Abstract
Objectives
The volume of prescribed antibiotics is associated with antimicrobial resistance and, unlike most other antibiotic classes, flucloxacillin prescribing has increased. We aimed to describe UK primary care flucloxacillin prescribing and factors associated with subsequent antibiotic prescribing as a proxy for non-response.
Patients and methods
Clinical Practice Research Datalink patients with acute prescriptions for oral flucloxacillin between January 2004 and December 2013, prescription details, associated Read codes and patient demographics were identified. Monthly prescribing rates were plotted and logistic regression identified factors associated with having a subsequent antibiotic prescription within 28 days.
Results
3 031 179 acute prescriptions for 1 667 431 patients were included. Average monthly prescription rates increased from 4.74 prescriptions per 1000 patient-months in 2004 to 5.74 (increase of 21.1%) in 2013. The highest prescribing rates and the largest increases in rates were seen in older adults (70+ years), but the overall increase in prescribing was not accounted for by an ageing population. Prescribing 500 mg tablets/capsules rather than 250 mg became more common. Children were frequently prescribed low doses and small volumes (5 day course) and prescribing declined for children, including for impetigo. Only 4.2% of new prescriptions involved co-prescription of another antibiotic. Age (<5 and ≥60 years), diagnosis of ‘cellulitis or abscess’ or no associated code, and 500 mg dose were associated with a subsequent antibiotic prescription, which occurred after 17.6% of first prescriptions.
Conclusions
There is a need to understand better the reasons for increased prescribing of flucloxacillin in primary care, optimal dosing (and the need to co-prescribe other antibiotics) and the reasons why one in five patients are prescribed a further antibiotic within 4 weeks.
Introduction
Flucloxacillin is the most common narrow-spectrum penicillinase-resistant penicillin used in the UK. It is primarily used to treat infections caused by Staphylococcus aureus and, in the community, use is almost exclusively for skin and soft-tissue infections (SSTIs).1 Skin conditions are one of the most common reasons for consulting in primary care and SSTIs are a common reason for antibiotic prescribing in this setting.2,3
Antimicrobial resistance is one of the most pressing public health concerns of our time,4 and resistance among S. aureus to flucloxacillin and other penicillinase-resistant penicillins, in the form of MRSA, is a key area of concern.5 However, while many antibiotics are being prescribed less often, flucloxacillin prescribing in UK primary care increased in the period up to 2006 overall6 and in adults7 and children.8 Higher volumes of antibiotic prescribing have been linked to greater levels of antibiotic resistance, both at the ecological9 and individual patient10 levels. Therefore, increasing levels of flucloxacillin prescribing, particularly if the trend has continued during more recent years, represent an important source of selection pressure for the development of antibiotic resistance and, therefore, an important threat to public health. However, recent trends have not been published and there has been little in the way of fine-grained analyses to help understand possible reasons for the observed trends. For example, some guidelines recommend co-treatment with phenoxymethylpenicillin (penicillin V) and flucloxacillin for cellulitis, particularly when severe or extensive;11,12 however, it is not known how frequently primary care clinicians co-prescribe other antibiotics with flucloxacillin. Furthermore, little is known about the appropriateness of flucloxacillin prescribing. The diagnosis of SSTIs can be problematic,13 the choice of antibiotic, route or dose may be inappropriate, or the causative organisms may be resistant. Treatment response is not routinely recorded in healthcare records, but the need for further antibiotics within the next few weeks can be used as a proxy for treatment non-response.
Trends in prescribing, particularly when linked to diagnoses and patient demographics, can be helpful for clinicians, policy makers and researchers in assessing the appropriateness or not of prescribing. Therefore, we set out to: (i) describe trends in prescribing of flucloxacillin by age, gender and indication; and (ii) describe patterns of prescribing, including co-prescription of other antibiotics, further antibiotic prescriptions within 28 days (as a proxy for non-response) and factors associated with prescribing a further antibiotic as a proxy measure for non-response to initial treatment.
Patients and methods
We analysed data from the Clinical Practice Research Datalink (CPRD) primary care dataset.14 CPRD developed from the General Practice Research Database and includes one of the largest and best validated primary care databases. The database contains data of over 20 years from over 650 general practices serving approximately 8% of the UK population. It includes anonymous data on patient demographics, prescribed medications, medical (Read) codes, consultations and the practices contributing to the database. CPRD conducts checks on data continuity and completeness and determines the date at which a practice as a whole is assessed as providing data that are of an acceptable research standard. In addition, patients are labelled as ‘acceptable’ for use in research by a process that identifies and excludes patients with non-contiguous follow-up or patients with poor data recording that raises suspicion as to the validity of that patient record.
We identified all patients who had been prescribed any oral flucloxacillin formulation during the 10 year period from 1 January 2004 to 31 December 2013 and were flagged as having data of acceptable quality. Prescriptions (and any related medial codes) were only included if: the prescription date was after the date that the practice was assessed as being ‘up to standard’ (in terms of research quality data); after the date that the patient registered; before the date that the patient died or transferred out of the practice; and before the date of the last data collection from the practice. Approximately 98% of the population of England and Wales are registered with a general practitioner (GP) practice. Prescriptions for flucloxacillin were identified based on product codes (please see the Supplementary data available at JAC Online).
Prescriptions are entered on general practice computer systems as either acute (single course) or repeat (a pre-set number of prescriptions can be issued). For this analysis, only acute prescriptions were used, as repeat prescriptions for flucloxacillin are uncommon and would only be used for chronic conditions.
