Abstract
In order to identify genetic components in flowering pathways of highbush blueberry (Vaccinium corymbosum L.), a transcriptome reference composed of 254,396 transcripts and 179,853 gene contigs was developed by assembly of 72.7 million reads using Trinity. Using this transcriptome reference and a query of flowering pathway genes of herbaceous plants, we identified potential flowering pathway genes/transcripts of blueberry. Transcriptome analysis of flowering pathway genes was then conducted on leaf tissue samples of transgenic blueberry cv. Aurora (‘VcFT-Aurora’), which overexpresses a blueberry FLOWERING LOCUS T-like gene (VcFT). Sixty-one blueberry transcripts of 40 genes showed high similarities to 33 known flowering-related genes of herbaceous plants, of which 17 down-regulated and 16 up-regulated genes were identified in ‘VcFT-Aurora’. All down-regulated genes encoded transcription factors/enzymes upstream in the signaling pathway containing VcFT. A blueberry CONSTANS-LIKE 5-like (VcCOL5) gene was down-regulated and associated with five other differentially expressed (DE) genes in the photoperiod-mediated flowering pathway. Three down-regulated genes, i.e., a MADS-AFFECTING FLOWERING 2-like gene (VcMAF2), a MADS-AFFECTING FLOWERING 5-like gene (VcMAF5), and a VERNALIZATION1-like gene (VcVRN1), may function as integrators in place of FLOWERING LOCUS C (FLC) in the vernalization pathway. Because no CONSTAN1-like or FLOWERING LOCUS C-like genes were found in blueberry, VcCOL5 and VcMAF2/VcMAF5 or VRN1 might be the major integrator(s) in the photoperiod- and vernalization-mediated flowering pathway, respectively. The major down-stream genes of VcFT, i.e., SUPPRESSOR of Overexpression of Constans 1-like (VcSOC1), LEAFY-like (VcLFY), APETALA1-like (VcAP1), CAULIFLOWER 1-like (VcCAL1), and FRUITFULL-like (VcFUL) genes were present and showed high similarity to their orthologues in herbaceous plants. Moreover, overexpression of VcFT promoted expression of all of these VcFT downstream genes. These results suggest that VcFT’s down-stream genes appear conserved in blueberry.
Introduction
Blueberry contains high amounts of antioxidants known to be important for human health [1]. The highbush blueberry (2n = 4x = 48) (Vaccinium corymbosum L.), including northern and southern ecotypes, is the major cultivated Vaccinium fruit crop [2, 3]. The northern highbush cultivars have better winter hardiness, but their adoption in warm areas is often limited by their requirement for more chill units (CU) (generally > 800) to break dormancy in the spring. In contrast, the southern highbush blueberry cultivars are derivatives of the northern highbush blueberry with additional genes from other southern Vaccinum species [e.g., V. darrowi (2n = 2x = 24), V. ashei (2n = 6x = 72), and V. tenellum (2n = 2x = 24)] [2]. These southern cultivars have better summer heat tolerance and require fewer chill units (150 to 600 CU) to induce flowering, but they are often less cold/freeze-tolerant. Developing new cultivars with different flowering times and chilling requirements, high cold/heat tolerances, and high yields are the top priorities in breeding for sustainable blueberry production, particularly in anticipation of climate changes.
Abundant genetic resources of the genus Vaccinium (i.e. 450 species) contain high genetic diversity but also lead to great complexity making it difficult to improve the cultivated tetraploid highbush cultivars through conventional breeding [2]. Alternatively, new genomic and biotechnological tools will facilitate studies on blueberry genetics and genomics and improve breeding efficiency [2, 4–10]. For blueberry genomic studies, a draft blueberry genome assembly with 358 million base pairs (Mb) of diploid blueberry accession ‘W8520’ was generated and used for genetic diversity studies of cultivated blueberry cultivars as well as marker-assisted breeding and comparative genomics of Vaccinium species [2]. Using RNA-Seq data and a draft blueberry genome assembly, candidate genes involved in fruit ripening and biosynthesis of bioactive compounds have been reported [7]. In addition, 22,401 blueberry (V. corymbosum) expressed sequence tags (ESTs) are available in GenBank. Generation and analyses of the transcriptomes of flower and fruit tissues of V. corymbosum ‘Northland’ yielded 34,464 NCBI Unigene clusters out of 64 million sequencing reads for identifying genes involved in antioxidant biosynthesis [8]. Transcriptomes of the major highbush blueberry cultivar Bluecrop were generated from leaves, flower buds at different stages of cold acclimation, and fruit tissues at different stages of development. About 15,000 contigs were derived from 600,000 reads and are considered to be useful for analyzing differentially expressed (DE) genes in flower bud formation, vernalization, cold acclimation, and fruit development [9].
Reliable and highly effective Agrobacterium tumefaciens-mediated transformation and regeneration methods have been developed for several highbush blueberry cultivars [11, 12]. The availability of blueberry transcriptome information and genetic transformation tools enables gene cloning and functional gene analysis. For example, overexpressing the endogenous blueberry C-repeat binding factor (CBF) gene (VcCBF) (GenBank AF234316) resulted in a significant increase of freeze tolerance in leaves and floral buds [10]. These results demonstrate the potential of genomics and genetic transformation for blueberry breeding.
Flowering is controlled and modulated by flowering genes and environmental signals (e.g., light and temperature) and is of importance for sustainable blueberry production. To date, the molecular basis of flowering in Arabidopsis thaliana (L.) and other herbaceous plants has been extensively studied through investigations into the networks of flowering-related genes [13–21]. In contrast, few similarly detailed studies have been conducted to reveal genetic control of seasonal flowering of woody perennials [14, 22]. Of the reported flowering-related genes of A. thaliana, the major ones include CONSTANS 1 (CO1), FLOWERING LOCUS C (FLC), FLOWERING LOCUS T (FT), SUPPRESSOR of Overexpression of Constans 1 (SOC1), LEAFY (LFY), APETALA1 (AP1), and FRUITFULL (FUL) [23]. To study the vernalization-mediated flowering mechanisms in blueberry, we isolated one blueberry FT-like gene (VcFT), three AP1-like genes, three SOC1-like genes and one LFY-like gene (VcLFY) from the highbush blueberry cvs. Bluecrop and Legacy. Overexpressing VcFT caused early and continuous flowering in in vitro shoots and in one-year old ‘Aurora’ plants [5]. However, our observation on two- and three-year old plants showed that the VcFT-overexpressing plants did not flower normally and the majority of the flower buds did not open under greenhouse conditions without chilling. Analyses of DE genes in VcFT-overexpressing blueberry plants should reveal VcFT-regulated flowering gene networks.
The aim of this study is to identify flowering-related gene networks of highbush blueberry. We developed a transcriptome reference of blueberry, assembled using Trinity [24], and annotated flowering pathway genes by comparative genomics of A. thaliana, rice (Oryza sativa), and some cereals, were used to identify flowering-related genes of blueberry [25]. DE analysis of flowering-related genes was subsequently conducted on the transcriptome from leaf tissues of transgenic ‘Aurora’ plants (herein ‘VcFT-Aurora’) overexpressing VcFT. In this way, we expected to identify functional flowering pathway genes of blueberry, which respond to the major flowering pathway integrator VcFT.
Materials and Methods
Plant materials
The northern highbush blueberry cv. Aurora and the southern highbush cv. Legacy were used. Both cultivars are tetraploid and require at least 800 CU to flower. The reference transcriptome was developed using eight ‘Legacy’ tissue samples derived from a wild type plant, one plant from each of two transgenic events containing an overexpressed VcCBF (herein ‘VcCBF-Legacy’) obtained in our previous study [10]. These tissue samples included unvernalized leaves and floral buds and flower tissues with corollas removed. Three plants of wild type ‘Aurora’ and three plants from one transgenic line of ‘VcFT-Aurora’, a transgenic line overexpressing VcFT obtained in our previous study [5], were used to analyze differential expression resulting from VcFT overexpression. Differential expression comparisons across tissue types (leaves, floral buds, and flower) utilized three plants of wild type ‘Legacy’.
