Abstract
Objectives
The testes are a potential viral sanctuary site for HIV-1 infection. Our study aims to provide insight into the expression and localization of key drug transporters and metabolic enzymes relevant to ART in this tissue compartment.
Methods
We characterized gene and protein expression of 12 representative drug transporters and two metabolic enzymes in testicular tissue samples obtained from uninfected (n = 8) and virally suppressed HIV-1-infected subjects on ART (n = 5) and quantified antiretroviral drug concentrations in plasma and testicular tissues using LC/MS/MS from HIV-1-infected subjects.
Results
Our data demonstrate that key ABC drug transporters (permeability glycoprotein, multidrug-resistance protein 1, 2 and 4, and breast cancer resistance protein), solute carrier transporters (organic anion transporting polypeptides 1B1 and 2B1, organic anion transporter 1, concentrative nucleoside transporter 1, equilibrative nucleoside transporter 2) and cytochrome P450 metabolic enzymes (CYP3A4 and CYP2D6) previously shown to interact with many commonly used antiretroviral drugs are expressed at the mRNA and protein level in the testes of both subject groups and localize primarily at the blood–testis barrier, with no significant differences between the two groups. Furthermore, we observed that PIs known to be substrates for ATP-binding cassette membrane transporters, displayed variable testicular tissue penetration, with darunavir concentrations falling below therapeutic values. In contrast, the NRTIs emtricitabine, lamivudine and tenofovir displayed favourable tissue penetration, reaching concentrations comparable to plasma levels. We also demonstrated that nuclear receptors, peroxisome proliferator-activated receptors α and γ exhibited higher gene expression in the testicular tissue compared with pregnane X receptor and constitutive androstane receptor, suggesting a potential regulatory pathway governing drug transporter and metabolic enzyme expression in this tissue compartment.
Conclusions
Our data suggest the testes are a complex pharmacological compartment that can restrict the distribution of certain antiretroviral drugs and potentially contribute to HIV-1 persistence.
Introduction
Current ART combines different classes of antiretroviral drugs (ARVs) to simultaneously target the different stages of the HIV-1 life cycle and has been very effective at suppressing viral replication as measured by plasma viral load.1 However, ART remains unable to completely eradicate the virus as several studies have shown that viral rebound occurs following treatment cessation.2 The source of this persistent infection remains unclear, but it is believed that sub-therapeutic ARV concentrations in both cellular reservoirs, such as CD4 memory T cells, and tissue sanctuary sites, such as the CNS, gastrointestinal and genitourinary tracts, can result in inadequate viral suppression and facilitate the evolution of drug-resistant virus.3
The extent of ARV penetration into viral reservoirs and sanctuary sites primarily depends on the physicochemical properties of the compound and a dynamic interplay between drug efflux, influx and metabolic processes.4 ARVs can be effluxed by ATP-binding cassette (ABC) membrane-associated transporters, influxed by solute carrier (SLC) transporters and metabolized by cytochrome P450 (CYP450) enzymes.5,6 Expression of these transporters and metabolic enzymes in cellular reservoirs such as CD4+ T cells has been shown to alter intracellular ARV concentrations relative to plasma.7,8 Similarly, the presence of these proteins at key blood–tissue barriers such as the blood–brain barrier (BBB) has been shown to limit ARV penetration into the CNS.9,10
Several studies have suggested that the testes can be infected by HIV-1 early during acute infection and remain infected during the asymptomatic stage.11,12 Furthermore, the blood–testis barrier (BTB), formed by columnar epithelial Sertoli cells that line the seminiferous tubules, creates one of the tightest cellular barriers in the body.13 Previous studies performed by our group as well as others have demonstrated the functional expression of ABC transporters, i.e. permeability glycoprotein (P-gp), multidrug-resistance protein 1 (MRP1) and breast cancer resistance protein (BCRP), in rodent and human Sertoli cell culture models.14–17 Furthermore, we demonstrated in an in vivo transgenic rodent model the role that these transporters play in restricting the distribution of atazanavir, a PI, into brain and testicular tissues.10 Together, these studies suggest that the testes could limit ARV penetration and act as a viral sanctuary site for HIV-1, and possibly contribute to persistent HIV-1 infection and the development of resistance. However, few studies have thoroughly characterized the expression of drug transporters and metabolic enzymes in human testes and to the best of our knowledge, none has examined this expression in HIV-1-infected subjects. Herein, we have investigated the expression of ABC transporters (P-gp, MRP1, MRP2, MRP4 and BCRP) and SLC transporters [organic anion transporting polypeptides (OATPs) 1A2, 1B1 and 2B1, organic anion transporter 1 (OAT1), organic cation transporter 1 (OCT1), concentrative nucleoside transporter 1 (CNT1) and equilibrative nucleoside transporter 2 (ENT2)] known to be involved in the disposition of several ARVs, as well as two CYP450 metabolic enzymes (CYP3A4 and CYP2D6), which play a major role in the metabolism of several ARVs. In addition, we also examined gene expression levels of four nuclear receptors, the peroxisome proliferator-activated receptors α (PPARα) and γ (PPARγ), the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR), which are known to regulate the expression levels of drug transporters and metabolic enzymes18 in other tissues. Our study provides novel insight into the expression and localization patterns of key drug transporters and metabolic enzymes in testicular tissues obtained from uninfected and HIV-1-infected individuals undergoing elective orchiectomy for gender reassignment. Furthermore, by combining the expression and localization data with ARV quantification data in plasma versus testicular tissues, we aimed to determine whether there is an association between drug transporter and metabolic enzyme expression in human testes, and ARV penetration in this tissue.
Methods
Sample population
Study participants undergoing elective orchiectomy for gender modification were voluntarily recruited at the Metropolitan Centre of Plastic Surgery in Montréal, Canada starting October 2012 and ending 31 May 2014 (REB no. 12-153 BMD). Participants were eligible for the study if they were males 18 years or older and willing to give informed consent, undergoing a transgender sex change after having obtained legal agreement, and receiving effective ART with a plasma viral load below level of detection (<50 copies/mL, applying a standard PCR-based diagnostic test) for at least 6 months (no restriction in CD4 T cell count). Participants were excluded if they had recent acute illness (<3 months), recent sexually transmitted infection, active cancer or uncontrolled coagulation disorder. This study obtained ethics approval from the McGill University Health Centre Ethical Review Board. All study subjects provided written informed consent for participation in the study.
Tissue processing
Blood and testicular samples were collected from all enrolled subjects. Two 8 mL blood samples were collected in EDTA tubes for ARV quantification from HIV-1-infected subjects immediately before surgery, ∼14–19 h after the last ARV dose. Testicular tissue samples for both biochemical assays and ARV quantification were stored in saline solution immediately after surgery and processed within 1–2 h by cutting into small pieces, snap frozen in liquid nitrogen and storing at −80°C until study analysis.
Cell culture
The HepG2 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Transient transfection of the HEK293T cell line with pEF/Amp-OATP1B1 and -OATP1A2 vectors provided by Dr Richard Kim (University of Western Ontario, London, Canada) was performed using Lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
All cell culture systems were maintained according to previously published protocols17,19 at 37°C humidified 5% CO2/95% air with fresh media replaced every 2–3 days. Cells were subcultured with 0.25% trypsin–EDTA upon reaching 80%–90% confluency.
