Highlight
Physiological and molecular mechanisms of cytosolic Ca2+ homeostasis and signalling in plant adaptive responses to hypoxia were investigated and the critical role of CAX11 exchangers in this process was revealed.
Key words: Ca2+-ATPase, calcium, Ca2+/proton exchanger, confocal microscopy, epidermis, hypoxia, potassium, sodium, stele, waterlogging.
Abstract
Waterlogging is a major abiotic stress that limits the growth of plants. The crucial role of Ca2+ as a second messenger in response to abiotic and biotic stimuli has been widely recognized in plants. However, the physiological and molecular mechanisms of Ca2+ distribution within specific cell types in different root zones under hypoxia is poorly understood. In this work, whole-plant physiological and tissue-specific Ca2+ changes were studied using several ACA (Ca2+-ATPase) and CAX (Ca2+/proton exchanger) knock-out Arabidopsis mutants subjected to waterlogging treatment. In the wild-type (WT) plants, several days of hypoxia decreased the expression of ACA8, CAX4, and CAX11 by 33% and 50% compared with the control. The hypoxic treatment also resulted in an up to 11-fold tissue-dependent increase in Ca2+ accumulation in root tissues as revealed by confocal microscopy. The increase was much higher in stelar cells in the mature zone of Arabidopsis mutants with loss of function for ACA8, ACA11, CAX4, and CAX11. In addition, a significantly increased Ca2+ concentration was found in the cytosol of stelar cells in the mature zone after hypoxic treatment. Three weeks of waterlogging resulted in dramatic loss of shoot biomass in cax11 plants (67% loss in shoot dry weight), while in the WT and other transport mutants this decline was only 14–22%. These results were also consistent with a decline in leaf chlorophyll fluorescence (F v/F m). It is suggested that CAX11 plays a key role in maintaining cytosolic Ca2+ homeostasis and/or signalling in root cells under hypoxic conditions.
Introduction
Waterlogging is a widespread abiotic stress to land plants, influencing nearly 10% of the global land area (Setter and Waters, 2003) and reducing up to 80% of crop yields (Shabala, 2011). There is a series of changes in physical, chemical, and biological properties in soil which ultimately inhibit growth of intolerant plants under waterlogging stress. The diffusivity of atmospheric oxygen (O2) into soil pores is reduced by ~10 000-fold under waterlogging, which exposes the plant roots to a hypoxic situation (Malik et al., 2001; Armstrong and Drew, 2002). The O2 deficiency in the root zone depresses plant shoot and root growth, dry matter accumulation, and yield (Malik et al., 2001; Colmer and Greenway, 2011; Shabala et al., 2014). In addition, hypoxia limits the supply of ATP to plant H+-ATPase pumps and impairs ion transport processes, cell metabolism, and nutrient acquisition (Bailey-Serres and Voesenek, 2008; Elzenga and van Veen, 2010; Shabala et al., 2014).
Cytosolic free Ca2+ ([Ca2+]cyt) has been widely recognized as the central regulatory signal for many processes in plant cells and as a key second messenger mediating plant adaptive responses to abiotic and biotic stimuli (Reddy et al., 2011; Steinhorst and Kudla, 2014; Zhu et al., 2015). These usually include multiple signal transduction pathways when plants are exposed to O2 deficiency, salinity, drought, osmotic, oxidative, heat, and cold stresses, pathogens, and bacterial and fungal signals (Sanders et al., 1999; McAinsh and Pittman, 2009; Bose et al., 2011). Hypoxic stress causes a rapid elevation of [Ca2+]cyt in the cells of Arabidopsis, maize, rice, and wheat (Subbaiah et al., 1994; Yemelyanov et al., 2011; Lindberg et al., 2012), and this elevation is fundamental for gene activation and acclimation responses at the cellular, tissue, as well as organismal levels (Subbaiah and Sachs, 2003; Shabala et al., 2014).
Potassium is not only essential for many plant metabolic processes, such as osmoregulation, enzyme function, stomatal movements, control of membrane polarization, and ionic balancing (Dreyer and Uozumi, 2011; Wang et al., 2013; Shabala and Pottosin, 2014), but also plays an important role in determining the fate of cells under stress conditions (Shabala, 2009; Demidchik et al., 2010; Anschutz et al., 2014; Demidchik et al., 2014). K+ homeostasis is essential to mediate plant adaptive responses to a broad range of abiotic and biotic stresses including drought, salinity, and oxidative stress (Chen et al., 2007; Cuin et al., 2008; Shabala et al., 2010; Bonales-Alatorre et al., 2013a; Anschutz et al., 2014; Demidchik, 2014). In rice seedlings, it was found that K+ effluxes in the coleoptile were down-regulated during prolonged anoxia (Colmer et al., 2001). The waterlogging-sensitive barley cultivar Naso Nijo showed a larger decline of K+ uptake in the mature zone after hypoxic treatment than waterlogging-tolerant TX9425 and CM72 cultivars. Also, the overall magnitude of the reduction of K+ efflux in the elongation zone was significantly higher in Naso Nijo than in TX9425 and CM72 (Pang et al., 2006; Zeng et al., 2014). Therefore, a root’s ability to retain K+ under hypoxic conditions is strongly correlated with waterlogging tolerance in barley, and highlights the root tissue specificity of plant-adaptive responses to hypoxia (Zeng et al., 2014). Salinity and waterlogging stress are abiotic stresses that often occur together in estuaries, river flood plains, agricultural land affected by secondary salinity (dryland and irrigated), or in areas irrigated with saline water (Smedema and Shiati, 2002; Qadir and Oster, 2004). When salinity occurs together with hypoxia, the transfer of Na+ and Cl− from roots to shoots increases, whereas K+ transport decreases even further compared with salinity under normoxic conditions (Barrett-Lennard and Shabala, 2013). Very few studies have elaborated Na+ and K+ distribution in specific cell types during combined salinity and hypoxia stress.
As a ubiquitous denominator of cellular signalling networks, [Ca2+]cyt signal is shaped by the balance of Ca2+ influx and efflux that is regulated by the membrane transport systems. The main Ca2+ sources entering into the cytoplasm are from the apoplast, vacuole, endoplasmic reticulum (ER), mitochondria, and chloroplast, through a large number of voltage-gated and ligand-gated Ca2+ channels at the plasma membrane, tonoplast, and endomembranes (Medvedev, 2005; Pottosin and Schnknecht, 2007; McAinsh and Pittman, 2009). Reducing [Ca2+]cyt to the resting level requires activation of Ca2+ transporters such as high-affinity Ca2+-ATPases [autoinhibited Ca2+-ATPases (ACAs) and ER-type Ca2+-ATPases)] and the low-affinity Ca2+/H+ antiporter (CAXs) which are localized in both the plasma membrane and endomembranes (Sanders et al., 2002; Hetherington and Brownlee, 2004; Manohar et al., 2011; Huda et al., 2013). The different subcellular locations of these Ca2+ circuits may contribute to the unique code of Ca2+ signals and provide a mechanism for creating a stimulus-specific Ca2+ signature (Boursiac et al., 2010).
As [Ca2+]cyt increases in response to environmental stress, the Ca2+ efflux system plays critical roles in restoring basal [Ca2+]cyt levels and terminating stress-induced [Ca2+]cyt signatures (Bose et al., 2011; Shabala, 2011). Analysis of Arabidopsis mutants, in particular aca4 and aca8, identified a variety of phenotypes including sensitivity to salt and cold stress, indicating these transporters play roles in plant stress responses (Geisler et al., 2000; Schiott and Palmgren, 2005). In yeast and tobacco BY-2 cells, a Ca2+/H+ antiporter, CAX4, is found to be localized on the tonoplast, and the CAX4 mRNA level increased after Mn2+, Na+, and Ni+ treatment (Cheng et al., 2002). The loss-of-function mutant (cax4-1) and CAX4 RNA interference (CAX4 RNAi) lines indicated altered root growth and development in response to Cd2+, Mn2+, and auxin (Mei et al., 2009). These results demonstrated that CAX4 functions in root growth under heavy metal stress conditions. The function of CAX11 was also characterized in yeast, in which the fusion of CAX11 and green fluorescent protein (GFP) was found in the plasma membrane and nuclear periphery. Expressing CAX11 in a yeast mutant showed its role of mediating high-affinity K+ uptake and Na+ transport (Zhang et al., 2011). However, the exact role of these transporters is still elusive.
An increase in Ca2+ concentration in the cytosol and nucleus is detected by many Ca2+-sensing proteins [e.g. calmodulin (CaM), calmodulin-like proteins (CMLs), Ca2+-dependent protein kinases (CDPKs), and calcineurin B-like (CBL)/Ca2+-independent protein kinases (CIPKs)] (Dodd et al., 2010; Reddy et al., 2011). These proteins are able to decode the information present within the different Ca2+ spikes or oscillations and process this information into alteration of the cell function. For example, ZmCAP1, encoding Ca2+-ATPase, from maize has been shown to bind to and be stimulated by CaM following heterologous expression in yeast (Subbaiah and Sachs, 2000). Following an elevation in [Ca2+]cyt, CaM will interact with an N-terminal autoinhibitory domain of the ACAs, leading to activation of Ca2+ transport activity (Baekgaard et al., 2005). These CaM-binding domains appear to be ubiquitous on all ACAs, but are highly divergent with little consensus sequence (Chung et al., 2000; Baekgaard et al., 2006). Different signalling pathways are regulated by the transcription machinery and expression of downstream target genes. The downstream reactions can cause an acclimation, helping the plant to survive the specific stress (Lindberg et al., 2012). Thus, it is of importance to carry out investigations and screening of the interaction of the Ca2+-sensing proteins with Ca2+-ATPase or Ca2+/H+ exchanger proteins under hypoxic stress.
Knowledge of hypoxia-induced transport processes is largely restricted to ion exchange at the root surface, which is easily accessible. However, little is known about the character of hypoxic signal transduction in specific cell types and tissues of roots (Pang et al., 2006; Zeng et al., 2014; Kotula et al., 2015). We hypothesize that radial and longitudinal O2 profiles reported in plant roots under conditions of hypoxia have a major impact on the energy availability in various cell types, thus affecting their ionic homeostasis. We have also postulated that a lack of functional Ca2+ efflux systems may be detrimental to plant acclimation to hypoxic conditions. Both these hypotheses were confirmed in the current study. The objective of our research was to investigate anoxia-induced changes of Ca2+, K+, and Na+ in meristem, epidermal, and stelar cells of Arabidopsis in physiologically different root zones in wild-type (WT), and aca and cax mutants to reveal essential roles of the CAX and ACA calcium transport systems. Overall, our results suggest that Ca2+ efflux systems and especially CAX11 are essential to mediate plant Ca2+ homeostasis under hypoxic conditions.