The number of prescriptions per study month was calculated and divided by the average number of patients registered in the database for that month. We then calculated monthly rates per 1000 registered patients and adjusted these for the number of days in each month. Monthly rates were plotted in a line graph and we used a simple linear regression model, with month of year included as a dummy variable, to calculate monthly (seasonal) effects as well as the underlying trend. This analysis used a data extract from early 2014 and, therefore, some encounters in the last couple of months of 2013 may have not been included. Therefore, we used the linear regression model to predict prescribing rates for December 2013. We then age-standardized the rates using the population data of England and Wales from 2004.15
Each prescription was classified into one of four categories: 500 mg tablets or capsules; 250 mg tablets or capsules; 250 mg/5 mL syrup or suspension; and 125 mg/5 mL syrup or suspension. The number and proportion falling into each category was calculated overall and by month, and line graphs used to display changes over time. The recorded quantity prescribed was identified. We excluded prescriptions where the quantity was zero or so large that it was assessed as likely to be a coding error. For tablets or capsules, we excluded quantities >112 (equivalent to more than two tablets four times a day for 2 weeks) and for liquids, where quantities are usually multiples of 100 mL, we excluded quantities >2000 mL (equivalent to more than 20 mL four times a day for 25 days). The quantity was used to estimate the length of the course and then multiplied by the dose to calculate the total amount of antibiotic prescribed.
Patient age (in years) at the date of prescription was calculated and classified into one of seven age bands (0–4, 5–9, 10–19, 20–59, 60–69, 70–79 and 80+ years) for age-specific rates and one of three age bands (0–19, 20–59 and 60+ years) for age- and gender-specific rates. Age- and age- and gender-specific rates were calculated as above, but using age- and gender-specific monthly denominator counts.
In the UK, primary care clinicians use Read codes to record diagnoses, symptoms, signs, procedures, etc. To describe the medical codes associated with prescriptions for flucloxacillin, we identified Read codes that were linked by consultation ID number. We searched for 2051 Read codes that could potentially indicate an SSTI. As we only included consultations in which flucloxacillin had been prescribed, we used an inclusive approach, for example, ‘breast lump’ as being indicative of a breast infection, ‘eczema’ indicative of infected eczema and ‘dressing change’ indicative of a wound infection. We included: 281 codes for SSTI diagnoses (cellulitis, abscess, impetigo, folliculitis, etc.); 42 codes for ingrowing nails, paronychia and other digital infections; 215 codes for breast, head and neck, bursitis, umbilicus and limb infections; 884 codes for insect bites, other bites, traumatic wounds and post-operative wounds; 524 codes for skin conditions that may become secondarily infected (eczema, seborrhoeic and contact dermatitis, psoriasis, viral or fungal infections, skin ulcers, cysts, warts, naevi, blisters, etc.); 29 codes for non-specific ‘SSTI’, rash and ‘skin symptoms’; and 76 codes for other skin conditions (erythrasma, phlebitis, thrombophlebitis, varicose veins, pyoderma, acne, rosacea). These were then classified into one of the following classifications: cellulitis or abscess, impetigo, boils/folliculitis, infected finger/toe, infection of other skin region, infected trauma/wound, secondary infection of skin lesion, non-specific SSTI and other skin infection. We also searched for Read codes for respiratory tract infections, urinary tract infections and osteomyelitis. Consultations with none of the above codes were then classified as having, ‘no relevant code’ or no Read code. The Read codes used are included in the Supplementary data available at JAC Online. We then calculated the proportion of prescriptions that were classified into each category and the three main subcategories for each major category. We also calculated monthly rates for each category of SSTI and plotted these as a line graph.
We identified all antibiotic prescriptions that occurred over the 10 year study period for all patients that had received one or more prescription for flucloxacillin. We then identified all first prescriptions of flucloxacillin (those that did not have another flucloxacillin prescription in the preceding 28 days) and then whether any additional antibiotics were co-prescribed at the same time or in the following 28 days. Prescriptions are not directly linked to a diagnosis in CPRD (but may be linked to Read codes via a consultation ID number) and, therefore, we took a pragmatic approach of including only prescriptions within the first 4 weeks as being likely to be related to the same condition. Where subsequent antibiotics were prescribed, we classified all subsequent antibiotic prescriptions that occurred within 28 days of each other as part of the same ‘episode’. We then calculated the amount of time each patient was included in the database by taking a start date as the later of 1 January 2004, the date they first registered with the practice or the date the practice was assessed as being up to standard, and the end date as the earlier of 31 December 2013, the date the patient transferred out of the practice, the date the patient died or the last data collection date for the practice. Patients with <90 days of observation were excluded and then the number of episodes per year of observation was calculated and converted into number per 10 years.
In order to identify factors associated with receiving a subsequent prescription of an antibiotic that might be used for an SSTI (oral penicillin, macrolide, tetracycline, cephalosporin, metronidazole, quinolone, clindamycin, sodium fusidate or vancomycin) (as a proxy for potential treatment failure) we used logistic regression. Patient age, gender, diagnostic category, dose formulation, study year and an interaction between study year and formulation (to determine whether changes in dose/formulation over time were associated with having a subsequent antibiotic) were included as covariates.
This study was approved by the Independent Scientific Advisory Committee (Ref: 14_096), the independent body that approves use of CPRD data.
Results
We identified 1 678 403 patients who received 3 114 366 prescriptions for flucloxacillin in 3 110 348 consultations over the 10 year period. A total of 82 399 (2.65%) prescriptions that were identified as being ‘repeat prescriptions’ and a further 788 (0.03%) prescriptions with dates that did not match the consultation date were removed from further analysis, leaving 3 031 179 prescriptions issued to 1 667 431 patients in 3 029 933 consultations.
