR, 5.89; P = 0.001). One-year estimated patient survival was 55% (95% confidence interval, 31%–73%), 72% (55%–84%), and 93% (89%–96%), for patients with CRKP, CSKP, and no Klebsiella pneumoniae infection, respectively. In multivariate analysis, CRKP (hazard ratio [HR], 6.92; P < 0.001) and CSKP infections (CSKP, HR, 3.84; P < 0.001), as well as bile leak (HR, 2.10; P = 0.03) were the strongest predictors of post-LT mortality. In an endemic area, post-LT CRKP infection is common, occurring in 6.6% of recipients, and is strongly associated with post-LT mortality. Improved strategies for screening and prevention of CRKP infection are urgently needed.
Bacterial infections are a major cause of morbidity and mortality after liver transplantation (LT),1–3 and in the last decade, there has been an important epidemiological shift toward more gram-negative infections postoperatively.4,5 These organisms generally harbor resistance to multiple classes of antibiotics and are frequently associated with poor outcomes, particularly after solid organ transplant.6–8 In this context, carbapenem-resistant Klebsiella pneumoniae (CRKP) infections have emerged as a major problem among LT recipients.
Although initially limited to the New York City area in the early 2000s,9,10 CRKP infections have since become a global problem.11 Its impact has been particularly strong among hospitalized and vulnerable populations, including solid organ transplant recipients. Several reports have documented extremely poor outcomes in patients with post-LT CRKP infections, with overall mortality ranging from 40% to 70%.12–16
As a result of the significant impact on patient outcomes, several investigators have identified risk factors for post-LT CRKP infection, though the causes are likely multifactorial and remain incompletely understood. Colonization may be a strong predictor of CRKP infection, as evidenced by a recent report describing a CRKP outbreak.17 In this report, among 9 LT recipients with CRKP colonization in the pre- and post-LT settings, 8 (89%) progressed to post-LT infection, with 78% in-hospital mortality. Other investigators have found that diabetes mellitus (DM), chronic kidney disease, and a Model for End-Stage Liver Disease (MELD) score greater than 20 at the time of LT were associated with post-LT CRKP infection. However, all of these data are the result of small, single-center studies with a small number of CRKP cases. In addition, important comparison groups including those with carbapenem-susceptible Klebsiella pneumoniae (CSKP) infections, and those without Klebsiella pneumoniae (KP) infection were not included in many of these studies, nor were patients with common sites of infection including pneumonia (PNA) and urinary tract infection (UTI).
In light of the poor outcomes associated with CRKP infection and the scarcity of donor organs, new strategies are needed to prevent and successfully treat CRKP infections. Thus, we aimed to define the incidence of post-LT CRKP infection, to identify clinical risk factors for this infection, and to assess the impact of CRKP infection on post-LT mortality.
PATIENTS AND METHODS
Patient Population
This is a retrospective cohort study of all adults (age ≥ 18 years) who underwent LT between January 1, 2010 and January 31, 2013 at Columbia University Medical Center. LT candidates were not routinely screened for CRKP colonization in this period. For patients who underwent more than 1 LT (n = 24), the most recent transplant was included as the LT of interest, as we hypothesized that retransplantation may be an important risk factor for CRKP infection.
Prophylaxis and Immunosuppression
Standard perioperative antibiotic prophylaxis consisted of ampicillin-sulbactam, or in the case of penicillin allergy, vancomycin and aztreonam, for 24 hours. In addition, all patients received standard post-LT prophylaxis with trimethoprim-sulfamethoxazole for 1 year and nystatin swish and swallow for 1 month. Valganciclovir was given to patients at intermediate and high risk as indicated by matched donor and recipient cytomegalovirus serologies, and prophylactic fluconazole was used in patients with significant risk factors for candida infection including significant intra-abdominal (IAB) bleeding and hemodialysis.
The immunosuppressive regimen consisted of corticosteroid bolus and taper, as well as calcineurin inhibitor (cyclosporine or tacrolimus) and mycophenolate mofetil. Occasionally, basilixmab induction was used for early post-LT calcineurin sparing in the case of significant renal or neurological impairment. Standard operative procedures did not change during this study period. Although the majority of patients underwent duct-to-duct biliary anastomoses, standard criteria were used to select a Roux-en-Y biliary reconstruction at the discretion of the surgeon (including a diagnosis of primary sclerosing cholangitis [PSC; n = 19], retransplantation [n = 9], or both [n = 5]). The study was approved by the institutional review board of Columbia University Medical Center (CUMC).
Microbial Testing
All cultures before LT and until 1-year post-LT year were assessed. The clinical microbiology laboratory at CUMC primarily uses the Vitek 2 microbial identification system (bioMérieux Inc., Durham, NC) for bacterial identification and antimicrobial susceptibility testing of KP isolates. Isolates resistant to ertapenem by Vitek 2 were also considered resistant to meropenem and imipenem. Etest (bioMérieux) was also performed upon clinician request. In this study, carbapenem resistance was defined as follows:
Ertapenem resistance (minimum inhibitory concentration ≥ 2 µg/mL by Vitek 2), or
Meropenem or imipenem (minimum inhibitory concentration ≥ 4 µg/mL by Vitek 2 or Etest).
Isolates that did not meet these criteria were deemed CSKP. If a patient had both CRKP and CSKP infections after LT, they were included in the CRKP group (n = 4).
Infection Assessment
Infections were defined as described in the Centers for Disease Control and Prevention’s National Healthcare Safety Network guidelines.18 UTI was defined as follows:
Documentation of at least 1 of the following—fever (temperature > 38°C), urgency, frequency, dysuria, or suprapubic tenderness with no other recognized cause, and
A urine culture with > 105 KP organisms per mL.
