Summary
Mast cell leukemia (MCL) is a rare form of systemic mastocytosis characterized by leukemic expansion of mostly immature mast cells, organ damage, drug-resistance, and a poor prognosis. Even when treated with chemotherapy, most patients have a life-expectancy of less than one year. However, there are rare patients with MCL in whom the condition is less aggressive and does not cause organ damage within a short time. In these patients, mast cells exhibit a more mature morphology when compared to acute MCL. A recently proposed classification suggests that these cases are referred to as chronic MCL. In the present article, we discuss clinical, histopathological and morphological aspects of acute and chronic MCL.
Keywords: mastocytosis, mast cells, chronic mast cell leukemia, KIT mutation
Introduction
According to the classification of the World Health Organization (WHO), mast cell leukemia (MCL) represents the leukemic manifestation and variant of systemic mastocytosis (SM) [1–4]. These patients have a particularly poor prognosis with short survival times [5–8]. MCL is characterized by a leukemic expansion of immature neoplastic mast cells (MCs) in hematopoietic tissues. The bone marrow (BM) is always affected, resulting in peripheral cytopenia [5,6,9–11]. The BM smear in these patients contains at least 20% atypical and mostly immature MCs [1–4]. In patients suffering from the classical variant of MCL, numerous circulating MCs are also found [5]. However, in a substantial number of patients with MCL, the leukemic spread into the peripheral blood is less extensive or is even absent. When MCs comprise less than 10% of all circulating blood leukocytes, the disease is termed ´aleukemic MCL´ [1–4]. In most patients with MCL, MCs are rather immature. Some of these cells have a blast-like morphology, also referred to as metachromatically granulated (metachromatic) blasts [6]. Other immature forms may contain bi- or multi-lobed nuclei and thus represent so-called atypical MCs type II or promastocytes [6].
During the past few years, more and more patients with MCL with a rather stable clinical course, absence of organ damage and a more mature MC morphology have been described [12–14]. For these patients the term chronic MCL has been proposed [14]. In the present article, we discuss histomorphological and cytological features of MCs in MCL, with special emphasis on the proposed delineation between acute and chronic MCL.
Diagnostic criteria defining chronic MCL
As mentioned above, MCL is the leukemic variant of SM [1–4]. Therefore, all patients with MCL have to fulfil SM criteria: in particular, if at least one major and one minor or at least three minor SM criteria are fulfilled, the diagnosis SM can be established [1–4]. The major SM criterion is the prominent multifocal clustering of MCs in the BM or in other internal organs [1–4]. Minor SM criteria include an atypical morphology of MCs, expression of CD2 and/or CD25 in MCs, the presence of an activating mutation at codon 816 of KIT and a serum tryptase level ≥20 ng/mL [1–4]. Once SM has been diagnosed, the next diagnostic step is to evaluate for the presence of MCs in good-quality BM smears. In those patients in whom at least 20% of all nucleated cells on BM smears are (immature or atypical) MCs, the diagnosis MCL can be established [1–4]. While in most patients with MCL, signs and symptoms of organ damage, so-called C Findings, are present, a recent proposal suggests that the absence of such C-Findings serves as a diagnostic criterion of chronic sub-variant of MCL [14]. Thus, chronic MCL is defined by at least 20% MCs on BM smears and absence of C-Findings [14]. In patients with chronic MCL in whom one (any type of) or more C-Findings develop, the diagnosis changes from chronic to acute MCL. Whether other clinical or laboratory findings are also prognostic in chronic MCL is currently under investigation. With regard to C-Findings it is important to state that organ damage only counts as C-Finding when the damage is caused by a local devastating MC infiltrate, and such MC infiltration needs to be confirmed by a biopsy and histologic examination of the affected organ. Table 1 provides a summary of clinical and laboratory features discriminating chronic MCL from acute MCL. Organomegaly may be found in both groups of patients.