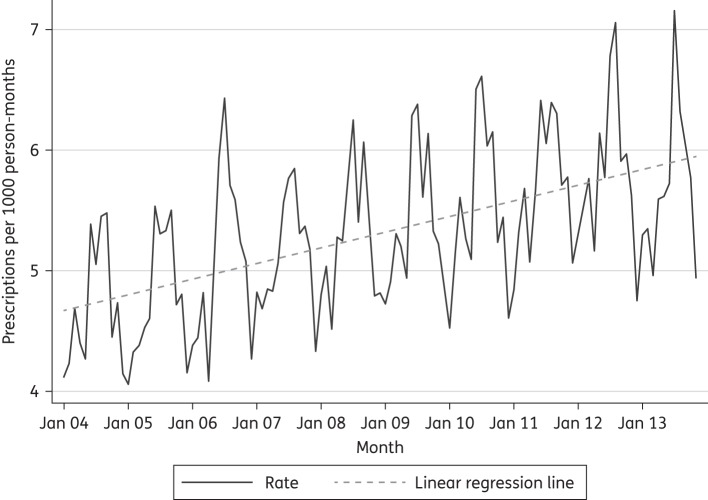
Monthly prescription rates with a monthly linear regression line are shown in Figure 1. The average monthly rates for each year are presented in Table 1. Over the 10 year period from 2004 to 2013 prescribing for flucloxacillin increased by 21.1% [(5.74−4.74)/4.74]. There was significant seasonal variation with the highest prescribing rates in the summer months and the lowest rates in the winter, and an overall trend of increasing prescribing over the study period. The relative influence of month of the year is shown in the regression analysis in Table 2. The lowest prescription rate was in December and the highest was in July, which had a coefficient of 1.61 (95% CI: 1.35, 1.87), which equates to a 33% increase compared with December [using the December 2008 rate (4.82) as the baseline].
Figure 1.
Flucloxacillin prescription rate per month: 2004–13.
Table 1.
Average monthly flucloxacillin prescribing rate by year
| Year | Prescribing rate (prescriptions per 1000 person-years)a |
|---|---|
| 2004 | 4.74 |
| 2005 | 4.81 |
| 2006 | 5.12 |
| 2007 | 5.17 |
| 2008 | 5.33 |
| 2009 | 5.44 |
| 2010 | 5.54 |
| 2011 | 5.72 |
| 2012 | 5.83 |
| 2013 | 5.74 |
aAge-standardized to 2004 population of England and Wales.
Table 2.
Linear regression of flucloxacillin prescribing rate on study month controlling for month of year
| Variable | Coefficient (95% CI) | P |
|---|---|---|
| Study month | 0.01 (0.01, 0.01) | 0.000 |
| Month of the year (reference: December) | ||
| January | 0.18 (0.08, 0.44) | 0.175 |
| February | 0.38 (0.12, 0.64) | 0.004 |
| March | 0.53 (0.27, 0.79) | 0.000 |
| April | 0.41 (0.15, 0.67) | 0.002 |
| May | 0.61 (0.35, 0.87) | 0.000 |
| June | 1.34 (1.08, 1.59) | 0.000 |
| July | 1.61 (1.35, 1.87) | 0.000 |
| August | 1.34 (1.08, 1.60) | 0.000 |
| September | 1.26 (1.00, 1.52) | 0.000 |
| October | 0.73 (0.47, 0.99) | 0.000 |
| November | 0.55 (0.29, 0.81) | 0.000 |
| Constant | −1.45 (−2.36, −0.55) | 0.002 |
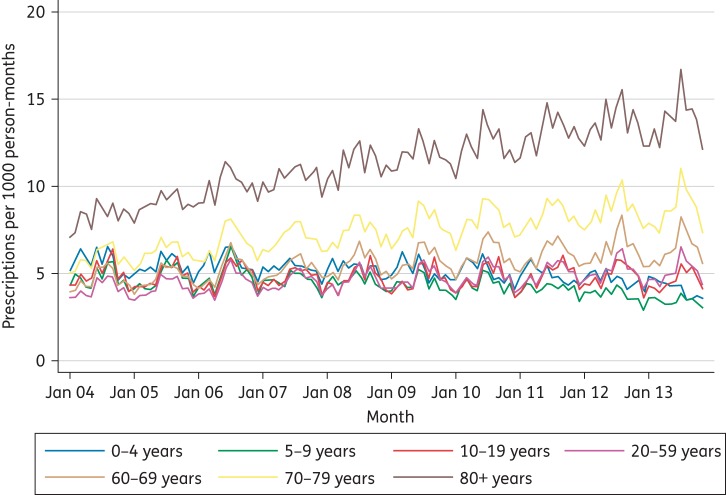
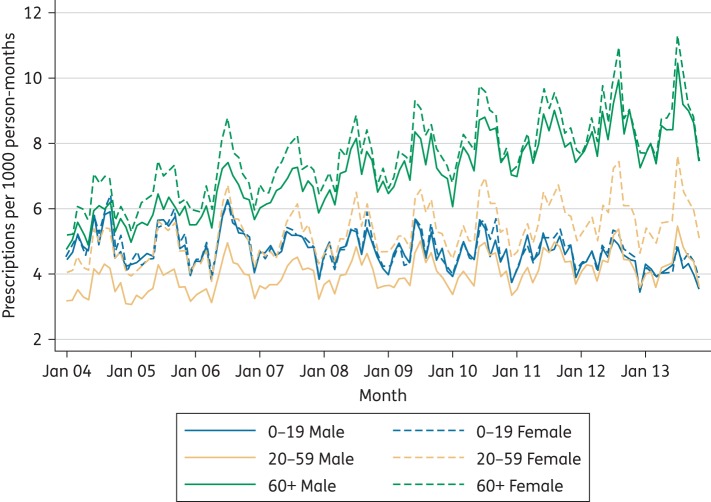
There were marked differences in prescribing rates by age band (Figure 2). The highest prescribing rates were in those aged 70 years and over, with a gradual increase in prescribing rates over the 10 year period in the 70–79 and 80+ age bands, a slight increase in the 60–69 year olds and a slight reduction in the 5–9 year olds. Prescribing rates by gender were similar for children (0–19) and older adults (60+), but for the 20–59 year age band prescribing rates were consistently higher for women than men (Figure 3).