Because of the extensive overlap between surgical site infections (SSIs) and IAB infections in the post-LT setting, these 2 categories were combined (SSI-IAB) and defined as follows:
A positive KP culture of purulent IAB material obtained during a surgical procedure subsequent to LT, or
A positive KP culture within 30 days of LT obtained from a drain placed through a stab wound into the organ/space during the LT procedure, or
A positive KP culture obtained via aseptic needle aspiration of an IAB fluid collection documented on imaging.
PNA was defined as follows:
Two or more serial chest radiographs (including computed tomography) with a new infiltrate or consolidation, and
Documentation of at least 1 of the following: fever (temperature > 38°C), purulent sputum, or increased secretions, and
A sputum culture only positive for KP.
A bloodstream infection (BSI) was defined as the presence of 1 or more positive blood cultures with KP not related to an infection at another site. When a positive KP blood culture occurred in the setting of another documented infection, the modifier “with bacteremia” was applied to that infection.
Definitions of cure were also dependent on the site of infection. For UTI, a cure was defined as follows:
A subsequent negative urine culture after antimicrobial therapy was discontinued, and
No renewal of active antimicrobial therapy for 14 days after they were discontinued.
For SSI-IAB, a cure was defined as follows:
Resolution of abdominal fluid collection by follow-up imaging, or
Negative abdominal cultures after active antimicrobial therapy was discontinued and no renewal of active antimicrobial therapy for 14 days after they were discontinued.
For PNA, a cure was defined as follows:
Radiographic improvement, and
Improvement in respiratory symptoms present at diagnosis, and
No renewal of active antimicrobial therapy for 14 days after they were discontinued.
Mortality due to KP was defined as death in the setting of persistent infection. Colonization with KP, as opposed to infection, was defined as a positive culture in nonsterile sites (sputum, urine, or rectal swab) in the absence of clinical or laboratory evidence of infection. Patients with colonization only were not classified as having infection. Assessment of infections and cures were separately adjudicated by 2 authors (M.R.P. and S.M.P.).
Statistical Analysis
Continuous variables were expressed as medians and interquartile ranges (IQRs), and they were compared using the nonparametric Mann-Whitney U test. Categorical variables were compared using the chisquare or Fisher’s exact test. Logistic regression models were then used to identify risk factors for post-LT CRKP infection using a backward stepwise approach. All covariates with P < 0.2 were included in the initial model, and nonsignificant predictors were sequentially eliminated. Bile leak and reoperation were not simultaneously included in the same multivariate model because of the correlation of these variables. A planned subanalysis was then performed evaluating risk factors for CRKP infection compared to CSKP infection in the post-LT period.
Finally, Kaplan-Meier survival analysis was performed to compare survival in LT recipients with CRKP infection, CSKP infection, or no KP infection. Cox proportional hazards modeling was then used to identify independent risk factors for post-LT mortality. Post-LT CRKP and CSKP infections were considered as time-varying covariates in the Cox models and the proportional hazards assumption was met for the final Cox model (P = 0.17). All statistical tests were 2-tailed with a threshold for statistical significance for P < 0.05. All statistics were performed using SAS 9.3 (SAS Institute, Cary, NC) and Stata 10.0 (StataCorp LP, College Station, TX).
RESULTS
Patient Characteristics
Over the 3-year study period, 305 adult patients underwent transplantation. One patient was excluded from the final analysis because of a KP infection experienced immediately before death but without the antimicrobial susceptibilities available to correctly classify the infection as CRKP or CSKP. Thus, 304 patients were included in the final analysis.
There were 63 patients with positive KP cultures after LT. Seven of these patients did not meet criteria for any infection and were therefore deemed to be colonized with KP (6 CSKP and 1 CRKP). Therefore, 20 (6.6%) and 36 (11.8%) patients experienced post- LT CRKP and CSKP infection, respectively. Eight patients in the CSKP group had KP isolates resistant to third and/or fourth generation cephalosporins.
The median age at LT was 58 years (IQR, 51–62 years); 67% were male; 38% had hepatitis C virus (HCV); and 14% received living donor allografts (Table 1). There were no significant differences in age, sex, indication for LT, graft type, body mass index, or history of DM among those with and without post-LT CRKP infections (Table 1). However, patients with post-LT CRKP infections were statistically more likely to be Caucasian, have undergone retransplantation or multiple organ transplantation, and had a higher laboratory MELD score at LT. The median (IQR) transplant hospitalization length of stay (40 [range, 23–82] versus 12 [range, 9–21] days; P < 0.001) and median surgical intensive care unit length of stay (9 [range, 3–20] versus 3 [range, 2–5] days; P = 0.001) were significantly longer in the patients who developed CRKP infection compared with those without CRKP, respectively.
TABLE 1.