Growth and flowering of non-transgenic ‘Aurora’ and transgenic ‘VcFT-Aurora’ plants were investigated using 12 non-transgenic plants and 12 plants from each of the five transgenic events obtained in our previous study [5]. Phenotypic data (i.e., plant height, flowering time, the number of floral buds that flowered, and flower number per flowered bud) were collected. For differential expression analyses, young leaf tissues of 3 two-year old, unvernalized ‘Aurora’ plants and 1 transgenic ‘VcFT-Aurora’ event of were sampled for RNA isolation and sequencing.
All plants were from in vitro cultured shoots and grown in the greenhouse (heated for winter) under natural light and ambient temperature conditions, and a regular schedule of irrigation and fertilization using 0.2 g/L fertilizer (Nitrogen: Phosphorus: Potassium = 21: 7: 7) [11]. To get full vernalization, one-year old plants were grown in controlled environment chambers at 4°C with a 12 hour photoperiod for two months; two- and three-year old plants were exposed to the natural environment in winter in a secured courtyard between our greenhouses.
RNA preparation and sequencing
Floral buds were collected in November 2013 before the plants were exposed to a non-heated greenhouse for chilling. Newly emerged young leaf and flower tissues of ‘Legacy’ were collected in February 2014. Flower tissues consisted of all parts from newly opened flowers except corollas. Young leaf tissues of two-year old ‘Aurora’ and ‘VcFT-Aurora’ plants were collected in June 2014 from unvernalized plants. All tissues collected were frozen immediately in liquid nitrogen and stored at -80°C.
Total RNA was isolated from 0.5 g tissues for each sample, using a cetyltrimethylammonium bromide (CTAB) method [26]. The samples were purified using RNeasy Mini Kit and On-Column DNase digestion with the RNase-free DNase Set (Qiagen, Valencia, CA, USA). Integrity of the RNA samples was assessed using the Agilent RNA 6000 Pico Kit (Agilent Technologies, Inc. Germany). All samples had a RNA quality score above 8.0 before submission for sequencing (100-bp paired-end reads) using the Illumina HiSeq2500 platform at the Research Technology Support Facility of Michigan State University (East Lansing, MI, USA).
de novo transcriptome assembly
FastQC program (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was used to assess the quality of sequencing reads for the per base quality scores range from 26–40. Generation of the transcriptome reference for highbush blueberry from RNA-seq was carried out using the Trinity platform (trinity/20140413p1)[24]. The command for running about 72 million reads (MR) was “Trinity—seqType fq—JM 50G —left reads_R1_CBFVF.fq—right reads_R2_CBFVF.fq—SS_lib_type RF—CPU 24”. The memory request was 250G and the wall-time request was 48 hr. Left/Right reads were a combination of the reads from eight samples of non-transgenic and transgenic ‘Legacy’. The quality of the assembly was assessed by alignment with existing EST singlets and contigs in the Unigene v1 library obtained from www.vaccinium.org. These sequences were derived from a variety of tissues and cultivars of highbush blueberry. BLASTplus version 2.2.27 was used to run blastn with default parameters except dust was off, percent identity ranged from 80–95%, with an evalue threshold of 1e-05. Unigene sequences were used as query with refTrinity as database. Results were filtered for completeness, calculated as (qend-(qstart-1))/qlen.
Differential expression analysis
RNA-seq reads of three biological replicates for each of the ‘Legacy’ tissues, plus ‘Aurora’ and ‘VcFT-Aurora’ leaves were analyzed. Two technical replicates were sequenced for each biological replicate and combined together for analysis. The paired reads, two sets for each biological replicate, were aligned to the transcriptome reference developed for ‘Legacy’ and the abundance of each read was estimated using the Trinity command “align_and_estimate_abundance.pl”. This generated 12 “genes.counts.matrix” and 12 “transcripts.counts.matrix” files. The Trinity command “abundance_estimates_to_matrix.pl—est_method RSEM” was then used to merge “genes.counts.matrix” files and “transcripts.counts.matrix” files, separately. The resultant two matrices were used for running DE analysis using the Trinity command “run_DE_analysis.pl—method edgeR”. The DE genes or transcripts with false discovery rate (FDR) values below 0.05 were used for further analyses of flowering pathway genes of blueberry.
For RT-PCR analysis of differentially expressed genes/transcripts, reverse-transcription of RNA to cDNA was performed using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The resulting cDNA was diluted (volume 1:10) in water, and 2 μl/sample, was used for each PCR reaction. Three RNA samples from leaf tissues for each of non-transgenic ‘Aurora’ and transgenic ‘VcFT-Aurora’ were used. The primers were included in S1 Table. The reaction conditions for all primer pairs were 94°C for 2 min, 35 cycles of 45 s at 94°C, 60 s at 60°C and 90 s at 72°C, with a final 10 min extension at 72°C. RT-PCR products were separated on 1.0% agarose gel containing ethidium bromide visualized, and photographed under UV light.
Sequence sources of plant flowering pathway genes
Three protein datasets of the flowering pathway genes of Arabidopsis, rice, and cereal crops [i.e., bread wheat (Triticum aestivum), einkorn wheat (T. monococcum), barley (Hordeum vulgare), and maize (Zea mays)] were developed according to the gene IDs reported by [25] (S2 Table). Each dataset was used for orthologue identification using tblastn against the transcriptome reference of blueberry. The top hits (i.e., transcripts and genes) with e-values less than e-20, were retained to do comparative genomics of blueberry flowering genes and to search for the DE profiles of VcFT-overexpressing ‘Aurora’.
Annotation of DE genes/transcripts
Trinotate was used to annotate the transcriptome reference and DE transcripts. Blueberry flowering pathway transcripts were also used as queries to search the A. thaliana protein database and GenBank using blastx. All Trinity and Trinotate analyses were performed using the resources at the High Performance Computing Center of Michigan State University.
Results
Phenotypes of VcFT overexpressing plants
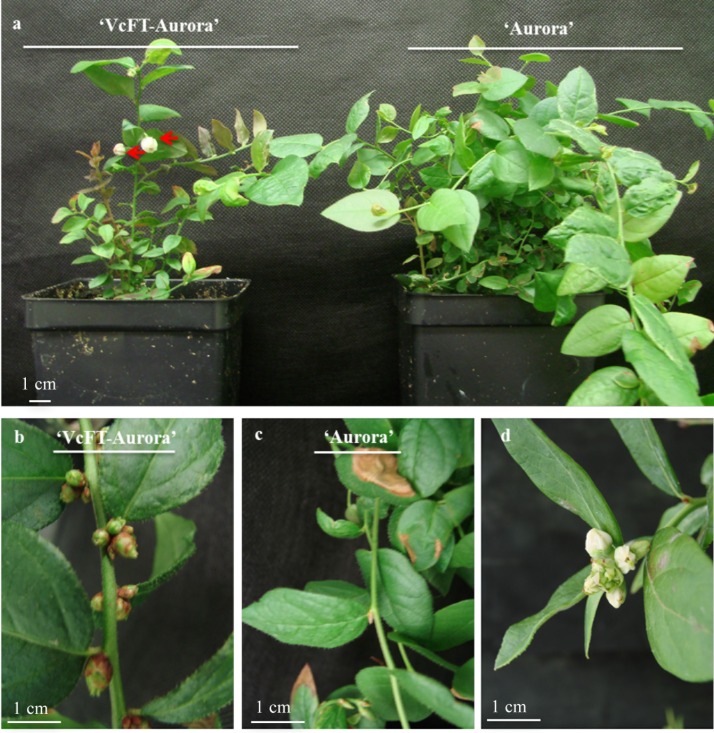
‘VcFT-Aurora’ constitutively expresses VcFT driven by the cauliflower mosaic virus (CaMV) 35S promoter [5]. Overexpressing VcFT in ‘VcFT-Aurora’ resulted in dwarf plants, early floral bud formation, and continuous flowering (Fig 1A–1C). For example, the height of one- or two-year old ‘VcFT-Aurora’ plants was about half that of non-transgenic ‘Aurora’ and some newly formed buds flowered during the growing season (Fig 1A). ‘Aurora’ plants did not develop floral buds until they were three-years old. Fully chilled plants flowered with a bloom period of about one week and each bud had 5–10 flowers, while unvernalized plants did not flower. In contrast, ‘VcFT-Aurora’ shoots started to flower while they were in in vitro culture [5]. Fully chilled ‘VcFT-Aurora’ plants flowered normally with 5–10 flowers/bud (Fig 1D). The plants then formed new shoots and reverted to continuous floral bud formation and flowering. For unvernalized ‘VcFT-Aurora’ plants, 20–50% of floral buds flowered with 2–3 flowers/bud (Fig 1A). These results suggest that over-expressing VcFT alone promotes early flowering, but is not sufficient to completely substitute the need for vernalization for blueberries to flower normally under non-chilling conditions.