Figure 2.
Flucloxacillin prescribing by age band: 2004–13. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Flucloxacillin prescribing by gender for three age bands: 2004–13. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
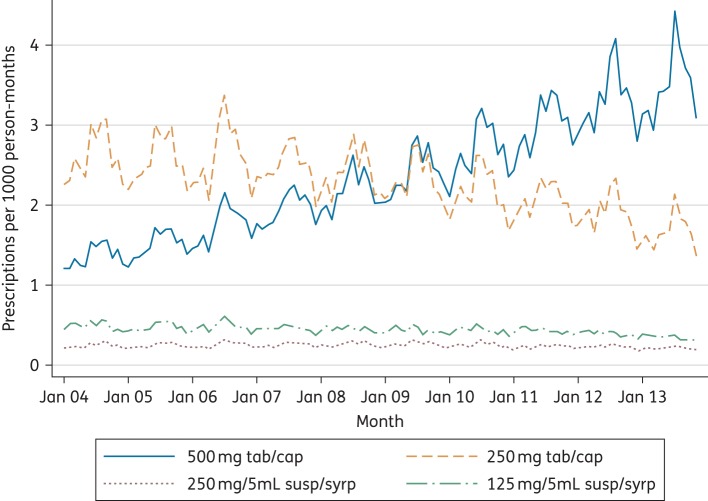
The majority (87.2%) of prescriptions were of tablets or capsules as opposed to suspensions or syrups. Over the 10 year period there was a substantial shift from prescribing predominantly 250 mg tablets or capsules to prescribing predominantly 500 mg tablets or capsules (Figure 4). A total of 2688 (0.09%) prescriptions were recoded as missing for the analysis of quantity because the quantities were over 112 (for tablets or capsules) or 2000 mL (for liquids). Overall, 80.8% of all tablet/capsule prescriptions were for 28 tablets/capsules, which equates to a 7 day course. Ten percent were for <28 and 9.2% were for >28. There was no significant variation in the quantity prescribed across adult age groups or by study year. As such, the shift in total amount of flucloxacillin prescribed in each course increased for all adult age groups during the period under study. For liquid preparations, 76.9% of prescriptions were for 100 mL, which is equivalent to a 5 day course of a 5 mL dose four times a day, with 1.5% being for <100 mL and 21.6% being for >100 mL. The total amount of antibiotic prescribed for children by age bands is shown in Table 3. No change was seen over the 10 year period in the total amount of antibiotic prescribed per course for younger children, but a small increase was seen in 10–19 year olds.
Figure 4.
Flucloxacillin prescribing by formulation: 2004–13. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 3.
Total amount of flucloxacillin prescribed by age group (0–10 years)
| Amount prescribed | 1–1250 g, n (row %) | 1251–2500 g, n (row %) | 2501–5000 g, n (row %) | 5001–7000 g, n (row %) | >7000 g, n (row %) | Total, n |
|---|---|---|---|---|---|---|
| Equivalent to (upper band) | 62.5 mg 4 times a day for 5 days | 125 mg 4 times a day for 5 days | 250 mg 4 times a day for 5 days | 250 mg 4 times a day for 7 days | >250 mg 4 times a day for 7 days | |
| Age band (years) | ||||||
| 0–1 | 1537 (3.3) | 40 661 (86.3) | 4661 (9.9) | 69 (0.2) | 173 (0.4) | 47 101 |
| 2–3 | 237 (0.3) | 54 985 (79.7) | 12 840 (18.6) | 361 (0.5) | 595 (0.9) | 69 018 |
| 4–5 | 38 (0.1) | 47 622 (72.6) | 15 809 (24.1) | 894 (1.4) | 1237 (1.9) | 65 600 |
| 6–7 | 34 (0.1) | 32 605 (56.9) | 20 254 (35.4) | 2177 (3.8) | 2206 (3.9) | 57 276 |
| 8–10 | 69 (0.1) | 25 918 (33.9) | 36 003 (47.0) | 9682 (12.7) | 4866 (6.4) | 76 538 |
| total | 1915 (0.6) | 201 791 (64.0) | 89 567 (28.4) | 13 183 (4.2) | 9077 (2.9) | 315 533 |
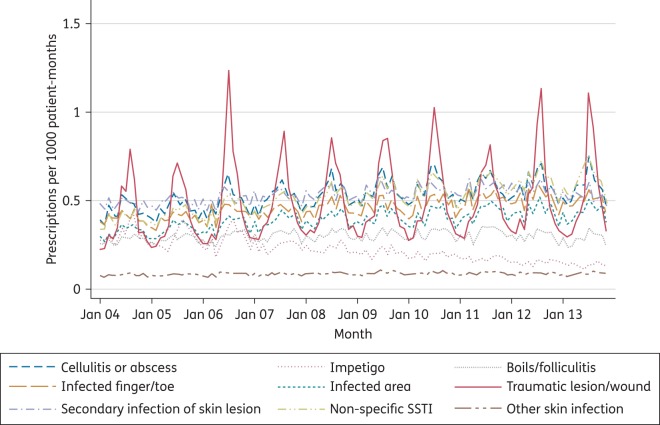
A wide range of Read codes were associated with consultations in which flucloxacillin was prescribed. Consultations were classified into 1 of 12 broad categories (including ‘No code’) and these, along with the most frequent diagnoses within each category, are presented in Table 4. The most frequent category (‘No relevant code’) represents consultations in which one or more Read code was used, but there are no codes that give any indication of an infection or any other likely reason for prescribing flucloxacillin. When this category is combined with those with no code at all, one-third of all prescriptions were not associated with any codes that indicated (or suggested) a diagnosis. The most frequent diagnostic categories were secondary infection of skin condition (10.2%), cellulitis or abscess (10.0%), non-specific SSTI (9.5%) and infected trauma/wound (9.2%). A very small proportion (0.3%) of prescriptions had codes for infections of other systems (osteomyelitis, urinary tract infections or respiratory tract infections) associated with them. Trends in the frequency of SSTI categories over time are presented in Figure 5. The most remarkable features are the marked seasonality for traumatic (primarily insect bite) infections and the gradual decline in flucloxacillin prescribing for impetigo.