Total RNA extraction, cDNA synthesis and quantitative PCR (qPCR)
Total RNA was isolated from testicular tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocols and treated with 1 U/mL DNAseI to remove contaminating genomic DNA. RNA concentration (absorbance at 260 nm) and purity (absorbance ratio 260/280 nm) was assessed using a DU Series 700 Scanning UV/VIS Spectrophotometer (Beckman Coulter, Brea, CA, USA). We then used 2 μg of total RNA for reverse transcription to cDNA using a high-capacity reverse transcription cDNA kit (Applied Biosystems, Waltham, MA, USA) according to the manufacturer's instructions. The reverse transcription reaction was initiated at 25°C for 10 min, followed by 37°C for 120 min and then 85°C for 5 min.
The mRNA expression levels of drug transporters, metabolic enzymes and nuclear receptors were assessed using TaqMan primers designed and validated by Life Technologies (Table S1, available as Supplementary data at JAC Online) and analysed by real-time qPCR on a Mastercycler ep Realplex 2S thermal cycler (Eppendorf, Hamburg, Germany) using TaqMan qPCR chemistry. All reactions were performed in triplicate with each 20 μL reaction containing 200 ng of cDNA, 1 μL of 20× primer mix and 10 μL of TaqMan qPCR mastermix. The expression level of each gene of interest is presented as normalized RNA expression levels (arbitrary units) relative to the human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene using the ΔCt method, where ΔCt is equal to the Ct value of the gene of interest minus the Ct value of GAPDH and the normalized value is equal to 2−ΔCt.
Immunoblot analysis
Immunoblotting was performed as described previously.17 Briefly, whole tissue lysates were extracted from frozen testicular samples using a modified radioimmunoprecipitation lysis buffer and manually homogenized by passing it repeatedly through 20G needles in a microfuge tube, followed by a 1 h incubation on ice. The samples were then centrifuged at 20 000 g for 10 min at 4°C and the supernatant protein concentration was quantified via a Bradford assay (Bio-Rad, Hercules, CA, USA) before being aliquoted and snap frozen in liquid nitrogen until ready for immunoblot analysis.
Proteins from the tissue lysate samples were separated on a 10% SDS/polyacrylamide gel and transferred on to a polyvinylidene fluoride membrane. The membranes were blocked for 2 h at room temperature in 5% skimmed milk/Tris-buffered saline containing 0.1% Tween 20 and incubated overnight with primary antibody (Table S2). The blots were then incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution). Signals were enhanced using a SuperSignal West Pico chemiluminescence system (Thermo-Fisher Scientific, South San Francisco, CA, USA) and detected by exposure to X-ray film. Quantitative comparisons were made using densitometric analysis with the AlphaDigiDoc RT2 software.
Localization of selected drug transporters and metabolic enzymes
Tissue sectioning and staining was performed by the Pathology Research Program at the University Health Network (Toronto, ON, Canada). Briefly, a testicular tissue sample from an uninfected and an HIV-1-infected, treated individual were fixed in 10% neutral buffered formalin for 48 h and fixed in paraffin wax before it was sectioned (8 μm) and mounted on to glass slides. Primary antibodies for the proteins of interest (see Table S2) were used to probe the mounted sections along with the appropriate corresponding fluorescently labelled secondary antibody (Figure S1a–c). Standard DAPI staining was used to identify the cell nuclei. Negative control slides stained with only secondary antibodies (see Table S2) were used to verify primary antibody signal specificity. Confocal microscopy work was completed at the Advanced Optical Microscopy Facility at the Toronto MARS Discovery Tower using a LSM700 confocal microscope (Zeiss, Oberkochen, Germany). Acquired images were processed using the Zen LE browser (Zeiss).
ARV quantification in plasma and testicular tissue
Testicular tissue lysates and plasma samples from HIV-infected, treated subjects were analysed in the Antiviral Pharmacology Laboratory, University of Nebraska Medical Center according to previously validated methods.20 Briefly, ARV concentrations in plasma were quantified directly and testicular tissue concentrations were determined from tissue homogenates. Atazanavir, darunavir, ritonavir, efavirenz, tenofovir, lamivudine and emtricitabine were extracted from testicular tissue and plasma using 70:30 methanol/water, mixed with 13C internal standards (ISs), and proteins were precipitated using an acetonitrile preparation step. To analyse tenofovir diphosphate, emtricitabine triphosphate and lamivudine triphosphate in testicular tissue, the phosphorylated drug was isolated from interferences, metabolites and unphosphorylated drug using an ion-exchange solid-phase extraction. Phosphorylated drug was then desalted with a reversed-phase extraction, dried and reconstituted before analysis.
Final sample extracts were separated and quantified using a Shimadzu Nexera ultrahigh-performance liquid chromatograph attached to an AB Sciex 5500 qTrap mass spectrometer. Ion pairs (tenofovir/emtricitabine/lamivudine/atazanavir/darunavir/ritonavir, positive; efavirenz, negative) were monitored in multiple reaction-monitoring mode. Analyte peaks were normalized to the corresponding IS peak, with the exception of efavirenz, for which no IS was available and was instead normalized to the emtricitabine-IS. The linear range of quantification for each analyte was 2–400 fmol total mass on column, with the exception of atazanavir, which was 0.2–40 fmol total mass on column. The interbatch %CV for the quality control samples in the phosphorylated NRTI methods (tenofovir diphosphate, emtricitabine triphosphate) and the PI/NNRTI methods (atazanavir, darunavir, ritonavir, efavirenz) were 3.8%–7.7% and 1.1%–7.4%, respectively. Absolute mean relative errors to the theoretical target quality control samples were <5.6% for phosphorylated NRTI methods and <8.1% for the PI/NNRTI methods. To compare between plasma and testicular tissue concentrations in ng/mL, we assumed that 1 g of tissue was equivalent to 0.96 mL.21
Statistical analyses
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Comparison between groups was performed applying the Mann–Whitney U non-parametric test. P < 0.05 was considered statistically significant.
Results
Demographics of study participants
Thirteen participants were enrolled in this study (Table 1). All of the HIV-1-infected, treated subjects were virally suppressed with plasma viral load <50 copies/mL. All study subjects were on hormone replacement therapy that was ceased 6 weeks before orchiectomy.
Table 1.
Patient characteristics
| Subject ID | Age (years) | Viral load (copies/mL) | ARVs |
Other medications | ||||
|---|---|---|---|---|---|---|---|---|
| NNRTIs | NRTIs | PIs | INSTIs | FIs | ||||
| HIV−, men, n = 8 | ||||||||
| 2 | 40 | — | — | — | — | — | — | — |
| 4 | 44 | — | — | — | — | — | — | — |
| 5 | 40 | — | — | — | — | — | — | — |
| 6 | 34 | — | — | — | — | — | — | — |
| 8 | 54 | — | — | — | — | — | — | — |
| 9 | 59 | |||||||
| 10 | 24 | — | — | — | — | — | — | — |
| 11 | 21 | — | — | — | — | — | — | — |
| median (range) | 40 (21–59) | |||||||
| HIV+, men, n = 5 | ||||||||
| 7 | 46 | <50 | — | — | DRV, RTV | DTG | MVC | citalopram |
| 12 | 27 | <50 | RPV | FTC, TFV | — | — | — | cyproterone acetatea, folic acida, oestradiola |
| 25 | NA | <50 | EFV | ABC, 3TC | — | — | — | levothyroxinea, oestradiola |
| 31 | 24 | <50 | — | 3TC, ZDV | DRV, RTV | RAL | — | |
| 39 | 47 | <50 | — | ABC, 3TC, TFV | ATV, RTV | — | — | |
| median (range) | 36.5 (24–47) | |||||||
INSTIs, integrase strand transfer inhibitors; FIs, fusion inhibitors; DRV, darunavir; RTV, ritonavir; DTG, dolutegravir; MVC, maraviroc; NA, not available; RPV, rilpivirine; FTC, emtricitabine; TFV, tenofovir; EFV, efavirenz; ABC, abacavir; 3TC, lamivudine; ZDV, zidovudine; RAL, raltegravir; ATV, atazanavir.