Materials and methods
Plant materials and treatments
Seeds of Arabidopsis thaliana WT Columbia-0 (Col-0) and loss-of-function mutants (all in the Col-0 background) aca8 (SALK_0578770), aca11 (SALK_002747), cax4 (SALK_119863), and cax11 (SALK_013040) were obtained from the Arabidopsis Biological Resource Centre (http://www.arabidopsis.org/abrc/). Seeds were surface sterilized with 1ml of commercial bleach [1% (v/v) NaClO] for 10min, and then washed five times with sterilized distilled water. Seeds were kept at 4 °C for 2 d and sown in Petri dishes containing 1% (w/v) phytogel, half-strength Murashige and Skoog (MS) medium, and 0.5% (w/v) sucrose at pH 5.7. Petri dishes containing seeds were sealed with 3M micro-pore tape and then transferred into a growth chamber with a 16h/8h day/night, 100 µmol m−2 s−1 photon flux density, and 22 °C. The Petri dishes were oriented upright, allowing the roots to grow down along the surface without penetrating into the medium. All chemicals were from Sigma-Aldrich (Castle Hill, NSW, Australia) in analytical grade unless individually specified.
Hypoxic treatment was imposed on the 10-day-old Arabidopsis seedlings submerged with 0.2% (w/v) agar solution pre-bubbled with high purity N2 (Coregas, Sydney, NSW Australia). For measurement of gene expression levels, WT Arabidopsis seedlings were treated with hypoxia for 1, 24, and 72h; for measurement of K+ and Ca2+ distribution in roots, the WT and the four mutants were treated with hypoxia for 24h. Combined hypoxia and salinity treatment was also imposed on the 10-day-old Arabidopsis seedlings in deoxygenated agar solution with addition of 50mM NaCl for 24h, with measurement of Na+ distribution in roots. The whole seedlings were submerged in the bubbled solution and kept in the growth chamber with a 16h/8h day/light, 100 µmol m−2 s−1 photon flux density, and at 22 °C. Compared with hypoxia treatment or combined hypoxia and salinity treatment, the control condition was normoxic, non-saline, and without agar solution.
In the phenotyping experiments, aca8, aca11, cax4, cax11, and WT plants were grown in 0.2 litre pots filled with peat moss, perlite, vermiculite, and coarse sand, with a ratio of 2:1:1:1 (v/v), and watered with half-strength Hoagland’s nutrient solution. Plants were grown in a growth chamber at a 12h/12h light/dark regime and at 21 °C for 3 weeks. Plants were then subjected to waterlogging treatment by immersing pots in water and maintaining the water level 0.5cm above the soil surface for another 3 weeks. The above-ground biomass of individual plants was determined as fresh weight, and dry weight was measured after drying in a Unitherm Drier (Birmingham, UK) for 2 d at 65 °C.
Chlorophyll fluorescence
Chlorophyll fluorescence was measured on the 6-week-old fully expanded leaves which had been subjected to the 3 week treatment with an OS-30p chlorophyll fluorometer (OPTI-Sciences, Hudson, USA). Plants were adapted in a dark chamber for 30min before measuring. The maximum quantum efficiency of photosystem II (PSII; F v/F m) was recorded at a saturating actinic light (660nm) intensity of 1100 µmol m−2 s−1 (Zeng et al., 2013). Measurements were taken in at least five replicates for each treatment.
Quantitative real-time PCR (RT-PCR)
Total RNA was extracted from 10-day-old Arabidopsis roots with TRIZOL reagent (Life Technologies, Mulgrave, VIC, Australia) (Liu et al., 2014; Chen et al., 2016). First-strand cDNA synthesis was performed using the sensiFAST Kit (Bioline, Alexandria, NSW, Australia) with 1 µg of total RNA. RT-PCR was performed with SensiMix SYBR No-ROX Kit (Bioline, Alexandria, NSW, Australia) using a Rotor-Gene Q6000 (QIAGEN, Hilden, Germany). The specific primers are listed in Supplementary Table S1 at JXB online. The PCR program was two steps: one cycle of 95 °C, 10min; and 40 cycles of 95 °C, 15s; 60 °C, 15s; and 72 °C, 15s. The amplification of the target genes was monitored every cycle by SYBR-green fluorescence. Three technical and biological replicates were performed for each experiment and treatment. The transcripts of target genes were normalized to the control gene RNA polymerase II subunit (RPB2).
Confocal laser scanning microscopy measurements
CoroNa Green acetoxymethyl (AM) ester (Invitrogen, Eugene, OR, USA), Calcium Green-5N, AM (Invitrogen), and Asante Potassium Green-2 (APG-2, TEFLabs, Austin, TX, USA) were employed to measure the Na+, Ca2+, and K+ concentration, respectively, in Arabidopsis root cells. The optimal CoroNa Green concentration was determined in our previous work (Bonales-Alatorre et al., 2013b; Wu et al., 2015). All five types of root cells showed optimal fluorescence intensity when exposed to 15 µM Calcium Green-5N (Supplementary Fig. S1) and 20 µM APG-2. The indicators were dissolved in DMSO (Sigma) to a stock concentration of 1mM. Then, 20 µM CoroNa Green, 20 µM Asante Potassium Green-2, and 15 µM Calcium Green-5N were added to measuring buffers (Na+, 10mM KCl, 5mM Ca2+-MES, pH 6.1; K+, 5mM NaCl, 5mM Ca2+-MES, pH 6.1; Ca2+, 10mM KCl, 5mM Na+-MES, pH 6.1), respectively. Arabidopsis seedlings were incubated in the dye-containing measuring buffers for 3h in the dark. The stained seedlings were washed in distilled water for 3min to remove residual dyes before measuring fluorescence intensity in meristem, elongation epidermal, elongation stelar, mature epidermal, and mature stelar root cells after 24h of hypoxic treatment. All dying steps in this work was done at room temperature.
A confocal laser scanning microscope fitted with a TCS SPII confocal head (SP5, Leica Microsystems, Heidelberg, Germany) was used to measure fluorescent signals from roots. We used the 488nm excitation line of an argon multiline laser and the tripledichroic TD 488/543/633nm beam splitter. CoroNa Green fluorescence emission was detected in the photomultiplier at 505–525nm. Calcium Green-5N fluorescence emission was detected in the photomultiplier at 520–550nm. APG-2 fluorescence emission was detected in the photomultiplier at 530–550nm. Images were analysed with Image J software (NIH, USA) to calculate cell fluorescence based on integrated density. The background signal was measured from an empty region with a similar size and subtracted from the whole-cell signal to obtain relative total cell fluorescence values (Bonales-Alatorre et al., 2013b). Relative total cell K+, Ca2+, and Na+ concentration data shown in Figs 3, 5, and 7 were divided by 1000.
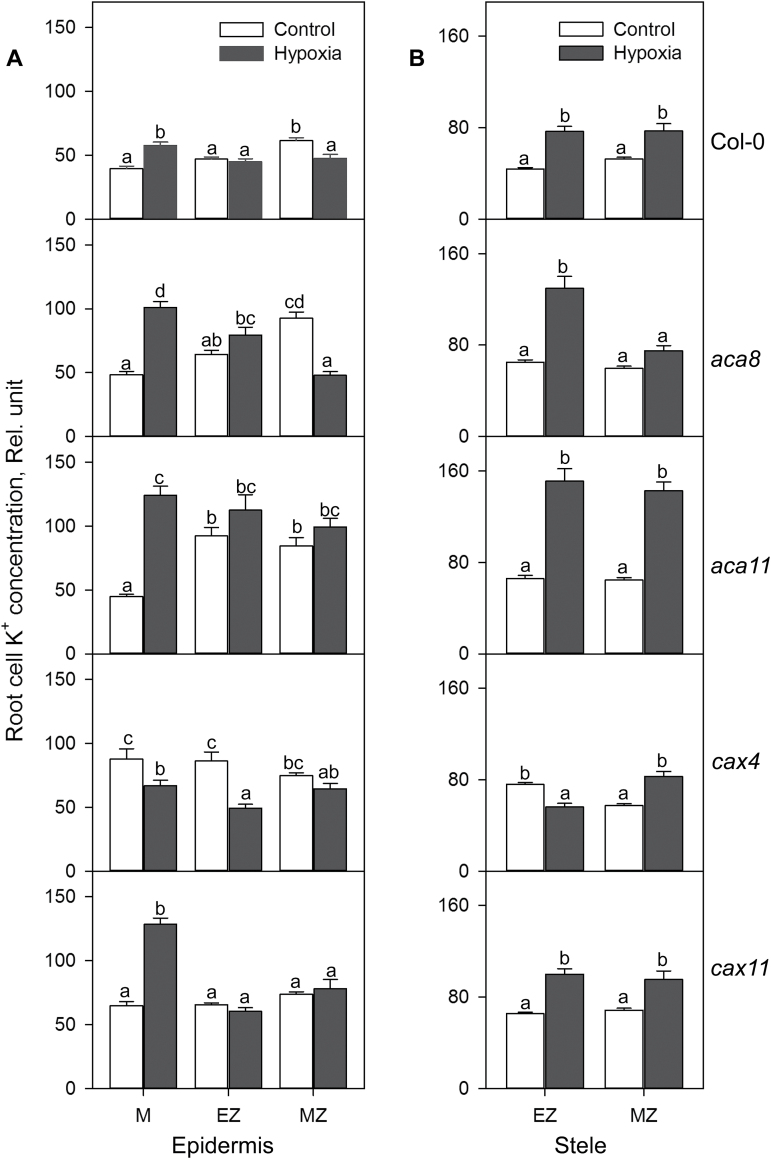
Fig. 3.
K+ concentrations in the meristem, elongation, and mature zone in root epidermis (A) and stele (B) of Col-0, aca8, aca11, cax4, and cax11 under normoxic or hypoxic treatments for 24h. The relative K+ concentration was calculated by the fluorescence integrated density using Image J software. Data are the mean ±SE (n=30–40 cells from one individual plant with at least nine replicate plants). Different lower case letters indicate significant differences at P<0.05.