Fig 1. Comparison of flowering between ‘VcFT-Aurora’ and ‘Aurora’ plants.
a, Continuous flowering of non-vernalized one-year old ‘VcFT-Aurora’ (left) alongside ‘Aurora’ plants with no flowers (right). Arrows show flowers. b, Floral bud formation on the new shoots of ‘VcFT-Aurora’. c, Vegetative buds, but not floral buds, appeared on new shoots of ‘Aurora’. d, Fully vernalized ‘VcFT-Aurora’ flowered normally.
Development of a transcriptome reference for blueberry
Approximately 8–10 million pair-end reads (MR) were generated for each sample. Using Trinity [24], 72.7 MR obtained from eight samples of leaf, floral bud, and flower tissues were assembled. The total Trinity ‘genes’ and ‘transcripts’ were 179,853 and 254,196, respectively (Table 1). All these transcript contigs, a total of 192 million bases, was designated as refTrinity and were used as the transcriptome reference for identifying candidate genes and differential expression analyses. The quality of the assembly was assessed by aligning to known EST and gene contig sequences obtained from the database at www.vaccinium.org. refTrinity aligned to 61% of 2,955 ESTs and 70% of 748 contig sequences with at least 90% identity and 90% completeness (S3 Table). Considering that the query sequences originated from varied cultivars, tissues, and treatments, the results suggest that Trinity is an efficient platform in generating transcriptome references for tetraploid blueberry plants.
Table 1. Statistic analysis of blueberry transcriptome reference developed using Trinity.
Nt: nucleotide.
| Statistics based on ALL transcript contigs | |
|---|---|
| Total trinity 'genes' | 179,853 |
| Total trinity transcripts | 254,196 |
| Percent GC | 41.58 |
| Contig N10 | 3323 nt |
| Contig N20 | 2528 nt |
| Contig N30 | 2018 nt |
| Contig N40 | 1620 nt |
| Contig N50 | 1256 nt |
| Median contig length | 423 nt |
| Average contig | 754 nt |
| Total assembled bases | 191,637,363 nt |
Identification of flowering pathway genes of blueberry
Abundant sequence data (i.e., DNA, cDNA, EST, and protein) of flowering pathway genes from many plant species are deposited in GenBank. To identify orthologues of flowering pathway genes of blueberry, we used protein sequences derived from the previous reports and a cutoff e-value of above e-20 to identify flowering pathway genes in blueberry (S2 Table) [25]. When 156 sequences of 81 flowering-related genes of Arabidopsis (TAIR 10) were used to blast against refTrinity, 127 sequences of 57 genes got hits. Of the query of 160 sequences of 103 flowering pathway genes of rice (RGAP 7), only OsF gene (LOC_Os02g08150.1) did not have a match. With the query of 49 flowering-related genes of cereal crops, 48 genes had matching sequences. Overall, we pooled 1,223 transcript contigs from 720 gene contigs that showed high similarities (e-values ≤ e-20) to the query sequences, which allowed us to generate a reference of potential flowering pathway genes of blueberry (herein Trinity_floral_ref) (S4 Table).
Orthologues of most common flowering pathway genes of Arabidopsis, rice, and cereal crops were found in blueberry (S4 Table). These included, important genes such as FRIGIDA-like (VcFRI), VcFT, APETALA 2-like (VcAP2), SUPPRESSOR of Overexpression of Constans 1-like (VcSOC1), LEAFY-like (VcLFY), APETALA1-like (VcAP1), CAULIFLOWER 1-like (VcCAL1), and FRUITFULL-like (VcFUL) genes. These results validated refTrinity as a reliable transcriptome reference for identification of flowering pathway genes of blueberry.
Arabidopsis CO1 and rice HEADING DATE (HD1) are the major integrator genes in their respective photoperiod-regulated flowering pathways [25]. Using the CO1 as a query to search online Vaccinium sequences, we did not get any sequence with a high similarity. In Trinity_floral_ref, we found three CONSTANS-LIKE (COL)-like genes (i.e., VcCOL2, VcCOL4, and VcCOL5), but no CO1-like (VcCO1) or HD1-like genes.
Arabidopsis FLC and cereal VRN(s) and their orthologues are among the main integrators of vernalization pathway genes in herbaceous plants. In blueberry, we did not find FLC orthologues by searching the online Vaccinium sequences. In our Trinity_floral_ref, only one transcript, which has the best hit to AGL79 (AT5G17690.1), showed 59.2% similarity (e-value = 2.00e-24) to FLC (AT5G10140.1). However, we found orthologues of other vernalization pathway genes, including a VcFRI (5.00e-58), a MADS AFFECTING FLOWERING 2 (MAF2)-like gene (VcMAF2) (4.00e-33), a MAF5-like gene (VcMAF5) (2.00e-21)], a VERNALIZATION 1-like gene (VcVRN1), and a VERNALIZATION 5-like gene (VcVRN5). Of these genes, VcVRN5 was the closest match (50.51% identity, e-value = 2.00e-168). These results suggest that blueberry (a perennial, woody plant) may have vernalization-pathways diverging from either the FLC-mediated pathway in Arabidopsis [27–29] or the VERNALIZATION genes (VRNs)-mediated pathways in cereals and their relatives [13, 16, 30]. Overall, of the major flowering pathway genes in ‘Aurora’, when compared to those in herbaceous plants, the genes up-stream of VcFT show less similarity than the down-stream genes (S4 Table).
Tissue-specific expression of different flowering pathway genes
Comparative analyses of three pools of Trinity transcripts (i.e., leaf vs. floral bud, flower vs. floral bud, and leaf vs. flower) of non-transgenic blueberry plants showed that 1,193 out of 1,223 transcript contigs of the Trinity_floral_ref were found in the combined contigs of leaf, bud, and flower tissues of non-transgenic ‘Legacy’. Furthermore, 1,122 (out of 1,193) contigs were shared among three tissue types, of which 397 contigs showed no differential expression between the tissues. The remaining 725 transcripts (69 genes) showed differential expression in the comparisons of leaf vs. bud, flower vs. bud, or leaf vs. flower (Table 2). For example, of the photoperiod pathway genes, VcCOL5 is a DE gene with high expression in leaf; but VcCOL2 and VcCOL4 did not show differential expression. Of the genes of the vernalization pathway, homologues of MAF2, VRN1, and TaVRN1 showed the highest expression in leaf tissues and VcVRN5 had the highest expression in buds; but VcFRI and VcMAF5 were not differentially expressed in the comparisons (Table 2). The results indicate that expression of some flowering-related transcripts/genes in blueberry is tissue specific. In addition, RNA-seq transcriptome analysis provides a very efficient approach to confirm tissue specificity of gene expression.
