Abstract
Hundreds of insect species are nowadays reared under laboratory conditions. Rearing of insects always implicates the risk of diseases, among which microbial infections are the most frequent and difficult problems. Although there are effective prophylactic treatments, the side effects of applied antibiotics are not well understood. We examined the effect of prophylactic antibiotic treatment on the overwintering success of wood tiger moth (Parasemia plantaginis) larvae, and the postdiapause effect on their life-history traits. Four weeks before hibernation larvae were treated with a widely used antibiotic (fumagillin). We monitored moths’ survival and life-history traits during the following 10 mo, and compared them to those of untreated control larvae. Prophylactic antibiotic treatment had no effect on survival but we show effects on some life-history traits by decreasing the developmental time of treated larvae. However, we also revealed relevant negative effects, as antibiotic treated individuals show a decreased number of laid eggs and also furthermore a suppressed immunocompetence. These results implicate, that a prophylactic medication can also lead to negative effects on life-history traits and reproductive success, which should be seriously taken in consideration when applying a prophylactic treatment to laboratory reared insect populations.
Keywords: mass-rearing, antibiotic, reproduction, immunocompetence, trade-off
Mass-rearing of insects under laboratory conditions is a widely used strategy in a variety of disciplines, but complications often arise while optimizing the procedure. Insect rearing is important for many different substantial research purposes such as the production of chemical insecticides, agricultural health research, pest management, genetic studies, and enhancement of domestic populations (Leppla 2009, Sørensen et al. 2012). Due to the raising interest on integrated and biological pest management methods, it is of special importance to maintain high quality laboratory populations with minimized workload (Singh 1982, Sørensen et al. 2012). However, it is also known that conditions in laboratory facilities can have negative effects on insects’ fitness and reproductive success, as insects’ performance can be affected by various behavioral, reproductive, or genetic factors (Singh 1982, Sørensen et al. 2012). The rearing of well-established laboratory model organisms like the confused flower beetle Tribolium confusum, the housefly Musca domestica, or the tobacco budworm Heliothis virescens is well recorded by freely available rearing protocols (Leppla 2009), though the number of scientifically based rearing protocols is humble (Cohen 2001). Establishing new insect species as laboratory populations is highly time consuming (Leppla 2009) due to work needed for the optimisation of rearing techniques, which is necessary to minimize the negative effects of the artificial rearing conditions.
The main limiting factor of artificially reared insect quality is, besides temperature and humidity conditions, a suboptimal nutrition that can consequently lead to microbial contaminations of the populations (Sikorowski and Lawrence 1994), which are known as major threats of low overwintering survival and reproduction success (Rull et al. 2005; Van Der Hoeven et al. 2008; Sørensen et al. 2012). Especially under laboratory conditions infections and diseases can easily establish and spread, affecting sensitive life stages such as overwintering, which is strongly influenced by light- and temperature conditions as well as by food quality (Bale and Hayward 2010, Xu et al. 2011, Spurgeon 2012, Sinclair 2014). Insect mating behavior is dependent on various environmental factors, but also on courtship behavior and fitness, and is furthermore affected by diseases (Leppla 2009). Production of high numbers of high quality insects might be achieved by including a prophylactic microbial control treatment (Singh 1982, Parker et al. 2005, Sørensen et al. 2012). Developing and understanding the importance of artificial diets and optimal rearing conditions is essential to guarantee high quality insect populations as well as the applicability of the results obtained from experiments (Cohen 2000, 2001; Leppla 2009; Sørensen et al. 2012).