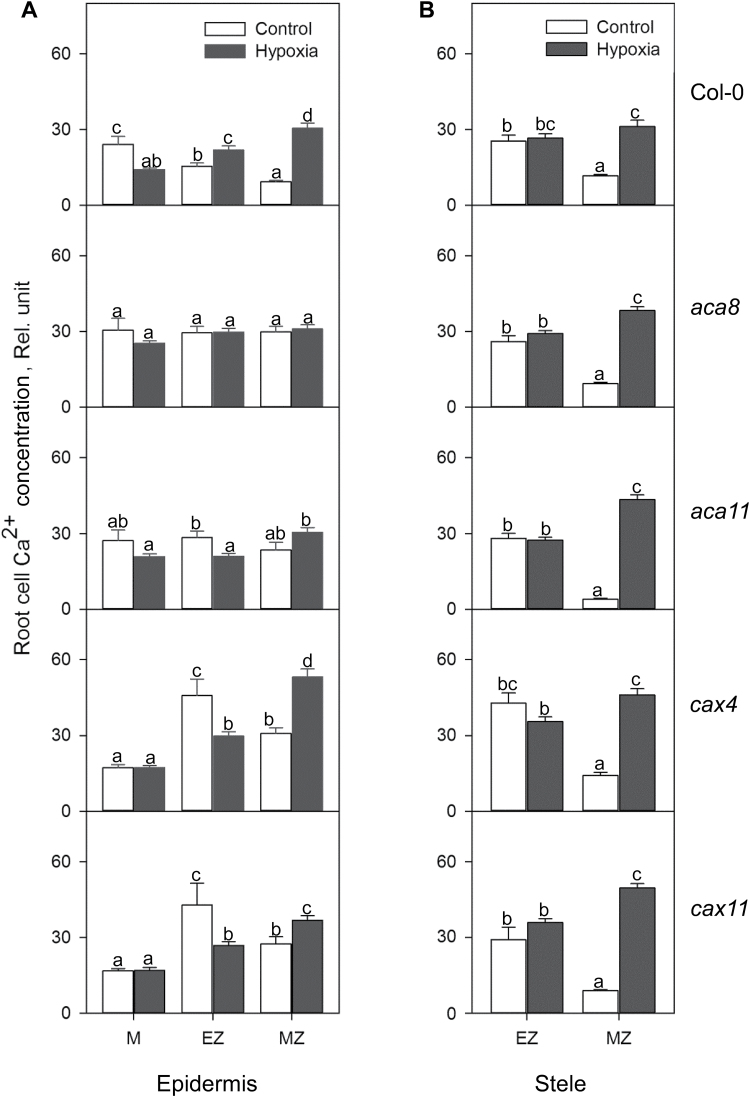
Fig. 5.
Relative Ca2+ concentration in the meristem, elongation, and mature zone in root epidermis (A) and stele (B) of Col-0, aca8, aca11, cax4, and cax11 under normoxic or hypoxic treatments for 24h. The relative Ca2+ concentration was calculated by the fluorescence integrated density using Image J software. Data are the mean ±SE (n=30–40 cells from one individual plant with at least nine replicate plants). Different lower case letters indicate significant differences at P<0.05.
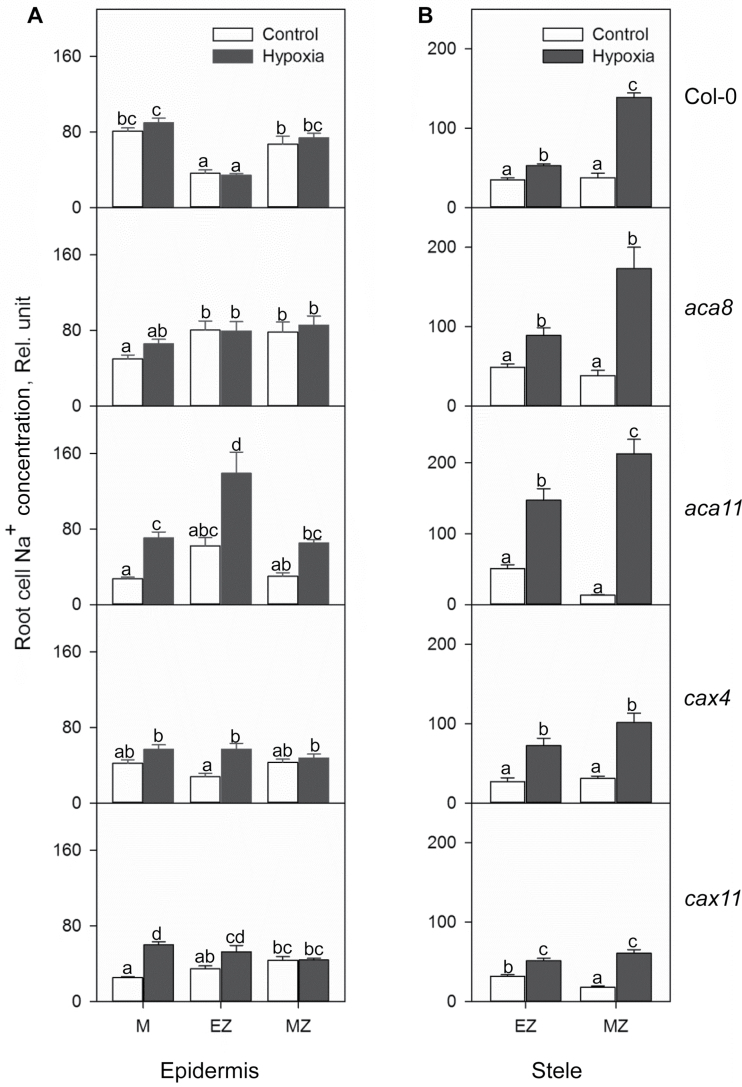
Fig. 7.
Relative Na+ concentration in the meristem, elongation, and mature zone in root epidermis (A) and stele (B) of Col-0, aca8, aca11, cax4, and cax11 under normoxic non-saline or combined salinity and hypoxia treatments for 24h. The relative Na+ concentration was calculated by the fluorescence integrated density using Image J software. Data are the mean ± SE (n=30–40 cells from one individual plant with at least nine replicate plants). Different lower case letters indicate the significant difference at P<0.05.
The cytosolic Ca2+ concentration in Arabidopsis root cells was also determined using the fluorescent dye Calcium Green. For image analysis, several lines were drawn across the region of interest as shown in Supplementary Fig. S2A in an appropriate zone with Leica Application Suite X software (Leica Microsystems). Continuous fluorescence was quantified in arbitrary units by LAS AF software based on intensity and plotted in an Excel file (Supplementary Fig. S2B) (Wu et al., 2015).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 21 (IBM, New York, USA). All data in the figures are given as means ±SE. The significant differences in relative gene expression level, relative total cell K+, Ca2+, and Na+ concentration, and phenotype were compared using Duncan’s multiple range test. Different lower case letters represent a significant difference between genotypes and treatments at P<0.05. The comparison of cytosolic Ca2+ concentration in different genotypes was done by paired samples t-test. The significance levels are *P<0.05, **P<0.01, and ***P<0.001.
Results
Hypoxia-induced changes in Ca2+ transporter transcript levels in Arabidopsis roots
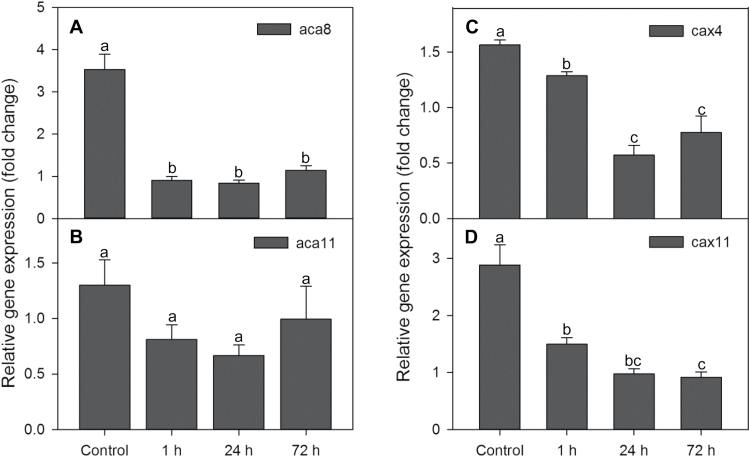
We investigated the effects of hypoxia on transcript levels of ACA8, ACA11, CAX4, and CAX11 in the roots of Arabidopsis WT after 1, 24, and 72h of hypoxia treatment (Fig. 1). With the exception of ACA11, all genes were down-regulated after 1h of hypoxia treatment (Fig. 1). Hypoxia had the highest impact on transcript levels of ACA8, CAX4, and CAX11 in Arabidopsis roots at 24h, with some transcripts reduced to only 33% of the control. Extending the hypoxia period to 72h produced no significant differences in expression levels of these genes in roots as compared with the 24h treatment.
Fig. 1.
Relative expression of ACA8, ACA11, CAX4, and CAX11 in roots of the Arabidopsis wild type (WT) under normoxic or hypoxic treatments for 1, 24, and 72h. RPB2 was used as a reference gene. Data are the mean ±SE (n=3 separate experiments each involving 3–5 biological replicates). Different lower case letters indicate significant differences between treatments at P<0.05.