Demographic and Transplant Characteristics of the Cohort
| Characteristic | All Patients (n = 304) |
CRKP Infection (n = 20) |
No CRKP Infection (n = 284) | P Value* | |
|---|---|---|---|---|---|
| CSKP Infection (n = 36) |
No KP Infection (n = 248) |
||||
| Age at transplant, years, median (IQR) |
58 (51–62) | 58 (50–63) | 58 (52–62) | 58 (51–63) | 0.85 |
| Sex, male, n (%) | 204 (67) | 16 (80) | 17 (47) | 171 (69) | 0.20 |
| Race/Ethnicity, n (%) | 0.03 | ||||
| Caucasian | 182 (60) | 14 (70) | 20 (56) | 148 (60) | |
| African American | 55 (18) | 1 (5) | 7 (19) | 47 (19) | |
| Hispanic | 53 (17) | 2 (10) | 6 (17) | 45 (18) | |
| Asian | 8 (3) | 3 (15) | 2 (6) | 3 (1) | |
| Other | 6 (2) | 0 (0) | 1 (3) | 5 (2) | |
| Etiology of liver disease, n (%) | 0.89 | ||||
| HCV | 114 (38) | 8 (40) | 14 (39) | 92 (37) | |
| Alcohol | 31 (10) | 4 (20) | 3 (8) | 24 (10) | |
| HCV and alcohol | 4 (1) | 1 (5) | 1 (3) | 2 (1) | |
| NAFLD/cryptogenic | 37 (12) | 2 (10) | 3 (8) | 32 (13) | |
| HBV | 22 (7) | 1 (5) | 2 (6) | 19 (8) | |
| Autoimmune hepatitis | 11 (4) | 1 (5) | 2 (6) | 8 (3) | |
| PSC | 26 (9) | 1 (5) | 1 (3) | 24 (10) | |
| PBC | 19 (6) | 1 (5) | 1 (3) | 17 (7) | |
| Acute liver failure | 8 (3) | 1 (5) | 1 (3) | 6 (2) | |
| Other | 32 (11) | 0 (0) | 8 (22) | 24 (10) | |
| HCC, n (%) | 103 (34) | 10 (50) | 11 (31) | 82 (33) | 0.12 |
| DM, n (%) | 103 (34) | 8 (40) | 13 (36) | 82 (33) | 0.55 |
| BMI at LT, kg/m2, median (IQR) | 27 (24–31) | 29 (25–33) | 27 (23–32) | 27 (24–31) | 0.35 |
| Laboratory MELD, median (IQR) | 17 (13–27) | 24 (18–32) | 22 (15–38) | 17 (12–25) | 0.02 |
| MELD with exceptions, median (IQR) |
25 (17–33) | 27 (19–35) | 31 (20–40) | 25 (17–31) | 0.39 |
| Living donor, n (%) | 42 (14) | 5 (25) | 6 (17) | 31 (12) | 0.13 |
| Retransplantation, n (%) | 24 (8) | 5 (25) | 8 (22) | 11 (4) | 0.003 |
| Multiple organ transplant, n (%) | 5 (2) | 2 (10) | 2 (6) | 1 (0.4) | 0.04 |
| Time on wait list, days, median (IQR) |
137 (39–340) | 93 (30–369) | 67 (12–200) | 162 (11–358) | 0.36 |
| Cold ischemia time, hours, median (IQR) |
6.58 (4.45–8.57) | 6.71 (2.95–7.91) | 5.98 (4.07–7.93 | 6.72 (4.58–8.77) | 0.49 |
P value for the comparison of CRKP versus no CRKP infection. Note: Some percentages may add up to 101 due to rounding.
Characteristics of the 20 patients with CRKP infection, the treatment they received, and their clinical outcomes are detailed in Table 2. Eight (40%) of these patients were infected in ≥ 2 sites; 13 (65%) had SSI-IAB; 12 (60%) had PNA; and 3 (15%) had a UTI. Antimicrobial treatment of CRKP infections was not uniform but generally consisted of multidrug regimens (75%). Polymyxin and tigecycline were the most common antimicrobials used 13 and 15 cases, respectively. The duration of antimicrobials was also variable, but often prolonged (14–71 days). Thirteen (65%) patients were successfully treated, whereas 7 (35%) patients died with ongoing CRKP infection.
TABLE 2.
Clinical Characteristics and Outcomes of CRKP Infections Following LT
| Patient Number |
Sex | Age at Transplant, Years |
Time From Transplant to CRKP Acquisition, Days |
Sites of CRKP Infection |
Antimicrobials Received (Duration in Days) |
Cure | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Female | 57 | 37 | SSI-IAB | Tigecycline (14) | Yes | Survived |
| 2 | Male | 64 | 29 | PNA with bacteremia |
Polymyxin (55) | No | Died from CRKP |
| Cefepime (17) | |||||||
| Amikacin (52) | |||||||
| Tigecycline (28) | |||||||
| Meropenem (23) | |||||||
| 3 | Male | 48 | 5 | SSI-IAB, PNA with bacteremia |
Polymyxin (44) | Yes | Survived |
| Tigecycline (34) | |||||||
| Meropenem (31) | |||||||
| Amikacin (44) | |||||||
| 4 | Female | 52 | 3 | SSI-IAB with bacteremia |