The use of prophylactic antibiotic treatments in laboratory-reared insect populations is a commonly used strategy to eliminate microbial infections and increase the quality of mass-reared insects (Sørensen et al. 2012). Laboratory-reared colonies of Lepidoptera (McLean-Cooper et al. 2008, Van Der Hoeven et al. 2008), Diptera (Dimou et al. 2010), and Coleoptera (Lehman et al. 2009) are fed on artificial diets complemented with antibiotics to eliminate epidemic infections such as contamination with obligate intercellular parasites, for example microsporidia (Higes et al. 2007). They can affect insect life-history traits negatively by a restricted larval development, decreased pupal weight, lower fecundity, and immune suppression, as detected for instance in Choristoneura fumiferana (Thomson 1958) and Apis mellifera (Antúnez et al. 2009). These effects could render insects more susceptible to secondary and/or opportunistic infections. Moreover, it is known that the adaptation to chemicals is associated with fitness costs, which may also include suppression of the immune response, as it is a costly mechanism (Coustau et al. 2000, Schmid-Hempel 2005). Insect immune system is a well-evolved defense against infections, consisting of complex multi-level interactions between specific detoxification enzymes and genes (Gillespie et al. 1997, Vilmos and Kurucz 1998). Up-regulating and maintaining an immune response is highly costly and thus trades off with other cost-associated factors, such as environmental stressors and diseases (Moret and Schmid-Hempel 2000, Schmid-Hempel 2003).
A commonly used antibiotic reagent for prophylactic therapy against microsporidia infection is fumagillin, isolated from the fungus Aspergillus fumigatus (Huang et al. 2013, Van Den Heever et al. 2014). For example the leading company of insect supplies (Frontier Agricultural Science) is offering ready mixed insect diets supplemented with antibiotics. However, there is little known about the long-term effects of the applied agents. Possible negative effects on life-history traits are suspected but still not well documented. Prolonged larval, pupal, and adult developmental times, as well as decreased reproductive success, are possible long-term consequences of a prophylactic treatment of laboratory insect populations (Wilkinson 1998). Wild insect populations are also facing increasing amounts of different xenobiotics. With the increased usage of antibiotics in human medical treatment, as well as in food animal production, insects might encounter their residuals, which stay active in environmental compounds (Daghrir and Drogui 2013).
The main objective of this study was to test the effect of a prophylactic antibiotic treatment on life-history traits and overwintering success of Lepidopteran larvae not showing obvious symptoms of an infection or disease, as well as the general long-term consequences of the medicine. Polyphagous larvae of the wood tiger moth, Parasemia plantaginis where used for this experiment. We treated the larvae by feeding them with the antibiotic fumagillin for a time span of 4 wk until they began to hibernate. We then monitored larval mortality before hibernation as well as 5 mo during hibernation. Furthermore, we examined the effect of the antibiotic on the immune response of the larvae before hibernation, by analyzing the activity of phenoloxidase. The possible long-time consequences of the medicine where assessed in several ways: 1) we measured the weight of the larvae before hibernation and after hibernation, as well as the pupal weight; 2) we monitored the developmental time from egg to pupa, and from egg to adult; 3) we examined the effect on egg laying success and on the number of laid eggs after mating the eclosed adults. Our findings offer a better understanding of the side effects that the treatment with prophylactic medicine has on laboratory-reared Lepidoptera larvae.
Material and Methods
Animals
The wood tiger moth, P. plantaginis, is a day active moth belonging to the Arctiinae subfamily (Conner 2008). They are herbivorous generalistic insects, which are able to consume and digest a variety of plant species. P. plantaginis is widely distributed over the northern hemisphere and is mostly studied for its warning coloration (Lindstedt et al. 2009, Hegna et al. 2015), but also for immunocompetence questions and host–parasite interactions (Ojala et al. 2005, Friman et al. 2009, Zhang et al. 2012, Nokelainen et al. 2013). Under natural conditions they overwinter as larvae and have one generation per year, whereas under laboratory conditions it is possible to have up to three generations per year (Ojala et al. 2007).
All P. plantaginis larvae used in this experiment were obtained from a laboratory stock population from the University of Jyväskylä, Finland (re) established in 2012. The laboratory stock is reared under greenhouse conditions of 25°C, a photoperiod of 18:6 (L:D) h, 80% RH, maintained in plastic boxes in groups of around 30 individuals, and fed with dandelion (reared following the methods from Lindstedt et.al [2009]). All larvae used for this experiment had the same hatching date, 16 September 2013. The individuals for the experiment were maintained continuing the above-mentioned rearing conditions.