Table 2. Differential expression of blueberry flowering pathway genes in leaf, bud, and flower tissues of ‘Legacy’.
| Orthologues of flowering pathway genes | FPKM (fragments per kilobase of transcript per million mapped reads) | Gene ID | Orthologues of flowering pathway genes | FPKM (fragments per kilobase of transcript per million mapped reads) | Gene ID | Orthologues of flowering pathway genes | FPKM (fragments per kilobase of transcript per million mapped reads) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bud | Leaf | Flower | Bud | Leaf | Flower | Bud | Leaf | Flower | |||||
| ABF3 | 10.6 | 4.8 | 4.2 | c99616_g2 | CRY2 | 5.8 | 40.0 | 9.9 | c92902_g3 | PIE1 | 24.4 | 23.0 | 31.3 |
| AGL14 | 3.2 | 45.8 | 0.0 | c92516_g1 | EFS | 35.7 | 20.2 | 21.9 | c95473_g3 | PIE1 | 12.9 | 7.6 | 11.3 |
| AGL19 | 4.9 | 20.5 | 5.6 | c94437_g2 | EFS | 39.7 | 25.0 | 20.4 | c95823_g6 | PIE1 | 18.7 | 11.6 | 11.9 |
| AGL19 | 129.5 | 85.1 | 15.2 | c99784_g1 | EFS | 20.1 | 13.3 | 10.4 | c96115_g2 | PIE1 | 26.5 | 23.1 | 24.8 |
| AGL19 | 2.9 | 19.0 | 4.7 | c99653_g1 | ELF3 | 22.8 | 27.9 | 47.1 | c96482_g1 | PIE1 | 27.4 | 15.4 | 12.8 |
| AGL19 | 30.3 | 0.2 | 86.8 | c93803_g1 | ELF4 | 23.8 | 30.2 | 47.4 | c97601_g1 | PIE1 | 31.4 | 16.6 | 13.1 |
| AGL19 | 17.1 | 72.0 | 2.0 | c95520_g1 | ELF4 | 6.4 | 8.9 | 4.9 | c98765_g4 | PIE1 | 23.0 | 16.5 | 20.0 |
| AGL19 | 5.1 | 25.2 | 3.8 | c91390_g4 | ELF5 | 56.8 | 94.4 | 44.2 | c99276_g1 | PIE1 | 36.5 | 17.1 | 7.5 |
| AP1 | 7.4 | 0.0 | 14.4 | c97312_g2 | ELF6 | 22.0 | 14.4 | 10.9 | c99993_g3 | PIE1 | 24.6 | 11.5 | 12.9 |
| AP1 | 91.4 | 0.5 | 15.6 | c98947_g5 | ELF6 | 50.5 | 21.1 | 22.0 | c98035_g1 | PRR 7 | 87.0 | 26.6 | 28.8 |
| AP2 | 12.7 | 5.1 | 3.2 | c99119_g2 | ELF6 | 28.6 | 17.4 | 14.1 | c91063_g2 | PRR5 | 2.0 | 0.3 | 1.3 |
| AP2 | 56.4 | 32.8 | 40.3 | c97265_g1 | FCA | 15.8 | 19.8 | 8.1 | c92222_g2 | PRR5 | 47.8 | 65.2 | 27.1 |
| AREB3 | 35.9 | 18.8 | 18.6 | c66694_g1 | FD | 5.9 | 3.7 | 0.1 | c92704_g6 | PRR5 | 53.4 | 2.2 | 0.8 |
| ARP | 11.7 | 10.0 | 27.0 | c96634_g1 | FKF1 | 2.1 | 3.8 | 5.3 | c96565_g2 | PRR5 | 77.8 | 51.0 | 32.8 |
| ARP6 | 35.0 | 21.1 | 17.7 | c96634_g2 | FKF1 | 84.3 | 112.7 | 175.8 | c94181_g4 | RAV1 | 22.6 | 10.6 | 3.6 |
| ARP6 | 382.0 | 437.9 | 397.7 | c96828_g1 | FKF1 | 66.0 | 31.4 | 11.0 | c88293_g2 | SOC1 | 5.5 | 27.0 | 0.0 |
| ARP6 | 26.9 | 15.5 | 13.6 | c97877_g1 | FKF1 | 23.3 | 60.6 | 23.9 | c89673_g5 | SOC1 | 20.9 | 87.9 | 13.2 |
| ARP6 | 76.4 | 133.2 | 212.4 | c89277_g1 | FLD | 12.7 | 16.5 | 28.9 | c99509_g2 | SPA2 | 35.0 | 27.6 | 17.1 |
| ATCOL5 | 29.6 | 10.6 | 16.8 | c97418_g1 | FLD | 11.1 | 4.1 | 2.5 | c97089_g4 | SPA3 | 48.6 | 72.0 | 38.4 |
| CAL1 | 0.4 | 0.6 | 14.2 | c81479_g2 | FLK | 61.4 | 39.8 | 37.8 | c79187_g1 | SPL | 104.7 | 34.4 | 23.0 |
| CDF2 | 2.8 | 13.4 | 5.2 | c91122_g1 | FLK | 39.0 | 25.0 | 20.9 | c80807_g1 | SPL | 283.3 | 294.3 | 133.4 |
| CDF2 | 25.8 | 37.1 | 26.6 | c92308_g1 | FLK | 12.4 | 11.4 | 12.7 | c87494_g1 | SPL | 4.7 | 3.8 | 6.4 |
| CDF3 | 16.2 | 15.7 | 35.2 | c97816_g1 | FLK | 44.5 | 28.0 | 25.7 | c91703_g1 | SPL | 122.3 | 89.5 | 73.5 |
| CDF3 | 1.8 | 3.9 | 1.1 | c99051_g1 | FPA | 9.6 | 11.0 | 10.4 | c92156_g2 | SPL | 64.1 | 48.0 | 24.9 |
| CDF3 | 20.1 | 15.5 | 9.2 | c99819_g1 | FPA | 20.4 | 15.5 | 12.4 | c93310_g3 | SPL | 253.3 | 114.1 | 130.7 |
| CDF3 | 57.9 | 22.5 | 11.4 | c84088_g2 | FT | 34.9 | 0.2 | 0.9 | c93493_g1 | SPL | 11.8 | 11.4 | 4.2 |
| CHE | 19.3 | 13.1 | 3.4 | c75036_g1 | FUL | 93.7 | 24.6 | 66.1 | c96319_g1 | SPL | 80.6 | 16.2 | 27.3 |
| CHE | 32.5 | 39.7 | 15.7 | c77424_g2 | FUL | 4.8 | 0.0 | 54.8 | c92144_g7 | SPY | 12.9 | 6.4 | 7.7 |
| CHE | 16.8 | 12.5 | 5.1 | c79125_g1 | FUL | 120.5 | 14.7 | 38.5 | c92144_g9 | SPY | 30.6 | 18.0 | 18.4 |