Table 1. Clinical and laboratory features discriminating chronic MCL from acute MCL.
| Disease-related Feature/Finding |
Chronic MCL | Acute MCL |
|---|---|---|
| MCs on BM smears ≥20% | + | + |
| Many MCs are mature MCs | + | − |
| Metachromatic blasts | − | + |
| MCs display KIT D816V | −/+ | +/− |
| MCs express CD25 | +/− | + |
| MCs express CD30 | +/− | +/− |
| MCs frequently express Ki-67 | − | +/− |
| C-Findings: | ||
| Marked anemia (Hb<10 g/dL) | − | +/− |
| Marked neutropenia (ANC<1,000/µL) | − | +/− |
| Marked thrombocytopenia (PLT<100,000) | − | +/− |
| Other C-Findings | − | +/− |
| Eosinophilia | +/− | +/− |
| Splenomegaly | +/− | + |
| Lymphadenopathy | −/+ | +/− |
MCL, mast cell leukemia; MCs, mast cells; BM, bone marrow; Hb, hemoglobin concentration; g/dL, gram per deciliter; ANC, absolute neutrophil count; PLT, platelet count.
Morphologic subtypes of MCs found on BM smears in patients with chronic MCL
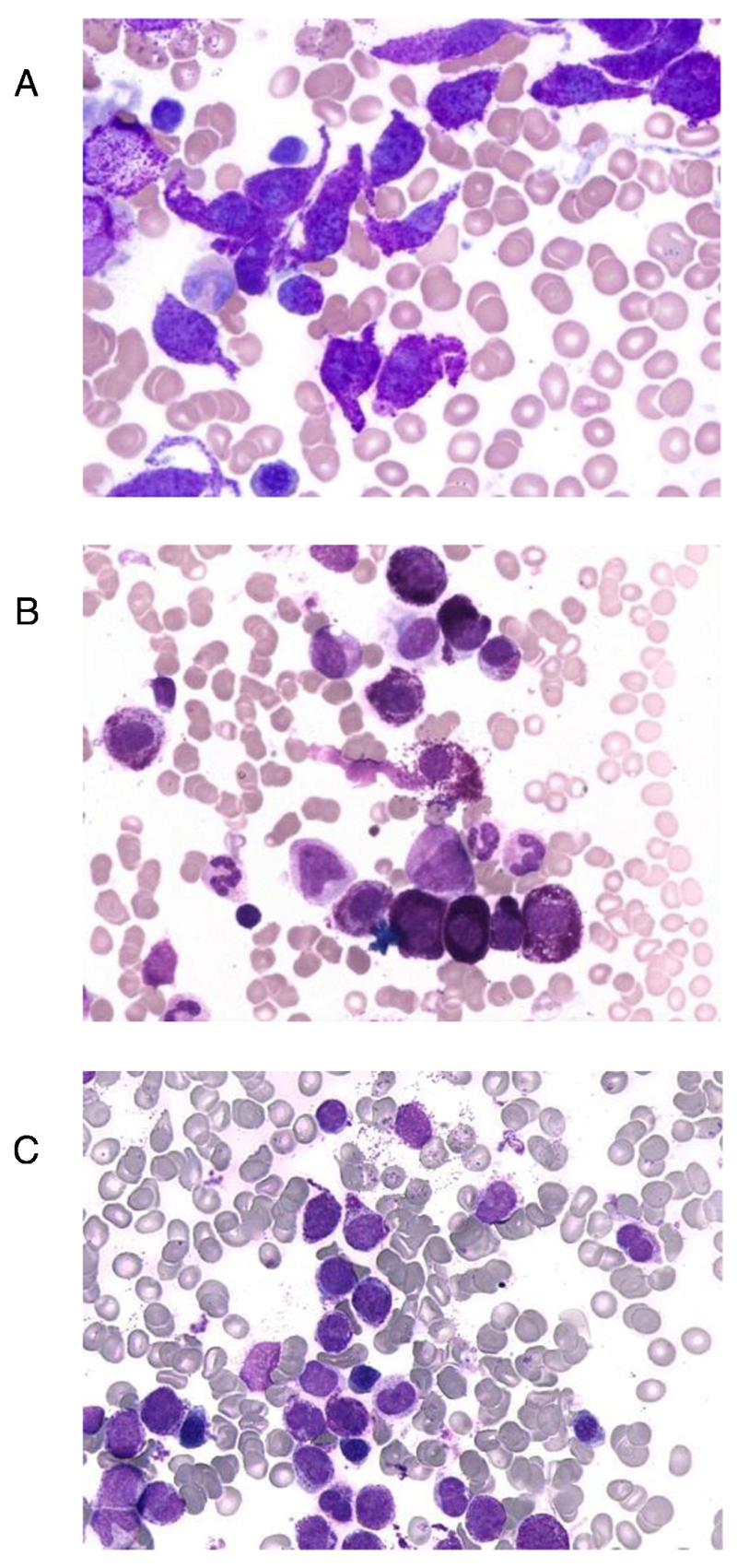
In the definition of MCL, at least 20% of all nucleated cells on BM smears are immature atypical MCs [1–4]. More recently, this definition has been extended to more mature (even round) MCs, and thus cases fulfilling criteria of the proposed new variant chronic MCL [14]. In fact, whereas in most patients with MCL, most MCs in BM smears are immature, there are also patients in whom a majority of all MCs have a more mature morphology, even when a leukemic infiltration is noted (Figure 1) [6,14]. Rarely, these cells even resemble mature, round tissue MCs which holds especially true for cases with well-differentiated SM. In other words, well-differentiated SM can manifest as, or progress into, chronic MCL. In some cases with chronic MCL, a majority of MCs may be spindle-shaped (Figure 1). By contrast, mature or spindle-shaped MCs are not found or are very rare in patients with acute MCL. Even in chronic MCL, MCs are usually immature. The most immature form, the metachromatically granulated blast, is often seen in acute MCL but is usually not detectable in patients with chronic MCL [14]. Immature MCs with bi- or multilobed nuclei may be detected in patients with acute MCL as well as in patients with chronic MCL [6]. All in all, a more mature morphology of MCs in MCL seems to correlate with a less aggressive (chronic) clinical course, consistent with the concept of a chronic leukemia (chronic MCL). However, this correlation needs to be confirmed in additional studies, and the detection of immature MCs does not exclude the presence of chronic MCL. On the other hand, the presence of metachromatic blasts makes the diagnosis ´chronic MCL´ very unlikely.