Polymyxin (37) | No | Died from CRKP |
| Cefepime (37) | |||||||
| Rifampin (18) | |||||||
| Tigecycline (29) | |||||||
| 5 | Male | 55 | 59 | SSI-IAB | Polymyxin (21) | Yes | Survived |
| Meropenem (21) | |||||||
| 6 | Female | 65 | 5 | SSI-IAB, PNA with bacteremia |
Polymyxin (13) | No | Died from CRKP |
| Meropenem (18) | |||||||
| Tigecycline (14) | |||||||
| 7 | Male | 62 | 29 | PNA, SSI-IAB | Polymyxin (71) | Yes | Survived |
| Tigecycline (71) | |||||||
| Cefepime (34) | |||||||
| Meropenem (20) | |||||||
| Rifampin (10) | |||||||
| 8 | Male | 53 | 27 | SSI-IAB | Tigecycline (38) | Yes | Survived |
| 9 | Male | 62 | 20 | UTI | Cefepime (14) | Yes | Survived |
| 10 | Male | 65 | 8 | SSI-IAB with bacteremia |
No treatment | No | Died from CRKP |
| 11 | Male | 38 | 5 | SSI-IAB, PNA with bacteremia |
Polymyxin (31) | Yes | Survived |
| Cefepime (29) | |||||||
| Tigecycline (31) | |||||||
| 12 | Male | 60 | 165 | PNA | Polymyxin (14) | Yes | Died from other reasons |
| Gentamicin (14) | |||||||
| 13 | Female | 41 | 9 | PNA with bacteremia |
Levaquin (15) | Yes | Survived |
| Tigecycline (14) | |||||||
| 14 | Male | 59 | 2 | SSI-IAB, PNA with bacteremia |
Polymyxin (24) | Yes | Survived |
| Tigecycline (22) | |||||||
| Gentamicin (37) | |||||||
| SMX/TMP (14) | |||||||
| 15 | Male | 49 | 12 | SSI-IAB, PNA with bacteremia |
Polymyxin (19) | No | Died from CRKP |
| Tigecycline (19) | |||||||
| Gentamicin (16) | |||||||
| 16 | Male | 62 | 43 | PNA | Polymyxin (2) | No | Died from CRKP |
| Meropenem (12) | |||||||
| 17 | Male | 46 | 51 | SSI-IAB, PNA with bacteremia |
Polymyxin (45) | Yes | Survived |
| Meropenem (21) | |||||||
| Tigecycline (45) | |||||||
| 18 | Male | 65 | 9 | UTI | Tigecycline (9) | Yes | Died from other reasons |
| 19 | Male | 69 | 4 | SSI-IAB, PNA with bacteremia |
Polymyxin (21) | No | Died from CRKP |
| Meropenem (11) | |||||||
| Tigecycline (21) | |||||||
| Rifampin (8) | |||||||
| 20 | Male | 51 | 8 | UTI | Tigecycline (1) | Yes | Survived |
| Fosfomycin (14) |
There were 6 patients with a CRKP infection before LT (3 UTIs, 2 BSIs, and 1 IAB infection), 5 (83%) of whom developed a CRKP infection after LT. Interestingly, 4 of those 5 post-LT infections were at the same site of pre-LT infection (1 pre-LT BSI had PNA after LT). The median time from infection to LT was 6.5 days (range, 1–231 days).
Predictors of CRKP Infection
Logistic regression was performed to identify predictors of post-LT CRKP infection (Table 3). Significant predictors in univariate analysis included the following: events before LT (the number of hospital admissions [odds ratio (OR), 1.16; 95% confidence interval (CI), 1.01–1.34; P = 0.03], KP infections [OR, 4.64; 95% CI, 1.64–13.19; P < 0.001], and CRKP infections [OR, 47.00; 95% CI, 8.42–262.50; P < 0.001]); transplant characteristics (retransplantation [OR, 4.65; 95% CI, 1.53–14.16; P = 0.01], simultaneous multiple organ transplant [OR, 10.41; 95% CI, 1.63–66.30; P = 0.01], and Roux-en-Y biliary choledochojejunostomy [OR, 2.76; 95% CI, 1.00–7.63; P = 0.05]); and postoperative complications (bile leak [OR, 5.05; 95% CI, 1.86–13.73; P < 0.001] and reoperation during the transplant hospitalization (OR, 8.47; 95% CI, 2.98–24.12; P < 0.001).
TABLE 3.
Risk Factors for CRKP Versus No CRKP Infection Following LT
| Characteristic | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age at transplant | 1.01 | 0.97–1.05 | 0.67 | |||
| Sex, male | 2.04 | 0.67–6.28 | 0.21 | |||
| Race/Ethnicity | ||||||
| White | 1.00 | Reference | ||||
| African American | 0.22 | 0.03–1.73 | 0.15 | |||
| Hispanic | 0.47 | 0.10–2.14 | 0.33 | |||
| Other | 3.27 | 0.82–13.12 | 0.09 | |||
| Indication for transplant | ||||||
| HCV | 1.00 | Reference | ||||