Table 4.
Diagnostic classifications recorded with flucloxacillin prescribing
| Coding category—n (%) | Main subcategories (up to three) | No. (% of total) |
|---|---|---|
| No relevant code—774 082 (25.5) | ‘patient reviewed’ | 106 458 (3.5) |
| ‘telephone encounter’ | 80 530 (2.7) | |
| ‘had a chat to patient’ | 34 691 (1.1) | |
| Secondary infection of skin conditions/lesions—309 968 (10.2) | infected eczema/dermatitis | 103 851 (3.4) |
| cysts, warts, naevi, blisters and other skin lesions | 76 682 (2.5) | |
| infected cyst/blister | 62 697 (2.1) | |
| Cellulitis or abscess—301 805 (10.0) | cellulitis | 189 359 (6.2) |
| cellulitis or abscess | 59 802 (2.0) | |
| abscess | 52 644 (1.7) | |
| Non-specific SSTI—286 647 (9.5) | SSTI not otherwise specified | 192 650 (6.4) |
| rash/skin symptoms | 70 106 (2.3) | |
| Traumatic lesion/wound—277 900 (9.2) | wound/dressing | 111 312 (3.7) |
| insect bite | 96 227 (3.2) | |
| post-operative infection | 40 137 (1.3) | |
| Infected digit/nail—259 261 (8.6) | ingrowing nail | 97 173 (3.2) |
| paronychia | 87 811 (2.9) | |
| infected toe/finger | 74 277 (2.5) | |
| No code—244 021 (8.1) | 244 021 (8.1) | |
| Infection classified by site (other than digits)—223 782 (7.4) | breast infection | 39 901 (1.3) |
| otitis externa | 35 008 (1.2) | |
| eye/eyelid infection | 31 667 (1.0) | |
| Boils/folliculitis—170 281 (5.6) | furuncle/carbuncle | 109 287 (3.6) |
| folliculitis | 60 994 (2.0) | |
| Impetigo—126 638 (4.2) | impetigo | 126 638 (4.2) |
| Other skin infection—49 026 (1.6) | superficial phlebitis | 14 849 (0.5) |
| pustules/septic spots | 13 781 (0.5) | |
| superficial lump/swelling | 7890 (0.3) | |
| Other infection—7768 (0.3) | respiratory | 6784 (0.2) |
| urinary | 590 (<0.1) | |
| osteomyelitis | 394 (<0.1) | |
| Total: 3031 179 (100) |
Figure 5.
Flucloxacillin prescribing by diagnostic category: 2004–13. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A total of 2 888 777 first (with no antibiotic prescription in the preceding 28 days) prescriptions of flucloxacillin were identified in 1 678 183 patients; 3670 patients were removed because they contributed <90 days of data, leaving 2 766 006 episodes of care (series of prescriptions with 28 days or less between them) in 1 674 513 patients. A total of 486 432 (17.6%) first prescriptions were followed by a subsequent antibiotic prescription within 28 days and, of these, 126 955 (4.6% of total) involved further subsequent antibiotics, resulting in episodes that involved three or more prescriptions. See Table 5. There was a median (IQR) of 2.0 (1.0, 3.0) episodes per patient per 10 years.
Table 5.
Number of prescriptions per episode of care
| Number of prescriptions in episode | Number of episodes | Proportion of episodes (%) |
|---|---|---|
| 1 | 2 279 574 | 82.4 |
| 2 | 359 477 | 13.0 |
| 3 | 83 013 | 3.0 |
| 4+ | 43 942 | 1.6 |
| Total | 2 766 006 | 100 |
Only 115 959 (4.2%) of 2 766 006 first prescriptions had co-antibiotics (in addition to flucloxacillin) prescribed during the same consultation. The most common co-antibiotics were penicillin V (58.6%), amoxicillin (14.4%), metronidazole (12.6%), co-amoxiclav (3.2%) and erythromycin (1.9%). The most frequently reported antibiotics prescribed at subsequent consultations (that involved prescription of any antibiotic) were flucloxacillin (44.6%), co-amoxiclav (10.2%), erythromycin (8.9%), amoxicillin (8.1%) and penicillin V (6.7%).
Younger (0–4 years) and older (60+) age (compared with the reference category of 20–59 years), having a diagnosis of cellulitis or abscess or having no Read code (compared with having a code that did not indicate an infection) and being prescribed 500 mg tablets (compared with 250 mg tablets) were all associated with a 10% or more increase in the odds of having a subsequent antibiotic prescription within the next 28 days (Table 6). Having a diagnosis of boils/folliculitis or ‘infection at other site’ (mostly breast infection or otitis externa) was associated with a 10% or more reduction in the odds of having a subsequent antibiotic prescription, when compared with having no relevant diagnostic code. There was no significant (in terms of size of effect) association with time (year) and no significant interaction between dose and time (year).
Table 6.