ARV compounds listed in bold were included in our drug quantification studies.
aOther medications were stopped at least 6 weeks prior to orchiectomy.
Relative mRNA expression of drug transporters and metabolic enzymes in the testes of uninfected and HIV-1-infected, treated subjects
We used TaqMan qPCR to analyse the expression of ABC transporters ABCB1 (P-gp), ABCC1 (MRP1), ABCC2 (MRP2), ABCC4 (MRP4) and ABCG2 (BCRP), SLC transporters SLCO1A2 (OATP1A2), SLCO1B1 (OATP1B1), SLCO2B1 (OATP2B1), SLC22A6 (OAT1), SLC22A1 (OCT1), SLC28A1 (CNT1) and SLC29A2 (ENT2) and metabolic enzymes CYP3A4 and CYP2D6 in the testes of both uninfected and HIV-1-infected, treated subjects. All genes analysed displayed interindividual differences in expression levels, with no significant differences in the average gene expression levels between the two study groups (Figure 1).
Figure 1.
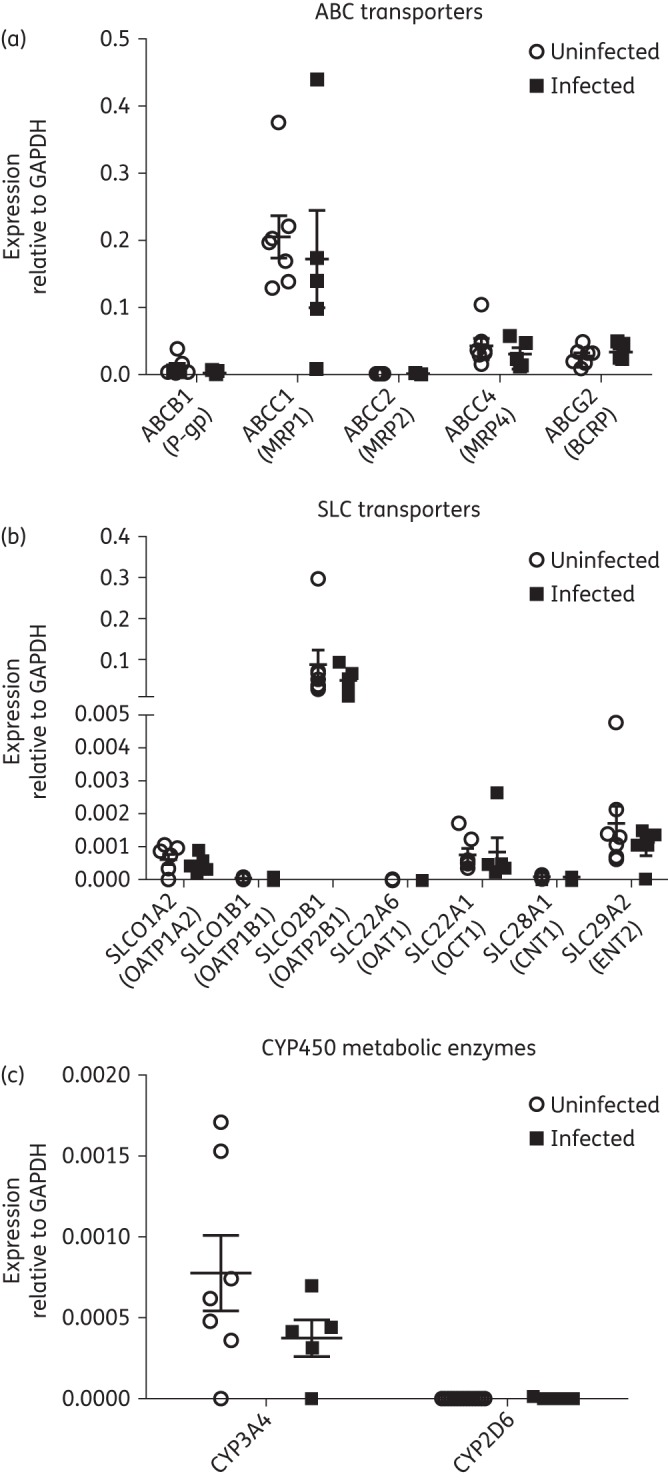
Relative mRNA expression of (a) ABC transporters, (b) SLC transporters and (c) drug metabolic enzymes in seven uninfected individuals and five HIV-1-infected, treated individuals assessed using quantitative real-time PCR. The results are expressed as mean relative mRNA expression ± SEM normalized to the housekeeping gene GAPDH. Differences between the two experimental groups were not statistically significant (P > 0.05).
For the ABC transporters, ABCC1 demonstrated the highest expression levels relative to GAPDH; ABCB1, ABCC4 and ABCG2 showed moderate expression levels, while ABCC2 showed nearly undetectable expression levels (Figure 1a). For the SLC transporters, we observed nearly undetectable mRNA expression levels for SLCO1B1, SLC22A6 and SLC28A1 relative to GAPDH, low-level expression for SLCO1A2 and SLC22A1 and high expression of SLCO2B1 (Figure 1b). We also observed low expression of CYP3A4 and nearly undetectable expression levels for CYP2D6 (Figure 1c).
Protein expression of drug transporters and metabolic enzymes in the testes of uninfected and HIV-1-infected, treated subjects
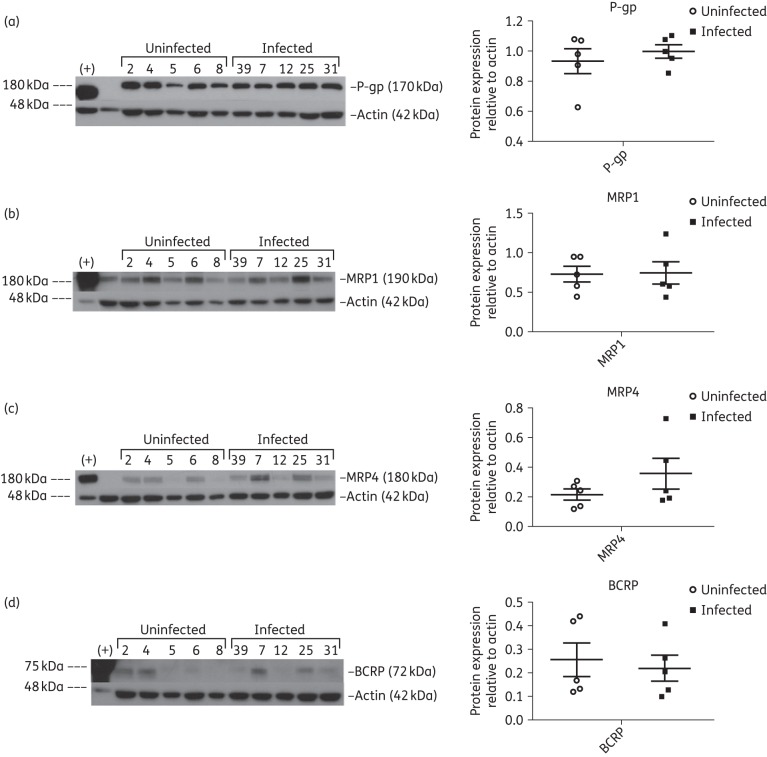
Our results demonstrate that ABC transporters P-gp (Figure 2a), MRP1 (Figure 2b), MRP4 (Figure 2c) and BCRP (Figure 2d) were expressed at the protein level in testicular tissues. While P-gp and MRP1 showed relatively consistent expression in the two study groups, MRP4 and BCRP showed more interindividual variability. Although, ABCC2 gene expression was low, robust bands corresponding to MRP2 (Figure S2a) were detected in both study groups.