Fumagillin
For conducting the experiment we obtained the product fumagilin-B (hereafter always referred as fumagillin), a soluble powder from Medivet (Medivet Pharmaceuticals Ldt., High River, Alberta, Canada), which is equivalent to 21 mg fumagillin base per gram. Fumagillin is a commonly used antimicrobial agent in bee-management and human medicine (Huang et al. 2013, Van Den Heever et al. 2014). It is a complex biomolecule isolated from the fungus A. fumigatus. Due to its ability to inhibit and block the enzyme methionine aminopeptidase-2 (MetAP2) it is widely used in human medicine to treat microsporidian infections (Fallon et al. 2011). MetAP2 is an essential enzyme in microsporidia and thus its inhibition by fumagillin kills microsporidian cells (Upadhya et al. 2006). The most relevant field of fumagillin application is in beekeeping management, as this substance is proved to be highly effective against nosema diseases in honeybees, A. mellifera. Both Nosema apis infection, as well as the microsporidian pathogen, Nosema ceranae, can be treated with a periodic fumagillin treatment (Webster 1994, Huang et al. 2013). Hives are treated in autumn and spring to ensure microsporidia-free colonies by prophylactically applying a recommended concentration of 25 mg/l of fumagilin in sugar syrup (Huang et al. 2013).
Treatments
Larvae for the experiment were taken from four families of the Finnish laboratory stock population in Jyväskylä with same hatching date (16 September 2013). Twenty-five-day-old larvae were divided into two treatment groups and placed individually in petri dishes (Sarstedt AG & Co, Nuernbrecht, Germany), resulting in 400 antibiotic treated and 400 control treated individuals. Because of uneven development within the families, the number of larvae per family could not be perfectly balanced, resulting in a sample size of 150, 330, 190, and 130 in families 1, 2, 3, and 4, respectively. In order to induce diapause, the temperature and light conditions of the growth chamber where stepwise decreased every week (first week 20°C and a photoperiod of 16:8 (L:D) h, second week 16°C and a photoperiod of 12:12 (L:D) h, third week 12°C and a photoperiod of 8:16 (L:D) h, fourth week 8°C and a photoperiod of 4:20 (L:D) h). For the antibiotic treatment we used 1% fumagilin solution in water. Larvae were orally treated with the antibiotic by dipping the food plant into the fumagillin solution. The treatment lasted for 4 wk. The procedure was repeated every second day to ensure a continuous exposure with the antibiotic. Remaining diet from the last inoculation was removed. Control larvae were treated with the same method, by using water as dipping solution for the food plant, to ensure similar leaf conditions. After 4 wk all larvae were transferred to individual overwintering containers, filled with moss and stored in a climate chamber with 4°C in complete darkness for hibernation. Larvae were kept for 5 mo under hibernation conditions and then placed in a warmer climate chamber (7°C) with increasing temperature and light conditions to slowly wake them up (7°C and a photoperiod of 8:16 (L:D) h cycle with low light intensity (2 out of 5, light intensity level), 4 d after waking up 15°C and a photoperiod of 16:8 (L:D) h cycle, 6 d after waking up 20°C and a photoperiod of 16:8 (L:D) h cycle with high light intensity (4 out of 5), 10 d after waking up greenhouse conditions of around 25°C). Individual rearing was then changed to group rearing, wherefore the larvae were placed in new bigger rearing containers according to their weight, family, and treatment. This grouping allowed further assignment of the larvae to its previous treatment group (fumagillin or control) as well as family. Overall 27 containers with control larvae and 28 containers with fumagillin treated larvae were kept for further observation. Gender was determined in pupa stage.
Survival
The survival was checked during the whole period of individual rearing. We monitored daily survival of individually reared larvae from both fumagillin and control treatments during the 4 wk prior to overwintering. During overwintering, larval mortality was checked every 2 wk and dead animals were removed. The survival monitoring ended with the start of group rearing after the 10 d waking-up period following the overwintering phase.
