SUMMARY
Objective
To determine if type III collagen is concentrated in the chymotrypsin-extractable collagen pool from osteoarthritic articular cartilage to assess its potential as a biomarker of Osteoarthritis (OA) pathogenic mechanisms.
Methods
Full thickness articular cartilage from grossly normal surfaces was analyzed from femoral heads, obtained at hip replacement surgery, from OA (n = 10) and fracture (n = 10) patients. Collagen, extracted by α-chymotrypsin, was characterized by SDS-PAGE/Western blot analysis, ELISA and immunohistochemistry using monoclonal antibodies specific to collagens types II and III.
Results
α-Chymotrypsin extracted more collagen from OA than control cartilage. The extractable pool included collagen types II and III from both OA and control hips. Importantly, OA cartilage contained 6-fold more collagen type III than control cartilage, based on ELISA. The estimated total tissue ratio of collagen III/II was in the 1–10% range for individual OA cartilage samples, based on pepsin-solubilized collagen using SDS-PAGE densitometry. Collagen type III N-propeptide trimers were the main molecular fragments seen on Western blot analysis of OA and control extracts. The chymotrypsin-extracted type II collagen gave primarily full-length α1(II) chains and chain fragments of α1(II) on Western blot analysis from both OA and control tissues. Immunohistochemistry showed that type III collagen was more concentrated in the upper half of OA cartilage and in the territorial matrix around individual chondrocytes and chondrocyte clusters.
Conclusions
The findings confirm that collagen type III deposition occurs in adult articular cartilage but significantly more pronounced in osteoarthritic joints, presenting a potential marker of matrix repair or pathobiology.
Keywords: Hip osteoarthritis, Articular cartilage, Collagen type II and type III
Introduction
Osteoarthritis (OA) is a multifactorial disease involving the whole joint in which loss of cartilaginous matrix is a fundamental feature. Chondrocytes have great potential to replace the proteoglycan components of the matrix. However, in the late stages of OA when collagen damage is far advanced, any repair attempt appears to be ineffective leading eventually to joint failure.
The collagen network of mature human articular cartilage develops from a heteropolymeric microfibril network of type II collagen polymerized on and covalently bound to a type XI collagen template and to type IX collagen molecules, which coat initial fibril surfaces1. Once laid down during development, there is little evidence that articular chondrocytes can recapitulate the overall collagen network architecture if the mature tissue is damaged by injury or degeneration. Collagen turnover in mature articular cartilage is very slow. A turnover time of 400 years for collagen of human femoral head cartilage was estimated from the synthetic rate of hydroxyproline2. However, some remodeling can occur3. It is possible that the chondrocytes can remodel micro-anatomical domains of collagenous matrix (e.g., replacing fibril-surface molecules, damaged fibrils or pericellular collagen) more rapidly than bulk collagen of the inter-territorial matrix1.
Several studies have shown increased synthesis of collagen in surgically induced OA in animals and in human OA cartilage4–6. Type II collagen was the major new product on radiolabeling in vivo and, though the data ruled out type I collagen6, left open the possibility of other collagen types being expressed including type III collagen. Since then, direct evidence has been provided for the appearance of type III collagen in the matrix of adult articular cartilage7.
Molecular analysis of the pool of extractable collagen showed the presence of collagen type III covalently linked to collagen type II in the matrix of human knee OA cartilage8. The findings indicated that pN-type III molecules were self-polymerized and covalently cross-linked to the surface of type II collagen fibrils in the extracellular matrix. This would be consistent with the concept that retained N-propeptides on the surface of procollagen prevents lateral growth of fibrils in the process of assembly9,10. Transmission electron-microscopy, using immunogold, showed type III collagen on the surface of banded type II collagen fibrils in human articular cartilage11. Low but increasing amounts of type III collagen were also detected in normal adult and OA human articular cartilage, where it was concentrated around chondrocytes throughout the depth7 or in the surface and upper mid-zones of OA cartilage12. Based on mRNA analysis, the expression of collagen type III was associated with expression of collagen type II but not collagen type I in OA cartilage12. Together these various findings indicate a metabolic response of chondrocytes to deposit collagen type III in regions of articular cartilage, presumably as a response to mechanical injury or other matrix damage. The effect may be akin to the wound-healing role of collagen type III in skin and other collagen type I-based connective tissues.
A previous study has shown that α-chymotrypsin digestion extracts more collagen from cartilage of OA than control joints13. α-Chymotrypsin is believed not to attack the native triple-helical domain of types I and II collagen molecules below the denaturation temperature of the triple-helix. Based on the immunochemical detection of type II collagen breakdown products in such extracts, it was concluded that chymotrypsin extracts a denatured pool of type II collagen, which may already be proteolytically cleaved14. However, it is known that native collagen type III, unlike collagen types I and II, is susceptible to cleavage by trypsin, and potentially chymotrypsin, in the domain of labile triple-helix which contains the site where tissue collagenase cleaves15. Chymotrypsin is also a candidate telopeptidase, so it could, in theory, depolymerize and solubilize native type II collagen molecules by crosslink breaking cleavages in telopeptide domains.