Figure 2.
Immunoblot analysis of ABC transporters P-gp (a), MRP1 (b), MRP4 (c) and BCRP (d) in testicular tissue lysates from five uninfected and five HIV-1-infected, treated subjects. Protein lysates from cell lines overexpressing P-gp, MRP1 and MRP4 were loaded in the first lane and used as positive controls (+) for their respective immunoblots while the breast cancer cell line MX100, known to overexpress BCRP, was used as the positive control (+) for BCRP. β-Actin was used as the loading control for each immunoblot. Densitometric analysis results are shown alongside their respective immunoblots. Differences between the two experimental groups were not statistically significant (P > 0.05).
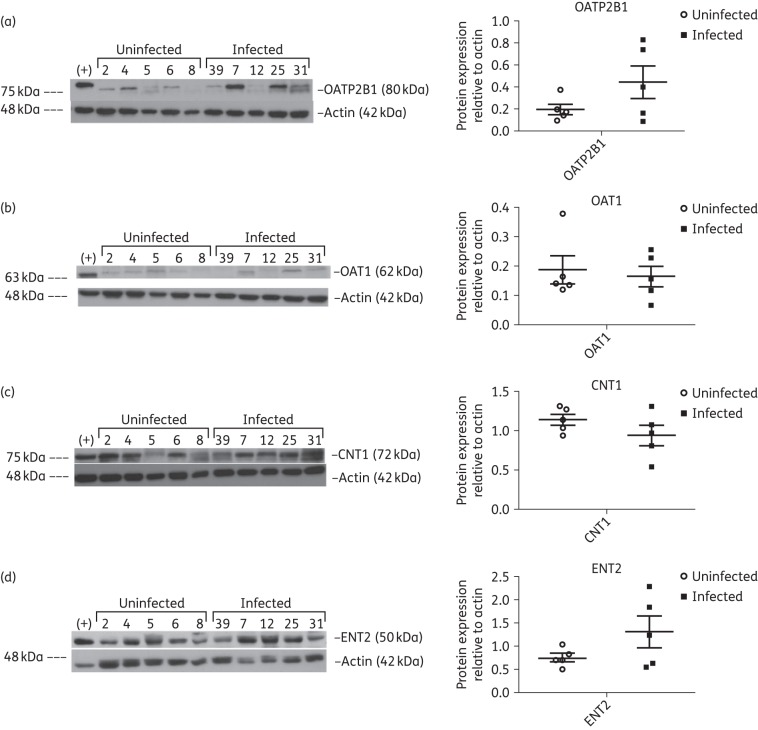
SLC transporters OATP2B1 (Figure 3a), OAT1 (Figure 3b), CNT1 (Figure 3c) and ENT2 (Figure 3d) were expressed at the protein level in testicular tissue and displayed interindividual variations in both study groups, whereas OATP1B1 (Figure S2b) expression was more consistent. We did not detect protein expression of OATP1A2 (Figure S2c) and OCT1 (Figure S2d) in the testicular tissues of either study groups, which correspond to our gene expression results indicating low expression of both transporters.
Figure 3.
Immunoblot analysis of SLC transporters OATP2B1 (a), OAT1 (b), CNT1 (c) and ENT2 (d) in testicular tissue lysates from five uninfected and five HIV-1-infected, treated subjects. Protein lysates isolated from the MDCK-OATP2B1 cell line were used as the positive control (+) for OATP2B1, whereas HepG2 cell protein lysate was used as the positive control (+) for OAT1, CNT1 and ENT2. β-Actin was used as the loading control for each immunoblot. Densitometric analysis results are shown alongside their respective immunoblots. Differences between the two experimental groups were not statistically significant (P > 0.05).
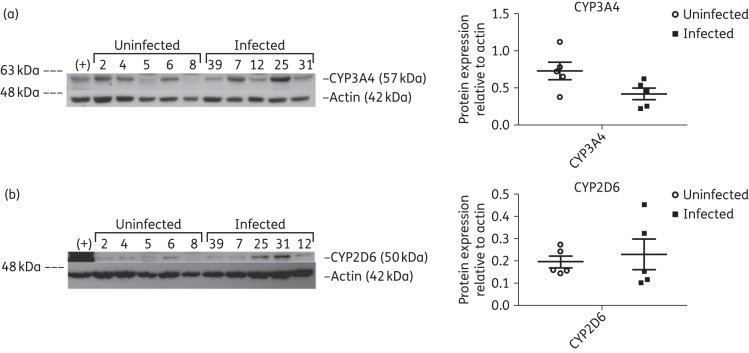
Lastly, despite nearly undetectable gene expression of both metabolic enzymes, we were able to detect protein expression of CYP3A4 (Figure 4a) and CYP2D6 (Figure 4b) in the testes and observed interindividual variability for both enzymes in the study groups.
Figure 4.
Immunoblot analysis of metabolic enzymes CYP3A4 (a) and CYP2D6 (b) in testicular tissue lysates from five uninfected and five HIV-1-infected, treated subjects. HepG2 cell protein lysate was used as the positive control (+) for both CYP3A4 and CYP2D6. β-Actin was used as the loading control for each immunoblot. Densitometric analysis results are shown alongside their respective immunoblots. Differences between the two experimental groups were not statistically significant (P > 0.05).
Overall, densitometric analysis comparisons between the uninfected and HIV-1-infected, treated patient groups revealed no significant differences in protein expression levels relative to actin for any of the studied drug transporters or metabolic enzymes.
Relative mRNA expression of nuclear receptors relevant to ART
We also assessed gene expression levels of four nuclear receptors relevant to ART. PPARα- and -γ were the most highly expressed nuclear receptors in human testicular tissue (Figure 5a), while PXR and CAR displayed very low levels of gene expression (Figure 5b). Overall, there were no significant differences between gene expression levels between the uninfected and HIV-1-infected, treated patient groups for any of the nuclear receptors we included in our study. However, we did observe a large degree of interindividual variation for PPARα and PXR.
Figure 5.