| Alcoholic liver disease | 1.80 | 0.51–6.27 | 0.36 | |||
| NAFLD/cryptogenic cirrhosis | 0.69 | 0.14–3.36 | 0.65 | |||
| Other | 0.54 | 0.17–1.65 | 0.28 | |||
| HCC | 2.05 | 0.83–5.11 | 0.12 | 3.19 | 1.18–8.59 | 0.02 |
| DM | 1.33 | 0.52–3.36 | 0.55 | |||
| Admissions within year before LT | 1.16 | 1.01–1.34 | 0.03 | |||
| ICU admissions within year before LT | 1.70 | 0.98–2.94 | 0.06 | |||
| TIPS | 1.16 | 0.25–5.32 | 0.85 | |||
| Klebsiella pneumoniae infection before LT | 4.64 | 1.64–13.19 | <0.001 | |||
| CRKP infection before LT | 47.00 | 8.42–262.50 | <0.001 | |||
| Pre-LT IR procedures (unitary increment) | 1.08 | 0.91–1.28 | 0.38 | |||
| Pre-LT ERCP (unitary increment) | 1.22 | 0.82–1.81 | 0.32 | |||
| Receipt of Klebsiella-active antimicrobial within 1 week of transplant |
2.46 | 0.98–6.14 | 0.05 | |||
| BMI | 1.02 | 0.95–1.11 | 0.56 | |||
| Laboratory MELD | 1.04 | 1.00–1.08 | 0.06 | 1.07 | 1.02–1.11 | 0.001 |
| MELD with exceptions points | 1.02 | 0.97–1.07 | 0.37 | |||
| Days on waiting list | 1.00 | 0.99–1.01 | 0.29 | |||
| Living donor | 0.45 | 0.15–1.31 | 0.14 | |||
| Retransplantation | 4.65 | 1.53–14.16 | 0.01 | |||
| Multiple organ transplant | 10.41 | 1.63–66.30 | 0.01 | |||
| Cold ischemia time (per minute) | 1.00 | 1.00–1.00 | 0.34 | |||
| Warm ischemia time (per minute) | 0.99 | 0.94–1.04 | 0.78 | |||
| Operative transfusion (per unit pRBC) | 1.00 | 1.00–1.00 | 0.92 | |||
| Intraoperative CVVH | 2.28 | 0.83–1.17 | 0.11 | |||
| Roux-en-Y biliary choledochojejunostomy | 2.76 | 1.00–7.63 | 0.05 | 3.15 | 1.05–9.40 | 0.04 |
| Bile leak | 5.05 | 1.86–13.73 | <0.001 | 5.89 | 2.02–17.18 | 0.001 |
| Reoperation during transplant admission | 8.47 | 2.98–24.12 | <0.001 | |||
| Duration of intubation (per day) | 1.08 | 0.93–1.25 | 0.34 | |||
| Cytomegalovirus viremia | 1.34 | 0.37–4.81 | 0.66 | |||
| Post-LT ERCP (unitary increment before infection) | 1.25 | 1.97–1.61 | 0.08 | |||
In the final multivariate model, laboratory MELD at LT (OR, 1.07; 95% CI, 1.02– 1.11; P = 0.001), hepatocellular carcinoma (HCC; OR, 3.19; 95% CI, 1.18–8.59; P = 0.02), Roux-en-Y biliary choledochojejunostomy (OR, 3.15; 95% CI, 1.05–9.40; P = 0.04), and bile leak (OR, 5.89; 95% CI, 2.02– 17.18; P = 0.001) remained significant predictors of post-LT CRKP infection. Pre-LT CRKP was not included in this model due to the very small number of cases and unacceptably wide CIs.
Predictors of Carbapenem Resistance Among Patients With KP Infection
We then compared patients who developed CRKP (n = 20) to those with CSKP infection (n = 36) in the post-LT period. The median (IQR) time from LT to KP infection was similar between the 2 groups (11 [range, 5–33] days in CRKP versus 30 [range, 7–57] days in CSKP; P = 0.10). Significant predictors of CRKP infections compared to CSKP infection included male sex (OR, 4.47; 95% CI, 3.96–6.23; P = 0.02), CRKP infection before LT (OR, 11.67; 95% CI, 1.25–108.56; P = 0.03), and Roux-en-Y choledochojejunostomy (OR, 4.71; 95% CI, 1.03–21.56; P = 0.046). Multivariate analysis of risk factors was not performed given the limited number of patients who experienced post-LT KP infection.
Impact of CRKP Infection on Post-LT Survival
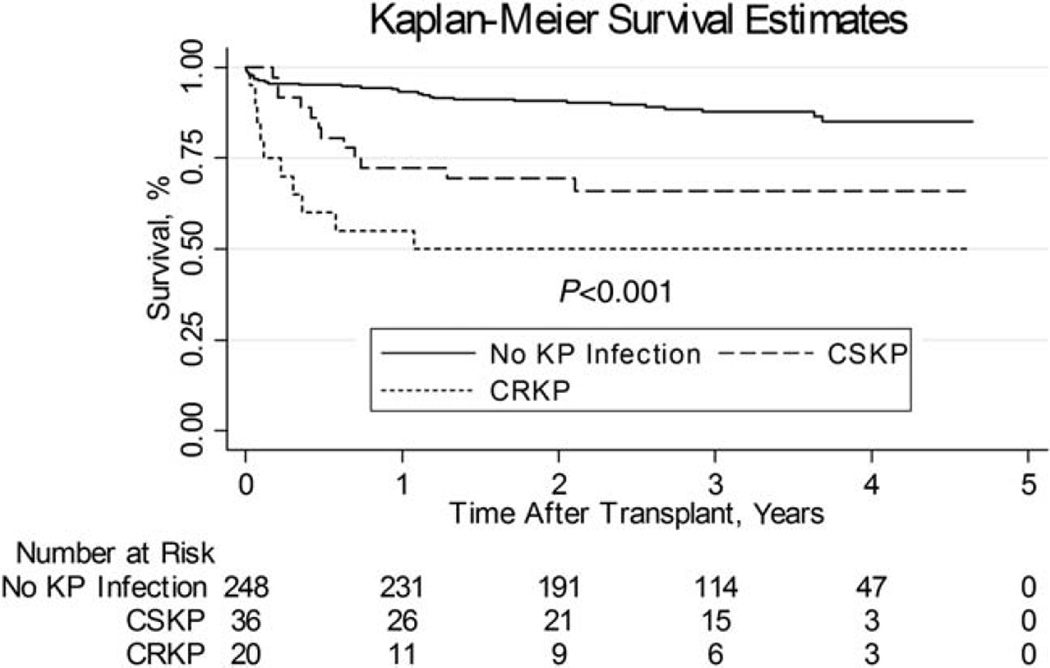
In the year following LT, 36 (12%) patients died. Overall survival by Kaplan-Meier analysis was significantly lower in patients with CRKP infection compared to those with CSKP and controls (log-rank P < 0.001; Fig. 1). The 1-year estimated survival was 55% (95% CI, 31%–73%), 72% (95% CI, 55%–84%), and 93% (95% CI, 89%–96%), for patients with CRKP, CSKP, and controls, respectively. Mortality due to KP infection was also significantly higher in patients with CRKP (35%) compared to CSKP (0%; P = 0.002).