Figure 1.
Morphology of mast cells in chronic mast cell leukemia (MCL)
Bone marrow smears obtained from patients with chronic MCL (A, B) and acute MCL (C) were stained with Wright-Giemsa solution. In chronic MCL, mast cells are more mature cells, sometimes with a spindle-shaped (atypical) morphology (A) but often also with a mature morphology resembling normal tissue mast cells (B). By contrast, in patients with acute MCL, mast cells are immature and often represent metachromatically granulated blast cells (C).
Histomorphologic and immunohistochemical properties of MCs in chronic MCL
In most patients with MCL, the BM is heavily infiltrated by atypical, immature MCs which is demonstrable by histological and immunohistochemical staining of paraffin-embedded BM sections [1–4,14–17]. The MC infiltration is usually multifocal and dense with an additional diffuse component (mixed infiltration pattern) [14–18]. In contrast to patients with indolent SM, the normal architecture of the BM is completely replaced by the leukemic infiltrate. Leukemic MCs in advanced SM and MCL are usually immature and often lack chloroacetate esterase (CAE) [16,17]. In all patients with MCL, MCs co-express KIT and tryptase, and in most cases, MCs display CD25, confirming the close relationship between MCL and other categories of SM [16–19]. In many patients with MCL, leukemic MCs also express cytoplasmic CD30 and CD52 [20,21]. CD30 is aberrantly expressed in neoplastic MCs in patients with SM, and strong cytoplasmic expression of CD30 favours the diagnosis of advanced SM/MCL over indolent SM [21]. However, in a smaller group of patients with indolent SM, CD30 may be expressed in MCs, and there are also (rare) cases of advanced SM in whom MCs do not express CD30. Similarly, CD52 seems to be a marker of neoplastic MCs in advanced SM and MCL, but is not specific for MCL. All in all, no immunohistochemical marker can discriminate between MCL and other variants of SM or between acute MCL and chronic MCL with certainty. However, more recent data suggest that the proliferation-associated antigen Ki-67 is expressed differentially in MCs in patients with acute MCL and chronic MCL. In fact, whereas in chronic MCL, most MCs stain negative for Ki-67, MCs in acute MCL frequently display Ki-67 [14,22,23].