Hypoxia-induced K+ distributions show tissue- and zone-specific responses in roots of Arabidopsis mutants
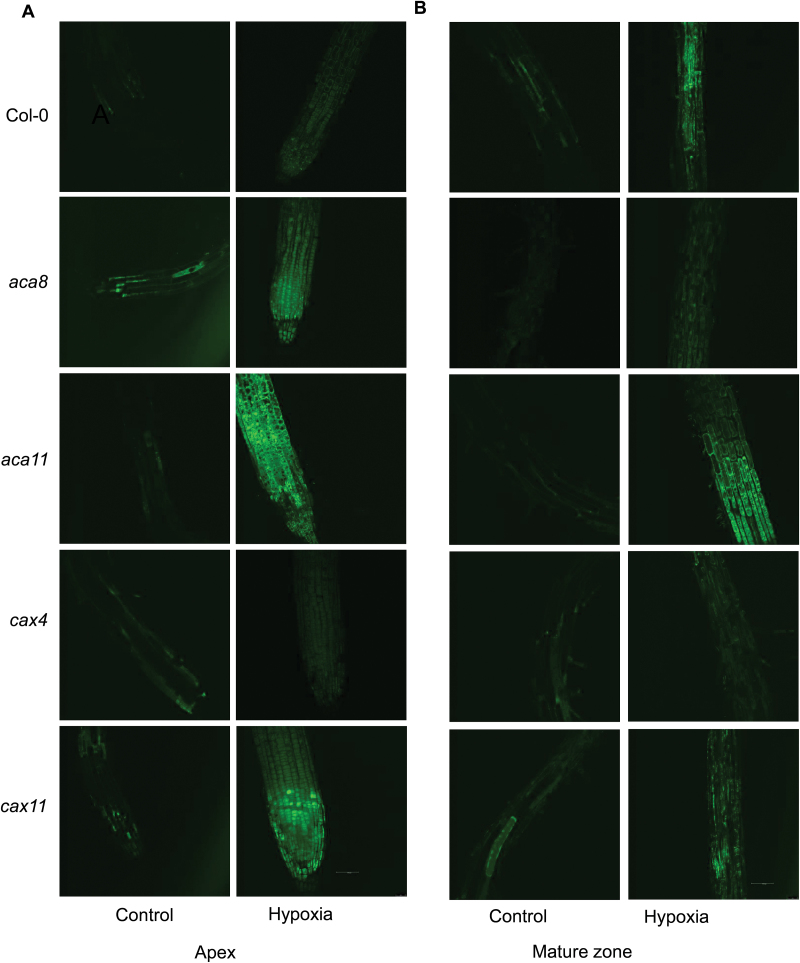
We studied changes of cellular K+ concentration in functionally different root zones (meristem, elongation, and mature) and tissues (epidermis and stele) in roots of Arabidopsis WT and four Ca2+ transporter mutants after 24h of hypoxia treatment (Figs 2, 3). In root epidermis, hypoxia led to an increase in the cellular K+ concentration in the root meristem but a decrease in the mature zone cells in WT Col-0 plants (Fig. 3A). In the root stele, the cellular K+ concentration was increased by 1.5- to 2-fold in both apex (elongation) and mature root tissues (Fig. 3B). Mutant plants showed similar trends to the WT, but with several exceptions. Hypoxia caused a dramatic increase in K+ concentration in meristem and stelar tissue in the elongation zone of roots of aca8, aca11, and cax11 (Fig. 3), but led to a significant decrease in K+ concentration in these cell types in cax4 (Fig. 3B). Interestingly, elongation zone cells in the epidermis in cax4 had a significant decrease in K+ concentration after hypoxia, while there was no significant difference among the other four genotypes. Compared with the control, there was a 20% decrease in K+ in the mature zone in root epidermis in the WT and a 50% decrease in K+ in aca8, and no significant difference among the other lines (Fig. 3A; Table 1). Also in the stelar cells of mature zones, after 24h of hypoxia treatment, the K+ concentration increased dramatically in theWT, aca11, cax4, and cax11, but was not changed in aca8 (Fig. 3B).
Fig. 2.
Distribution of K+ in root meristem, elongation, and mature zone in Arabidopsis wild type (Col-0), aca8, aca11, cax4, and cax11 under normoxic or hypoxic treatments for 24h. (A) Representative images of the root apex in control and hypoxic treatment. (B) Representative images of root mature zone in control and hypoxic treatment. The relative K+ concentration in roots of 10-day-old seedlings was visualized using a confocal imaging system with Asante Potassium Green-2 fluorescent dye. One out of nine typical images is shown for each line. Scale bar=50 µm.
Table 1.
Effects of 24h of hypoxia on quantitative characteristics of K+, Ca2+, and Na+ concentration measured from the meristem, elongation epidermal cell, elongation stelar cell, mature epidermal cell, and mature stelar cell in Arabidopsis wild type (WT), and mutants aca8, aca11, cax4, and cax11 (ratio of hypoxia ion concentration to control ion concentration)
| Meristem | Elongation epidermis | Mature epidermis | Elongation stele | Mature stele | ||
|---|---|---|---|---|---|---|
| WT | K+ | 1.5 | 1.0 | 0.8 | 1.7 | 1.5 |
| Ca2+ | 0.6 | 1.4 | 3.3 | 1.0 | 2.7 | |
| Na+ | 1.1 | 0.9 | 1.1 | 1.5 | 3.7 | |
| aca8 | K+ | 2.1 | 1.2 | 0.5 | 2.0 | 1.3 |
| Ca2+ | 0.8 | 1.0 | 1.0 | 1.1 | 4.1 | |
| Na+ | 1.3 | 1.0 | 1.1 | 1.8 | 4.5 | |
| aca11 | K+ | 2.8 | 1.2 | 1.2 | 2.3 | 2.2 |
| Ca2+ | 0.8 | 0.7 | 1.3 | 1.0 | 10.8 | |
| Na+ | 2.6 | 2.2 | 2.2 | 2.9 | 16.1 | |
| cax4 | K+ | 0.8 | 0.6 | 0.9 | 0.7 | 1.4 |
| Ca2+ | 1.0 | 0.6 | 1.7 | 0.8 | 3.3 | |
| Na+ | 1.4 | 2.0 | 1.1 | 2.7 | 3.3 | |
| cax11 | K+ | 2.0 | 0.9 | 1.1 | 1.5 | 1.4 |
| Ca2+ | 1.0 | 0.6 | 1.3 | 1.2 | 5.6 | |
| Na+ | 2.4 | 1.5 | 1.0 | 1.6 | 3.4 | |
Data are the ratio of hypoxia over control. Measurements were collected from the meristem, elongation zone epidermal cell, elongation zone stele cell, mature epidermal cell, and mature stele cell of five Arabidopsis lines.
Hypoxia induces an elevation of Ca2+ in the mature root zone
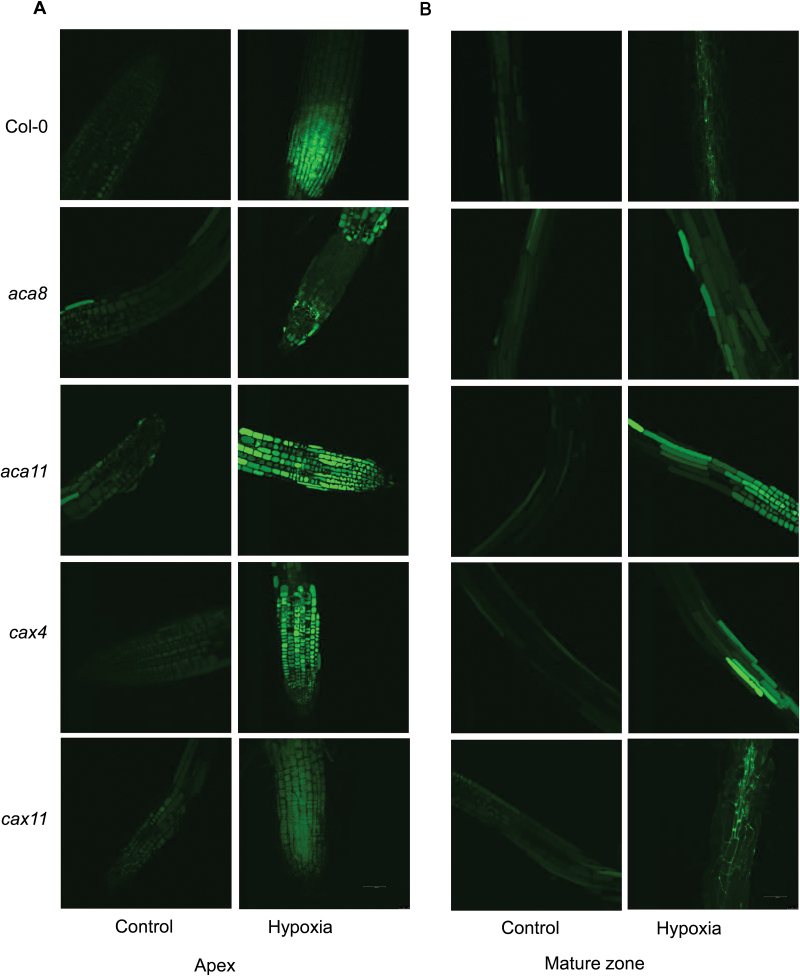
A clear difference in Ca2+ concentration was found in cells of the meristem, elongation zone, and mature zone after 24h of hypoxia treatment, showing genotypic difference after O2 deprivation (Figs 4, 5). In the root meristem (Fig. 5A), epidermal cells of WT plants showed a 40% loss of Ca2+ concentration under 24h of hypoxic conditions. However, no significant changes in Ca2+ concentration were detected in aca8, aca11, cax4, and cax11 mutants compared with normoxic conditions (Fig. 5A; Table 1). In epidermal cells of the elongation zone (Fig. 5A), the Ca2+ concentration in the WT was much higher under hypoxic conditions, while a 30–40% decrease was measured in aca11, cax4, and cax11 mutants, and no changes in aca8. Epidermal cells in the mature zone showed a dramatic increase in Ca2+ level in the WT (3.3-fold) and cax mutants (a 1.3- to 1.7-fold increase) and no changes in the aca mutants (Fig. 5A; Table 1). Comparing the stelar cells in the elongation and mature zones, hypoxia had no significant effect on Ca2+ in the elongation zone of any genotype, but resulted in marked increases in Ca2+ for stelar cells in the mature zone of aca11, with a 10.8-fold rise of Ca2+ compared with the control and a 5.6-fold increase in cax11, while the increase was only 2.7-fold in the WT (Fig. 5B).
Fig. 4.
Distribution of Ca2+ in the root meristem, elongation, and mature zone in Arabidopsis wild-type (Col-0), aca8, aca11, cax4, and cax11 under normoxic or hypoxic treatments for 24h. (A) Representative images of the root apex in control and hypoxic treatment. (B) Representative images of the root mature zone in control and hypoxic treatment. The relative Ca2+ concentration in roots of 10-day-old seedlings was visualized using a confocal imaging system with Calcium Green fluorescent dye. One out of nine typical images is shown for each line. Scale bar=50 µm.