Figure 1.
Kaplan-Meier analysis comparing recipient survival between patients with post-LT CRKP infection, CSKP infection, and those without KP infection.
Post-LT KP infection (CRKP, hazard ratio [HR], 8.36; 95% CI, 4.04–17.33; P < 0.001; CSKP, HR, 4.72; 95% CI, 2.37–9.39; P < 0.001), retransplantation (HR, 2.22; 95% CI, 1.05–4.72; P = 0.04), laboratory MELD at LT (HR, 1.02; 95% CI, 1.00–1.05; P = 0.03), bile leak (HR, 3.21; 95% CI, 1.71–6.03; P < 0.001), and reoperation during the transplant admission (HR, 2.94; 95% CI, 1.70–5.06; P < 0.001) were significantly associated with mortality in univariate analysis (Table 4). In the final multivariate proportional hazards model, KP infection (CRKP, HR, 6.92; 95% CI, 3.24–14.79; P < 0.001; CSKP, HR, 3.84; 95% CI, 1.86–7.94; P < 0.001), and bile leak (HR, 2.10; 95% CI, 1.08–4.08; P = 0.03) remained significant predictors of death.
TABLE 4.
Risk Factors for Mortality Following LT
| Characteristic | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
|
Klebsiella pneumoniae infection within 1-year post-LT |
||||||
| None | — | — | — | — | — | — |
| CSKP | 4.72 | 2.37–9.39 | <0.001 | 3.84 | 1.86–7.94 | <0.001 |
| CRKP | 8.36 | 4.04–17.33 | <0.001 | 6.92 | 3.24–14.79 | <0.001 |
| Age at LT | 1.01 | 0.98–1.03 | 0.64 | |||
| Sex, male | 1.04 | 0.58–1.85 | 0.90 | |||
| HCV | 1.67 | 0.97–2.86 | 0.06 | |||
| HCC | 1.28 | 0.74–2.21 | 0.39 | |||
| Retransplantation | 2.22 | 1.05–4.72 | 0.04 | |||
| DM | 1.28 | 0.74–2.23 | 0.37 | |||
| Laboratory MELD | 1.02 | 1.00–1.05 | 0.03 | |||
| Living donor | 1.58 | 0.63–3.96 | 0.33 | |||
| Multiple organ transplant | 1.22 | 0.17–8.84 | 0.20 | |||
| Cold ischemia time | 1.00 | 1.00–1.00 | 0.31 | |||
| Bile leak | 3.21 | 1.71–6.03 | <0.001 | 2.10 | 1.08–4.08 | 0.03 |
| Reoperation during transplant admission | 2.94 | 1.70–5.06 | <0.001 | |||
DISCUSSION
Here we describe a large retrospective cohort of LT recipients and their incidence of CRKP and KP infections over a 3-year period. Our data confirm the profound impact of CRKP infection on post-LT mortality. CRKP infection led to an almost 7-fold increase in post-LT mortality and was the strongest predictor of patient outcomes in multivariate models. In addition, we identified important clinical predictors of post-LT CRKP infection including pre-LT infection, as well as advanced hepatic impairment as measured by MELD at LT, the type of biliary anastomosis used, and the occurrence of bile leaks.
Solid organ transplant recipients are at high risk of bacterial infections due to surgical interventions, posttransplant immunosuppression, and frequent antibiotic exposures.15,19 The emergence of multidrug resistant gram-negative bacterial infections, including KP, in LT recipients has been demonstrated in multiple studies, and it is associated with increased morbidity and mortality ranging from 42% to 71%. Kalpoe et al.14 found a staggering 71% mortality among LT recipients infected with CRKP, usually in the first 30 days after LT. Similarly, Mouloudi et al.16 identified 10 patients with post-LT CRKP infections and found an overall mortality of 60%, with an attributable mortality rate of 30%.
Here we observed a 1-year mortality of 50% in patients with CRKP infection, a finding consistent with previous reports. This current analysis, however, includes a larger number of LT recipients with CRKP than previously reported, as well as the use of comparator groups with CSKP and no KP infection to identify clinical predictors of post-LT CRKP infection. Additionally, our study included patients with UTIs and PNAs, which were not included in the previously reported studies.
This study identified several important risk factors for post-LT CRKP infection: Roux-en-Y choledochojejunostomy during transplant surgery, postoperative bile leak, HCC, and high MELD score at time of LT. Of the 44 recipients in our study who underwent a Roux-en-Y biliary anastomoses, 6 had a post-LT CRKP infection. Roux-en-Y at the time of transplantation has been previously associated with an increased risk of ascending cholangitis, peritonitis, and infected bilomas.20–22 There are many possible reasons for this association, including the need for complex biliary and enteric manipulation at the time of transplant, the development of biliary strictures after transplant, or the loss of a hepatopancreatic sphincter, leading to enteric colonization of the biliary tree. Additionally, this association could be due to the underlying conditions leading to a Roux-en-Y, such as PSC, rather than the procedure itself. Patients with PSC have frequent episodes of cholangitis and may require repeated courses of antibiotics as well as endoscopic procedures before LT. However, whereas PSC was the most common indication for a Roux-en-Y in this study, only 1 of these patients developed a post-LT CRKP infection, and pre-LT endoscopic retrograde cholangiopancreatography was not associated with an increased risk of infection.
