Abstract
KIT D816V is present in a majority of patients with systemic mastocytosis (SM). We determined the KIT D816V allele burden by quantitative real-time PCR in bone marrow and peripheral blood of 105 patients with mastocytosis. KIT D816V was detected in 92/105 patients (88%). Significant differences in the median allele burden were observed among disease subgroups; cutaneous mastocytosis (0.042%), indolent SM (0.285%), smoldering SM (5.991%), aggressive SM (9.346%) and SM with associated hematologic non-mast cell lineage disease (3.761%) (P<0.001). The KIT D816V burden also correlated with serum tryptase (R=0.5, P<0.005) but not with mast cell infiltration in bone marrow or mediator symptoms. Moreover, the allele burden was of prognostic significance regarding survival (P<0.01). Patients responding to cytoreductive therapy showed a significant decrease in KIT D816V (P<0.03). To conclude, the KIT D816V burden correlates with the variant of mastocytosis, predicts survival, and is a valuable follow-up parameter in SM.
Keywords: mastocytosis, allele burden, KIT D816V, survival, treatment response
Introduction
The great majority of patients with systemic mastocytosis (SM) harbor the somatic point mutation KIT D816V (1–4). Kristensen et al. reported a highly sensitive quantitative real-time PCR (qPCR) for KIT D816V (5). Recently the importance of allele burden measurements by qPCR techniques has been confirmed applying different methods (6). Moreover, differences in KIT D816V allele burden among indolent and aggressive forms have been observed (6, 7). The aims of the present study were to evaluate the impact of KIT D816V allele burden on the clinical course and survival in SM.
Patients and methods
A total of 105 adult patients with mastocytosis i.e. cutaneous mastocytosis (CM, N=12), mastocytosis in the skin (MIS, N=3) indolent SM (ISM, N=67), smoldering SM (SSM, N=5), aggressive SM (ASM, N=7), mast cell leukemia (MCL, N=1), and SM with an associated hematologic non-MC-lineage disease (SM-AHNMD, N=10) were examined. Patients´ characteristics are shown in Table S1. KIT D816V allele burden was quantified from genomic DNA of bone marrow (BM) aspirates and peripheral blood using allele-specific qPCR as described (5).
Results
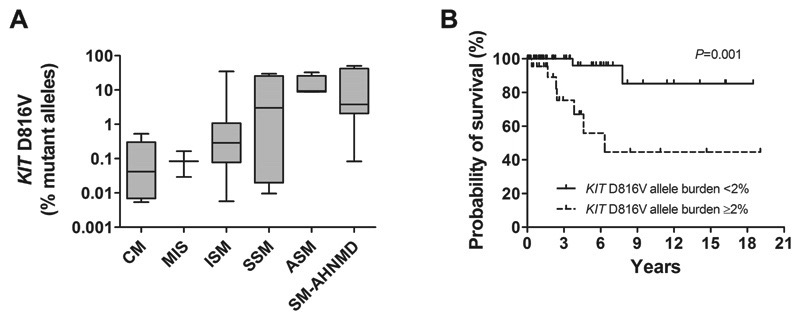
At diagnosis, KIT D816V was detectable in 92/105 patients (88%) with a median KIT D816V allele burden of 0.315%, (range: 0.005%-50.183%). In KIT D816V+ patients (N=92), we compared the mutant allele burden in the different WHO-subgroups. In CM the median KIT D816V allele burden was 0.042% (range: 0.005%-0.530%), in MIS 0.084% (range: 0.029%-0.165%), in ISM 0.285% (range: 0.006%-34.585%), in SSM 5.991% (range: 0.023%-29.620%), in ASM 9.346% (range: 8.816%-32.480%), and 3.761% (range 0.083%-50.183%) in SM-AHNMD. These differences were highly significant (Kruskal-Wallis test; P<0.001; Fig. 1A).
Fig. 1. KIT D816V allele burden in different WHO-subtype of mastocytosis and its impact on survival.