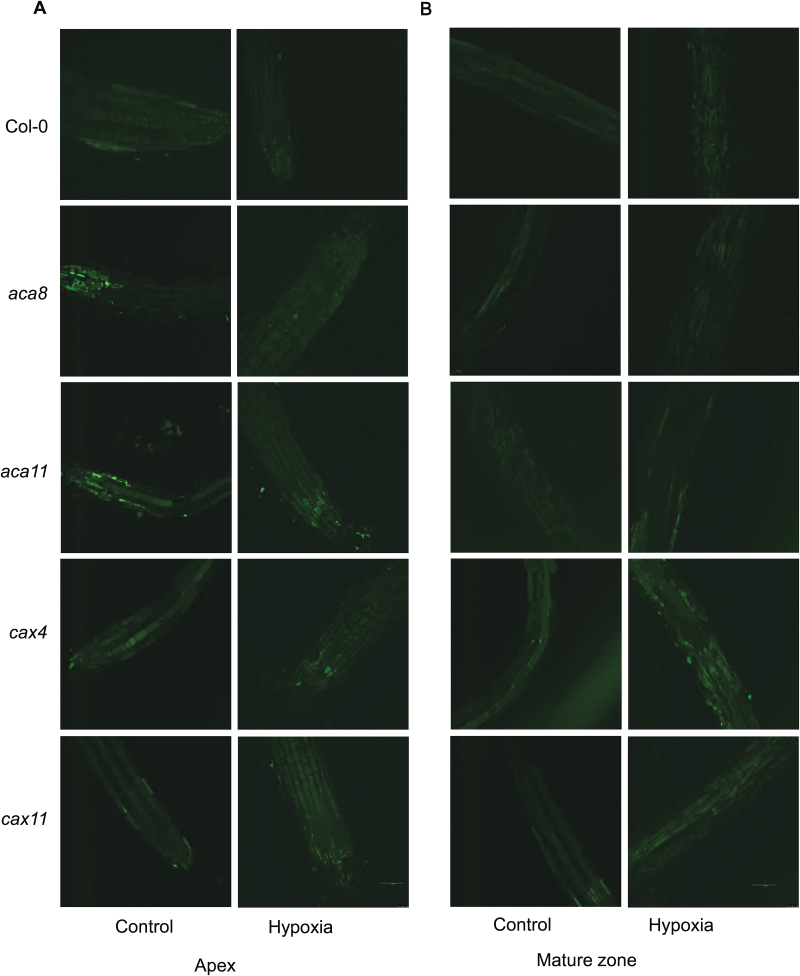
Combined hypoxia and salinity stress cause higher Na+ accumulation in stelar cells of the elongation zone and mature zone
NaCl treatment (50mM) with hypoxia caused dramatic increases in the Na+ concentration, especially in the root stele in both the elongation zone and mature zone (Fig. 7B). aca11 showed a huge 16.1-fold increase in Na+ concentration in the mature zone in the root stele, while the changes for the WT and other mutants were from 3.3- to 4.5-fold higher than in the control. Also in stele, the Na+ concentration increased moderately from 1.5- to 2.9-fold in the elongation zone of all these genotypes (Fig. 7B). Hypoxia and salinity stress caused complex changes in the root epidermis (Figs 6, 7A). In root meristem cells, no significant differences of Na+ accumulation were found among WT, aca8, and cax4, while there were 2.6- and 2.4-fold higher levels of Na+ in aca11 and cax11, respectively (Fig. 7A; Table 1). In epidermal cells of the elongation zone, there were no changes of Na+ distribution in the WT and aca8, but 2.2-, 2-, and 1.5-fold increases were found in aca11, cax4, and cax11. Interestingly, in the mature zone in the root epidermis, all the five genotypes showed no significant Na+ concentration changes compared with the control (Fig. 7A).
Fig. 6.
Distribution of Na+ in the root meristem, elongation, and mature zone in Arabidopsis wild type (Col-0), aca8, aca11, cax4, and cax11 under normoxic or hypoxic treatments for 24h. (A) Representative images of the root apex in control and hypoxic treatment. (B) Representative images of the root mature zone in control and hypoxic treatment. The relative Na+ concentration in roots of 10-day-old seedlings was visualized using a confocal imaging system with CoroNa Green fluorescent dye. One out of nine typical images is shown for each line. Scale bar=50 µm.
More Ca2+ is accumulated in the cytosol of stelar cells in the mature zone
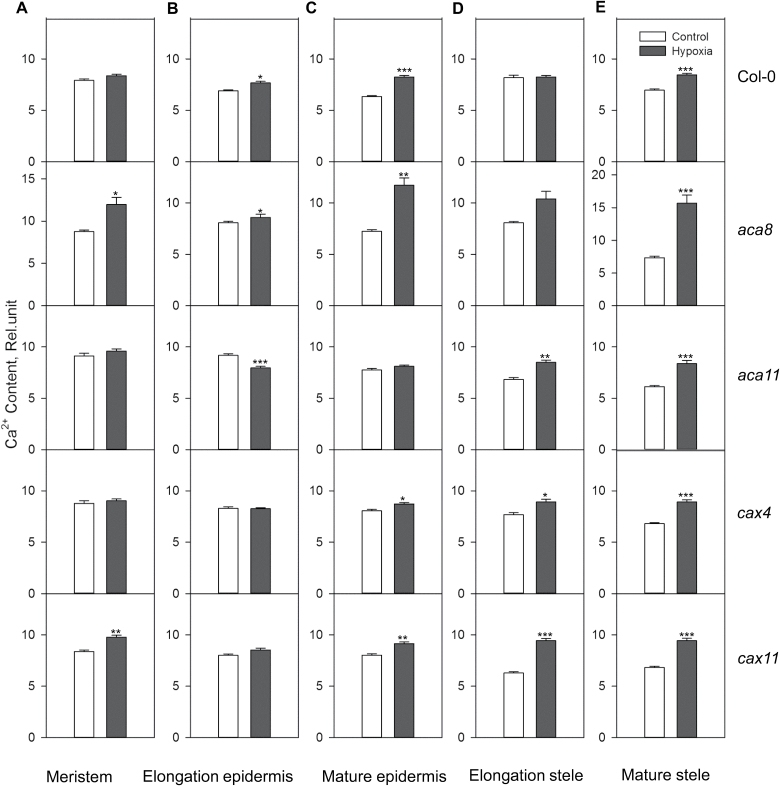
Hypoxic stress-induced changes in the [Ca2+]cyt have been rarely considered in the context of the tissue-specific expression and regulation of appropriate Ca2+ transporters. Significantly (P<0.01) higher quantities of Ca2+ were accumulated in the cytosol of meristematic cells in aca8 and cax11 after 24h of hypoxia, while no changes were observed in the WT, aca11, and cax4 (Fig. 8A). In the elongation zone in root epidermis, [Ca2+]cyt increased significantly in the WT (P<0.05) and aca8 (P<0.05) and highly significantly in aca11 (P<0.001), but no changes were found in cax4 and acax11 (Fig. 8B). Epidermal cells in the mature zone showed significant elevation in [Ca2+]cyt in all genotypes except aca11 (Fig. 8C). Stelar root cells in the elongation zone had a higher Ca2+ concentration in the cytosol under hypoxic conditions in three of the mutants but not in the WT and aca8 (Fig. 8D). In stelar cells of the mature zone, a significant (P<0.001) increase in the [Ca2+]cyt was found in all the five genotypes (Fig. 8E).
Fig. 8.
Relative Ca2+ concentration in the cytosol in the meristem, elongation, and mature zone in root epidermis and stele of Col-0, aca8, aca11, cax4, and cax11 under normoxic or hypoxic treatments for 24h. Data are the mean ±SE (n=60 cells from one individual plant with at least nine replicate plants). Asterisks indicate significant differences at *P<0.05, **P<0.01, and ***P<0.001.
A loss of CAX11 results in a sensitive phenotype to waterlogging stress
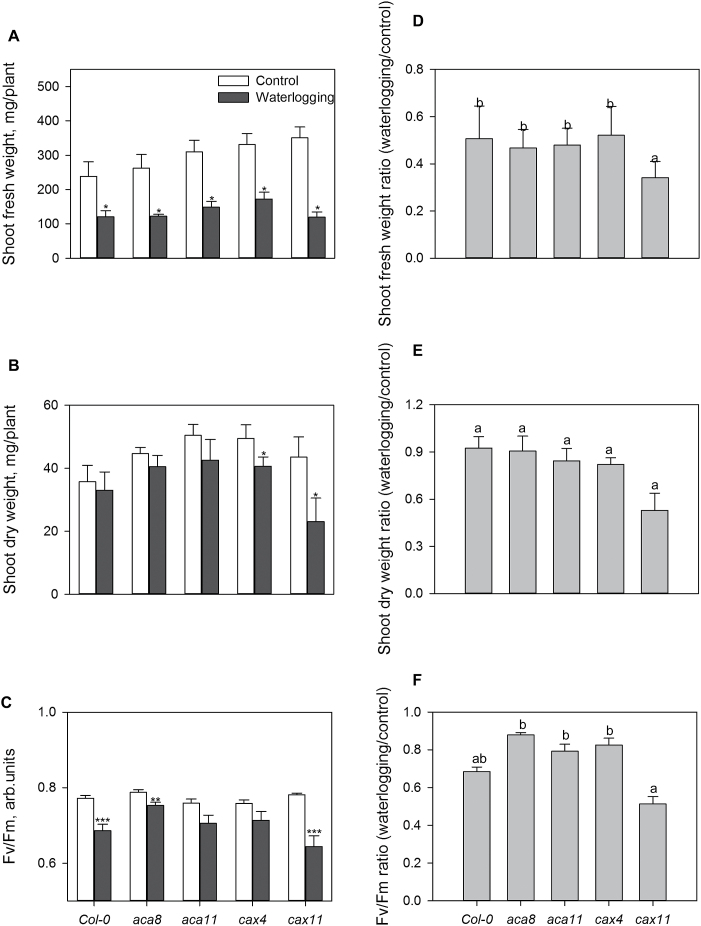
Growth of the Arabidopsis WT and all the mutants was significantly (P<0.05) reduced after 3 weeks of waterlogging stress (Fig. 9). The observed decline in the shoot fresh weight, dry weight, and shoot F v/F m was highly genotype specific, with cax11 more sensitive to waterlogging stress than the other genotypes. The knock-out mutant cax11 showed the strongest growth phenotype and exhibited a 60% reduction in shoot fresh weight and 67% loss in shoot dry weight after 3 weeks of waterlogging. Meanwhile, the average waterlogging-induced reductions in the shoot fresh and dry weight in the WT and other mutants were only 42% and 18%, respectively (Fig. 9A, B, D, E). These results were consistent with the significant decrease in the maximal photochemical efficiency of PSII (F v/F m chlorophyll fluorescence values) in leaves of cax11 (Fig. 9C, F).