In a study of cartilage from osteoarthritic femoral heads, more than twice as much collagen was extracted by chymotrypsin than from non-osteoarthritic femoral heads13. The molecular nature of this extractable collagen has not been characterized. Therefore, the present study was designed to examine the possibility that collagen type III was prominent in it, to determine the size of the molecular fragments and to explore the potential for insights in OA pathogenesis and the potential for a novel biomarker of the OA process. The availability of well-characterized sets of femoral heads from clinical OA and osteoporotic fracture patients undergoing hip replacement surgery made this collaborative study possible.
Methods
Patient tissue source
Femoral heads (10 OA and 10 controls) were obtained at total hip replacement surgery from either patients with OA or femoral neck fracture (i.e., controls). After surgery all femoral heads were kept frozen at −80°C. The femoral neck fracture patients were mostly elderly females and had typical osteoporotic neck fractures. Cases of known RA and secondary OA were excluded from the study. The cartilage of OA samples, obtained from the OA patients at total hip replacement surgery, had Kellgren and Lawrence grade 2 or more.
Cartilage samples from five OA (patient age-range 60–80 yr, four female) and five control femoral heads (patient age-range 78–87 yr, five female) were analyzed for total collagen content and chymotrypsin-extractable collagen (Experiment 1) (Table I). Cartilage samples from another five OA (patient age-range 50–80 yr, four female) and five control heads (patient age-range 53–87 yr, two female) were used to study pepsin-extracted collagen (Experiment 2) (Table II). The sampled area of cartilage was roughly 10 mm2 taking full-depth from visibly intact articular surfaces of both fracture control and OA heads (osteophytes and joint margins were avoided).
Table I.
Yields of chymotrypsin-extractable collagen from OA and control cartilage
| OA (n = 5) | Non-OA (n = 5) | P-value* | Difference OA & Non-OA | Age adjusted P-value |
Age adjusted-estimate of difference |
|
|---|---|---|---|---|---|---|
| Age (year) | 69 (66.7–74.7) | 81 (79.5–85.5) | 0.024 | −12 (−1 to 21) | ||
| Total hydroxyproline (µg/mg wet wt. cartilage) | 23.8 (14.5–24.3) | 23.6 (21.5–28.2) | 0.548 | −4.04 (−13.26–6.78) | 0.66 | −2.06 (−12.7–8.6) |
| % Extracted hydroxyproline | 0.7 (0.6–0.9) | 0.2 (0.2–0.4) | 0.008 | 0.45 (0.18–0.83) | 0.097† | 0.28 (−0.0066–0.57) |
| Relative type III collagen content of extracts by ELISA (µg human collagen type III enriched standard/100 mg cartilage) |
81 (51.3–134.8) | 14.7 (10.1–19.5) | 0.008 | 73.4 (30.2–130) | 0.005† | 80.7 (42.2–119) |
Values are medians (25–75 percentiles).
Unadjusted; using exact Wilcoxon–Mann–Whitney test.
Weighted model.
Table II.
Proportion of collagen III in OA and control cartilage
| OA (n = 5) | Non-OA (n = 5) | P-value | Difference OA & Non-OA | Age-adjusted P-value | Age-adjusted difference | |
|---|---|---|---|---|---|---|
| Age (year) | 70 (62–79.2) | 80 (72.5–85.5) | 0.198 | −8 (−30–17) | ||
| % Collagen type III (III/II + III in pepsin extract) |
5 (0.8e8.1) | 0† | 0.025 | 5 (0–9.1) | 0.049 | 3.8 (0.025–7.6)* |
Values are medians (25–75 percentiles).
NOTE: Difference is the Lehmann–Hodges estimator of the shift in medians; whereas the adjusted difference is difference in means adjusted for baseline. Both differences are with 95% confidence intervals in both Tables I and II.
Adjusted for age by group.
No detectable band with NIH ImageJ software.
The study was approved by the Ethical Review Board at Lund University and each patient's informed consent was obtained.
Collagen extraction procedures
Experiment 1
Five OA and five control cartilage samples were finely diced and digested with α-chymotrypsin (1 mg/50 mg wet weight cartilage) in 0.05 M Tris/HCl buffer, pH 7.6 containing protease inhibitors (1mMEDTA,1mMiodoacetamide and 10 µg/ml Pepstatin-A) at 32°C for 16 h to extract susceptible collagen, essentially as previously described13. The supernatants were removed, clarified by centrifugation and aliquots of extracts and tissue residues were acid hydrolyzed and assayed for hydroxyproline, as described13,16. Additional aliquots of supernatants were freeze-dried and used for competition ELISA, SDS-PAGE and Western blot analysis using antibodies specific to collagen types II and III.
Experiment 2
Five full thickness OA and five control cartilage samples were powdered under liquid N2, then extracted in 4 M guanidine HCl, 0.05MTris/HCl, pH 7.5, at 4°C for 24 h to remove proteoglycans and other matrix proteins. The residues were stored at −20°C for pepsin digestion. Collagen was solubilized by pepsin in 3% (v/v) acetic acid for 24 h at 4°C and then run on SDS-6%PAGE to resolve collagen types II and III for quantitation by densitometry.