Factors associated with a subsequent antibiotic prescription within 28 days
| OR | 95% CI (lower) | 95% CI (upper) | |
|---|---|---|---|
| Age band (years) | |||
| 0–4 | 1.27 | 1.24 | 1.30 |
| 5–9 | 0.91 | 0.88 | 0.93 |
| 10–19 | 0.93 | 0.92 | 0.94 |
| 20–59 (reference) | 1 | ||
| 60–69 | 1.37 | 1.35 | 1.38 |
| 70–79 | 1.57 | 1.55 | 1.58 |
| 80+ | 1.67 | 1.66 | 1.69 |
| Gender | |||
| male (reference) | 1 | ||
| female | 1.04 | 1.03 | 1.04 |
| Diagnostic category | |||
| no SSTI code (reference) | 1 | ||
| cellulitis or abscess | 1.32 | 1.31 | 1.34 |
| impetigo | 1.08 | 1.06 | 1.10 |
| boils/folliculitis | 0.90 | 0.89 | 0.91 |
| infected digit/nail | 0.95 | 0.94 | 0.96 |
| infection classified by site (other than digits) | 0.88 | 0.87 | 0.89 |
| traumatic lesion/wound | 0.91 | 0.89 | 0.92 |
| secondary infection of skin lesion | 1.00 | 0.99 | 1.01 |
| non-specific SSTI | 0.95 | 0.94 | 0.96 |
| other skin infection | 0.99 | 0.96 | 1.01 |
| no code | 1.28 | 1.27 | 1.30 |
| Dose | |||
| 500 mg tablets | 1.29 | 1.27 | 1.31 |
| 250 mg tablets (reference) | 1 | ||
| suspension | 1.00 | 0.97 | 1.02 |
| Year (continuous) | 1.00 | 1.00 | 1.00 |
| Dose × year interaction | |||
| 500 mg | 1.00 | 1.00 | 1.00 |
| 250 mg (reference) | 1 | ||
| liquid | 1.01 | 1.00 | 1.01 |
| Constant | 0.17 | 0.15 | 0.16 |
Discussion
The prescription rate for flucloxacillin in primary care in the UK increased by 21.1% between 2004 and 2013, with marked peaks in the summer. Over the same period, there was a marked shift toward prescribing higher-dose (500 mg tablets) over low-dose (250 mg tablets) flucloxacillin. The highest prescribing rates and the largest increases in rates were seen in older adults (70+). There were few differences by gender. The quantity/duration of antibiotics prescribed (typically 7 days for tablets and 5 days for liquid) did not change significantly and, therefore, the increasing use of higher-dose tablets led to an increase in the average total amount of antibiotic prescribed between 2004 and 2013 for adults. Many children were prescribed lower doses than recommended for their age (for example, one-third of 8–10 year olds were prescribed the equivalent of 125 mg 4 times a day for 5 days or less). Cellulitis or abscess, secondary infection of skin conditions and infected trauma/wound were the most prevalent diagnostic categories. However, over a third of prescriptions had no associated relevant medical diagnostic code and a further 10% were associated with a non-specific SSTI code. Most (82.4%) new prescriptions for flucloxacillin were not followed by an antibiotic prescription within the next 28 days. There was a median of 2 (IQR 1, 3) episodes (clusters of antibiotic prescriptions within 28 days of each other that started with a prescription of flucloxacillin) per 10 years per patient. Being <5 or ≥60 years, having a diagnostic code for cellulitis or abscess or no diagnostic code, and being prescribed higher-dose flucloxacillin, were all associated with an increased odds of being prescribed an antibiotic in the 28 days following an initial flucloxacillin prescription. Only 4.2% of first prescriptions of flucloxacillin had another antibiotic prescribed at the same time and 58.6% of these were penicillin V.
This study used a large, well-validated database with research quality data.16 By using data going back to 2004 we were able to examine trends over time and, by using such a large dataset, we were able to describe precisely the rates by month (and, therefore, describe the seasonality of use) and by important subgroups. Prescriptions in UK general practice are almost universally generated via the computer and, therefore, recording of prescribing data is very accurate. However, it is possible that some prescriptions, particularly those generated during home visits, will not be included. Home visits are a small proportion of the workload, particularly for a condition that does not often incapacitate (SSTIs), but it is possible that housebound patients, who are at risk of cellulitis, are under-represented.
The main weakness of this study is that prescriptions are not directly linked to diagnosis in CPRD. Linking of diagnostic codes can only be achieved at the level of the consultation (i.e. Read codes entered during the same consultation in which a prescription is issued are likely to be linked). However, we found that even at the consultation level a high proportion of prescriptions had no associated diagnostic codes. There are also few data available to measure the accuracy of the diagnostic codes used and, given the large number of codes within the Read system, there will inevitably be variations in coding practices between GPs. Fortunately, flucloxacillin is an antibiotic that is only indicated for infections likely to be caused by S. aureus and, in UK primary care, this is almost exclusively SSTIs. Therefore, we can have confidence in the overall levels of prescribing being robust, but the subdivision by diagnoses may need to be treated with caution. In our analysis of subsequent prescribing, we identified prescriptions for any antibiotic that could be used for SSTI and did not limit inclusion to those that were associated with an SSTI code. It is possible that some of these prescriptions were for alternative (new) diagnoses, such as respiratory tract infections; however, we restricted the follow-up period to 4 weeks and found that the most commonly prescribed subsequent antibiotic was also flucloxacillin. Therefore, we believe that we are predominantly identifying subsequent prescribing for SSTIs.
Previous analyses of flucloxacillin prescribing in primary care have shown rates increasing up to 20066–8 and we were able to demonstrate that this trend continued up to the end of 2013 and that increases were primarily in older adults. We used age-specific population denominators and the rates have been age-standardized to the 2004 population of England and Wales, and so the increases do not simply relate to an ageing population. However, the changes in population demographics, with significant ongoing increases in the proportion of the population that are older, do make these data particularly concerning and worthy of further investigation.