Cell surface phenotype of MCs in chronic MCL
The cell surface phenotype of MCs in MCL includes various myeloid determinants and is quite similar when compared to the cell surface membrane phenotype of MCs in other variants of SM [9,10,24–27]. Notably, leukemic MCs in MCL usually express CD9, CD25, CD33, CD44 and CD117 (KIT) [9,10,25–27]. In addition, MCs in MCL may express CD123 and HLA-DR. The CD2 antigen (LFA-2) is also detectable on MCs in a subset of patients with MCL [9,27]. However, compared to patients with indolent SM, the number of ´CD2-positive´ cases among MCL patients is low, and if detected on the surface of MCs, the levels of CD2 are usually lower compared to that found on MCs in indolent SM [25–27]. These observations suggest that CD2 expression may decrease during progression of SM into MCL which has been confirmed in individual patients with SM [27] as well as by an in vitro model of human MCL [28]. Other cell surface markers may be expressed at higher levels on MCs in MCL compared to indolent SM [25–27]. These surface antigens include, among others, CD52 and CD123 [25–27]. The CD30 antigen (Ki-1) can also be detected on MCs in MCL [29]. However, contrasting its potential diagnostic value as cytoplasmic antigen, cell surface expression of CD30 does not correlate with a particular type of SM [29]. An interesting aspect is that several of these cell surface antigens (CD30, CD52) serve as potential targets of therapy. So far, no cell surface markers that would safely discriminate between the acute and chronic form of MCL have been identified. However, sometimes chronic MCL seems to develop in patients with well differentiated SM (WDSM). In these patients, neoplastic MCs may be CD25-negative cells, contrasting other patients with MCL.
Molecular markers detectable in chronic MCL
In a vast majority of all patients with SM, activating point mutations in KIT are detectable [1–4,30–32]. In most patients with indolent SM, MCs display the KIT point mutation D816V [1–4]. In advanced SM, including aggressive SM and SM with an associated hematologic non-MC-lineage disease (SM-AHNMD), MCs also express KIT D816V in a high proportion of cases [1–4,32]. In patients with MCL, KIT mutations are also detected quite frequently. However, in these patients, neoplastic MCs display KIT D816V in only 50-70% of the cases. In the remaining MCL cases, other KIT mutations or no KIT mutations are found [33–36]. Other mutations in KIT include mutations in codon 816, such as D816H [22], but also mutations outside of codon 816 [33–36]. In patients with chronic MCL, only a few mutations have been described so far. In some of these patients, MCs again express KIT D816V. In other patients, rare mutations are detected. Some of these mutations are also detectable in the well-differentiated variants of SM. Another interesting aspect is that these mutations are sometimes found in the germline in these patients [37]. In one case, the somatic mutation KIT S476I was described [23]. This is a remarkable finding because the same mutation has also been reported in patients with indolent childhood SM [38]. From these observations one may conclude that chronic MCL may sometimes develop from a more indolent long-lasting pre-phase of (childhood-onset) SM. At the same time, this observation suggests that additional lesions in other genes must be responsible for the leukemic expansion of MCs in these patients which is consistent with recent studies [39–41]. However, whereas in chronic MCL a few additional lesions may be sufficient, many more lesions may be required to cause acute MCL. Deep sequencing studies are currently performed in order to test this hypothesis. This concept would also be supported by the observation that chronic MCL may progress into acute MCL after a few months or years [23].