| CIB1 | 0.9 | 1.2 | 5.4 | c88293_g4 | FUL | 56.7 | 29.1 | 162.4 | c90289_g1 | SVP | 12.9 | 28.7 | 0.5 |
| CIB1 | 0.4 | 0.1 | 65.3 | c90323_g1 | FUL | 7.8 | 0.0 | 1.2 | c90829_g2 | SVP | 1.6 | 35.7 | 1.7 |
| CIB1 | 8.4 | 19.8 | 5.4 | c92912_g1 | FY | 69.0 | 37.9 | 37.4 | c91377_g1 | SVP | 52.4 | 57.9 | 29.5 |
| CIB1 | 15.3 | 10.6 | 36.7 | c95783_g1 | FY | 24.4 | 13.3 | 15.2 | c95674_g3 | SWN | 22.6 | 30.4 | 30.1 |
| CIB1 | 16.0 | 14.6 | 82.9 | c99108_g3 | GI | 72.0 | 27.3 | 34.6 | c75317_g1 | TaVRN1 | 11.8 | 7.1 | 0.3 |
| CKA1 | 35.7 | 26.7 | 35.3 | c91613_g4 | GRF3 | 312.1 | 281.0 | 285.4 | c94181_g3 | TEM2 | 17.8 | 21.6 | 0.6 |
| CKA1 | 12.2 | 8.1 | 1.3 | c93155_g2 | GRF3 | 1.7 | 0.7 | 29.6 | c94930_g3 | TOC1 | 68.6 | 1.3 | 2.4 |
| CKA1 | 54.2 | 61.9 | 42.3 | c87227_g1 | HAP3B3 | 12.1 | 24.1 | 8.7 | c52913_g1 | TOE1 | 0.9 | 3.4 | 0.1 |
| CKA1 | 29.3 | 11.6 | 16.9 | c98146_g5 | HAP3B3 | 33.1 | 106.5 | 30.0 | c85378_g3 | TOE1 | 0.6 | 0.3 | 2.9 |
| CKA1 | 50.1 | 34.2 | 33.4 | c95551_g2 | HAP5C | 71.7 | 31.6 | 83.9 | c87192_g2 | TOE1 | 8.1 | 1.5 | 4.8 |
| CKA2 | 22.7 | 28.4 | 28.5 | c59813_g1 | HvFT5 | 0.1 | 1.5 | 5.8 | c87192_g5 | TOE1 | 14.1 | 7.5 | 3.2 |
| CKA2 | 113.3 | 14.6 | 14.9 | c98104_g1 | LD | 14.8 | 6.7 | 5.1 | c87982_g1 | TOE1 | 6.2 | 0.3 | 1.3 |
| CKA2 | 156.2 | 107.6 | 120.1 | c98104_g2 | LD | 15.2 | 8.4 | 7.8 | c91054_g4 | TOE1 | 1.1 | 32.6 | 0.5 |
| CKA2 | 54.0 | 31.0 | 34.9 | c96427_g2 | LFY | 44.4 | 1.8 | 0.2 | c96694_g1 | TOE1 | 12.9 | 1.1 | 1.2 |
| CKA3 | 54.4 | 33.7 | 29.0 | c90643_g1 | LHP1 | 50.7 | 30.5 | 27.9 | c96979_g5 | TOE1 | 23.5 | 3.0 | 0.4 |
| CKA3 | 4.2 | 1.2 | 38.4 | c58074_g2 | LHY | 0.6 | 3.6 | 0.0 | c98453_g2 | TOE1 | 56.1 | 16.7 | 5.8 |
| CKA3 | 15.3 | 15.5 | 9.7 | c92088_g3 | LHY | 63.8 | 62.3 | 69.6 | c91085_g1 | TOE3 | 11.1 | 3.9 | 4.0 |
| CKA3 | 74.7 | 49.3 | 47.2 | c99092_g1 | LHY | 76.6 | 223.5 | 137.8 | c96979_g6 | TOE3 | 28.6 | 13.3 | 8.3 |
| CKA3 | 59.8 | 45.3 | 94.5 | c88534_g2 | LKP2 | 19.3 | 8.4 | 13.0 | c91926_g8 | TSF3 | 8.7 | 0.7 | 0.2 |
| CKA3 | 29.6 | 14.5 | 2.5 | c96650_g1 | LUX | 10.2 | 12.7 | 8.5 | c92401_g3 | VEL1 | 1.5 | 13.4 | 4.9 |
| CKA4 | 80.5 | 78.9 | 81.1 | c95303_g7 | MAF2 | 8.7 | 7.5 | 17.7 | c92401_g6 | VEL1 | 4.3 | 17.7 | 6.6 |
| CKA4 | 6.8 | 13.2 | 9.0 | c93556_g1 | OsEhd1 | 59.0 | 28.0 | 21.8 | c98995_g1 | VRN1 | 30.7 | 10.5 | 23.7 |
| CLF | 13.8 | 9.9 | 4.4 | c97066_g3 | OsLFL1 | 11.4 | 8.2 | 6.4 | c93433_g2 | VRN5 | 7.5 | 11.0 | 11.6 |
| COP1 | 14.4 | 28.7 | 4.4 | c82061_g1 | OsSPY | 63.1 | 35.8 | 50.6 | c82411_g2 | ZmID1 | 16.0 | 24.9 | 11.8 |
| COP1 | 13.9 | 26.0 | 3.9 | c87872_g1 | PAF2 | 162.6 | 112.9 | 155.3 | c92595_g6 | ZmID1 | 109.4 | 40.8 | 33.3 |
| COP1 | 9.8 | 22.5 | 3.9 | c95421_g1 | PHYC | 6.7 | 8.8 | 9.7 | c93923_g1 | ZmID1 | 35.9 | 15.5 | 16.1 |
| CRY1 | 39.2 | 89.8 | 43.8 | c84580_g2 | PIE1 | 26.1 | 20.5 | 25.1 | c94665_g2 | ZmID1 | 16.1 | 21.8 | 4.8 |
| CRY1 | 20.1 | 38.3 | 26.0 | c89213_g2 | PIE1 | 43.9 | 24.3 | 25.1 | c96507_g4 | ZmID1 | 23.6 | 36.2 | 18.2 |
| CRY1 | 34.4 | 76.5 | 45.6 | c89266_g3 | PIE1 | 30.0 | 19.2 | 15.1 | c88070_g1 | ZTL | 22.1 | 44.2 | 58.1 |
| CRY2 | 4.2 | 13.1 | 4.7 | c92275_g1 | PIE1 | 17.5 | 8.1 | 10.6 | |||||
DE analyses of transgenic blueberry overexpressing VcFT
124,560 gene contigs (69.3% of the 179,853 refTrinity genes) and 187,779 transcript contigs (73.8% of the 254,396 refTrinity genes) were present in young leaves of ‘VcFT-Aurora’ plants. In addition, 1,130 transcript contigs (92.4% of the 1,223) of blueberry flowering-related genes were identified. The absence of some gene/transcript contigs is attributed to either tissue-specific or genotype-dependent expression.