One question is whether the increase in flucloxacillin prescribing is caused by an increase in the incidence of consultations for SSTI or if it relates to a reduction in the threshold for prescribing. We recently identified SSTIs as a common infection and a common reason for antibiotic prescription in older adults in care homes.17 However, data on how the incidence of SSTIs in this age group has changed over time is somewhat conflicting. Fleming et al.7 reported reductions in the incidence of skin infections in primary care for both adults and children between 1999 and 2005, but did not look specifically at older adults. Hayward et al.,6 on the other hand, used Hospital Episode Statistics data to explore trends in hospital admissions for community-onset staphylococcal diseases and found an increase between 1989–90 and 2003–04, with the highest rates, and greatest increases, in older adults.
Although any trend toward increased antibiotic prescribing is potentially concerning, the implications of increasing flucloxacillin prescribing on antimicrobial resistance (in the form of MRSA) are unclear. A recent study of the prevalence of MRSA in commensals identified from the anterior nares of individuals in the community found low levels in all nine European countries studied (including the UK).18
We were not able to identify any other studies that highlighted the shift from prescribing lower-dose to higher-dose flucloxacillin over the past decade. The higher dose is certainly recommended for cellulitis or other infections where group A streptococci are the likely causative organisms. However, there remains little empirical evidence to guide the dose or duration of treatment. The use of lower than recommended unit doses for flucloxacillin in children has been previously described,19 but we took this further by identifying that most children are prescribed only enough for 5 days. As with adults, there is little empirical evidence supporting an optimal dose or duration. However, our data make it apparent that children are frequently prescribed low doses and short courses.
We found that nearly one in five patients who are prescribed a course of flucloxacillin are prescribed a subsequent course of antibiotics within 4 weeks, suggesting that treatment failure may be a significant problem. Currie et al.3 reported a treatment failure rate for SSTIs, using a similar approach, but identifying consultations where any antibiotic was associated with a diagnosis of SSTI (rather than flucloxacillin prescribing), of 12.8%. However, the diagnosis of bacterial infection is not always straightforward in primary care and prescribing a subsequent antibiotic is not necessarily an indication that the first antibiotic ‘failed’. For example, in our study subsequent antibiotic prescribing may have been for another condition or for colonized skin ulcers, eczema flares or hidradenitis suppurativa, and may not be an indication that the first antibiotic ‘failed’. Nevertheless, most antibiotic treatment is intended to be delivered as a single course and, therefore, our finding that 17.6% received a subsequent course is of concern. We found that being prescribed a higher dose of flucloxacillin increased the risk of being prescribed a subsequent antibiotic. This probably represents confounding by indication, with those having more severe infections prescribed higher doses and thus being more likely to re-consult. Our finding that children were less likely (than adults) to require a subsequent course, despite often receiving doses that are below guideline recommendations, suggests that either dosing guidelines need revisiting or that many of these initial prescriptions were unnecessary. Resistance to flucloxacillin may be a reason for the high rate of further prescriptions; however, MRSA prevalence in commensal organisms in the community is relatively low in the UK.18 Diagnosis of skin infections has been shown to be problematic,13 and misdiagnosis may also be a contributing factor.
Seasonal variation has been previously described in both the incidence of impetigo20 and hospital admissions for non-necrotizing cellulitis,21 and Fleming et al.7 have previously shown increased flucloxacillin prescribing in the third quarter. However, we have provided a more detailed analysis and been able to show that prescribing peaks in July and that seasonal variation is more pronounced for some diagnostic categories (infected traumatic lesions, cellulitis and non-specific SSTI) than for others (impetigo and ‘other SSTI’).
Cellulitis is the main serious SSTI and, therefore, it is not surprising that cellulitis or abscess was identified as one of the most common diagnostic categories. However, we found that many prescriptions were associated with other codes or not associated with any useful code. A previous study of indications for antibiotic prescribing in primary care identified ‘skin’, ‘other’, ‘abscess/boil’ and ‘impetigo’ as the most common categories.1 It is difficult to make direct comparisons due to differences in the way conditions were classified, but we found that ‘boils’ and ‘impetigo’ were uncommon diagnoses associated with flucloxacillin prescribing and that ‘no relevant code’ and ‘secondary infection of skin conditions/lesions’ were the most common categories. Part of the explanation for the differences lies in the complexities of Read coding; some Read codes are for ‘cellulitis or abscess’ and, therefore, as it was impossible to disentangle these diagnoses completely, we grouped them together. There is clearly an important need to improve coding in primary care medical records. NHS funders should work closely with electronic medical record suppliers to optimize their systems to make it easier to apply codes accurately, and primary care staff should be trained and incentivized to code more accurately. Finally, the UK should consider moving away from the Read code system, which is repetitive and allows for vague and unhelpful coding, to a more standardized and universally accepted coding system such as the International Classification of Primary Care.
Previous research has highlighted higher levels of antibiotic prescribing (particularly flucloxacillin) in patients identified as having chronic wounds.22 While we did not specifically identify this group, codes associated with wounds/dressings accounted for only 3.7% of prescribing in our study, suggesting that while high levels of prescribing may have important implications for the individuals involved, chronic wounds do not seem to be a major contributor to overall levels of acute prescribing for flucloxacillin.
S. aureus and group A streptococci are both important causes of skin infections and guidelines sometimes recommend using flucloxacillin to cover the Staphylococcus and penicillin V for the streptococci.11 We found that co-prescribing occurred in only a small minority of consultations and we are not aware of any other evidence on the frequency of co-prescribing penicillin V with flucloxacillin.