(A) Highly significant differences in the KIT D816V allele burden were found between patients with cutaneous mastocytosis (CM), mastocytosis in the skin (MIS), indolent systemic mastocytosis (ISM), smoldering systemic mastocytosis (SSM), aggressive systemic mastocytosis (ASM), mast cell leukemia (MCL), and systemic mastocytosis with an associated hematologic non-mast cell lineage disease (SM-AHNMD) (P<0.001, Kruskal Wallis test). (B) Kaplan-Meier plot for overall survival of mastocytosis patients with a KIT D816V mutation burden <2% and patients with a KIT D816V burden of ≥ 2% at diagnosis. The difference in the probability of survival was significant (P=0.001).
The KIT D816V allele burden correlated significantly with serum tryptase levels (Pearson’s correlation; R=0.50, P<0.005) and age (R=0.56, P<0.005), but only roughly with mast cell (MC) infiltration in the BM (R=0.369) or the percentage of MC in BM smears (R=0.334) (Fig. S1). No significant differences in the allele burden were found when comparing patients with or without mediator-related symptoms (Fig. S2).
The KIT D816V mutation burden was a significant predictor for survival in our 92 KIT D816V+ patients (Cox-regression, P=0.015). Using a cut-off level of 2% mutant alleles, two prognostically distinct groups were separated. In the group with <2% mutant allele burden (N=66) the median survival was not reached whereas in patients with a KIT D816V allele burden of ≥2% (N=26) the median survival was 6.3 years (Log-rank test; P=0.001; Fig. 1B).
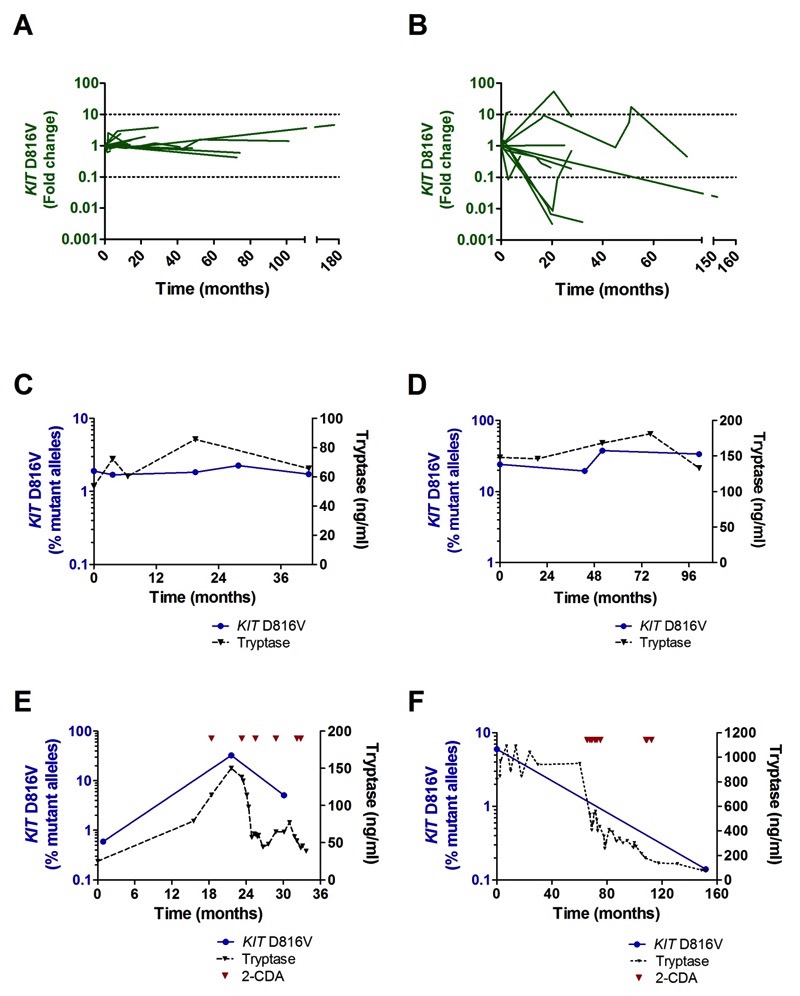
In 30 patients, the KIT D816V allele burden could be followed over a median observation time of 21 months (range 3-178 months). In patients with stable clinical course (N=19), no substantial increase or decrease of the KIT D816V allele burden was observed (Fig. 2A, C-D; Fig. S3). In cases with advanced SM receiving cytoreductive therapy (N=11), marked changes in the mutant allele burden were observed (Fig. 2B, E-F; Fig. S4). In patients with a good or partial response to cytoreductive treatment (cladribine N=4, hydroxyurea N=1, interferon N=1) a significant decrease in the KIT D816V burden (91.6% median reduction) was observed (paired t-test; P=0.027).