SDS-polyacrylamide electrophoresis was run according to Laemmli17 with 6% and 12.5% gels. Proteins bands were stained with Coomassie Brilliant Blue or transblotted to PVDF membrane for Western blot analysis, using the anti-type II and anti-type III collagen-specific mouse monoclonal antibodies. mAb 1C10 binds to a sequence-specific epitope in the denatured α1(II) triple-helical domain (res 934–945)18 and mAb 4G9 binds to a conformational epitope in the collagen type III N-propeptide globular domain8. Westerns were developed after primary antibody incubation using alkaline phosphatase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, Avondale, PA) and 5-bromo-4chloro-3-indolyl phosphate/nitro blue tetrazolium as substrate for alkaline phosphatase.
Competition ELISA
Type III collagen was quantified in α-chymotrypsin extracts by competition ELISA in 96-well plates (Nunc MaxiSorp from Thermo Scientific). The coating antigen was a 1 µg/ml solution of pepsinsolubilized and denatured human type III collagen. Wells were blocked with 50 mM HEPES, 0.2% BSA, 0.2% Tween. Standards were serial dilutions of the same collagen type III-enriched preparation in 50 mM HEPES, 0.2% BSA, 0.2% Tween. Equal volumes of mAb 4G99 were added and the plates rocked for 20 h at 4°C. Goat antimouse secondary antibody conjugated to horseradish peroxidase was added for 2 h and then developed using tetramethylbenzidine. Plates were read at 450 nm after acidification with1 M H2SO4 (Titertek Multiskan-plus plate reader). Results were expressed as relative units using the equivalent concentration of total collagen in the standard/coat solution.
Gel electrophoresis and mass spectrometry
Stained protein bands resolved by SDS-PAGE gel electrophoresis under reducing conditions were cut out and subjected to in-gel trypsin digestion19. Electrospray mass spectrometry was performed on the tryptic peptides using an LCQ Deca XP ion-trap mass spectrometer equipped with in-line microbore LC (Thermo-Finnigan) using a C8 capillary column (0.3 × 150 mm; Grace Vydac 208MS5.315) eluted at 4 µl/min. The LC mobile phase consisted of Buffer A (0.1% formic acid in MilliQ water) and Buffer B (0.1% formic acid in 3:1 acetonitrile:n-propanol, v/v). Sequest search software (ThermoFinnigan) was used for peptide identification using the NCBI protein database.
Immunohistochemistry
For immunohistochemical localization of collagen type III, 4 µm thick cartilage sections were pre-treated (deparaffinization, rehydration and epitope retrieval) using the EnVisionFlex High pH-Kit (DAKO, Copenhagen, Denmark) and then stained in an Autostainer Plus (DAKO; Glostrup, Copenhagen, Denmark) with the mAb 4G9 antibody diluted 1:1000.
Statistical analysis
Exact Wilcoxon–Mann–Whitney tests were used to compare outcomes20. The Lehmann–Hodges–Sen estimator was used for median difference between the two groups, presented with confidence intervals. Age-adjusted analyses were made with linear regression. In the case of obvious deviations from homoscedasticity, we used a weighted model where increasing variances with increasing means were adjusted for in a generalized least-squares model21,22. P-values less than or equal to 0.05 were considered significant.
Results
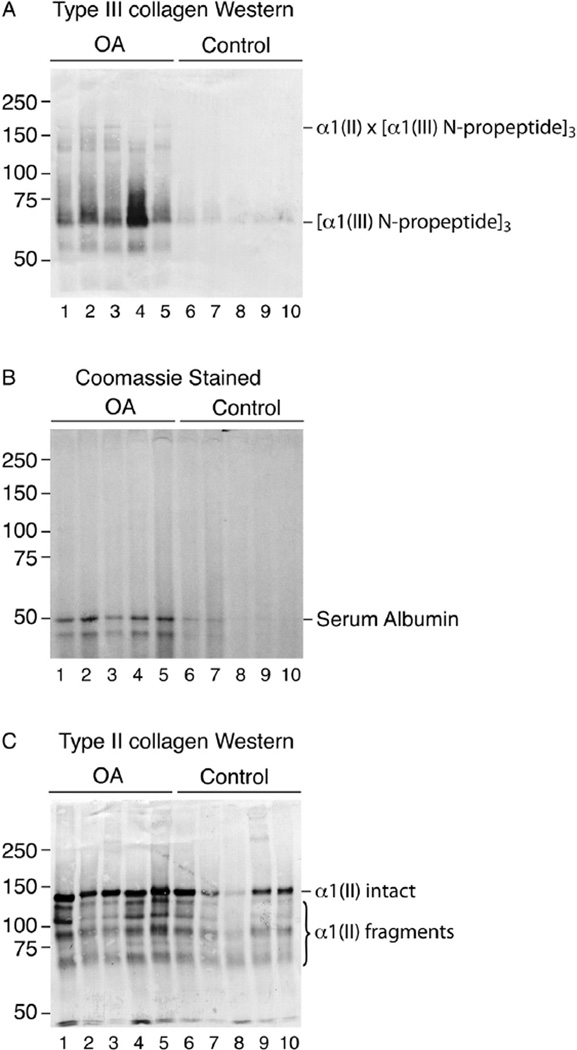
Table I shows similar amounts of total hydroxyproline in the OA and non-OA cartilages. Based on SDS-PAGE/Western blot analyses, fragments of collagen types II and III were a prominent finding in chymotrypsin extracts of OA cartilage (Fig.1). Control cartilage from non-OA femoral heads of hip fracture cases gave lower yields of total extractable collagen and strikingly less type III collagen on Western blot analysis (Table I). Figure 1 shows the difference between five control and five OA patient samples on Western blotting. An α1(III) N-propeptide trimer band dominates in the mAb 4G9 Western patterns [Fig. 1(A)]. The prominent sharp bands that run close to 150 kDa consist of N-propeptide trimers cross-linked to intact α1(II) chains8.