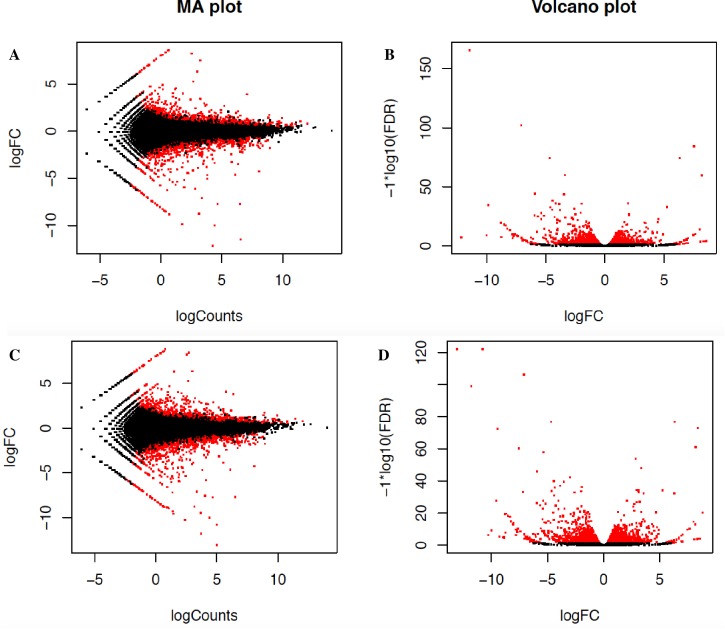
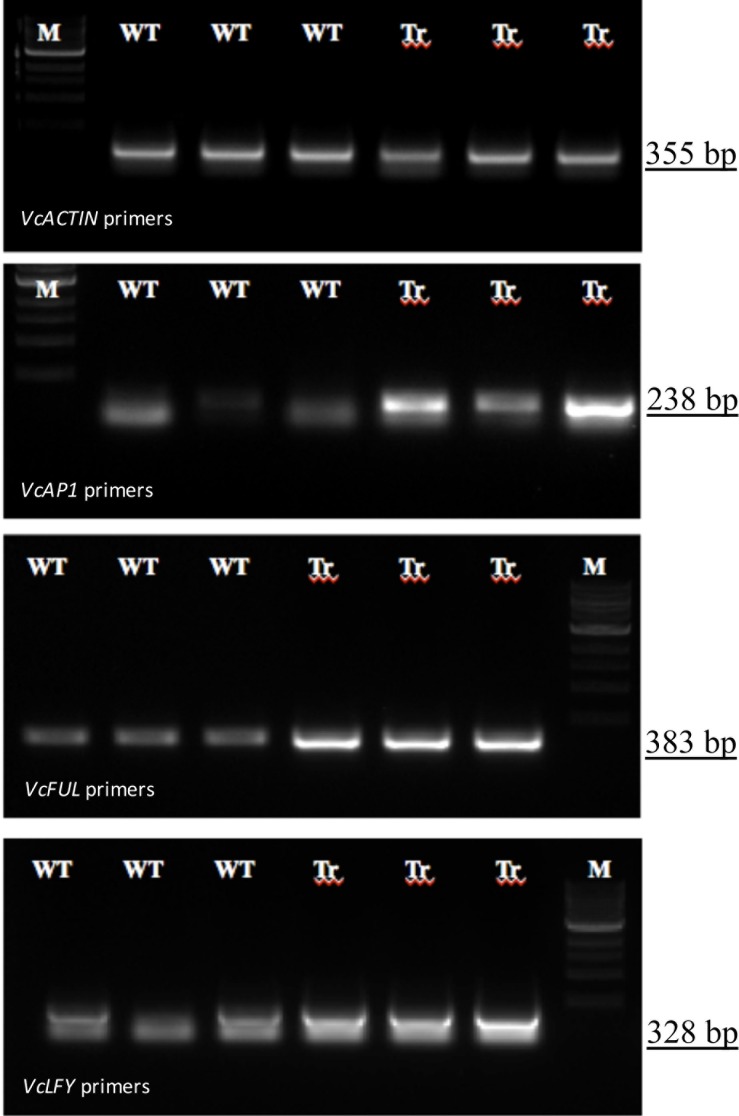
Differential expression analyses of transcriptome of ‘VcFT-Aurora’ indicated that over-expressed VcFT had a broad impact on global gene expression. We found expression levels of 3,023 Trinity gene contigs (2.4% of 124,560) were significantly altered in ‘VcFT-Aurora’ plants (p-value<0.001 and FDR<0.05), including 1,498 up-regulated and 1,525 down-regulated gene contigs (Fig 2). At the transcript level, 4,844 Trinity transcripts (2.6% of 187,779) were significantly altered, of which 2,270 were up-regulated and 2,574 were down-regulated (Fig 2). Sixty-one transcripts of 40 gene contigs showed high similarities to 33 known flowering-related genes, of which 17 were down-regulated and 16 were up-regulated (Table 3). All down-regulated flowering-related genes were the upstream genes of VcFT. The 35S promoter drove high expression of the VcFT, which was associated with up-regulated TWIN SISTER of FT (TSF)-like (VcTSF) as well as the downstream genes VcAP1, VcFUL, SEPALLATA-like (VcSPL), VcLFY, and VcSOC1. The up-regulation of the selected VcAP1, VcLFY, and VcFUL was confirmed in our RT-PCR analysis (Fig 3). Interestingly, nine VcFT upstream genes were also up-regulated (Table 3).
Fig 2.
Comparison of transcript expression profiles between ‘VcFT-Aurora’ and ‘Aurora’ to identify differentially expressed genes (a, b) and isoforms (c, d) in leaf tissues. a, c, MA plot for differential expression analysis generated by EdgeR: for each gene or isoform, the log2(fold change) (log2(Aurora/VcFT-Aurora)) between the two samples is plotted (A, y axis) against the gene’s log2(average expression) in the two samples (M, x axis). b, d, Volcano plot reporting false discovery rate (−logFDR, y axis) as a function of log2(fold change) between the samples (logFC, x axis). Genes or isoforms that are identified as significantly differentially expressed at most 0.1% FDR are colored in red.
Table 3. Differential expression of blueberry flowering pathway genes in ‘VcFT-Aurora’ leaves and their putative pathways.
| Putative Pathway | Gene | Log2(fold change) = Log2(VcFT-Aurora/Aurora) | FDR |
|---|---|---|---|
| Photoperiod & Temperature | VcPRR5_g1 | 1.18 | 1.15E-02 |
| VcPRR7_g1 | 0.58 | 2.67E-03 | |
| VcPRR9_g1 | -1.82 | 1.62E-02 | |
| VcLHY_g1 | 0.89 | 1.75E-08 | |
| VcCHE_g1 | 0.85 | 3.00E-03 | |
| VcCHE_g2 | -0.74 | 1.96E-03 | |
| VcCHE_g3 | -0.95 | 5.62E-04 | |
| VcCOL5_g1 | -1.14 | 6.26E-11 | |
| Hormone | VcABF4_g1 | 0.70 | 4.29E-03 |
| VcCKA3_g1 | 3.03 | 2.64E-02 | |
| VcCKA3_g2 | 1.48 | 2.77E-06 | |
| VcCKA2_g1 | -0.50 | 9.16E-03 | |
| VcAGL14_g1 | -1.30 | 2.39E-02 | |
| VcAGL14_g2 | -2.94 | 1.31E-03 | |
| Age | VcFLD_g1 | 1.01 | 2.66E-02 |
| VcTOE3_g1 | 0.76 | 3.51E-03 | |
| VcAGL19_g1 | 0.61 | 8.61E-03 | |
| VcFLK_g1 | -1.62 | 5.45E-02 | |
| VcFCA_g1 | -0.54 | 5.61E-03 | |
| VcFCA_g2 | -0.57 | 9.11E-03 | |
| VcTOE1_g1 | -1.17 | 1.15E-02 | |
| VcHAP5C3_g1 | -0.58 | 1.56E-02 | |
| VcSPL_g1 | 1.55 | 1.65E-09 | |
| VcSPL_g2 | 1.01 | 6.39E-03 | |
| VcSPL_g3 | 0.85 | 3.31E-03 | |
| VcSPL_g4 | 0.45 | 3.72E-02 | |
| VcSPL_g5 | -1.11 | 6.11E-05 | |
| VcCIB1_g1 | 0.77 | 1.21E-04 | |
| Vernalization | VcFRI_g1 | 0.65 | 2.53E-02 |
| VcVRN1_g1 | 2.02 | 3.43E-03 | |
| VcVRN1_g2 | 0.79 | 2.64E-03 | |
| VcSUF4_g1_i1* | -0.50 | 1.20E-02 | |
| VcPAF2_g1 | -0.44 | 3.72E-02 | |
| VcHUA2_g1 | -0.47 | 3.20E-02 | |
| VcARP6_g1 | 0.63 | 4.50E-02 | |
| VcARP6_g2 | -0.65 | 1.70E-03 | |
| VcMAF2_g1/VcMAF5_g1 | -2.07 | 4.53E-04 | |
| Conserved Flowering | VcFT_g1 | 11.46 | 1.94E-166 |
| VcFUL_g1 | 4.73 | 6.87E-14 | |
| VcSOC1_g1 | 1.35 | 1.03E-10 | |
| VcTSF3_g1 | 3.54 | 1.32E-09 | |
| VcAP1_g1 | 5.77 | 3.52E-02 | |
| VcAP1_g2 | 3.87 | 3.59E-36 | |
| VcAP1_g3 | 3.79 | 4.30E-31 | |
| VcAP1_g4 | 3.45 | 2.96E-08 | |
| VcLFY_g1 | 2.26 | 7.30E-07 | |
| VcLFY_g2 | 1.39 | 3.47E-07 |
* Isoform was differentially expressed, but not at gene level.