Immunity
Four weeks after the first fumagillin treatment (see Supp Fig. 1 [online only]) hemolymph from 100 individuals from both treatment groups was sampled. Hemolymph was collected by puncturing the larvae with a sterile needle. Four microliters of hemolymph were immediately mixed with 100 µl chilled phosphate buffered saline buffer and stored at −80°C until further use. For estimating the Phenoloxidase activity samples were thawed on ice and then centrifuged at 4°C for 7 min at full speed to obtain the supernatant. The assay was performed in a 96-well plate. Twenty-four microliters of each supernatant were mixed with 200 µl 3 mM L-Dopa (Sigma Aldrich, Helsinki, Finland). To analyze the phenoloxidase activity, changes in absorbance where measured at 30°C and 490 nm for 90 min with a Victory X4 2030 plate reader (Perkin Elmer, Waltham, MA, USA).
Development
Developmental time
To control the effect of fumagillin on developmental time of P. plantaginis, every developmental stage was monitored and dates documented. The egg-laying date was used as the starting date for development. During the whole experimental process larval hatching date, pupation date, as well as adult eclosion date were monitored and recorded. These data were further used to calculate the developmental time from egg to pupa and from egg to adult and thus the effect of the fumagillin treatment on the development of P. plantaginis larvae could be examined.
Weight
Larval weight was measured on three different time points before pupation; pupa weight was measured too (see Supp Fig. 1 [online only]). The first weight measurement was taken on 25-d-old larvae before the treatment started; the second one was taken 4 wk later, and the third one right after the overwintering period. Pupa weight was measured 1 d after pupation.
Egg laying and hatching
To examine the egg laying and hatching- success single-pair matings were performed with the emerged adults, within families and within the treatment groups. In total, 24 matings were conducted within the antibiotic treatment group and 15 matings in the control group. Pairs were placed together in plastic boxes (12 by 10 by 10 cm) under above-mentioned rearing conditions (25°C, a photoperiod of 18:6 (L:D) h, 80% RH). After 3 d all eggs were counted. Additionally hatched larvae were counted after 21 d (18 d after egg counting).
Statistical Analyses
All statistical analyses were performed with R 3.1.1 (R Foundation for Statistical Computing, 2014, Vienna, Austria).
All data were checked for normality and homogeneity of variance, and family was used as a random factor. Reported error terms are standard deviations, unless specified otherwise. We analyzed the survival upon treatment separately for the time before and during hibernation with Cox Proportional Hazard-Models. Treatment was added as a fixed factor and family as a random effect. We used analysis of variance (ANOVA) to test for differences in larval weight gain across treatment and developmental time point (day). To account for differences in larval weight before the experiment, we subtracted the weight of first weight measurement from the weights of second and third weight measurement after treatment. Larval development was calculated as timespan between egg laying date and pupation date; overall development was calculated as timespan between egg laying date and adult date. We analyzed each development characteristic with a separate ANOVA, with treatment and gender as fixed factors, and an interaction term of the two factors. Reproductive success was analyzed as the amount of laid eggs per mating couple with a Zero inflated Count Model. The Zero inflated Count Model performed a binomial test on the probability to lay eggs among treatment and a Poisson regression on the amount of laid eggs per treatment. Furthermore a generalized linear mixed model via penalized quasi-likelihood (PQL) was used for examining the proportion of hatched larvae depending on the amount of laid eggs and treatment. Data of immune parameters were analyzed with a Mann–Whitney test, using treatment as factor.