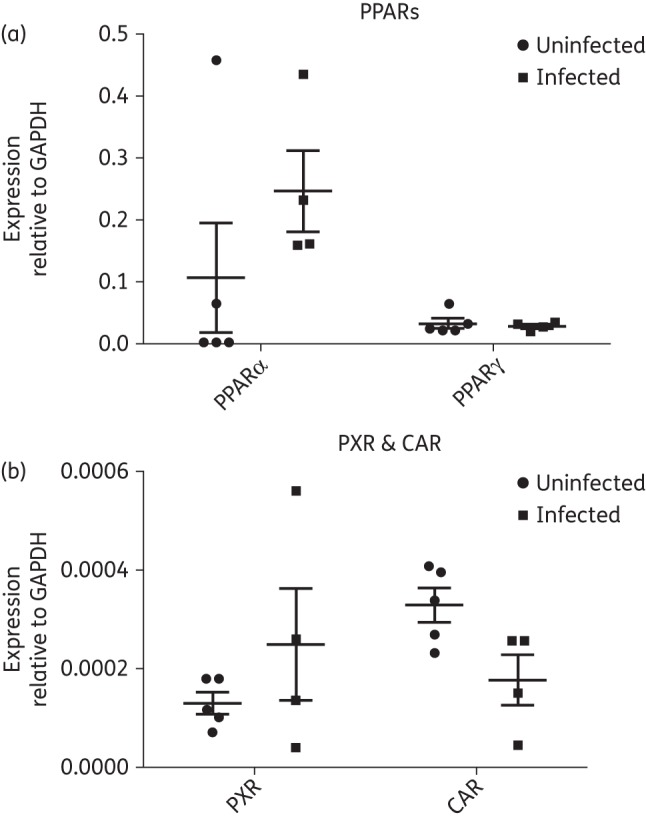
Relative mRNA expression of (a) PPARα- and -γ and (b) PXR and CAR in five uninfected individuals and four HIV-1-infected, treated individuals assessed using quantitative real-time PCR. The results are expressed as mean relative mRNA expression ± SEM normalized to the housekeeping gene GAPDH. Differences between the two experimental groups were not statistically significant (P > 0.05).
Localization of drug transporters and metabolic enzymes in testes of uninfected and HIV-1-infected, treated subjects
To gain further insight on how drug transporters and metabolic enzymes could modulate ARV disposition in this tissue, we used immunofluorescence confocal microscopy to assess drug transporter localization in the testis of uninfected and HIV-1-infected, treated subjects. Overall, we did not observe a difference in localization pattern for the drug transporters and metabolic enzymes between the two subject groups (Figure 6 and Figure S3).
Figure 6.
Immunofluorescence imaging of selected transporters in fixed testicular tissue sections from a single HIV-1-infected, treated subject with representative images showing the localization pattern of (a) ABC transporters, (b) SLC transporters and (c) CYP450 metabolic enzymes. Tissue sections were stained with DNA dye, DAPI (blue) and examined by immunofluorescence using respective primary antibodies corresponding to our proteins of interest (green). Anti-Na+/K + ATPase-α (red) antibody was used as a marker for the plasma membrane. Cells were stained with Alexa Fluor-conjugated secondary antibodies 488/555 alone to verify the signal specificity of the primary antibodies (Figure S1a–c). Scale bar, 20 μm. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
We found that P-gp, MRP1 and BCRP (Figure 6a) localized throughout the testicular tissue at the seminiferous epithelium, as well as throughout the interstitial space, similar to previous immunofluorescence and immunohistochemistry results in rat and human testes.14,15 For MRP4 (Figure 6a), we observed a stronger localization signal towards the basolateral side of the seminiferous epithelium, as well as at the level of the testicular endothelium. Similarly for BCRP, we also detected a stronger localization signal at the basolateral side of the seminiferous epithelium and at the testicular endothelium. We also found that BCRP displayed some immunofluorescence signal within the seminiferous epithelium and at its apical side, similar to the BCRP localization previously reported in rat testes.16 Lastly, we identified MRP2 (Figure 6a) localization in the testicular interstitium contrary to previous studies indicating the absence of MRP2 in human testicular tissue.15
For the SLC transporters, we found that OATP1B1, OATP2B1, OAT1, CNT1 and ENT2 (Figure 6b) localized at the seminiferous epithelium and throughout the testicular interstitium. We detected a stronger localization signal for CNT1 and ENT2 at the level of the seminiferous epithelium compared with the testicular interstitium, whereas OAT1 showed strong localization at the testicular endothelium. In contrast, we did not detect fluorescence from OATP1A2 and OCT1 (Figure S4), which reflects our immunoblotting results showing undetectable protein expression.
For CYP450 drug metabolic enzymes, we found that both CYP3A4 and CYP2D6 (Figure 6c) localized throughout the testicular tissue, with CYP2D6 showing a stronger localization signal at the seminiferous epithelium and testicular endothelium. Antibodies used to detect the proteins of interest in immunofluorescence experiments were specific, because we have not detected any signal when tissues were incubated with secondary antibodies alone (negative control, Figure S1).
ARV quantification in plasma and testicular tissue
We performed drug quantification analysis in both the plasma and testicular tissues of HIV-infected, treated subjects (Table 2). Overall, we observed similar plasma versus testicular concentrations for tenofovir, lamivudine and emtricitabine, higher testicular concentrations for ritonavir and lower testicular concentrations for efavirenz, atazanavir and darunavir. Notably, we observed that darunavir concentrations in testicular tissue were consistently lower than those observed in plasma and below previously reported trough concentrations from clinical trials with HIV-1-infected subjects.22 NRTI concentrations in most HIV-1-infected, treated subjects reached plasma and testicular tissue concentrations approximating those previously reported in pharmacokinetic studies in plasma.23 However, we observed large interindividual variability in lamivudine plasma and testicular tissue concentrations, with one subject's testicular lamivudine concentration falling below its target therapeutic range. We also quantified the active phosphorylated metabolite of the NRTIs included in this study, which displayed testicular tissue concentrations above those previously reported in PBMCs isolated from virally suppressed, HIV-1-infected patients on a tenofovir disoproxil fumarate/emtricitabine-containing regimen24 and in healthy subjects given 300 mg lamivudine.25
Table 2.
Plasma and testicular tissue ARV quantification data
| Patient ID | Drug | Plasma (ng/mL) | Testes (ng/mL) | Testes/plasma ratio |
|---|---|---|---|---|
| 7 | DRV | 2043.70 | 396.59 | 0.19 |
| RTV | 48.90 | 389.29 | 7.96 | |
| 12 | TFV | 61.70 | 44.71 | 0.72 |
| TFV-DP | — | 5.83 | — | |
| FTC | 214.40 | 251.79 | 1.17 | |
| FTC-TP | — | 691.63 | — | |
| 25 | 3TC | 207.80 | 147.10 | 0.71 |
| 3TC-TP | — | 174.69 | — | |
| EFV | 7448.90 | 1856.45 | 0.25 | |
| 31 | 3TC | 125.80 | 140.36 | 1.12 |
| 3TC-TP | — | 108.80 | — | |
| DRV | 2375.80 | 523.11 | 0.22 | |
| RTV | 236.00 | 683.82 | 2.90 | |
| 39 | TFV | 51.01 | 43.51 | 0.85 |
| TFV-DP | — | 13.94 | — | |
| 3TC | 136.70 | 21.34 | 0.16 | |
| 3TC-TP | — | 3227.47 | — | |
| ATV | 1300.20 | 1029.76 | 0.79 | |
| RTV | 271.80 | 523.81 | 1.93 |
DRV, darunavir; RTV, ritonavir; TFV, tenofovir; TFV-DP, tenofovir-diphosphate; FTC, emtricitabine; FTC-TP, emtricitabine-triphosphate; 3TC, lamivudine; 3TC-TP, lamivudine-triphosphate; EFV, efavirenz; ATV, atazanavir.