Our findings highlight a need to understand better the reasons for the ongoing increase in prescribing of flucloxacillin in UK primary care. Although we identified older age and diagnosis of cellulitis, and not a younger age and diagnosis of impetigo, as areas to focus on, problems with diagnostic coding limited our ability to draw firm conclusions. Prospective observational studies or analyses of routine data where the diagnosis is confirmed (by contacting participating clinicians or through an analysis of the ‘free text’) could provide greater clarity. It is unclear how generalizable our results are to other settings (secondary care and other countries) and there are limited data on MRSA. There is also a need for trials to explore the optimal use of oral antibiotics in the management of SSTIs, including optimal dose and duration and the role of co-prescription of penicillin V, or other antibiotics, with flucloxacillin. Finally, there is a need to understand why the prescribing of further antibiotics within 4 weeks of an initial prescription of flucloxacillin is so prevalent. Suboptimal dosing or incorrect choice of antibiotic may play a role, but misdiagnosis of other inflammatory skin conditions as infection could also be a reason. Therefore, there is a need to evaluate (and seek to improve) the diagnostic accuracy of the clinical diagnosis of cellulitis in primary care.
Funding
This work was supported by a fellowship for N. A. F. from the National Institute for Social Care and Health Research (NISCHR) Wales (now Health and Care Research Wales) (grant number: HF-11-01).
The study funder reviewed the study proposal, but played no role: in the study design; in the collection, analysis and interpretation of the data; or in the writing of the report.
K. H. receives funding from Health and Care Research Wales as part of the South East Wales Trials Unit. R. L. and C. C. B. receive funding through the Farr Institute of Health Informatics Research. The Farr Institute is supported by a consortium of ten UK research organisations: Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the MRC, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Government) and the Chief Scientist Office (Scottish Government Health Directorates). MRC Grant No: MR/K006525/1. C. C. B. also receives support from the National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at the University of Oxford.
Transparency declarations
None to declare.
Supplementary data
Supplementary data are available at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We would like to thank Sara Jenkins-Jones from Pharmatelligence for extracting the data.
References
- 1.Petersen I, Hayward AC, on behalf of the SACAR Surveillance Subgroup. Antibacterial prescribing in primary care. J Antimicrob Chemother 2007; 60 Suppl 1: i43–7. [DOI] [PubMed] [Google Scholar]
- 2.Schofield JK, Fleming DM, Grindlay D et al. . Skin conditions are the commonest new reason people present to general practitioners in England and Wales. Br J Dermatol 2011; 165: 1044–50. [DOI] [PubMed] [Google Scholar]
- 3.Currie CJ, Berni E, Jenkins-Jones S et al. . Antibiotic treatment failure in four common infections in UK primary care 1991–2012: longitudinal analysis. BMJ 2014; 349: g5493–3. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Antimicrobial Resistance: Global Report on Surveillance. 2014.
- 5.Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 2014; 58 Suppl 1: S20–7. [DOI] [PubMed] [Google Scholar]
- 6.Hayward AC, Knott F, Petersen I et al. . Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerging Infect Dis 2008; 14: 720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming DM, Elliot AJ, Kendall H. Skin infections and antibiotic prescribing: a comparison of surveillance and prescribing data. Br J Gen Pract 2007; 57: 569–73. [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena S, Thompson P, Birger R et al. . Increasing skin infections and Staphylococcus aureus complications in children, England, 1997–2006. Emerging Infect Dis 2010; 16: 530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens H, Ferech M, Vander Stichele R et al. . Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365: 579–87. [DOI] [PubMed] [Google Scholar]
- 10.Costelloe C, Metcalfe C, Lovering A et al. . Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. [DOI] [PubMed] [Google Scholar]
- 11.Anon. Dilemmas when managing cellulitis. Drug Ther Bull 2003; 41: 43–6. [DOI] [PubMed] [Google Scholar]
- 12.Quirke M, O′Sullivan R, McCabe A et al. . Are two penicillins better than one? A systematic review of oral flucloxacillin and penicillin V versus oral flucloxacillin alone for the emergency department treatment of cellulitis. Eur J Emerg Med 2014; 21: 170–4. [DOI] [PubMed] [Google Scholar]
- 13.Levell NJ, Wingfield CG, Garioch JJ. Severe lower limb cellulitis is best diagnosed by dermatologists and managed with shared care between primary and secondary care. Br J Dermatol 2011; 164: 1326–8. [DOI] [PubMed] [Google Scholar]
- 14.Kousoulis AA, Rafi I, de Lusignan S. The CPRD and the RCGP: building on research success by enhancing benefits for patients and practices. Br J Gen Pract 2015; 65: 54–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.www.ons.gov.uk.
- 16.Herrett E, Thomas SL, Schoonen WM et al. . Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hood K, Nuttall J, Gillespie D et al. . Probiotics for Antibiotic-Associated Diarrhoea (PAAD): a prospective observational study of antibiotic-associated diarrhoea (including Clostridium difficile-associated diarrhoea) in care homes. Health Technol Assess 2014; 18: doi:10.3310/hta18630doi:10.3310/hta18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Heijer CDJ, van Bijnen EME, Paget WJ et al. . Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S aureus, in nine European countries: a cross-sectional study. Lancet Infect Dis 2013; 13: 409–15. [DOI] [PubMed] [Google Scholar]
- 19.Saxena S, Ismael Z, Murray ML et al. . Oral penicillin prescribing for children in the UK: a comparison with BNF for Children age-band recommendations. Br J Gen Pract 2014; 64: e217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loffeld A, Davies P, Lewis A et al. . Seasonal occurrence of impetigo: a retrospective 8-year review (1996–2003). Clin Exp Dermatol 2005; 30: 512–4. [DOI] [PubMed] [Google Scholar]
- 21.Haydock SF, Bornshin S, Wall EC et al. . Admissions to a U.K. teaching hospital with nonnecrotizing lower limb cellulitis show a marked seasonal variation. Br J Dermatol 2007; 157: 1047–8. [DOI] [PubMed] [Google Scholar]
- 22.Howell-Jones RS, Price PE, Howard AJ et al. . Antibiotic prescribing for chronic skin wounds in primary care. Wound Repair Regen 2006; 14: 387–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.