Results
Survival
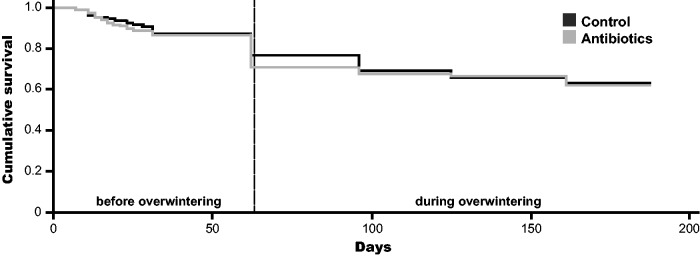
Treatment effect on larval survival before and during hibernation. A prophylactic treatment with fumagillin did not increase P. plantaginis survival during overwintering in comparison to control larvae (Fig. 1: Cox proportional-hazard regression; β = 0.06 ± 0.14 (se), χ2 = 0.17, P = 0.68). Furthermore, the treatment did not affect the survival before the start of hibernation (Fig. 1: Cox proportional-hazard regression; β = 0.09 ± 0.2 (se) χ2 = 0.2, P = 0.65)
Fig. 1.
Effect of fumagillin treatment on larval survival of P. plantaginis before hibernation (50 d) and during hibernation compared to survival of control larvae. Dashed line separates survival curve in before overwintering mortality (left) and mortality during overwintering (right).
Immunity
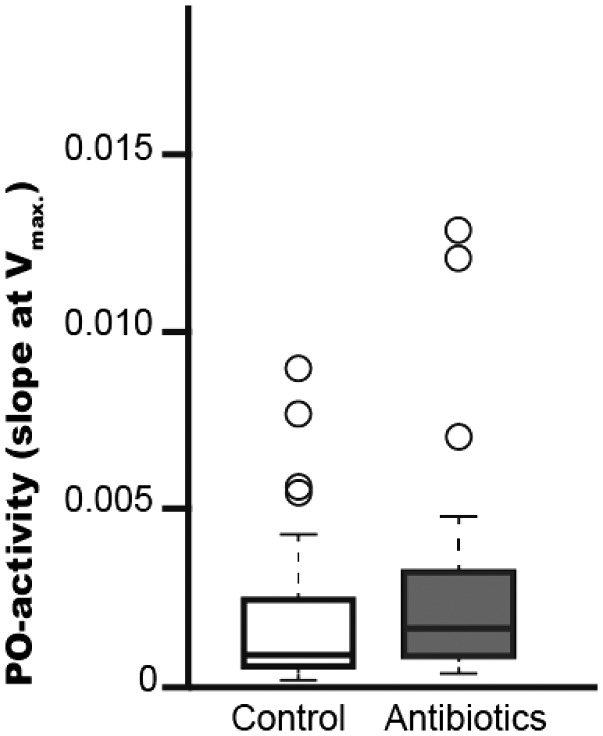
Phenoloxidase activity. Larval phenoloxidase activity in the hemolymph was significantly higher in the fumagillin treatment group than in the control group (Fig. 2: Wilcox-test: W = 2271, P = 0.007).
Fig. 2.
Phenoloxidase activity in the hemolymph of P. plantaginis larvae, fed on fumagillin, 4 wk after treatment exposure, compared to control treated larvae of same age. Phenoloxidase activity (slope at Vmax.) measured from hemolymph samples, comparing the activity of antibiotic treated larvae and control larvae.
Development
Effect of fumagillin on developmental time of P. plantaginis Developmental time was calculated as the larval development (timespan from egg-laying date until pupation date) and also as the overall developmental time (timespan from egg-laying date until adult eclosion date). Development differs between the genders, with males showing a shorter developmental time (Table 1: mean days to pupation: males = 237.95 ± 2.63; females = 240.46 ± 2.92). Fumagillin treated larvae develop faster than control treated larvae (Table 1: mean days to pupation: control = 239.49 ± 3.0; fumagillin = 238.53 ± 2.95). However, there is no effect of fumagillin on the overall developmental duration from egg to adult (Table 1: mean days to eclosion: control = 248.73 ± 3.59; fumagillin = 248.05 ± 3.54). There is no difference in sex ratio (appearance of male and female pupae) and fumagillin does not affect the survival of male and female pupae differently (χ2 test: x2 = 0.003, df = 1, P = 0.955).