Fig. 1.
Western blot analysis of collagen type III degradation products in α-chymotrypsin extracts of OA and control femoral head cartilage samples (A). Extracts of full thickness tissue samples from individual hips were run on SDS-6%PAGE, blotted to PVDF membrane and Westerns developed using anti-type III collagen antibody (A) or stained with Coomassie blue to visualize total protein (B). Serum albumin is the only prominent protein by staining (confirmed by mass spectrometry) and is more prominent in the OA cartilage, presumably because of increased penetration from synovial fluid. Collagen type III N-propeptide trimers are consistently enriched in α-chymotrypsin extracts of OA cartilage compared with controls. Western blot analysis of collagen type II degradation products in chymotrypsin extracts of OA and control femoral head cartilage samples (C). A replicate SDS-6%PAGE gel to that shown in Fig. 1(A) was blotted to PVDF for Western development using mAb 1C10, which recognizes a sequence-specific epitope in the α1(II) chain. The main bands are essentially an intact α1(II) chain and large fragments of it.
Quantitation using the same mAb 4G9 in ELISA format gave a median of almost six fold more type III collagen in extracts from 5 OA than in 5 control tissue samples (Table I). On analysis of the same extracts by SDS-PAGE using an antibody against type II collagen, intact α1(II) chains dominated together with large chain fragments of α1(II) that contain the sequence-specific epitope [Fig. 1(C)].
The antibody mAb 1C10 recognizes a sequence-specific epitope (residues 934–945) in the triple-helical domain of α1(II), so all chain fragments containing this sequence are detected.
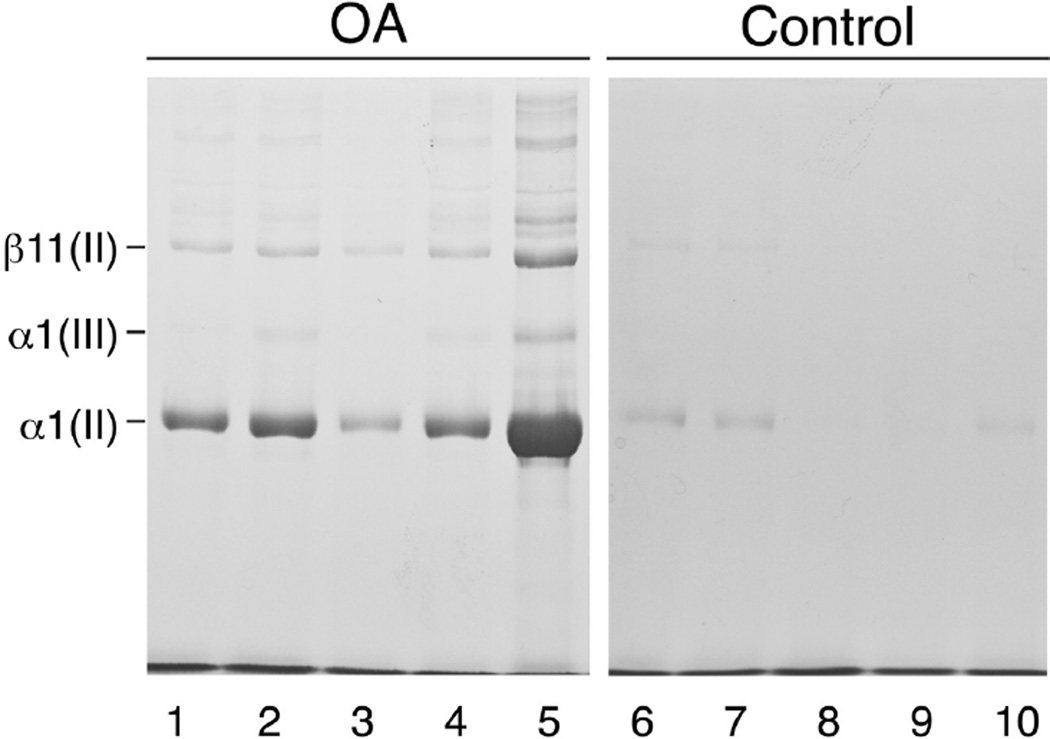
The ratio of type III to type II collagen in full thickness OA cartilage samples (second experiment) was estimated by densitometry of bands after SDS-PAGE of the pepsin-solubilized collagen pool. Cartilage was powdered using liquid N2 to maximize yields. Figure 2 shows the results of interrupted electrophoresis (DTT was added to sample wells after 20 min) to resolve α1(III) and α1(II) chains. Based on the α1(III)/α1(II) chain ratio by densitometry, type III collagen ranged from 1 to 8% of the collagen content of OA samples and below the limit of quantitation (<1%) in fracture control samples (Table II). In-gel trypsin digestion followed by iontrap mass spectrometry confirmed the identity of the human α1(III) chain (data not shown). Figure 2 also shows no detectable type I collagen as evidenced by lack of a detectable α2 (I) chain in the pepsin-solubilized collagen pools. This confirms a lack of any fibrocartilage repair tissue in the samples consistent with the normal surface appearance of the full-thickness sites from which articular cartilage was sampled.