Postoperative bile leaks were also associated with post-LT CRKP infections. Of the 34 recipients with post-LT bile leaks, 7 experienced CRKP infection. The majority of these cases occurred in recipients with duct-to-duct anastomoses and were significantly more common in recipients of living donor grafts, which is consistent with previous literature.23,24 Although bile leak is a known risk factor for bacterial and candidal infections,25,26 it is not clear why LT recipients with bile leaks are at increased risk of resistant infections in particular. One could postulate that the greater exposure to antibiotics, the need for invasive biliary procedures, and a prolonged hospital stay could account for some of this increased risk, but none of these factors were found to be significant in this study.
The presence of HCC was also associated with post-LT CRKP infection, which has not previously been reported. Although the reasons for this association are uncertain, perhaps for those patients who undergo transarterial chemoembolization therapy while on the wait list, the frequent exposure to periprocedure antibiotics and repeated induction of tissue necrosis could predispose to biliary infection and colonization with drug-resistant organisms. However, this finding should be confirmed in larger studies.
Finally, a history of pre-LT CRKP infection before LT was also associated with post-LT CRKP infection in univariate analysis, although this was not included in multivariate models because of the very small number of cases and wide CIs generated. Given the difficulties in successful treatment of CRKP infection after LT, there has been great interest in screening LT candidates for CRKP colonization, at least in an outbreak setting, and implementing possible preventative strategies.17 Prevention of CRKP infections should include aggressive control of hospital CRKP outbreaks as well as the judicious use of antibiotics and invasive procedures that might predispose patients to infection. In addition, the knowledge of pre-LT CRKP colonization could prompt several actions, including attempted CRKP decolonization. Although limited data are available, some studies on selective digestive decolonization with oral colistin and gentamicin have shown a significant decline in CRKP carriage rates.27 However, this approach carries the risk for a significant rise in secondary resistance to those agents in posttreatment isolates.28 Another possibility is to adjust perioperative antibiotics to include agents with activity against CRKP for those candidates who are colonized. Although there are no data on this approach in LT recipients, 1 single-center report in renal transplant recipients found that adding perioperative gentamicin was associated with a decline in postoperative CRKP infections.29 In our study, 2 of 6 patients with CRKP cultures before LT received targeted perioperative prophylaxis (polymyxin), and 1 of them still developed a post-LT CRKP infection. Finally, another approach could be to deny LT to candidates with pre-LT CRKP infection or colonization; however, with the small numbers of patients currently in the literature, this approach cannot currently be advocated.
This study has several limitations. First, the small study population only allowed for detection of large effect sizes in risk factor analyses, and potential risk factors for CRKP infection may therefore have not met statistical significance. However, the risk factors identified for CRKP infection and mortality appear to carry a higher degree of plausibility than those that were not statistically significant. In addition, our center did not universally screen LT candidates for CRKP; and thus, we are unable to make statements about the impact of pre-LT CRKP colonization as a risk factor for post-LT infection or regarding optimal prophylactic antibiotic strategies. Finally, this study was conducted at a high-volume single center in a region of CRKP endemicity, and our findings may not be applicable in areas where the prevalence of CRKP is low.
In conclusion, CRKP infection following LT is an independent risk factor for mortality at 1 year after LT. Providers should exercise caution when proceeding with LT in patients with a known history of CRKP infection. Further prospective studies examining the efficacy of CRKP surveillance for colonization in potential recipients as well as individualized perioperative antimicrobial prophylaxis are warranted. Better measures to prevent and treat these serious infections are necessary.
Acknowledgments
Grants and financial support: Nothing to report.
Abbreviations
- BMI
body mass index
- BSI
bloodstream infection
- CI
confidence interval
- CRKP
carbapenem-resistant Klebsiella pneumoniae
- CSKP
carbapenem-susceptible Klebsiella pneumoniae
- CUMC
Columbia University Medical Center
- CVVH
continuous venous-venous hemofiltration
- DM
diabetes mellitus
- ERCP
endoscopic retrograde cholangiopancreatography
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IAB
intra-abdominal
- ICU
intensive care unit
- IQR
interquartile range
- IR
interventional radiology
- KP
Klebsiella pneumoniae
- LT
liver transplantation
- NAFLD
nonalcoholic fatty liver disease
- MELD
Model for End-Stage Liver Disease
- OR
odds ratio
- PBC
primary biliary cirrhosis
- PNA
pneumonia
- pRBC
packed red blood cell
- PSC
primary sclerosing cholangitis
- TIPS
transjugular intrahepatic portosystemic shunt
- SSI
surgical site infection
- UTI
urinary tract infection.