Table 1.
Effect of fumagillin treatment on the developmental time of P. plantaginis
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Developmental time | ||||
| egg to pupa | ||||
| treatment | 1 | 58.3 | 7.296 | 0.007 |
| gender | 1 | 333.7 | 45.274 | 1.556e–10 |
| treatment:gender | 1 | 2.6 | 0.354 | 0.552 |
| residuals | 213 | 7.4 | ||
| egg to adult | ||||
| treatment | 1 | 12.35 | 1.463 | 0.228 |
| gender | 1 | 55.48 | 6.573 | 0.011 |
| treatment:gender | 1 | 4.63 | 0.548 | 0.460 |
| residuals | 198 | 8.44 | ||
Results of ANOVA testing for the effect of treatment, gender and their interactions on developmental time measured as time in days from egg to pupa and egg to adult (df = degrees of freedom; MS = Mean Square, F = F-value; P = significance probability).
Effect of fumagillin on larval and pupal weight
Compared to control larvae, there is no significant weight gain or loss of fumagillin treated larvae neither during overwintering (Table 2: standardized larval weight: control = 1.44 ± 8.81 g; fumagillin = 1.31 ± 9.78 g), nor at the two time points (Table 2: standardized larval weight: second weighing = −1.36 ± 6.96g; 3rd weighing = 3.27 ± 10.19 g). The weight of P. plantaginis pupa is not affected by a prophylactic fumagillin treatment of the larvae before overwintering (Table 2: mean pupa weight: control = 198.03 ± 48.97 g; fumagillin = 203.06 ± 49.96 g), although male pupae are heavier than female pupae (Table 2: mean pupa weight: males = 181.89 ± 35.62 g; females = 236.56 ± 52.61 g).
Table 2.
Effect of fumagillin on weight of P. plantaginis larvae and pupa
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Weight | ||||
| larva | ||||
| treatment | 1 | 44.59 | 0.550 | 0.458 |
| day | 1 | 69.33 | 0.856 | 0.355 |
| treatment:day | 1 | 10.96 | 0.135 | 0.713 |
| residuals | 1,162 | 81.01 | ||
| pupa | ||||
| treatment | 1 | 0 | 0.000 | 0.997 |
| day | 1 | 156,974 | 83.159 | <2e–16 |
| treatment:day | 1 | 2,616 | 1.386 | 0.240 |
| residuals | 213 | 1,888 | ||
Results of ANOVA testing for the effect of treatment on larva weight as well as pupa weigh. Analysis for larva weight also use day (before and after hibernation) and the interaction between treatment and day as factors. The ANOVA testing for the effect of treatment on pupa weight also tests for effect of gender and the interaction between treatment and gender (df = degrees of freedom; MS = Mean Square, F = F-value; P = significance probability).