Discussion
A major obstacle in the pharmacological treatment of HIV-1 infection is the presence of viral sanctuary sites, which are characterized by poor ARV permeability and could contribute to ineffective viral suppression, development of viral drug resistance and persistent infection.3,4 The extent of ARV distribution into tissues is primarily dependent on the drug's physicochemical properties, drug serum protein binding as well as presence of drug transporters and metabolic enzymes as demonstrated in studies published by our group and others at the BBB.9,10 However, very limited data are available on the expression of drug transporters and metabolic enzymes relevant to ART in human testicular tissues and to the best of our knowledge, none has investigated their expression in the context of HIV-1 infection.
In this study, we detected gene expression of ABC and SLC drug transporters in testicular samples from both uninfected and HIV-1-infected, treated subjects that generally reflected trends seen previously using pooled human testicular samples.26 We also detected protein expression and localization patterns of the ABC transporters P-gp, MRP1, MRP4 and BCRP in both of our experimental groups that largely paralleled our gene expression data as well as previously published data in uninfected human testes.14,15 To our knowledge, our study is also the first to provide a comprehensive survey of protein expression and localization data for SLC transporters relevant to ART in the testes of both uninfected and HIV-1-infected, treated subjects, which could have a significant impact on ARV penetration into this compartment since many commonly used ARV compounds can act as both substrates and inhibitors for these transporters (Tables S3–S5). For example, tenofovir is a known substrate of OAT1,27 and its uptake by OAT1 at the basolateral membrane of the renal proximal tubule cell has been proposed as a key step in tenofovir excretion,28 demonstrating the key role that SLC transporters can play in mediating ARV disposition. Although there have yet to be studies demonstrating the functional expression of SLC transporters in ART in human testicular tissues, studies examining nucleoside transport in rat Sertoli cells have reported that ENT1 and ENT2 can actively transport the pyrimidine nucleoside analogue uridine across the BTB,29 and studies examining penetration of the contraceptive adjudin into rodent testes have shown direct evidence that drug uptake transporters are required for its transport across the BTB.30 We showed that drug metabolic enzymes CYP3A4 and CYP2D6 are expressed at the protein level in testicular tissues and localize throughout this tissue, where they may contribute to local drug metabolism within the testes similar to that observed in the brain.31 Furthermore, if CYP450 enzymes are functionally active in the testes, they could potentially mediate drug–drug interactions within testicular tissue since many PIs act as substrates and inhibitors of metabolic enzymes (Table S5). Interestingly, we were able to detect protein expression for MRP2, OATP1B1, OAT1, CNT1, ENT2, CYP3A4 and CYP2D6 despite low to undetectable levels of gene expression for these drug transporters and metabolic enzymes, which suggest possible post-transcriptional modifications negatively affecting mRNA stability of these genes.32
Our immunofluorescence data indicate that SLC transporters and drug metabolic enzymes are expressed throughout the testicular tissue, which likely reflect their role not only in mediating xenobiotic uptake into tissues, but also their role in regulating transport/metabolism of endogenous substances. For example, CNT1 and ENT2 play a major role in the uptake of nucleosides into tissue compartments,33 as a result, their localization throughout the testicular tissue is expected to support germ cell replication. On the other hand, we observed a more specific localization pattern for the ABC transporters. The localization of P-gp, MRP1 and MRP4 at the seminiferous epithelium is likely a reflection of their protective role in limiting xenobiotic entry into key tissue compartments and/or subcompartments.
Overall, we did not observe a significant difference between gene or protein expression levels of drug transporters and metabolic enzymes in our two experimental groups. There has been evidence that HIV-associated inflammation is likely responsible for the downregulation of efflux transporter expression via NFκB activation by proinflammatory cytokines in the brain.34 However, HIV-associated inflammation is unlikely to affect transporter and metabolic enzyme expression levels in the testes due to the immuno-privileged status of this tissue.35 The testes possess a naturally attenuated inflammatory response as evidenced by the lack of an IL-1β and TNFα response to LPS stimulation in rodents.36 Recent studies in primate models of HIV-1 infection have also revealed a lack of leucocyte and neutrophil influx into the testicular tissue and observed little damage to the testicular architecture,37 suggesting an attenuated inflammatory response to HIV-1 infection in the testes. Furthermore, the HIV-1-infected subjects included in our study were all on ART and virally suppressed, which should reduce HIV-associated inflammation. Indeed, in our previous study in the sigmoid colon, we observed a return to basal levels of protein expression of the ABC transporters P-gp, MRP1, MRP2 and MRP4 when comparing the uninfected with the HIV-1-infected, treated subjects.38
Although we did not observe a significant difference between subject groups, we detected more than a 10-fold difference between maximum and minimum expression values for MRP1, MRP2 and OCT1 in HIV-1-infected, treated patients and P-gp and OATP2B1 in uninfected patients. These results appear consistent with interindividual variations previously observed at the gene level in the small intestine.39 These differences may be a result of varying levels of transcriptional factor activation, inducing differential expression levels of drug transporters and metabolic enzymes. In contrast, interindividual differences in transporter expression at the protein level were not as pronounced as at the gene level. A recent study reported an 18-fold difference in MRP1 expression in lung tissue isolated from seven deceased male and female donors,40 suggesting the possibility for both tissue- and gender-specific differences in drug transporter expression. Long-term ART has been reported to have the potential to modulate transporter expression in HIV-1-infected subjects via transcription factor activation (i.e. nuclear receptors),18 as ARV compounds are known to induce drug transporter and metabolic enzyme regulation by acting as ligands of nuclear receptors such as PXR41 and CAR.42 For example, efavirenz has been demonstrated to be an inducer of P-gp expression and activity via the PXR and CAR receptors in a human brain microvessel endothelial cell line.42 However, gene expression studies on healthy, pooled human testicular tissue samples have indicated that PXR and CAR display very low expression levels in the testicular tissue and that the dominant nuclear receptors in the testes are the liver-X receptors α and β, PPARα- and -γ, and glucocorticoid receptor.43 Similarly, our gene expression studies on these nuclear receptors in both subject groups demonstrated the same expression pattern. Although these nuclear receptors have the potential to modulate transporter and metabolic enzyme expression, it remains unknown whether ART can induce their activation and whether they are functionally expressed in the testicular tissue. In addition, factors unique to each individual subject such as smoking, diet, environmental toxin exposure,44–46 as well as ARV regimens, which can also contribute to nuclear receptor regulation of drug transporter and metabolic enzyme expression, are likely responsible for the variability we observed. Finally, there has been evidence that oestrogen receptor α induction is capable of upregulating drug transporter expression. In particular, mitoxantrone has been shown to induce Abcg2 expression via oestrogen receptor α in a rat placental cell line,47 and the synthetic oestrogen 17α-ethynyloestradiol has also been shown to induce MRP3 expression in HepG2 cells via oestrogen receptor α.48 In contrast, studies on BCRP function in isolated rodent brain capillaries demonstrated that 17β-oestradiol reduced BCRP transport activity via the oestrogen receptors,49 providing direct evidence of the role that the oestrogen receptors and their natural ligands could play in modulating drug transporter activity. However, it remains unclear what effect 17β-oestradiol activation of oestrogen receptor α has on drug transporter expression levels in the testes and it appears unlikely that it could contribute to the expression patterns we observed since the patients included in this study ceased hormone therapy at least 6 weeks before surgery.