Fig. 2. KIT D816V allele burden during the follow-up.
Change in KIT D816V mutation positive allele fraction during the follow-up in patients without (A; N=19) and with cytoreductive treatment (B; N=11). The allele burden was normalized to the mutant burden at diagnosis. The value of 1 corresponds to no change in allele fraction over time and the values of 0.1 and 10 (dotted lines) correspond to 10 fold decreases and increases. (C-F) Follow-up of KIT D816V allele burden (blue) and serum tryptase (black) in individual mastocytosis patients with an uneventful course (C, ISM; D, SSM), a patient initially diagnosed as ISM with disease progression to ASM-CMML and response to cytoreductive therapy with cladribine (2-CDA) (E), and a patient with SSM receiving cytoreductive therapy due to recurrent episodes of life threatening vascular instability requiring epinephrine support (F).
Discussion
Recently qPCR has been shown to be a reliable test for the quantification of KIT D816V in patients with mastocytosis (5-11). In line with these results the median KIT D816V allele burden was 0.315% in our patients. The different subgroups of SM are considered to differ substantially with regard to the burden of neoplastic MC (12). Indeed, the lowest KIT D816V allele burden was observed in CM followed by ISM and SSM, the highest in ASM and SM-AHNMD. Similar results have recently been reported in a study investigating indolent SM but not cases with ASM (7). The higher KIT D816V allele burden in advanced SM, including SM-AHNMD, could also indicate that non-MC-lineage cells harbor the mutation in these patients. In this regard it is noteworthy that multi-lineage involvement is frequent in advanced SM (13, 14). Multilineage involvement may also explain why the KIT D816V allele burden correlated only moderately with serum tryptase levels and failed to correlate with the number of MC in BM biopsies and smears. Another explanation would be that other oncogenic mutations are involved in proliferation and/or survival of MC in the BM (15).
Since the KIT mutation D816V triggers autonomous KIT activation, we were interested whether mediator-related clinical symptoms would correlate with the KIT D816V mutant burden. However, we were unable to demonstrate such a correlation which also confirms results obtained by Broesby-Olsen et al (8) in a cohort including advanced SM. Together, these data suggest that mediator-related symptom occur regardless of the WHO-subgroup and independently of the burden of KIT-mutated MC.
Recently an impact of the KIT D816V allele burden on survival has been suggested in a cohort with a very high percentage (52%) of advanced SM (6). In our cohort of consecutively diagnosed patients with only 17% advanced SM, thereby reflecting a less selected patient population, the KIT D816V allele burden was of prognostic significance regarding survival. Using a cut-off level of 2% mutant allele burden in our study, two prognostically different groups of patients with clearly different survival times could be separated. However, the value of a particular cut-off needs to be confirmed in future prospective trials.
At present, only a few laboratory parameters are available to monitor treatment response in advanced SM (4). So far, follow-up data of KIT D816V allele burden was only published for patients with ISM (8) and 4 patients with advanced SM (6). In our cohort, patients with indolent disease showed a stable allele burden during the follow-up whereas in cases with disease progression a marked increase in KIT D816V was found. Patients responding to cytoreductive therapy showed a significant decrease in the KIT D816V burden. Thus, the KIT D816V allele burden may be employed for response evaluation in clinical trials.
Together, we confirm the diagnostic value of KIT D816V qPCR in mastocytosis. Moreover, our data show that the mutation burden differs significantly among patients with different WHO-subgroups and is of prognostic significance concerning survival. Finally, the KIT D816V allele burden is a valuable follow-up parameter in untreated and drug-treated patients with advanced SM.