Differential diagnosis to chronic MCL
The most obvious differential diagnosis to chronic MCL is acute MCL. In some of the patients with chronic MCL, C-Findings may develop rapidly, so that the final conclusion is the disease was acute MCL at first presentation but was diagnosed before any C-Findings developed. Another important differential diagnosis to consider is indolent SM with a well-differentiated morphology (also termed well-differentiated SM, WDSM). In fact, in several cases of WDSM, the numbers of MCs in BM smears are elevated. However, in most of these cases, MCs do not exceed 20%. If MCs in these cases exceed 20% of all BM cells on BM smears, the diagnosis is (or changes to) chronic MCL. Finally, it is important to differentiate chronic MCL from smouldering SM, aggressive SM, SM-AHNMD, myelomastocytic leukemia, tryptase+ acute myeloid leukemia (AML), chronic basophilic leukemia and reactive MC hyperplasia [14,42]. Table 2 provides a summary of differential diagnoses that have to be considered in patients with suspected chronic MCL.
Table 2.
Differential diagnoses to chronic MCL
| Differential diagnosis | Discriminating feature(s) |
|---|---|
| Acute MCL | C-Findings and presence of immature MCs and metachromatic blast cells Very high and rapidly increasing tryptase KIT mutations, often KIT D816V, are found |
| Smouldering SM | MCs are less than 20% in BM smears High or very high but stable tryptase levels KIT D816V is detected in almost all cases |
| Myelomastocytic leukemia | Immature MCs and metachromatic blast cells, C-Findings and underlying myeloid neoplasm Slightly to moderately elevated tryptase KIT D816V is not detectable by definition |
| Chronic basophilic leukemia | Expression of BB1, 2D7 and CD123 on neoplastic cells and absence of KIT (CD117) Slightly to moderately elevated tryptase KIT D816V is not detectable in neoplastic cells |
| Tryptase+ AML | Myeloblasts predominate, MCs are less than 10% in BM smears Tryptase levels vary from low to very high KIT D816V is not detectable in most cases |
MCL, mast cell leukemia; MCs, mast cells; BM, bone marrow; AML, acute myeloid leukemia.
Clinical course in chronic MCL
The clinical course of patients with chronic MCL is indolent and thus resembles the course in patients with smouldering SM, a subtype of indolent SM characterized by a huge burden of MCs and organomegaly [1–4]. Indeed, smouldering SM and chronic MCL have several features in common, such as the huge burden of MCs, splenomegaly or a high but stable tryptase level. In the proposed chronic form of MCL, first clinical signs may be non-characteristic so that it may take some time until the diagnosis is established. This is also because in contrast to indolent SM and smouldering SM, patients with chronic MCL usually have no skin lesions. In other patients, a well-differentiated SM is diagnosed several months or years (of prephase) before the diagnosis of chronic MCL is established. Signs of organ damage, such as cytopenia, liver involvement with ascites or osteolyses (C-Findings) are not recorded in chronic MCL. As soon as such C-findings are detected, the diagnosis changes from chronic to acute MCL. The clinical course in patients with chronic MCL is unpredictable. Some of these patients have an indolent course for several months or even years. Other patients progress to acute MCL within short time. In most cases reported so far, progression to acute MCL was seen. Whether some of these patients can also progress to MCL-AHNMD, especially acute myeloid leukemia (SM-AML) remains at present unknown. Mediator-related symptoms are usually found in patients with chronic MCL. Like in other SM variants, the type and impact of these symptoms vary from patient to patient.