Fig 3. RT-PCR analysis of differentially expressed transcripts in leaf tissues of non-transgenic ‘Aurora’ (WT) and transgenic ‘VcFT-Aurora’ (Tr).
VcACTIN is the internal control. M: 1 Kb ladder.
In ‘VcFT-Aurora’, VcCOL5 is a major down-regulated flowering pathway gene associated with five other DE genes in the photoperiod-regulated flowering pathway (Table 3). VcMAF2 and VcMAF5 or VcVRN1 may function as integrators of the vernalization pathway, in which six other genes [i.e., VcFRI, VcHUA2, SUPPRESSOR OF FRIGIDA4-like (VcSUF4), 20S PROTEASOME SUBUNIT PAF2-like (VcPAF2), and ACTIN-RELATED PROTEIN 6 (ARP6)-like gene (VcARP6)] were involved (Table 3). No VcCO1 or VcFLC were found in blueberry. These results suggest VcCOL5 and VcMAF2/VcMAF5 or VcVRN1 in blueberry may be the major integrator(s) in the photoperiod and vernalization pathways, respectively.
Our comparative analyses of flowering pathway genes of blueberry, in addition to the profile of DE flowering-related genes in VcFT-over-expressing ‘Aurora’ plants (Table 3), suggest that the VcFT down-stream genes (e.g., VcSOC1, VcFUL, VcLFY, and VcAP1) are functionally conserved.
Discussion
The genetic nature of many fruit crops, e.g., polyploidy, high heterozygosity, long juvenility, and clonal propagation, makes traditional breeding a lengthy and expensive process. Although a few genetic markers have been developed to facilitate the selection process [9] blueberry breeding is mainly guided by breeders’ experiences. Manipulation of flowering pathway genes is anticipated to change the regime of blueberry flowering and to increase fruit production [4, 5]. To this end, we here have used comparative genomics and biotechnology tools to investigate flowering pathway genes and flowering mechanisms of blueberry.
Transcriptome reference of blueberry
In a previous study of anthocyanin biosynthesis in blueberry, over 64 million RNA-sequencing reads were generated from blueberry skin and pulp, 34,464 unigenes were obtained through de novo assembly [8]. The first whole genome sequence of blueberry with 15,129 scaffolds for 358 Mb was reported on a diploid V. corymbosum accession ‘W8520’ [2, 7]. Using this draft genome reference and RNA-seq data, great efforts have been made to identify genes involved in blueberry ripening and over 400 biosynthetic pathways (e.g., ethylene and anthocyanin) [7]. Currently, there is an online blueberry genomics database (BBDG) that contains a collection of EST sequences (http://bioinformatics.towson.edu/BBGD/)), and some published RNA-seq data are available in the GenBank [8]. To date, no blueberry genome reference has been made available to the public.
We demonstrated that the Trinity platform [24] is amenable to use and is very efficient in generating transcriptome references for tetraploid blueberry. With 72.7 MR (8–10 MR/sample), we believe our transcriptome reference (refTrinity) covers the majority of transcripts in leaves, flowers, and flower buds. Although this transcriptome reference does not include root- and fruit-specific transcripts, it provides a good coverage of the majority of blueberry flowering pathway genes for studying gene expression in blueberry plants.
Conserved flowering pathway genes in blueberry
In this study, it is not surprising that the orthologues of the majority of the flowering pathway genes found in Arabidopsis, rice, and cereals crops were identified in blueberry, although some of these orthologues may have distinctive functions to meet the specific needs of blueberry floral transitions or flowering. Meanwhile, there could be other genes (e.g., VcCO1 and VcFLC) that might have eluded our comparative genomic analysis due to their high specificity or uniqueness to blueberry.
Flowering gene network of blueberry
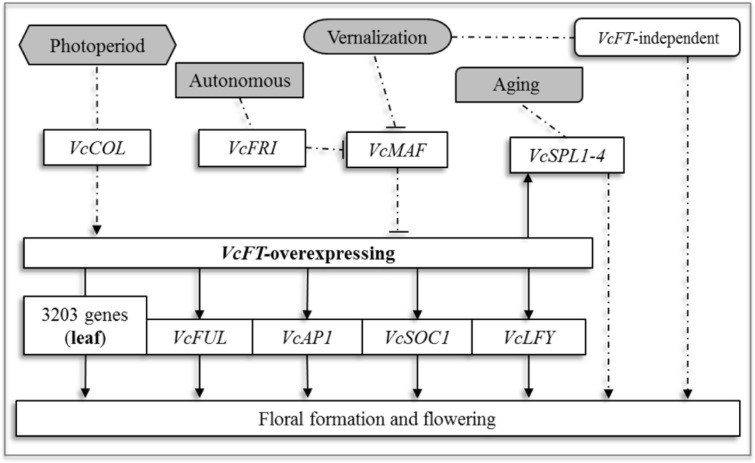
Based on profiles of DE flowering-related genes in ‘VcFT-Aurora’, we propose a flowering gene network in blueberry (Fig 4). In this network, blueberry has a universal FT-mediated flowering pathway, where the photoperiod pathway (e.g., VcCOL2, and VcCOL5), and the vernalization and autonomous pathways (e.g., VcFRI, VcMAF2, VcMAF5, or VcVRN1) function through VcFT and its downstream integrators VcSOC1 and VcLFY, although no orthologues of CO1 and FLC were identified. In this universal pathway, over-expressing VcFT promotes floral bud formation and flowering. VcFT also appears to promote expression of several SPL (SQUAMOSA PROMOTER BINDING LIKE) homologs in the aging pathway. However, VcFT over-expression is not sufficient to completely reverse the influence of environmental stimuli for normal plant flowering; for example, ‘VcFT-Aurora’ plants did not flower normally when grown under non-chilling conditions. It is possible that there are additional genes/pathways independent of VcFT expression that regulate vernalization-mediated blueberry flowering.
Fig 4. Flowering pathways in blueberry.
Arrows: promotion. T-signs: inhibition. Solid lines: results of this study. Dashed lines: proposed correlation.
In conclusion, we developed a transcriptome reference of highbush blueberry using RNA sequencing data and Trinity. By searching this transcriptome using known flowering pathway gene sequences of Arabidopsis, rice and cereal, we developed a reference of blueberry flowering-related genes. Comparative genomics of flowering pathway genes in blueberry and herbaceous plants revealed a conserved VcFT-mediated flowering through its down-stream VcSOC1, VcAP1, VcLFY, and VcFUL genes. Interestingly, neither VcCO1 nor VcFLC are present in our transcriptome reference. The profile of DE flowering pathway genes in leaf tissues of VcFT-overexpressing plants suggests VcCOL5 and VcMAF2/VcMAF5 may be the major integrator(s) in the photoperiod and vernalization pathway, respectively.
Supporting Information
(DOCX)
(DOCX)
Data from blastn of BLASTplus with e-value threshold of 1e-5 using indicated query and refTrinity as database.