Effect of fumagillin on egg laying- and hatching success
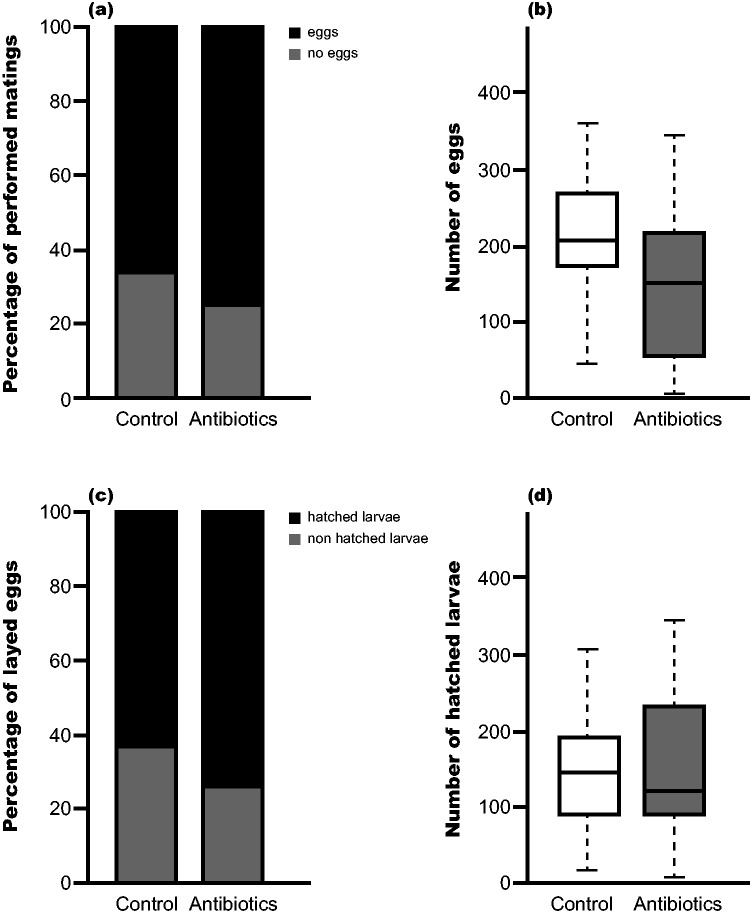
Egg laying success was evaluated by comparing the pairings that did not lay eggs (0 eggs produced 3 d post mating date) as well as the pairs that successfully laid eggs (at least one egg laid 3 d post mating date). We found no significant effect of antibiotic treatment on egg laying success (Fig. 3a: Zero-inflated Count Data Regression: estimate = −0.405, z-value = −0.561, Pr(>|z|) = 0.575), but mating pairs treated with the antibiotic laid less eggs than control mating pairs (Fig. 3b: Zero-inflated Count Data Regression: z-value = 236.13, Pr(>|z|) = <2e-16). The proportion of hatched larvae per number of laid eggs is not significantly different between the control and antibiotic treatment groups, even though hatching success is higher in the antibiotic treated group (Fig. 3c: Generalized Linear Mixed Model via PQL: P = 0.246 and Fig. 3d: Linear mixed effects model: t-value = 0.357).
Fig. 3.
Effect of fumagillin on egg laying and hatching success in P. plantaginis. Egg laying success was evaluated by (a) presence/absence of eggs 3 d post mating for control and antibiotic treated larvae; and (b) amount of laid eggs, counted 3 d post mating for control and antibiotic treated larvae. Hatching success was evaluated by (c) hatched and nonhatched larvae in proportion to the amount of laid eggs and also as (d) total number of hatched larvae, counted 21 d post egg laying date for control and antibiotic treated larvae.
Discussion
We found significant effects of fumagillin treatment on central life- history traits in wood tiger moths but at the same time antibiotic treatment did not affect either larval overwintering survival or weight gain. Antibiotics are commonly added to artificial diets of mass-reared insects to suppress diseases and infections, which can easily spread in laboratory-reared colonies. However, there is limited knowledge on how the antibiotic itself might influence important life-history traits like overwintering performance and reproductive success. The observed negative effects in our experiment are related to reproduction and immune responses, and are important both in lab and wild insect populations. The lack of negative effects on larval survival can however be misleading to users of antibiotics in insect mass-rearing and skew the population fitness characteristics.
Guaranteeing a good overwintering performance is an important factor for mass-rearing techniques to maintain a functioning insect population. Overwintering is a highly sensitive life stage, affected by various factors, and can greatly influence the insects’ quality (Bale and Hayward 2010, Xu et al. 2011, Spurgeon 2012). Our results show that the antibiotic treatment does not affect the larval overwintering success. Survival and weight gain were also similar between treated and nontreated individuals. It is known that antibiotics can interact with intestinal microbiota, resulting in growth promoting effects by changing the gut flora, and thus promote better weight gain (Lin 2011). We did not see a growth promoting effect, initiated by fumagillin, on larval weight gain performance, but in this study it remains unanswered, whether this is caused by the antibiotic not affecting the insects’ microbiota. Thus, the 4-wk continuous fumagilin treatment seems not to affect the larval ability to prepare for hibernation. A prophylactic antibiotic treatment might help to limit the risk of infection without affecting the larval ability to overwinter.