Fig. 2.
Electrophoretic resolution of pepsin-solubilized collagen types II and III from OA and control femoral head cartilage samples. SDS-6%PAGE was run using delayed DTT addition to separate α1(III) and α1(II) chain components. Protein was stained using Coomassie blue, revealing only type II and III collagen chains. Collagen type I is absent based on the lack of α2(I) which would run below α1(II).
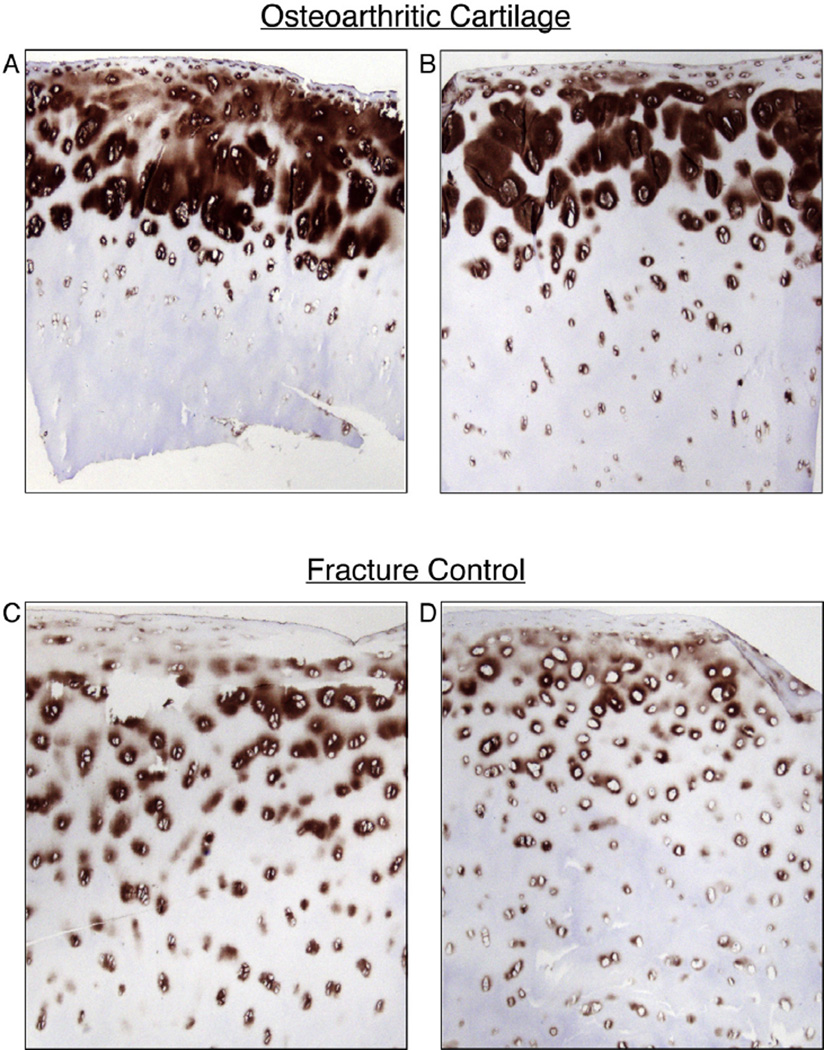
Immunohistochemistry using mAb 4G9 was applied to tissue sections from all femoral heads used in the biochemical analyses and from additional OA and control heads. Figure 3(A) and (B) shows representative sections of cartilage from two OA heads and Fig. 3(C) and (D) from two control heads. The results consistently showed that type III collagen was most concentrated in the upper third of OA femoral head articular cartilage and predominantly in the territorial matrix surrounding individual chondrocytes and chondrocyte clusters [Fig. 3(A) and (B)]. The lower concentration in articular cartilage from control heads seen by Western blot was mirrored consistently in the immunohistochemistry analysis. Here type III collagen was more sparsely distributed but still focally around cells and in the top half of the tissue [Fig. 3(C) and (D)]. This focal distribution strongly implies biosynthesis and deposition by the chondrocytes themselves.