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Jeff Landgraf and Mr. Kevin Carr at the Michigan State University Research Technology Support Facility for RNA sequencing. We thank Dr. Vance Baird and Dr. Wayne Loescher for their review of our manuscript. The authors have no conflict of interest to report.
Data Availability
All relevant data are contained in the Supporting Information within this paper and supporting files.
Funding Statement
This work was supported by Michigan State University, AgBioResearch Project GREEEN, URL: http://agbioresearch.msu.edu/project_greeen.
References
- 1.Ehlenfeldt MK, Prior RL. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbus blueberry. J Agr Food Chem. 2001;49(5):2222–7. 10.1021/jf0013656 . [DOI] [PubMed] [Google Scholar]
- 2.Bian Y, Ballington J, Raja A, Brouwer C, Reid R, Burke M, et al. Patterns of simple sequence repeats in cultivated blueberries (Vaccinium section Cyanococcus spp.) and their use in revealing genetic diversity and population structure. Mol Breeding. 2014;34(2):675–89. 10.1007/s11032-014-0066-7 [DOI] [Google Scholar]
- 3.Chavez DJ, Lyrene PM. Effects of Self-pollination and Cross-pollination of Vaccinium darrowii (Ericaceae) and Other Low-chill Blueberries. Hortscience. 2009;44(6):1538–41. . [Google Scholar]
- 4.Song GQ, Walworth A, Zhao DY, Hildebrandt B, Leasia M. Constitutive expression of the K-domain of a Vaccinium corymbosum SOC1-like (VcSOC1-K) MADS-box gene is sufficient to promote flowering in tobacco. Plant Cell Reports. 2013;32(11):1819–26. 10.1007/S00299-013-1495-1 . [DOI] [PubMed] [Google Scholar]
- 5.Song GQ, Walworth A, Zhao DY, Jiang N, Hancock JF. The Vaccinium corymbosum FLOWERING LOCUS T-like gene (VcFT): a flowering activator reverses photoperiodic and chilling requirements in blueberry. Plant Cell Reports. 2013;32(11):1759–69. 10.1007/S00299-013-1489-Z . [DOI] [PubMed] [Google Scholar]
- 6.Die JV, Rowland LJ. Superior cross-species reference genes: a blueberry case study. PLoS One. 2013;8(9):e73354 10.1371/journal.pone.0073354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta V, Estrada AD, Blakley IC, Reid R, Patel K, Meyer MD, et al. RNASeq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. GigaScience. 2015; 4:5 10.1101/010116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Sun H, Pei J, Dong Y, Wang F, Chen H, et al. De novo sequencing and comparative analysis of the blueberry transcriptome to discover putative genes related to antioxidants. Gene. 2012;511(1):54–61. 10.1016/j.gene.2012.09.021 . [DOI] [PubMed] [Google Scholar]
- 9.Rowland LJ, Alkharouf N, Darwish O, Ogden EL, Polashock JJ, Bassil NV, et al. Generation and analysis of blueberry transcriptome sequences from leaves, developing fruit, and flower buds from cold acclimation through deacclimation. BMC Plant Biol. 2012;12:46 10.1186/1471-2229-12-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walworth AE, Rowland LJ, Polashock JJ, Hancock JF, Song GQ. Overexpression of a blueberry-derived CBF gene enhances cold tolerance in a southern highbush blueberry cultivar. Mol Breeding. 2012;30(3):1313–23. 10.1007/S11032-012-9718-7 . [DOI] [Google Scholar]
- 11.Song GQ. Blueberry (Vaccinium corymbosum L.). Methods Mol Biol. 2015;1224:121–31. 10.1007/978-1-4939-1658-0_11 . [DOI] [PubMed] [Google Scholar]
- 12.Song GQ, Sink KC. Agrobacterium tumefaciens-mediated transformation of blueberry (Vaccinium corymbosum L.). Plant Cell Rep. 2004;23(7):475–84. Epub 2004/08/10. 10.1007/s00299-004-0842-7 . [DOI] [PubMed] [Google Scholar]
- 13.Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science. 2007;12(8):352–7. Epub 2007/07/17. S1360-1385(07)00156-2 [pii] 10.1016/j.tplants.2007.06.010 . [DOI] [PubMed] [Google Scholar]
- 14.Wilkie JD, Sedgley M, Olesen T. Regulation of floral initiation in horticultural trees. J Exp Bot. 2008;59(12):3215–28. Epub 2008/07/26. ern188 [pii] 10.1093/jxb/ern188 . [DOI] [PubMed] [Google Scholar]
- 15.Zeevaart JA. Leaf-produced floral signals. Curr Opin Plant Biol. 2008;11(5):541–7. Epub 2008/08/12. S1369-5266(08)00112-X [pii] 10.1016/j.pbi.2008.06.009 . [DOI] [PubMed] [Google Scholar]
- 16.Greenup A, Peacock WJ, Dennis ES, Trevaskis B. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Annals of Botany. 2009;103(8):1165–72. Epub 2009/03/24. mcp063 [pii] 10.1093/aob/mcp063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaels SD. Flowering time regulation produces much fruit. Curren Opinion in Plant Biology. 2009;12(1):75–80. Epub 2008/10/22. S1369-5266(08)00161-1 [pii] 10.1016/j.pbi.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61(6):1001–13. Epub 2010/04/23. TPJ4148 [pii] 10.1111/j.1365-313X.2010.04148.x . [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Lee I. Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot. 2010;61(9):2247–54. Epub 2010/04/24. erq098 [pii] 10.1093/jxb/erq098 . [DOI] [PubMed] [Google Scholar]
- 20.Wellmer F, Riechmann JL. Gene networks controlling the initiation of flower development. Trends in Genetics. 2010;26(12):519–27. Epub 2010/10/16. S0168-9525(10)00187-3 [pii] 10.1016/j.tig.2010.09.001 . [DOI] [PubMed] [Google Scholar]
- 21.Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJL, et al. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science. 2010;330(6009):1397–400. 10.1126/science.1197004 [DOI] [PubMed] [Google Scholar]
- 22.Fan Z, Li J, Li X, Wu B, Wang J, Liu Z, et al. Genome-wide transcriptome profiling provides insights into floral bud development of summer-flowering Camellia azalea. Scientific Reports. 2015;5(9729). 10.1038/srep09729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fornara F, de Montaigu A, Coupland G. SnapShot: Control of Flowering in Arabidopsis. Cell. 2010;141(3). 10.1016/j.cell.2010.04.024 . [DOI] [PubMed] [Google Scholar]
- 24.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protocols. 2013;8(8):1494–512. 10.1038/Nprot.2013.084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JA, Bailey PC, Laurie DA. Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS One. 2010;5(4):e10065 10.1371/journal.pone.0010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamboni A, Pierantoni L, De Franceschi P. Total RNA extraction from strawberry tree (Arbutus unedo) and several other woodyplants. Iforest-Biogeosciences and Forestry. 2008;1:122–5. 10.3832/Ifor0465-0010122 . [DOI] [Google Scholar]
- 27.Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. PLANT CELL. 1999;11(5):949–56. Epub 1999/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, et al. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. PLANT CELL. 1999;11(3):445–58. Epub 1999/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proceedings of the National Academy of Sciences of the United States of America. 2000;97(7):3753–8. Epub 2000/03/15. 10.1073/pnas.060023597 060023597 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexandre CM, Hennig L. FLC or not FLC: the other side of vernalization. J Exp Bot. 2008;59(6):1127–35. Epub 2008/04/09. ern070 [pii] 10.1093/jxb/ern070 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data from blastn of BLASTplus with e-value threshold of 1e-5 using indicated query and refTrinity as database.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are contained in the Supporting Information within this paper and supporting files.