Therapeutic options
The prognosis of patients with MCL is poor which is mostly because MCs in these patients are largely resistant against conventional drugs and targeted drugs, including tyrosine kinase inhibitors (TKI) directed against KIT D816V such as PKC412 (midostaurin) [4–10,43–45]. In patients with acute MCL, poly-chemotherapy followed by allogeneic stem cell transplantation (SCT) is usually recommended as only curative therapy-option for young and fit patients [46]. For older patients, chemotherapy, cladribine (2CdA) and targeted drugs are considered. In patients with chronic MCL, targeted drugs (alone or combined with chemotherapy) may be an option. Some of these patients respond to KIT-targeting TKI. In each case, it is important to define the KIT mutation status before TKI therapy. In those with KIT D816V, PKC412 is usually recommended. Other mutant forms of KIT may respond to imatinib or other KIT blockers [33–37]. One strategy may be to induce remission with chemotherapy and to maintain the response with TKI therapy as long as possible. Some of these patients may still relapse and progress to acute MCL. For these patients, re-induction-chemotherapy and SCT may be an option. In each case, debulking should be performed prior to SCT if possible. Palliative therapy should be performed using hydroxyurea. Several of these patients may benefit from a combination of hydroxyurea and prednisolone.
Conclusions and future perspectives
Chronic MCL is a recently proposed sub-variant of MCL that has to be differentiated from acute MCL based on the absence of severe organ damage (C-Findings). Some of these patients have a stable clinical course for several years, whereas others progress to acute MCL within a few months. There is also an apparent correlation between the silent clinical course and the relatively mature morphology of MCs in these patients. So far, little is known about the molecular basis of chronic MCL. However, it seems as if the number of pro-oncogenic lesions is lower in these patients compared to acute MCL. The proposed delineation of chronic from acute MCL may have clinical implications. However, further studies are required to confirm this newly proposed concept and its clinical value.
Acknowledgements
This study was supported by the Austrian Science Fund (FWF) grant #SFB-F4704-B20 and a research grant of The Mastocytosis Society (TMS). The authors declare that they have no conflict of interest in this study and paper.
Supported by: Austrian Science Fund (FWF) grant #SFB-F4704-B20 and a research grant of The Mastocytosis Society (TMS)
Footnotes
Contributions
All co-authors contributed by establishing the concept, by participating in essential discussions, by writing parts of the manuscript and by correcting and approving the final version of the manuscript.
Conflict of Interest Statements
The authors declare that they have no conflict of interest in this study and paper.
References
- 1.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Horny H-P, Li CY, Longley JB, Metcalfe DD, Parwaresch RM, Bennett JM. Mastocytosis (mast cell disease) In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization (WHO) Classification of Tumours Pathology & Genetics Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon France: 2001. pp. 291–302. [Google Scholar]
- 3.Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, Valent P, Bain B. Mastocytosis (mast cell disease) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization (WHO) Classification of Tumours. Pathology & Genetics Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 54–63. [Google Scholar]
- 4.Valent P, Akin C, Sperr WR, Horny HP, Metcalfe DD. Mast cell proliferative disorders: current view on variants recognized by the World Health Organization. Hematol Oncol Clin North Am. 2003;17:1227–41. doi: 10.1016/s0889-8588(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Li CY, Hoagland HC, Travis LB, Banks PM. Mast cell leukemia: report of a case and review of the literature. Mayo Clinic Proceedings. 1986;61:957–66. doi: 10.1016/s0025-6196(12)62636-6. [DOI] [PubMed] [Google Scholar]