Fig. 3.
Immunohistochemical localization of collagen type III in full-thickness sections of femoral head cartilage from OA and fracture control hip joints. The mAb 4G9 was used as primary antibody to detect collagen III in full depth sections of frozen articular cartilage from OA and control joints. Representative sections from two OA samples, A (84 yr, female), B (62 yr, male) and two controls, C (59 yr, male), D (79 yr, female) are shown. Collagen type III is more prominent in cartilage from OA than control femoral heads consistent with the extracted collagen findings. In both it is most concentrated in territorial matrix surrounding cells in the upper half of the tissue.
In order to examine possible impact of age on molecular contents, the OA and non-OA cartilage results were age adjusted (Tables I and II). Results did not suggest that age was a significant factor affecting molecular contents.
Discussion
An inherent difficulty with biochemical studies of articular cartilage in human OA is in obtaining well-defined tissue samples and appropriate control tissue. The strength of the present findings lies in comparing cartilage with no signs of OA, obtained from fracture patients, with cartilage obtained from visibly intact articular surfaces of OA patients. In a previous study we have shown that the average Mankin grades of full-depth, intact looking cartilage from control and osteoarthritic were two and four, on a scale of 13, respectively13. In the present study it was, therefore, possible to compare normal appearing cartilage from both OA and fracture control hips as others have studied biochemically for noncollagenous matrix components23.
The findings in the present study build on previous reports that type III collagen can make an appearance with aging in articular cartilage of mature joints7. We extend previous findings by establishing a significant type III collagen increase in OA cartilage compared with control samples. This was obvious initially on Western blot analysis of the small pool of collagen extracted by αchymotrypsin (Fig. 1). More collagen was extracted from OA than control cartilage (Table I), as also previously reported13. Quantitation of the extracted collagen III by mAb ELISA showed a median of almost six fold higher yield from OA than control tissue samples (Table I). The antibody used for both the ELISA assay and the Western blot analysis recognizes a conformation-specific epitope in the collagen type III N-propeptide globular cysteine-rich domain (disulfide cleavage destroys the epitope – data not shown)8. The molecular size of the main bands on the Western blot (Fig. 1) is consistent with disulfide-bonded trimers of the α1(III) N-propeptide, presumably cleaved from intact molecules N-terminal to their N-telopeptide cross-linking sites. In a previous report, we showed that full length molecules of pN type III collagen were present covalently bonded by lysyl oxidase-mediated cross-links to type II collagen fibrils in the matrix of articular cartilage from an osteoarthritic human knee8.
Using a type II collagen-specific antibody, mAb 1C10, the αchymotrypsin extracts were also shown to contain type II collagen whole α-chains and large chain fragments. This confirms previous studies in which collagen type II immunoreactive peptides were quantified in chymotrypsin digests of articular cartilage and a concept that a denatured sub-pool of type II collagen was a feature of OA joints24,25. However, in those early studies the molecular size of the immunoreactive collagen fragments was not examined. The dominance of intact α1(II) chains on Western blot analysis (Fig. 1) suggests that telopeptide cleavage by α-chymotrypsin had depolymerized and released a pool of largely intact molecules of type II collagen, which give intact α-chains on SDS-PAGE.
Nevertheless, the presence of smaller fragments [Fig. 1(C)] may reflect proteolytic nicks already present in individual chains of some native molecules that predispose such a molecular pool to chymotrypsin cleavage. The findings in Fig. 1(C) do indicate, however, that most of the solubilized type II collagen is of large molecular size not small peptides. It should also be noted that the antibody used in the earlier study24, which recognizes a sequence-specific epitope in α1(II), was apparently only tested for lack of cross-reactivity against bovine, not human, α1(III) collagen. The latter differs in sequence at the homologous and potentially cross-reacting epitopic site [GenBank:ACZ58371.1]. It is notable that the immunohistochemical location of this epitope, reported for human OA and control cartilage25, is remarkably similar to the observed distribution of type III collagen (Fig. 3).
The biochemical findings are consistent with the localization of type III collagen on the surfaces of type II collagen fibrils in articular cartilage, as previously seen by immunogold electron microscopy11. In addition, Aigner and colleagues showed that type III collagen was expressed in OA articular cartilage independently of collagen type I, but in conjunction with type II collagen expression12. The expression of collagen type III by articular chondrocytes appears to be relatively early in the OA process, at least before overt destruction of all articular surfaces in an OA joint has occurred. The earliest microscopic changes observed in animal models are swelling of the collagen network, as evidenced by hydration studies and transmission electron microscopy26. In our previous human hip cartilage study, cartilage swelling was indicated by increased water content, taking place before apparent macro- and microscopical cartilage changes13. Collagen type II biosynthesis is stimulated globally in joint cartilage at this early stage just 2 weeks after inducing OA surgically as seen in ACL or meniscectomy models4,5. Such radiolabeling studies could rule out type I collagen, but not type III collagen expression in conjunction with the stimulated expression and deposition of type II collagen.