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Blair JE, Kusne S. Bacterial, mycobacterial, and protozoal infections after liver transplantation--part I. Liver Transpl. 2005;11:1452–1459. doi: 10.1002/lt.20624. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Gayowski T, Wagener M, Yu VL. Infectious complications in liver transplant recipients on tacrolimus. Prospective analysis of 88 consecutive liver transplants. Transplantation. 1994;58:774–778. [PubMed] [Google Scholar]
- 3.Wagener MM, Yu VL. Bacteremia in transplant recipients: a prospective study of demographics, etiologic agents, risk factors, and outcomes. Am J Infect Control. 1992;20:239–247. doi: 10.1016/s0196-6553(05)80197-x. [DOI] [PubMed] [Google Scholar]
- 4.Bert F, Larroque B, Paugam-Burtz C, Janny S, Durand F, Dondero F, et al. Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transpl. 2010;16:393–401. doi: 10.1002/lt.21991. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Wagener MM, Obman A, Cacciarelli TV, de Vera ME, Gayowski T. Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver Transpl. 2004;10:844–849. doi: 10.1002/lt.20214. [DOI] [PubMed] [Google Scholar]
- 6.Shi SH, Kong HS, Xu J, Zhang WJ, Jia CK, Wang WL, et al. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis. 2009;11:405–412. doi: 10.1111/j.1399-3062.2009.00421.x. [DOI] [PubMed] [Google Scholar]
- 7.Linares L, García-Goez JF, Cervera C, Almela M, Sanclemente G, Cofán F, et al. Early bacteremia after solid organ transplantation. Transplant Proc. 2009;41:2262–2264. doi: 10.1016/j.transproceed.2009.06.079. [DOI] [PubMed] [Google Scholar]
- 8.Bodro M, Sabé N, Tubau F, Lladó L, Baliellas C, Roca J, et al. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation. 2013;96:843–849. doi: 10.1097/TP.0b013e3182a049fd. [DOI] [PubMed] [Google Scholar]
- 9.Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, et al. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis. 2004;39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 10.Bratu S, Mooty M, Nichani S, Landman D, Gullans C, Pettinato B, et al. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother. 2005;49:3018–3020. doi: 10.1128/AAC.49.7.3018-3020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58:1274–1283. doi: 10.1093/cid/ciu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergamasco MD, Barroso Barbosa M, de Oliveira Garcia D, Cipullo R, Moreira JC, Baia C, et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl Infect Dis. 2012;14:198–205. doi: 10.1111/j.1399-3062.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 13.Huprikar S. Update in infectious diseases in liver transplant recipients. Clin Liver Dis. 2007;11:337–354. doi: 10.1016/j.cld.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kalpoe JS, Sonnenberg E, Factor SH, del Rio Martin J, Schiano T, Patel G, Huprikar S. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2012;18:468–474. doi: 10.1002/lt.23374. [DOI] [PubMed] [Google Scholar]
- 15.Linares L, Cervera C, Hoyo I, Sanclemente G, Marco F, Cofán F, et al. Klebsiella pneumoniae infection in solid organ transplant recipients: epidemiology and antibiotic resistance. Transplant Proc. 2010;42:2941–2943. doi: 10.1016/j.transproceed.2010.07.080. [DOI] [PubMed] [Google Scholar]
- 16.Mouloudi E, Massa E, Piperidou M, Papadopoulos S, Iosifidis E, Roilides I, et al. Tigecycline for treatment of carbapenem-resistant Klebsiella pneumoniae infections after liver transplantation in the intensive care unit: a 3-year study. Transplant Proc. 2014;46:3219–3221. doi: 10.1016/j.transproceed.2014.09.160. [DOI] [PubMed] [Google Scholar]
- 17.Lübbert C, Becker-Rux D, Rodloff AC, Laudi S, Busch T, Bartels M, Kaisers UX. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis. Infection. 2014;42:309–316. doi: 10.1007/s15010-013-0547-3. [DOI] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Patel G, Huprikar S. Infectious complications after orthotopic liver transplantation. Semin Respir Crit Care Med. 2012;33:111–124. doi: 10.1055/s-0032-1301739. [DOI] [PubMed] [Google Scholar]
- 20.Said A, Safdar N, Lucey MR, Knechtle SJ, D’Alessandro A, Musat A, et al. Infected bilomas in liver transplant recipients, incidence, risk factors and implications for prevention. Am J Transplant. 2004;4:574–582. doi: 10.1111/j.1600-6143.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 21.Pandanaboyana S, Bell R, Bartlett AJ, McCall J, Hidalgo E. Meta-analysis of Duct-to-duct versus Roux-en-Y biliary reconstruction following liver transplantation for primary sclerosing cholangitis. Transpl Int. 2015;28:485–491. doi: 10.1111/tri.12513. [DOI] [PubMed] [Google Scholar]
- 22.Pungpapong S, Alvarez S, Hellinger WC, Kramer DJ, Willingham DL, Mendez JC, et al. Peritonitis after liver transplantation: Incidence, risk factors, microbiology profiles, and outcome. Liver Transpl. 2006;12:1244–1252. doi: 10.1002/lt.20801. [DOI] [PubMed] [Google Scholar]
- 23.Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253–265. doi: 10.1111/ajt.12034. [DOI] [PubMed] [Google Scholar]
- 24.Verna EC, De Martin E, Burra P, Neri D, Gaglio PJ, Emond JC, Brown RS, Jr, et al. The impact of hepatitis C and biliary complications on patient and graft survival following liver transplantation. Am J Transplant. 2009;9:1398–1405. doi: 10.1111/j.1600-6143.2009.02649.x. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Wen TF, Mi K, Wang C, Yan LN, Li B. Analysis of infections in the first 3-month after living donor liver transplantation. World J Gastroenterol. 2012;18:1975–1980. doi: 10.3748/wjg.v18.i16.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nafady-Hego H, Elgendy H, Moghazy WE, Fukuda K, Uemoto S. Pattern of bacterial and fungal infections in the first 3 months after pediatric living donor liver transplantation: an 11-year single-center experience. Liver Transpl. 2011;17:976–984. doi: 10.1002/lt.22278. [DOI] [PubMed] [Google Scholar]
- 27.Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33:14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 28.Lübbert C, Faucheux S, Becker-Rux D, Laudi S, Dürrbeck A, Busch T, et al. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: a single-centre experience. Int J Antimicrob Agents. 2013;42:565–570. doi: 10.1016/j.ijantimicag.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Abboud CS, Bergamasco MD, Sousa EE, Zandonadi Ede C, Cortez D. Successful use of gentamycin as an antibiotic prophylaxis regimen to reduce the rate of healthcare-associated infections after renal transplantation. Braz J Infect Dis. 2013;17:254–255. doi: 10.1016/j.bjid.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]