Fig. 9.
Effects of waterlogging stress on growth and maximum photochemical efficiency of PSII (F v/F m) of 6-week-old Arabidopsis plants. Three-week-old Arabidopsis plants were subjected to waterlogging treatment with water maintained at 0.5cm above the soil surface for 3 weeks. F v/F m (D) was measured after 3 weeks of treatment and plants were used for morphological (A) and biomass analysis (B, C). Data are the mean ±SE (n=6). The ratio of shoot fresh weight loss (E), shoot dry weight loss (F), and F v/F m loss (G) were calculated.
Discussion
Ca2+ efflux systems play important roles in hypoxic stress
A higher concentration of cytosolic Ca2+ can cause cytotoxicity owing to the precipitation of Ca2+ with phosphate and other anions, or binding to negatively charged macromolecules in the cytosol (Williams, 2006); it may also interfere with the cell’s ability to respond to various stimuli (Bose et al., 2011). To restore the low resting concentration in the cytosol, Ca2+ has to be transported out against an electrochemical gradient into the apoplast, or into intracellular organelles by Ca2+-ATPases and/or Ca2+/H+ antiporters. It is suggested that Ca2+-ATPases with high-affinity (K m=0.1–2 µM) and low-capacity transporters are responsible for termination of [Ca2+]cyt signalling; while Ca2+ exchangers with low-affinity (K m=10–15 µM) and high-capacity transporters may be involved in the removal of [Ca2+]cyt when its elevations are high (Sze et al., 2000; Bose et al., 2011; Shabala et al., 2015). Recently, CAX11 was reclassified as CCX5 (Cation Calcium eXchanger) due to higher homology to mammalian K+-dependent Na+/Ca2+ antiporters which catalyse the electrogenic countertransport of four Na+ for one Ca2+ and one K+ (Dong et al., 2001; Cai and Lytton, 2004; Shigaki and Hirschi, 2006). Expression of AtCCX5 in a yeast mutant restored its growth in low K+ (0.5mM) medium and also suppressed its Na+ sensitivity (Zhang et al., 2011). These findings suggest that CAX11 not only has the ability to remove Ca2+ into the vacuole but also plays a role in Na+ transportation. In the present study, cax11 was more sensitive to 3 weeks of waterlogging stress than the WT and other mutants tested (Fig. 9). We speculate that mutation of ACA8, ACA11, CAX4, and CAX11 in Arabidopsis potentially reduces the ability to terminate [Ca2+]cyt signalling or to remove [Ca2+]cyt disturbance of Ca2+ homeostasis. Knock out of the multiple functional CAX11 might lead to sensitivity to waterlogging stress.
Ca2+ efflux systems play important roles in stress tolerance (Qudeimat et al., 2008; Bose et al., 2011). The role of ACAs in shaping [Ca2+]cyt signatures under salinity stress was first demonstrated in yeast (Anil et al., 2008). Knocking out AtACA4 and AtACA2 resulted in an increased NaCl sensitivity, while their overexpression in Arabidopsis seedlings led to increased stress tolerance in comparison with WT plants (Geisler et al., 2000). After 2–4h of low O2 treatment, ZmCAP1 encoding Ca2+-ATPase in maize roots showed a 2- to 3-fold increase in expression levels (Subbaiah and Sachs, 2000). In contrast, 1h and 24h hypoxic treatments reduced the expression of ACA8, CAX4, and CAX11 in WT roots to 33–50% of the control (Fig. 1), but not for ACA11. Reports on the functional role of ACA11 in plants are rather scarce, to say nothing of its modulation by hypoxic stress. ACA11 was identified as localized on the tonoplast and preferentially expressed in Ca-rich mesophyll; the simultaneous knock out of ACA4 and ACA11 does not appear to reveal a phenotype associated with perturbed apoplastic Ca2+ homeostasis compared with cax1/cax3 (Conn et al., 2011). In the current study, after 3 weeks of waterlogging stress, the aca11 mutant did not show significant changes in shoot dry weight and maximum photochemical efficiency of PSII (chlorophyll fluorescence) compared with the control, consistent with the insignificant changes of ACA11 expression in the WT, which suggests that ACA11 is unlikely to play an important role in hypoxic stress responses. In rice, Ca2+ was reported to act as a physiological transducer for the expression of the alternative oxidase 1a (AOX1a) gene under O2 deficiency (Tsuji et al., 2000). Here we suggest that hypoxia induces a rapid elevation of Ca2+ concentration as a signal, which probably inhibits the expression of Ca2+ efflux transporters. The Atcax1 mutant showed enhanced tolerance to freezing following cold acclimation (Catala et al., 2003). In contrast, Atcax3 has increased sensitivity to salt stress (Zhao et al., 2008). In our present work, cax11 was more sensitive to waterlogging than the WT (Fig. 9). Ca2+/H+ exchangers may be involved in resetting the [Ca2+]cyt elevation following different kinds of stress induction with specific characteristics (Dodd et al., 2010). The differential stress sensitivities of the cax mutants may be a consequence of specific responses by AtCAX1, AtCAX3, and AtCAX11 to the individual stresses (McAinsh and Pittman, 2009).
Hypoxia stress leads to cell- and tissue-specific changes in K+, Ca2+, and Na+ homeostasis
Over the last few decades, increasing numbers of reports showed that plant stress tolerance is conferred by cell type-dependent processes (Roberts and Tester, 1995; Nylander et al., 2001; Ahmad and Maathuis, 2014). In this study, hypoxia had significant impacts on tissue distributions and cellular concentrations of K+ and Ca2+ in roots of Arabidopsis WT and Ca2+ transporter mutants. The combined hypoxia and salinity stress also had significant effects on the distribution of Na+ in different tissues and cell types in Arabidopsis WT and Ca2+ transporter mutants. Correspondingly, O2 gradients have been found along both longitudinal (Pang et al., 2006) and radial (Armstrong, 1979; Armstrong et al., 1994; Gibbs et al., 1998) root profiles under hypoxic stress. It was demonstrated that with the shoot in the air, different barley root zones had different O2 requirements for uptake from the medium, with higher O2 influx in the elongation zone than in the mature zone (Pang et al., 2006). An excised maize root showed a steep O2 concentration decline from the outer layers of cells to the stelar cells when in hypoxic solution (Gibbs et al., 1998), showing more severe hypoxic stress within the stele than in the cortex. Intact barley roots in deoxygenated stagnant solution and relying on internal O2 movement into and along the root aerenchyma had slightly higher O2 in various tissues in the subapical region than just behind the root tip. At the subapical region clear radial gradients in O2 were seen, with the stele having the lowest O2 concentration (Kotula et al., 2015). Therefore, it is suggested that different cell types in roots with diverse access to, and potential sensitivity to, O2 operate differently under hypoxic stress. Our present data further support this suggestion that various cell types in different zones regulate concentrations of K+, Ca2+, and Na+ in distinct ways when faced with hypoxia.
Calcium is a universal secondary messenger response to abiotic and biotic stress (Chen et al., 2010; Shabala et al., 2011; Scherzer et al., 2015), but the changes in Ca2+ in different tissue-specific cells in roots under hypoxia required elucidation. The interesting finding in our data is that in the WT and all of the four Ca2+ transporter mutants (aca8, aca11, cax4, and cax11), the stelar cells in the elongation zone showed no changes in Ca2+ concentration, while in the mature zone they had higher Ca2+ than in normoxic controls (Fig. 5). This situation is similar to the stelar cells in the mature zone which also had significant increases of [Ca2+]cyt in these five genotypes when treated with hypoxia (Fig. 8E). There have been very few studies on the Ca2+ concentration changes in different tissue-specific cells in roots under hypoxia. Studies on Arabidopsis leaf showed that there is more Ca2+ accumulated in the vacuoles of mesophyll cells then in those of the epidermis (Conn et al., 2011), which has been consistently observed across all eudicots so far examined. Conversely, the cereal monocots barley and wheat preferentially accumulate Ca2+ in epidermal cells and not within mesophyll or vascular bundle cells (Conn and Gilliham, 2010). In general, the simple presence or absence of ion transporters could not explain cell type-specific differences in ion accumulation. However, we might suggest that the higher concentration of Ca2+ observed in stelar cells in the mature zone than in stelar cells in the elongation zone in hypoxic roots provides evidence for the necessity for energetically expensive Ca2+ accumulation in the root stele tissue (Subbaiah and Sachs, 2003).
There are numerous studies reporting perturbation in intracellular K+ homeostasis in response to hypoxia in plants (Wiengweera and Greenway, 2004; Mugnai et al., 2011; Barrett-Lennard and Shabala, 2013). Hypoxia increased K+ efflux in 6-day-old wheat roots due to membrane depolarization and the increase of membrane permeability of K+ (Pang and Shabala, 2010). In Vitis riparia roots, an anoxia-tolerant variety, severe K+ imbalance is avoided during anoxia stress by decreasing membrane permeability of K+; whereas Vitis rupestris, an anoxia-sensitive variety, had a strong decrease of K+ (Mancuso and Marras, 2006). Accordingly, in aerobic roots, xylem loading of ions from the external medium is achieved with energy provided by the plasma membrane H+-ATPase (De Boer and Volkov, 2003) through the depolarization-activated outwardly rectifying K+ (SKOR) channels. Since the stele is the first root tissue suffering O2 deficiency, the H+-ATPase activities in the xylem would be inhibited, leading to the closure of SKOR channels for a substantial decline of xylem K+ concentration (Colmer and Greenway, 2011). However, the present data in our experiment showed that there was more K+ accumulation in the stele than in the epidermis in both the elongation zone and mature zone in the WT, aca11, and cax11, which is contrary to the former theory. We speculate that the K+ accumulation in stele could probably flow via NORCs (non-selective outward-rectifying channels) (Chen et al., 2007; Zepeda-Jazo et al., 2008). Unfortunately, the molecular identity of NORCs (as other non-selective cation channels) remains elusive (Demidchik and Maathuis, 2007), and their expression levels cannot thus be thus directly measured. In addition, cells in hypoxic tissues would still have produced some ATP by oxidative phosphorylation when O2 was above zero, or from glycolysis linked to ethanolic fermentation (Kotula et al., 2015).