Supplementary Material
Acknowledgments
The authors would like to thank Matthias Mayerhofer (Hanusch Hospital, Vienna, Austria) for critical reading of the manuscript.
Financial Support
This study was supported by Austrian National Science Fund (FWF) project P26079-B13 and SFB project F4704-B20.
Abbreviations
- MC
mast cell
- SM
systemic mastocytosis
- CM
cutaneous mastocytosis
- ISM
indolent systemic mastocytosis
- SSM
smoldering systemic mastocytosis
- ASM
aggressive systemic mastocytosis
- SM-AHNMD
systemic mastocytosis with an associated hematologic non-mast cell lineage disease
- MCL
mast cell leukemia
- AML
acute myeloid leukemia
- CMML
chronic myelomonocytic leukemia
- BM
bone marrow
- qPCR
quantitative real-time PCR
Footnotes
Author Contribution
G.H., G.E.D., G.G., G.M., and C.M. performed molecular tests and analyzed the data. G.H., K.V.G., F.W., E.H., P.V., and W.R.S. obtained and analyzed clinical data. G.H. and P.V. and W.R.S. designed the study and wrote the paper. All authors revised and approved the manuscript.
Conflict of Interest
P.V. is a consultant in a global Novartis trial investigating the effects of PKC412 in patients with advanced systemic mastocytosis. In addition, P.V. received honoraria and a research grant from Novartis. The authors declare no other competing financial interests.
References
- 1.Longley BJ, Jr, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu SZ, et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA. 1999;96(4):1609–1614. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92(23):10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435–453. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 4.Gotlib J, Pardanani A, Akin C, Reiter A, George T, Hermine O, et al. International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European Competence Network on Mastocytosis (ECNM) consensus response criteria in advanced systemic mastocytosis. Blood. 2013;121(13):2393–2401. doi: 10.1182/blood-2012-09-458521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen T, Vestergaard H, Moller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13(2):180–188. doi: 10.1016/j.jmoldx.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erben P, Schwaab J, Metzgeroth G, Horny HP, Jawhar M, Sotlar K, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93(1):81–88. doi: 10.1007/s00277-013-1964-1. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen T, Vestergaard H, Bindslev-Jensen C, Moller MB, Broesby-Olsen S. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014 doi: 10.1002/ajh.23672. [DOI] [PubMed] [Google Scholar]
- 8.Broesby-Olsen S, Kristensen T, Vestergaard H, Brixen K, Moller MB, Bindslev-Jensen C, et al. KIT D816V mutation burden does not correlate to clinical manifestations of indolent systemic mastocytosis. J Allergy Clin Immunol. 2013;132(3):723–728. doi: 10.1016/j.jaci.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen T, Broesby-Olsen S, Vestergaard H, Bindslev-Jensen C, Moller MB. KIT D816V Mutation-Positive Cell Fractions in Lesional Skin Biopsies from Adults with Systemic Mastocytosis. Dermatology. 2013;226(3):233–237. doi: 10.1159/000349986. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen T, Broesby-Olsen S, Vestergaard H, Bindslev-Jensen C, Moller MB. Mastocytosis Centre Odense University H. Circulating KIT D816V mutation-positive non-mast cells in peripheral blood are characteristic of indolent systemic mastocytosis. Eur J Haematol. 2012;89(1):42–46. doi: 10.1111/j.1600-0609.2012.01789.x. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen T, Broesby-Olsen S, Vestergaard H, Bindslev-Jensen C, Moller MB. Mastocytosis Centre Odense University H. Serum tryptase correlates with the KIT D816V mutation burden in adults with indolent systemic mastocytosis. Eur J Haematol. 2013;91(2):106–111. doi: 10.1111/ejh.12128. [DOI] [PubMed] [Google Scholar]
- 12.Sperr WR, Jordan JH, Fiegl M, Escribano L, Bellas C, Dirnhofer S, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128(2):136–141. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 13.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94(7):2333–2342. [PubMed] [Google Scholar]
- 14.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 15.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.