Our ARV quantification studies in the testicular tissues of HIV-1-infected, treated individuals revealed for the first time (to our knowledge) that nucleoside analogues, emtricitabine, lamivudine and tenofovir, penetrated effectively into this tissue, while their phosphorylated metabolites displayed lower but still virally effective concentrations when compared with previous data in PBMCs.24,25 This may reflect compartment differences in kinase activity and cellular activation states.23 Despite the variability we observed between tissue and plasma concentrations of ritonavir and atazanavir, concentrations of both compounds in these compartments were above the median therapeutic plasma concentrations reported in HIV-1-infected patients.50 However, darunavir appeared to consistently display a testicular concentration below the therapeutic trough concentrations reported from clinical trials in HIV-1-infected patients22 and may result in inadequate viral suppression and lead to the selection and perpetuation of drug-resistant virus.51 Overall, the small sample size of our study combined with the paucity of therapeutic efficacy data in tissue compartments makes it difficult to draw a direct conclusion as to whether the ARV concentrations we observed in testicular tissue were sufficient to achieve effective viral suppression.
Previous studies quantifying ARV concentrations in seminal plasma found high accumulation of emtricitabine (440%), lamivudine (316%–420%) and tenofovir (510%) and poor accumulation of efavirenz (undetectable–9%) and darunavir (9%–11%) relative to blood plasma,52 which reflect the concentration patterns we observed in testicular tissue. Favourable penetration of NRTIs into tissues and genital tract secretions are likely a result of their small molecular weight and low protein binding, which may be able to counteract their interactions with efflux transporters. Penetration of nucleoside analogues may also be enhanced by expression of the OAT1 we observed in the testes, which is a known SLC uptake transporter for tenofovir and lamivudine.53 Our results indicate a stronger localization signal for OAT1 at the level of testicular endothelium, which suggests a potential uptake pathway for tenofovir and lamivudine. On the other hand, the unfavourable physicochemical properties of certain ARVs such as atazanavir, darunavir and efavirenz combined with their affinity for efflux transporters could drastically limit their accumulation in the testes. Furthermore, these ARVs all display substantial protein binding (>85%)23 that could also contribute to the low testicular tissue concentrations we observed relative to plasma. Interestingly, we found that ritonavir accumulation in testicular tissue was higher than in plasma, contrary to data from seminal plasma,52 suggesting a possibility for local drug–drug interactions preventing efflux of ritonavir from testicular tissue through inhibition of P-gp, MRP1 or MRP2. Although our data suggest a potential association between decreased concentrations of certain ARVs and drug transporter expression in the testes, due to the relatively small number of subjects included in this study, our results should be interpreted with caution and further studies are necessary to confirm these observations in a larger sample population. Interestingly, studies on chemotherapy for childhood lymphoma relapse in the testes have indicated that methotrexate, which is a substrate for P-gp, MRP1, MRP4 and BCRP,54 accumulates at 2–4-fold lower concentrations in the testicular interstitial space and at 15–20-fold lower concentrations in the seminiferous tubule lumen compared with plasma.55 This provides further evidence of the potential role that ABC drug transporters can play in restricting drug penetration into the testes.
Although the data reported in our study are limited due to the small sample size, they nevertheless suggest a strong possibility that it may be difficult for not only ARVs, but also other pharmacological agents to achieve effective concentrations in testicular tissue due to extensive expression of ABC drug transporters and drug metabolic enzymes. Therefore, novel molecular delivery systems and/or methods may be necessary to achieve the goal of adequate/safe delivery of therapeutic compounds into the testicular compartment. For example, transporter-dependent delivery mechanisms may take advantage of pharmaceutical excipients that are known inhibitors of major drug efflux transporters. Cremophor, an emulgator used in oral, topical and parenteral solutions, has been shown to increase the systemic bioavailability of saquinavir in healthy male subjects via inhibition of intestinal P-gp activity.56 Transporter-independent delivery methods involving nanoparticles may also be an option, as a recent study using a trans-activating transcription peptide conjugated to a nanoparticle encapsulating saquinavir was able to cross the BBB effectively in an in vivo mouse model when given as an intravenous injection.57
Overall, our data suggest the testes are a complex pharmacological compartment that has the potential to limit the entry of ARVs known to be substrates for the ABC transporters found in the testes. This knowledge could assist clinicians in selecting ART regimens that are more effective in suppressing viral replication in this tissue and help the development of alternative delivery methods to enhance drug penetration into tissue compartments. Further work is necessary to understand better what role the testicular compartment plays in both ARV disposition and HIV-1 persistence.
Funding
This project was funded by a Canadian Institute of Health Research (CIHR) Catalyst grant (Grant #296635) and by the Canadian HIV Cure Enterprise Team Grant HIG-133050 (to J.-P. R. and R. B.) from the CIHR in partnership with CANFAR and IAS. Y. H. is a recipient of the Ontario Graduate Scholarship (OGS) award.
Transparency declarations
None to declare.
Author contributions
Y. H., T. H., R. B., M.-A. J., N. L. S., N. C. and J.-P. R. were responsible for the overall design of the study. P. B. and M. B. were responsible for patient enrolment and tissue collection. K. V. assisted with patient sample preparation and shipment. C. V. F. contributed LC/MS/MS analytical tools. J.-P. R. and R. B. contributed new reagents. S.-K. W. contributed data on nuclear receptor gene expression and helped with manuscript revision. Y. H. performed the biochemical experiments and data analyses. Y. H. and R. B. wrote the manuscript.
Supplementary data
Acknowledgements
Parts of this work have been presented previously as a poster at the Fifteenth International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, Washington, DC, USA, 2014 (Poster P_52), and as an oral poster at the 2015 Conference on Retroviruses and Opportunistic Infections Seattle, WA, USA (Abstract 534) and at the Seventh International Workshop on HIV Persistence during Therapy, Miami, FL, USA, 2015 (Abstract OP6.3).
We would like to thank Lee C. Winchester and Dr Anthony Podany from the University of Nebraska Medical Center, for their help and guidance with the LC/MS/MS drug quantification experiments. We also thank Mrs Angie Massicotte for her clerical assistance and coordination, and Mrs Anne Dubé for her valuable assistance in patient recruitment.
R. B. is a Career Scientist of the Ontario HIV Treatment Network (OHTN). J.-P. R. is a holder of the Louis Lowenstein Chair in Hematology & Oncology, McGill University.