- 6.Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, Valent P. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–36. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 7.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 8.Pardanani A, Lim KH, Lasho TL, Finke C, McClure RF, Li CY, Tefferi A. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114(18):3769–72. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 9.Dalton R, Chan L, Batten E, Eridani S. Mast cell leukaemia: evidence for bone marrow origin of the pathologic clone. British Journal of Haematology. 1986;64(2):397–406. doi: 10.1111/j.1365-2141.1986.tb04133.x. [DOI] [PubMed] [Google Scholar]
- 10.Baghestanian M, Bankl HC, Sillaber C, Beil WJ, Radaszkiewicz T, Füreder W, Preiser J, Vesely M, Schernthaner G, Lechner K, Valent P. A case of malignant mastocytosis with circulating mast cell precursors: biologic and phenotypic characterization of the malignant clon. Leukemia. 1996;10(1):159–166. [PubMed] [Google Scholar]
- 11.Teodosio C, García-Montero AC, Jara-Acevedo M, Sánchez-Muñoz L, Alvarez-Twose I, Núñez R, Schwartz LB, Walls AF, Escribano L, Orfao A. Mast cells from different molecular and prognostic subtypes of systemic mastocytosis display distinct immunophenotypes. J Allergy Clin Immunol. 2010;125:719–726. doi: 10.1016/j.jaci.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Pagano L, Valentini CG, Caira M, Rondoni M, Van Lint MT, Candoni A, et al. Advanced mast cell disease: an Italian Hematological Multicenter experience. Int J Hematol. 2008;88:483–8. doi: 10.1007/s12185-008-0166-4. [DOI] [PubMed] [Google Scholar]
- 13.Chan EC, Bai Y, Kirshenbaum AS, Fischer ER, Simakova O, Bandara G, et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus propos. Ann Oncol. 2014 doi: 10.1093/annonc/mdu047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horny HP, Parwaresch MR, Lennert K. Bone marrow findings in systemic mastocytosis. Hum Pathol. 1985;16:808–14. doi: 10.1016/s0046-8177(85)80252-5. [DOI] [PubMed] [Google Scholar]
- 16.Horny HP, Sillaber C, Menke D, Kaiserling E, Wehrmann M, Stehberger B, et al. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol. 1998;22:1132–40. doi: 10.1097/00000478-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001;25:543–51. doi: 10.1016/s0145-2126(01)00021-2. [DOI] [PubMed] [Google Scholar]
- 18.Krokowski M, Sotlar K, Krauth MT, Födinger M, Valent P, Horny HP. Delineation of patterns of bone marrow mast cell infiltration in systemic mastocytosis: value of CD25, correlation with subvariants of the disease, and separation from mast cell hyperplasia. Am J Clin Pathol. 2005;124:560–68. doi: 10.1309/CX45R79PCU9HCV6V. [DOI] [PubMed] [Google Scholar]
- 19.Sotlar K, Horny HP, Simonitsch I, Krokowski M, Aichberger KJ, Mayerhofer M, Printz D, Fritsch G, Valent P. CD25 indicates the neoplastic phenotype of mast cells: a novel immunohistochemical marker for the diagnosis of systemic mastocytosis (SM) in routinely processed bone marrow biopsy specimens. Am J Surg Pathol. 2004;28:1319–25. doi: 10.1097/01.pas.0000138181.89743.7b. [DOI] [PubMed] [Google Scholar]
- 20.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, Valent P, Horny HP. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585–95. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 21.Hoermann G, Blatt K, Greiner G, Putz EM, Berger A, Herrmann H, et al. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J. 2014 doi: 10.1096/fj.14-250894. in press. [DOI] [PubMed] [Google Scholar]
- 22.Valent P, Blatt K, Eisenwort G, Herrmann H, Cerny-Reiterer S, Thalhammer R, et al. FLAG-induced remission in a patient with acute mast cell leukemia (MCL) exhibiting t(7;10)(q22;q26) and KIT D816H. Leuk Res Rep. 2013;3:8–13. doi: 10.1016/j.lrr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valent P, Berger J, Cerny-Reiterer S, Eisenwort G, Peter B, Hoermann G, et al. A case of chronic mast cell leukemia: an extremely rare entity defined by mast cell maturation and absence of severe organ damage. Ann Hematol. 2014 doi: 10.1007/s00277-014-2207-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escribano L, Orfao A, Díaz-Agustin B, Villarrubia J, Cerveró C, López A, Marcos MA, Bellas C, Fernández-Cañadas S, Cuevas M, Sánchez A, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998;91:2731–36. [PubMed] [Google Scholar]
- 25.Escribano L, Díaz-Agustín B, Bellas C, Navalón R, Nuñez R, Sperr WR, Schernthaner GH, Valent P, Orfao A. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk Res. 2001;25:563–70. doi: 10.1016/s0145-2126(01)00050-9. [DOI] [PubMed] [Google Scholar]