Although waterlogging is a widespread stress in its own right, its impact on a plant performance is often confounded by soil salinity (Barrett-Lennard and Shabala, 2013). Even a brief period of root O2 deprivation might result in a prolonged Na+ accumulation even after return to normoxic conditions (Marschner, 2011). Thus, it was of interest to measure the Na+ concentration under both hypoxia and salinity conditions. After 24h of combined salt and hypoxic treatment, there was a significant increase in Na+ in the elongation zone stele and a dramatic increase in mature stelar cells in the WT and all of the four Ca2+ transporter mutants (Fig. 7B). H+-ATPase pumps are responsible for the maintenance of the highly negative membrane potential at the root plasma membrane, and the low energy in O2-deficient tissues leads to a substantial depolarization of the plasma membrane potential and the closure of SKOR, which is highly selective for K+ over Na+ (Dietz et al., 2001; Koizumi et al., 2011; Zeng et al., 2014). In contrast, the NORC, transporting K+, Na+, and anions could open during hypoxia, and NORC is also considered as the main transporter for loading Na+ into the xylem (Wegner and Raschke, 1994; Wegner and DeBoer, 1997; Pang and Shabala, 2010), which probably explains the increase of Na+ in cells of both the elongation zone and mature stele. Interestingly, we also found that there was a 2.6-fold increase in Na+ concentration in mature stelar cells relative to elongation zone stelar cells under hypoxia, while there was no difference between the two kinds of cells in the WT under normoxic conditions (Supplementary Table S2). This may be suggestive that even under hypoxia there is still Na+ flow in the xylem from the root to the shoot via other pathways. Some studies found that protein kinase SOS2 (Salt Overly Sensitive 2)/CIPK24, involved in salt tolerance (Cheng et al., 2004), could bind to the autoinhibitory domain of CAX1 to activate the Ca2+ transport into the yeast vacuole, making the cells tolerant to the high Ca2+ in the media. This result implies the co-regulation of CAX1 and the SOS1 antiporter by SOS2/CIPK24 in the signalling pathway for salinity tolerance (Shigaki and Hirschi, 2006). Therefore, a possible convergence of salinity and hypoxia tolerance via the link to Ca2+ signalling and Ca2+ efflux systems is highly likely. This question requires a detailed study in the future.
Conclusions
The results presented here allow a new hypothesis concerning the hypoxic signal transduction in Arabidopsis roots linked with CAX and ACA Ca2+ transporter systems. The tendency in past research on hypoxic stress has been to focus on whole roots, but the key challenge now is to try to explain root function in terms of the different root zones (meristem, elongation, and mature), tissues (epidermis and stele), and specific cell types. In this study, unique cell types in different zones regulate concentrations of K+, Ca2+, and Na+ in distinct ways when faced with hypoxia. In the WT and all of the four Ca2+ transporter mutants, the stelar cells in the elongation zone showed no changes in Ca2+ concentration, while in the mature zone they had higher Ca2+ than normoxic controls. We also speculate that more K+ and Na+ accumulation in the stele than in the epidermis in both the elongation zone and mature zone could probably flow via NORC which could open during hypoxia. In addition, Ca2+ efflux systems and in particular CAX11 were shown to mediate plant Ca2+ homeostasis under hypoxic conditions, with an influence on waterlogging tolerance.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Dose dependence of Calcium Green loading into five types of Arabidopsis root cells.
Figure S2. Illustration of the quantification procedure for Ca2+ distribution in the cytosol.
Table S1. The primers for quantitative RT-PCR experiments.
Table S2. Effect of 24h of hypoxia on Na+ relative concentrations in stelar cells of the elongation zone and mature zone in Arabidopsis WT, aca8, aca11, cax4, and cax11 (ratio of Na+ concentration in mature stelar cells relative to elongation stelar cells under control or hypoxic conditions).
Acknowledgrments
This project is supported by an Australian Research Council (ARC) Discovery Project to TDC and SS. Z-HC is supported by an ARC Discovery Early Career Researcher Award (DE140101143) and a 1000-Plan project. XL is the recipient of a China Scholarship Council award. We thank Dr Rong Liu, Linda Westmoreland, Liz Kabonoff, Dr Sumedha Dharmaratne, and Rosemary Freeman for their technical assistance. We thank especially Dr Anya Salih for her support on imaging at the Confocal Bio-Imaging Facility at Western Sydney University.
References
- Ahmad I, Maathuis FJ. 2014. Cellular and tissue distribution of potassium: physiological relevance, mechanisms and regulation. Journal of Plant Physiology 171, 708–714. [DOI] [PubMed] [Google Scholar]
- Anil VS, Rajkumar P, Kumar P, Mathew M. 2008. A plant Ca2+ pump, ACA2, relieves salt hypersensitivity in yeast. Modulation of cytosolic calcium signature and activation of adaptive Na+ homeostasis. Journal of Biological Chemistry 283, 3497–3506. [DOI] [PubMed] [Google Scholar]
- Anschutz U, Becker D, Shabala S. 2014. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology 171, 670–687. [DOI] [PubMed] [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants . In: Woolhouse HWW , ed. Advances in Botanical Research , Vol. 7 Academic Press, 225–332. [Google Scholar]
- Armstrong W, Drew M. 2002. Root growth and metabolism under oxygen deficiency. In: Waisel Y, Eshel A, Kafkafi U, eds. Roots: the hidden half , 3rd edn. New York: Marcel Dekker Inc, 729–761. [Google Scholar]
- Armstrong W, Strange M, Cringle S, Beckett P. 1994. Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany 74, 287–299. [Google Scholar]
- Baekgaard L, Fuglsang AT, Palmgren MG. 2005. Regulation of plant plasma membrane H+- and Ca2+-ATPases by terminal domains. Journal of Bioenergetics and Biomembranes 37, 369–374. [DOI] [PubMed] [Google Scholar]
- Baekgaard L, Luoni L, De Michelis MI, Palmgren MG. 2006. The plant plasma membrane Ca2+ pump ACA8 contains overlapping as well as physically separated autoinhibitory and calmodulin-binding domains. Journal of Biological Chemistry 281, 1058–1065. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LA. 2008. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59, 313–339. [DOI] [PubMed] [Google Scholar]
- Barrett-Lennard EG, Shabala SN. 2013. The waterlogging/salinity interaction in higher plants revisited—focusing on the hypoxia-induced disturbance to K+ homeostasis. Functional Plant Biology 40, 872–882. [DOI] [PubMed] [Google Scholar]
- Bonales-Alatorre E, Pottosin I, Shabala L, Chen Z, Zeng F, Jacobsen S, Shabala S. 2013. a Plasma and vacuolar membrane transporters conferring genotypic difference in salinity tolerance in a halophyte species, Chenopodium quinoa . International Journal of Molecular Sciences 14, 9267–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonales-Alatorre E, Shabala S, Chen Z-H, Pottosin I. 2013. b Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa. Plant Physiology 162, 940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S. 2011. Calcium efflux systems in stress signaling and adaptation in plants. Frontiers in Plant Science 2, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, Harper JF. 2010. Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiology 154, 1158–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Lytton J. 2004. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. Journal of Biological Chemistry 279, 5867–5876. [DOI] [PubMed] [Google Scholar]
- Catala R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM, Salinas J. 2003. Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. The Plant Cell 15, 2940–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR. 2010. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+concentration and protein phosphorylation. The Plant Journal 61, 816–825. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Pottosin II, Cuin TA, et al. 2007. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology 145, 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Wang Y, Wang JW, Babla M, Zhao C, García-Mata C, Sani E, Differ C, Mak M, Hills A. 2016. Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytologist 209, 1456–1469. [DOI] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Hirschi KD. 2002. Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiology 128, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Zhu JK, Hirschi KD. 2004. The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. Journal of Biological Chemistry 279, 2922–2926. [DOI] [PubMed] [Google Scholar]
- Chung WS, Lee SH, Kim JC, et al. 2000. Identification of a calmodulin-regulated soybean Ca2+-ATPase (SCA1) that is located in the plasma membrane. The Plant Cell 12, 1393–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Greenway H. 2011. Ion transport in seminal and adventitious roots of cereals during O2 deficiency. Journal of Experimental Botany 62, 39–57. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Huang SB, Greenway H. 2001. Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. Journal of Experimental Botany 52, 1507–1517. [DOI] [PubMed] [Google Scholar]
- Conn S, Gilliham M. 2010. Comparative physiology of elemental distributions in plants. Annals of Botany 105, 1081–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, et al. 2011. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. The Plant Cell 23, 240–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Betts SA, Chalmandrier R, Shabala S. 2008. A root’s ability to retain K+ correlates with salt tolerance in wheat. Journal of Experimental Botany 59, 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer AH, Volkov V. 2003. Logistics of water and salt transport through the plant: structure and functioning of the xylem. Plant, Cell and Environment 26, 87–101. [Google Scholar]
- Demidchik V. 2014. Mechanisms and physiological roles of K+ efflux from root cells. Journal of Plant Physiology 171, 696–707. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. 2010. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJ. 2007. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175, 387–404. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V. 2014. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. Journal of Experimental Botany 65, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Tavakoli N, Kluge C, Mimura T, Sharma SS, Harris GC, Chardonnens AN, Golldack D. 2001. Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. Journal of Experimental Botany 52, 1969–1980. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. 2010. The language of calcium signaling. Annual Review of Plant Biology 61, 593–620. [DOI] [PubMed] [Google Scholar]
- Dong H, Light PE, French RJ, Lytton J. 2001. Electrophysiological characterization and ionic stoichiometry of the rat brain K+-dependent Na+/Ca2+ exchanger, NCKX2. Journal of Biological Chemistry 276, 25919–25928. [DOI] [PubMed] [Google Scholar]