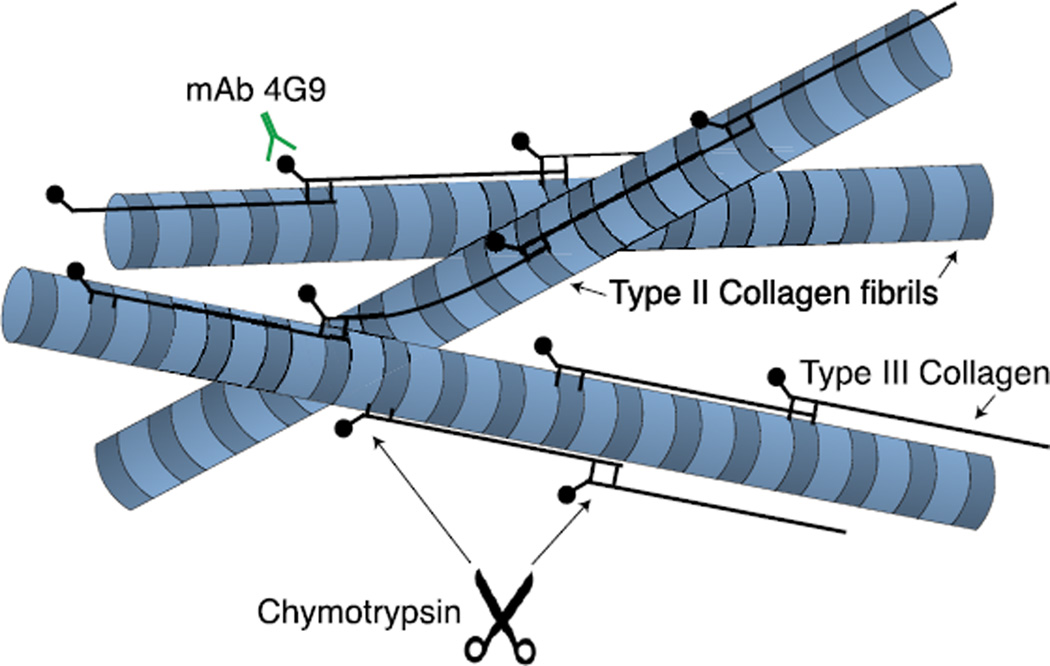
We speculate, therefore, that type III collagen could act as a collagen network glue expressed by articular chondrocytes. This appears to be part of an attempt to repair and constrain the existing collagen network of the extracellular matrix from further damage. This response may be triggered by molecular or mechanical signals, resulting from the joint trauma. The concept is illustrated in Fig. 4. The role of type III collagen may therefore be considered similar to that in wound-healing and the response to damage in tissues that are based on type I collagen, for example skin and tendon27,28.
Fig. 4.
Illustrated molecular concept of polymeric pN type III collagen filaments deposited between and covalently attached to the surface of type II collagen fibrils (modified from reference8).
In summary, the findings imply that collagen type III expression is part of an early response repertoire of articular chondrocytes in intact articular surfaces of joints undergoing progressive osteoarthritic molecular failure. Such expression also occurs in adult normal joints, but it clearly becomes more pronounced in OA cartilage. Whether such expression reflects inherently pathological activity, an active repair response or both, will be important to establish in order to develop molecular treatments.
Acknowledgments
We thank Håkan Axelson, Professor at molecular tumor biology and Elise Nilsson at section of pathology of Lund University for immunohistochemistry work in this study.
Role of the funding source
Research reported in this publication was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers AR036794 and AR037318 to DRE and from Lund University, The Swedish Research Council, Skåne county Council's Research and Development Foundation, Swedish National Centre for Research in Sports, The Swedish Rheumatism Association, The Alfred Österlund Foundation, The Herman Järnhardt Foundation, Stiftelsen för bistånd åt rörelsehindrade i Skåne, The Greta and Johan Kock Foundation and The Skåne University Hospital Funds to LED.
Footnotes
Author contributions
Shahrzad Hosseininia: conception and design, analysis and interpretation of the data, critical revision for important intellectual content, drafting of the study article, final approval of the article, collection and assembly of data.
Mary-Ann Weis: analysis and interpretation of the data, critical revision for important intellectual content, final approval of the article, collection and assembly of data.
Jyoti Rai: analysis and interpretation of the data, critical revision for important intellectual content, final approval of the article.
Lammy Kim: analysis and interpretation of the data, critical revision for important intellectual content, final approval of the article.
Sarah Funk: analysis and interpretation of the data, critical revision for important intellectual content, final approval of the article.
Leif E. Dahlberg: conception and design, interpretation of the data, drafting of the article, final approval of the article, provision of study materials or patients, obtaining of funding.
David R. Eyre: conception and design, interpretation of the data, drafting of the article and critical revision for important intellectual content, final approval of the article, obtaining of funding.
Conflict of interest
There is no conflict of interest for any of the authors.
Contributor Information
S. Hosseininia, Email: shahrzad.hosseininia@med.lu.se.
M.A. Weis, Email: maweis@uw.edu.