References
- 1.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014; 384: 258–71. [DOI] [PubMed] [Google Scholar]
- 2.Davey RT, Bhat N, Yoder C et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA 1999; 96: 15109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MZ, Wightman F, Lewin SR. HIV reservoirs and strategies for eradication. Curr HIV/AIDS Rep 2012; 9: 5–15. [DOI] [PubMed] [Google Scholar]
- 4.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS 2013; 8: 190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kis O, Robillard K, Chan GNY, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci 2010; 31: 22–35. [DOI] [PubMed] [Google Scholar]
- 6.Walubo A. The role of cytochrome P450 in antiretroviral drug interactions. Expert Opin Drug Metab Toxicol 2007; 3: 583–98. [DOI] [PubMed] [Google Scholar]
- 7.Janneh O, Owen A, Chandler B et al. Modulation of the intracellular accumulation of saquinavir in peripheral blood mononuclear cells by inhibitors of MRP1, MRP2, P-gp and BCRP. AIDS 2005; 19: 2097–102. [DOI] [PubMed] [Google Scholar]
- 8.Janneh O, Hartkoorn RC, Jones E et al. Cultured CD4T cells and primary human lymphocytes express hOATPs: intracellular accumulation of saquinavir and lopinavir. Br J Pharmacol 2008; 155: 875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RB, Fromm MF, Wandel C et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 1998; 101: 289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robillard KR, Chan GNY, Zhang G et al. Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract. Antimicrob Agents Chemother 2014; 58: 1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Tortorec A, Le Grand R, Denis H et al. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One 2008; 3: e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roulet V, Satie A-P, Ruffault A et al. Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. Am J Pathol 2006; 169: 2094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012; 64: 16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bart J, Hollema H, Groen HJM et al. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer 2004; 40: 2064–70. [DOI] [PubMed] [Google Scholar]
- 15.Klein DM, Wright SH, Cherrington NJ. Localization of multidrug resistance-associated proteins along the blood-testis barrier in rat, macaque, and human testis. Drug Metab Dispos 2013; 42: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian X, Mruk DD, Wong EWP et al. Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis. Am J Physiol 2013; 304: E757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robillard KR, Hoque T, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther 2012; 340: 96–108. [DOI] [PubMed] [Google Scholar]
- 18.Chan GNY, Hoque MT, Bendayan R. Role of nuclear receptors in the regulation of drug transporters in the brain. Trends Pharmacol Sci 2013; 34: 361–72. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee N, Allen C, Bendayan R. Differential role of organic anion-transporting polypeptides in estrone-3-sulphate uptake by breast epithelial cells and breast cancer cells. J Pharmacol Exp Ther 2012; 342: 510–9. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher CV, Staskus K, Wietgrefe SW et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA 2014; 111: 2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mardirossian G, Tagesson M, Blanco P et al. A new rectal model for dosimetry applications. J Nucl Med 1999; 40: 1524–31. [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services, 2015; 1–267. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 23.Bazzoli C, Jullien V, Le Tiec C et al. Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin Pharmacokinet 2010; 49: 17–45. [DOI] [PubMed] [Google Scholar]
- 24.Adams JL, Sykes C, Menezes P et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence? J Acquir Immune Defic Syndr 2013; 62: 260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuen GJ, Lou Y, Bumgarner NF et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother 2004; 48: 176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 2005; 20: 452–77. [DOI] [PubMed] [Google Scholar]
- 27.Ray AS, Cihlar T, Robinson KL et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 2006; 50: 3297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagle MA, Truong DM, Dnyanmote AV et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem 2011; 286: 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein DM, Evans KK, Hardwick RN et al. Basolateral uptake of nucleosides by Sertoli cells is mediated primarily by equilibrative nucleoside transporter 1. J Pharmacol Exp Ther 2013; 346: 121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su L, Mruk DD, Lee WM et al. Drug transporters and blood–testis barrier function. J Endocrinol 2011; 209: 337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci 2011; 32: 708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012; 13: 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young JD, Yao SYM, Baldwin JM et al. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med 2013; 34: 529–47. [DOI] [PubMed] [Google Scholar]
- 34.Ashraf T, Ronaldson PT, Persidsky Y et al. Regulation of P-glycoprotein by human immunodeficiency virus-1 in primary cultures of human fetal astrocytes. J Neurosci Res 2011; 89: 1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol 2011; 335: 60–8. [DOI] [PubMed] [Google Scholar]
- 36.O'Bryan MK, Gerdprasert O, Nikolic-Paterson DJ et al. Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am J Physiol 2005; 288: R1744–55. [DOI] [PubMed] [Google Scholar]
- 37.Winnall WR, Lloyd SB, De Rose R et al. Simian immunodeficiency virus infection and immune responses in the pig-tailed macaque testis. J Leukoc Biol 2015; 97: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Rosa MF, Robillard KR, Kim CJ et al. Expression of membrane drug efflux transporters in the sigmoid colon of HIV-infected and uninfected men. J Clin Pharmacol 2013; 53: 934–45. [DOI] [PubMed] [Google Scholar]
- 39.Albermann N, Schmitz-Winnenthal FH, Z'graggen K et al. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol 2005; 70: 949–58. [DOI] [PubMed] [Google Scholar]
- 40.Sakamoto A, Matsumaru T, Yamamura N et al. Quantitative expression of human drug transporter proteins in lung tissues: analysis of regional, gender, and interindividual differences by liquid chromatography-tandem mass spectrometry. J Pharm Sci 2013; 102: 3395–406. [DOI] [PubMed] [Google Scholar]
- 41.Zastre J, Chan G, Ronaldson P et al. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res 2009; 87: 1023–36. [DOI] [PubMed] [Google Scholar]
- 42.Chan GNY, Patel R, Cummins CL et al. Induction of P-glycoprotein by antiretroviral drugs in human brain microvessel endothelial cells. Antimicrob Agents Chemother 2013; 57: 4481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura M, Naito S, Yokoi T. Tissue-specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metab Pharmacokinet 2004; 19: 135–49. [DOI] [PubMed] [Google Scholar]
- 44.Chang TKH, Waxman DJ. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR). Drug Metab Rev 2006; 38: 51–73. [DOI] [PubMed] [Google Scholar]
- 45.Lamba V, Yasuda K, Lamba JK et al. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol 2004; 199: 251–65. [DOI] [PubMed] [Google Scholar]
- 46.Molina-Molina J-M, Amaya E, Grimaldi M et al. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 2013; 272: 127–36. [DOI] [PubMed] [Google Scholar]
- 47.Oda K, Nishimura T, Higuchi K et al. Estrogen receptor α induction by mitoxantrone increases Abcg2 expression in placental trophoblast cells. J Pharm Sci 2013; 102: 3364–72. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz ML, Rigalli JP, Arias A et al. Estrogen receptor-α mediates human multidrug resistance associated protein 3 induction by 17α-ethynylestradiol. Role of activator protein-1. Biochem Pharmacol 2013; 86: 401–9. [DOI] [PubMed] [Google Scholar]
- 49.Hartz AMS, Mahringer A, Miller DS et al. 17-β-Estradiol: a powerful modulator of blood–brain barrier BCRP activity. J Cereb Blood Flow Metab 2010; 30: 1742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Avolio A, Carcieri C, Cusato J et al. Intracellular accumulation of atazanavir/ritonavir according to plasma concentrations and OATP1B1, ABCB1 and PXR genetic polymorphisms. J Antimicrob Chemother 2014; 69: 3061–6. [DOI] [PubMed] [Google Scholar]
- 51.Ghosn J, Chaix M-L, Peytavin G et al. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS 2004; 18: 1958–61. [DOI] [PubMed] [Google Scholar]
- 52.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 2011; 16: 1149–67. [DOI] [PubMed] [Google Scholar]
- 53.Burckhardt G. Drug transport by organic anion transporters (OATs). Pharmacol Ther 2012; 136: 106–30. [DOI] [PubMed] [Google Scholar]
- 54.Chan LMS, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci 2004; 21: 25–51. [DOI] [PubMed] [Google Scholar]
- 55.Riccardi R, Vigersky RA, Barnes S et al. Methotrexate levels in the interstitial space and seminiferous tubule of rat testis. Cancer Res 1982; 42: 1617–9. [PubMed] [Google Scholar]
- 56.Martin-Facklam M, Burhenne J, Ding R et al. Dose-dependent increase of saquinavir bioavailability by the pharmaceutic aid cremophor EL. Br J Clin Pharmacol 2002; 53: 576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao KS, Reddy MK, Horning JL et al. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials 2008; 29: 4429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.