- Dreyer I, Uozumi N. 2011. Potassium channels in plant cells. FEBS Journal 278, 4293–4303. [DOI] [PubMed] [Google Scholar]
- Elzenga JTM, van Veen H. 2010. Waterlogging and plant nutrient uptake. In: Mancuso S, Shabala S, eds. Waterlogging signalling and tolerance in plants . Berlin: Springer, 23–35. [Google Scholar]
- Geisler M, Frangne N, Gomes E, Martinoia E, Palmgren M. 2000. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiology 124, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H. 1998. Response to oxygen deficiency in primary maize roots. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Australian Journal of Plant Physiology 25, 745–758. [Google Scholar]
- Hetherington AM, Brownlee C. 2004. The generation of Ca2+ signals in plants. Annual Review of Plant Biology 55, 401–427. [DOI] [PubMed] [Google Scholar]
- Huda KMK, Banu MSA, Tuteja R, Tuteja N. 2013. Global calcium transducer P-type Ca2+-ATPases open new avenues for agriculture by regulating stress signalling. Journal of Experimental Botany 64, 3099–3109. [DOI] [PubMed] [Google Scholar]
- Koizumi Y, Hara Y, Yazaki Y, Sakano K, Ishizawa K. 2011. Involvement of plasma membrane H+-ATPase in anoxic elongation of stems in pondweed (Potamogeton distinctus) turions. New Phytologist 190, 421–430. [DOI] [PubMed] [Google Scholar]
- Kotula L, Clode PL, Striker GG, Pedersen O, Lauchli A, Shabala S, Colmer TD. 2015. Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare). New Phytologist 208, 1114–1125. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Kader MA, Yemelyanov V. 2012. Calcium signalling in plant cells under environmental stress. In: Parvaiz A, Prasad MNV, eds Environmental adaptations and stress tolerance of plants in the era of climate change . Berlin: Springer, 325–360. [Google Scholar]
- Liu XH, Mak M, Babla M, Wang FF, Chen G, Veljanoski F, Wang G, Shabala S, Zhou MX, Chen ZH. 2014. Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Frontiers in Plant Science 5, 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. 2001. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Australian Journal of Plant Physiology 28, 1121–1131. [Google Scholar]
- Mancuso S, Marras AM. 2006. Adaptative response of Vitis root to anoxia. Plant and Cell Physiology 47, 401–409. [DOI] [PubMed] [Google Scholar]
- Manohar M, Shigaki T, Hirschi KD. 2011. Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biology (Stuttgart) 13, 561–569. [DOI] [PubMed] [Google Scholar]
- Marschner H. 2011. Marschner’s mineral nutrition of higher plants . Academic Press. [Google Scholar]
- McAinsh MR, Pittman JK. 2009. Shaping the calcium signature. New Phytologist 181, 275–294. [DOI] [PubMed] [Google Scholar]
- Medvedev SS. 2005. Calcium signaling system in plants. Russian Journal of Plant Physiology 52, 249–270. [Google Scholar]
- Mei H, Cheng NH, Zhao J, Park S, Escareno RA, Pittman JK, Hirschi KD. 2009. Root development under metal stress in Arabidopsis thaliana requires the H+/cation antiporter CAX4. New Phytologist 183, 95–105. [DOI] [PubMed] [Google Scholar]
- Mugnai S, Marras AM, Mancuso S. 2011. Effect of hypoxic acclimation on anoxia tolerance in Vitis roots: response of metabolic activity and K+ fluxes. Plant and Cell Physiology 52, 1107–1116. [DOI] [PubMed] [Google Scholar]
- Nylander M, Svensson J, Palva ET, Welin BV. 2001. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana . Plant Molecular Biology 45, 263–279. [DOI] [PubMed] [Google Scholar]
- Pang J, Shabala S. 2010. Membrane transporters and waterlogging tolerance. In: Mancuso S, Shabala S, eds.Waterlogging signalling and tolerance in plants . Berlin: Springer, 197–219. [Google Scholar]
- Pang JY, Newman I, Mendham N, Zhou M, Shabala S. 2006. Microelectrode ion and O2 fluxes measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant, Cell and Environment 29, 1107–1121. [DOI] [PubMed] [Google Scholar]
- Pottosin II, Schnknecht G. 2007. Vacuolar calcium channels. Journal of Experimental Botany 58, 1559–1569. [DOI] [PubMed] [Google Scholar]
- Qadir M, Oster JD. 2004. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Science of the Total Environment 323, 1–19. [DOI] [PubMed] [Google Scholar]
- Qudeimat E, Faltusz AMC, Wheeler G, Lang D, Brownlee C, Reski R, Frank W. 2008. A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens . Proceedings of the National Academy of Sciences, USA 105, 19555–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ASN, Ali GS, Celesnik H, Day IS. 2011. Coping with stresses: roles of calcium-and calcium/calmodulin-regulated gene expression. The Plant Cell 23, 2010–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SK, Tester M. 1995. Inward and outward K+-selective currents in the plasma membrane of protoplasts from maize root cortex and stele. The Plant Journal 8, 811–825. [Google Scholar]
- Sanders D, Brownlee C, Harper JF. 1999. Communicating with calcium. The Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. 2002. Calcium at the crossroads of signaling. The Plant Cell 14, S401–S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer S, Bohm J, Krol E, et al. 2015. Calcium sensor kinase activates potassium uptake systems in gland cells of Venus flytraps. Proceedings of the National Academy of Sciences, USA 112, 7309–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiott M, Palmgren MG. 2005. Two plant Ca2+ pumps expressed in stomatal guard cells show opposite expression patterns during cold stress. Physiologia Plantarum 124, 278–283. [Google Scholar]
- Setter TL, Waters I. 2003. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil 253, 1–34. [Google Scholar]
- Shabala S. 2009. Salinity and programmed cell death: unravelling mechanisms for ion specific signalling. Journal of Experimental Botany 60, 709–711. [DOI] [PubMed] [Google Scholar]
- Shabala S. 2011. Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytologist 190, 289–298. [DOI] [PubMed] [Google Scholar]
- Shabala S, Baekgaard L, Shabala L, Fuglsang A, Babourina O, Palmgren MG, Cuin TA, Rengel Z, Nemchinov LG. 2011. Plasma membrane Ca2+ transporters mediate virus-induced acquired resistance to oxidative stress. Plant, Cell and Environment 34, 406–417. [DOI] [PubMed] [Google Scholar]
- Shabala S, Bose J, Fuglsang AT, Pottosin I. 2015. On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. Journal of Experimental Botany (in press). [DOI] [PubMed] [Google Scholar]
- Shabala S, Pottosin I. 2014. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum 151, 257–279. [DOI] [PubMed] [Google Scholar]
- Shabala S, Shabala L, Barcelo J, Poschenrieder C. 2014. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant, Cell and Environment 37, 2216–2233. [DOI] [PubMed] [Google Scholar]
- Shabala S, Shabala L, Cuin TA, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner LH. 2010. Xylem ionic relations and salinity tolerance in barley. The Plant Journal 61, 839–853. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD. 2006. Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biology (Stuttgart) 8, 419–429. [DOI] [PubMed] [Google Scholar]
- Smedema LK, Shiati K. 2002. Irrigation and salinity: a perspective review of the salinity hazards of irrigation development in the arid zone. Irrigation and Drainage Systems 16, 161–174. [Google Scholar]
- Steinhorst L, Kudla J. 2014. Signaling in cells and organisms—calcium holds the line. Current Opinion in Plant Biology 22, 14–21. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Sachs MM. 2000. Maize cap1 encodes a novel SERCA-type calcium-ATPase with a calmodulin-binding domain. Journal of Biological Chemistry 275, 21678–21687. [DOI] [PubMed] [Google Scholar]
- Subbaiah CC, Sachs MM. 2003. Molecular and cellular adaptations of maize to flooding stress. Annals of Botany 91, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Zhang JK, Sachs MM. 1994. Involvement of intracellular calcium in anaerobic gene-expression and survival of maize seedlings. Plant Physiology 105, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. 2000. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annual Review of Plant Physiology and Plant Molecular Biology 51, 433–462. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Nakazono M, Saisho D, Tsutsumi N, Hirai A. 2000. Transcript levels of the nuclear-encoded respiratory genes in rice decrease by oxygen deprivation: evidence for involvement of calcium in expression of the alternative oxidase la gene. FEBS Letters 471, 201–204. [DOI] [PubMed] [Google Scholar]
- Wang FF, Deng SR, Ding MQ, et al. 2013. Overexpression of a poplar two-pore K+ channel enhances salinity tolerance in tobacco cells. Plant Cell, Tissue and Organ Culture 112, 19–31. [Google Scholar]
- Wegner LH, DeBoer AH. 1997. Two inward K+ channels in the xylem parenchyma cells of barley roots are regulated by G-protein modulators through a membrane-delimited pathway. Planta 203, 506–516. [Google Scholar]
- Wegner LH, Raschke K. 1994. Ion channels in the xylem parenchyma of barley roots—a procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiology 105, 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H. 2004. Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. Journal of Experimental Botany 55, 2121–2129. [DOI] [PubMed] [Google Scholar]
- Williams RJP. 2006. The evolution of calcium biochemistry. Biochimica et Biophysica Acta 1763, 1139–1146. [DOI] [PubMed] [Google Scholar]
- Wu H, Shabala L, Liu X, Azzarello E, Zhou M, Pandolfi C, Chen ZH, Bose J, Mancuso S, Shabala S. 2015. Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Frontiers in Plant Science 6, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemelyanov VV, Shishova MF, Chirkova TV, Lindberg SM. 2011. Anoxia-induced elevation of cytosolic Ca2+ concentration depends on different Ca2+ sources in rice and wheat protoplasts. Planta 234, 271–280. [DOI] [PubMed] [Google Scholar]
- Zeng FR, Konnerup D, Shabala L, Zhou MX, Colmer TD, Zhang GP, Shabala S. 2014. Linking oxygen availability with membrane potential maintenance and K+ retention of barley roots: implications for waterlogging stress tolerance. Plant, Cell and Environment 37, 2325–2338. [DOI] [PubMed] [Google Scholar]
- Zeng FR, Shabala L, Zhou MX, Zhang GP, Shabala S. 2013. Barley responses to combined waterlogging and salinity stress: separating effects of oxygen deprivation and elemental toxicity. Frontiers in Plant Science 4, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda-Jazo I, Shabala S, Chen Z, Pottosin II. 2008. Na+–K+ transport in roots under salt stress. Plant Signaling and Behavior 3, 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Takano T, Liu S. 2011. Characterization of an AtCCX5 gene from Arabidopsis thaliana that involves in high-affinity K+ uptake and Na+ transport in yeast. Biochemical and Biophysical Research Communications 414, 96–100. [DOI] [PubMed] [Google Scholar]
- Zhao J, Barkla BJ, Marshall J, Pittman JK, Hirschi KD. 2008. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227, 659–669. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Dunand C, Snedden W, Galaud JP. 2015. CaM and CML emergence in the green lineage. Trends in Plant Science 20, 483–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.