J. Rai, Email: jyotirai@uw.edu.
L. Kim, Email: lammykim@gmail.com.
S. Funk, Email: funks@uw.edu.
L.E. Dahlberg, Email: leif.dahlberg@med.lu.se.
D.R. Eyre, Email: deyre@uw.edu.
References
- 1.Eyre DR, Weis MA, Wu JJ. Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 2.Maroudas A. Adult Articular Cartilage. 2nd. London: Pitman Medical; 1979. Physicochemical properties of articular cartilage. [Google Scholar]
- 3.Dahlberg L, Billinghurst RC, Manner P, Nelson F, Webb G, Ionescu M, et al. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1) Arthritis Rheum. 2000;43:673–682. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Eyre DR, McDevitt CA, Billingham ME, Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980;188:823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floman Y, Eyre DR, Glimcher MJ. Induction of osteoarthrosis in the rabbit knee joint: biochemical studies on the articular cartilage. Clin Orthop Relat Res. 1980:278–286. [PubMed] [Google Scholar]
- 6.Lippiello L, Hall D, Mankin HJ. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977;59:593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wotton SF, Duance VC. Type III collagen in normal human articular cartilage. Histochem J. 1994;26:412–416. doi: 10.1007/BF00160053. [DOI] [PubMed] [Google Scholar]
- 8.Wu JJ, Weis MA, Kim LS, Eyre DR. Type III collagen, a fibril network modifier in articular cartilage. J Biol Chem. 2010;285:18537–18544. doi: 10.1074/jbc.M110.112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes RJ, Schmid TM, Eyre DR. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur J Biochem. 2003;270:3243–3250. doi: 10.1046/j.1432-1033.2003.03711.x. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmajer R, Olsen BR, Timpl R, Perlish JS, Lovelace O. Collagen fibril formation during embryogenesis. Proc Natl Acad Sci USA. 1983;80:3354–3358. doi: 10.1073/pnas.80.11.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young RD, Lawrence PA, Duance VC, Aigner T, Monaghan P. Immunolocalization of collagen types II and III in single fibrils of human articular cartilage. J Histochem Cytochem. 2000;48:423–432. doi: 10.1177/002215540004800312. [DOI] [PubMed] [Google Scholar]
- 12.Aigner T, Bertling W, Stoss H, Weseloh G, von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993;91:829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseininia S, Lindberg LR, Dahlberg LE. Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis; a case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet Disord. 2013;14:18. doi: 10.1186/1471-2474-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollander AP. Collagen degradation assays. Methods Mol Biol. 2001;151:473–484. doi: 10.1385/1-59259-046-2:473. [DOI] [PubMed] [Google Scholar]
- 15.Miller EJ, Finch JE, Jr, Chung E, Butler WT, Robertson PB. Specific cleavage of the native type III collagen molecule with trypsin. Similarity of the cleavage products to collagenase-produced fragments and primary structure at the cleavage site. Arch Biochem Biophys. 1976;173:631–637. doi: 10.1016/0003-9861(76)90300-3. [DOI] [PubMed] [Google Scholar]
- 16.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Eyre DR, Pietka T, Weis MA, Wu JJ. Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J Biol Chem. 2004;279:2568–2574. doi: 10.1074/jbc.M311653200. [DOI] [PubMed] [Google Scholar]
- 19.Weis MA, Hudson DM, Kim L, Scott M, Wu JJ, Eyre DR. Location of 3-hydroxyproline residues in collagen types I, II, III, V/XI implies a role in fibril supramolecular assembly. J Biol Chem. 2010;285:2580–2590. doi: 10.1074/jbc.M109.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hothorn T, Hornik K, van de Wiel MA, Zeileis A. A Lego system for conditional inference. Am Statistician. 2006;60:257–263. [Google Scholar]
- 21.Pinheiro J, Bates D. Mixed-effects Models in S and S-PLUS. New York: Springer; 2006. [Google Scholar]
- 22.Team RC. R: a Language and Environment for Statistical Computing. 2015 [Google Scholar]
- 23.Burkhardt D, Michel BA, Baici A, Kissling R, Theiler R. Comparison of chondroitin sulphate composition of femoral head articular cartilage from patients with femoral neck fractures and osteoarthritis and controls. Rheumatol Int. 1995;14:235–241. doi: 10.1007/BF00262089. [DOI] [PubMed] [Google Scholar]
- 24.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunham J, Shackleton DR, Nahir AM, Billingham ME, Bitensky L, Chayen J, et al. Altered orientation of glycosaminoglycans and cellular changes in the tibial cartilage in the first two weeks of experimental canine osteoarthritis. J Orthop Res. 1985;3:258–268. doi: 10.1002/jor.1100030302. [DOI] [PubMed] [Google Scholar]
- 27.Barnes MJ, Morton LF, Bennett RC, Bailey AJ, Sims TJ. Presence of type III collagen in guinea-pig dermal scar. Biochem J. 1976;157:263–266. doi: 10.1042/bj1570263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pingel J, Lu Y, Starborg T, Fredberg U, Langberg H, Nedergaard A, et al. 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: evidence of tenocyte and matrix buckling. J Anat. 2014;224:548–555. doi: 10.1111/joa.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]