- 26.Escribano L, Orfao A, Villarrubia J, Martín F, Madruga JI, Cuevas M, Velasco JL, Rios A, San Miguel JF. Sequential immunophenotypic analysis of mast cells in a case of systemic mast cell disease evolving to a mast cell leukemia. Cytometry. 1997;30:98–102. doi: 10.1002/(sici)1097-0320(19970415)30:2<98::aid-cyto4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Teodosio C, García-Montero AC, Jara-Acevedo M, Sánchez-Muñoz L, Pedreira CE, Álvarez-Twose I, Matarraz S, Morgado JM, Bárcena P, Matito A, Mayado A, et al. Gene expression profile of highly purified bone marrow mast cells in systemic mastocytosis. J Allergy Clin Immunol. 2013;131:1213–24. doi: 10.1016/j.jaci.2012.12.674. [DOI] [PubMed] [Google Scholar]
- 28.Schernthaner GH, Jordan JH, Ghannadan M, Agis H, Bevec D, Nuñez R, et al. Expression, epitope analysis, and functional role of the LFA-2 antigen detectable on neoplastic mast cells. Blood. 2001;98:3784–92. doi: 10.1182/blood.v98.13.3784. [DOI] [PubMed] [Google Scholar]
- 29.Morgado JM, Perbellini O, Johnson RC, Teodósio C, Matito A, Álvarez-Twose I, et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63:780–7. doi: 10.1111/his.12221. [DOI] [PubMed] [Google Scholar]
- 30.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci (USA) 1995;92:10560–4. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P, Födinger M. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357–64. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 32.Bibi S, Langenfeld F, Jeanningros S, Brenet F, Soucie E, Hermine O, Damaj G, Dubreuil P, Arock M. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol Allergy Clin North Am. 2014;34:239–62. doi: 10.1016/j.iac.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Mital A, Piskorz A, Lewandowski K, Wasag B, Limon J, Hellmann A. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur J Haematol. 2011;86:531–35. doi: 10.1111/j.1600-0609.2011.01598.x. [DOI] [PubMed] [Google Scholar]
- 34.Joris M, Georgin-Lavialle S, Chandesris MO, Lhermitte L, Claisse JF, Canioni D, Hanssens K, Damaj G, Hermine O, Hamidou M. Mast Cell Leukaemia: c-KIT Mutations Are Not Always Positive. Case Rep Hematol. 2012;2012:517546. doi: 10.1155/2012/517546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgin-Lavialle S, Lhermitte L, Suarez F, Yang Y, Letard S, Hanssens K, et al. Mast cell leukemia: identification of a new c-Kit mutation, dup(501-502), and response to masitinib, a c-Kit tyrosine kinase inhibitor. Eur J Haematol. 2012;89:47–52. doi: 10.1111/j.1600-0609.2012.01761.x. [DOI] [PubMed] [Google Scholar]
- 36.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 37.Chan EC, Bai Y, Kirshenbaum AS, Fischer ER, Simakova O, Bandara G, et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodemer C, Hermine O, Palmérini F, Yang Y, Grandpeix-Guyodo C, Leventhal PS, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–815. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 39.Tefferi A, Levine RL, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. Frequent TET2 mutations in systemic mastocytosis: clinical, KITD816V and FIP1L1-PDGFRA correlates. Leukemia. 2009;23:900–904. doi: 10.1038/leu.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson TM, Maric I, Simakova O, Bai Y, Chan EC, Olivares N, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–63. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–66. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 42.Sperr WR, Horny HP, Lechner K, Valent P. Clinical and biologic diversity of leukemias occurring in patients with mastocytosis. Leuk Lymphoma. 2000;37:473–86. doi: 10.3109/10428190009058500. [DOI] [PubMed] [Google Scholar]
- 43.Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, Bennett JM, Metcalfe DD. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27:635–41. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 44.Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–70. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121:1285–95. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 46.Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic Stem-Cell Transplantation for Advanced Systemic Mastocytosis. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.55.2018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]