While previous studies have focused only on how fumagillin is affecting infected insects, we examined the possible negative effect of the antibiotic on the lifespan development of noninfected lepidopteran larvae. A study with the mosquito Anopheles stephensi has revealed possible negative effects of the toxin fumagillin on insects; the larval developmental period was prolonged, rising with increasing antibiotic dose (Rutledge 1970). Our results did not display a similar effect on noninfected Lepidoptera, as the development of P. plantaginis larvae was actually shortened by the fumagillin treatment, whereas the overall developmental time from egg to adult was neither shortened nor prolonged. A prophylactic antibiotic treatment in mass-reared colonies could thus be used without expecting negative effects on the overall insects’ development, even in the absence of a disease.
Fumagillin treatment increased the immune response of larvae, measured as activity of phenoloxidase in the hemolymph, meaning that the antibiotic affects immune- related enzymes. Antibiotics might be recognized as nonself by the insects’ immune system. Consequently, the immune response will be up-regulated, resulting in increased enzyme levels. On the other hand, Fallon et al. (2011) found a limited hemocyte activity in hemolymph of Galleria mellonella larvae treated with fumagillin (Fallon et al. 2011). This showed, that the ability to fight infections decreases after being exposed to the antibiotic, resulting in a lower survival. A treatment of not obviously infected or sick individuals does not reveal the same results, as the survival was not affected and at the same time the immune response was activated. Thus, we propose that the antibiotic is recognized as nonself by the immune system. Because up-regulation of immune response is related with high costs as well as with the production of reactive oxygen species, this can result in a decrease of other fitness traits, especially when exposed to an infection.
Our study shows a significantly reduced number of laid eggs by fumagillin treated adults. These results, together with those from studies on the effect of fumagillin on Nosema infections in Bombyx occidentalis (Whittington and Winston 2003) and A. mellifera (Webster 1994), reveal negative consequences on brood levels, indicating that antibiotics negatively affect insects’ reproduction success. Establishing new wild populations as a lab colony, however, implies mainly the maintenance of the insects’ reproductive success. It is known that maternal stress can affect offspring quality by altering fitness traits such as hatching success, growth, or development (Mousseau et al. 1991, Kyneb and Toft 2006) as shown for instance in rove beetles (Kyneb and Toft 2006) and Trichoplusia ni larvae (Freitak et al. 2009). Offspring quantity and quality could also trade-off, such that large egg clutches might result in smaller offspring, whereas the hatching success and fitness of larvae from smaller egg clutches might be higher (Koch and Meunier 2014). Interestingly, the antibiotic treatment decreased the number of laid eggs while slightly increasing the number of hatched larvae. Still the proportion of hatched larvae per eggs laid did not differ between treatments. A prophylactic fumagillin treatment does, however, limit reproductive success. How this might affect long-term fitness, measured as decreased population size in the following generations, remains unanswered.
Our findings offer a better, more holistic understanding of prophylactic medicine treatments for laboratory-reared Lepidoptera larvae. Even though the application of antibiotics as prophylaxis is a common strategy in mass-rearing of insects, there was no recent study about possibly negative side effects. In conclusion, we show that although a prophylactic fumagillin treatment does not adversely affect larval development, it has a negative effect on reproductive success. The application of antibiotics in mass-reared insect colonies should thus be carefully considered, and possible negative side effects taken into account.
Acknowledgments
We would like to thank everyone who helped with rearing the individuals: Kaisa Suisto, Jimi Kirvesoja, Liisa Hämäläinen, Iida Heikkilä, Diana Abondano and Morgan Brain. We also thank Dimitri Stucki and Sebastiano De Bona for excellent help with the statistical analysis, and Bibiana Rojas for helpful comments. The Finnish Cultural Foundation and Finnish Centre of Excellence in Biological Interactions (project number SA-252411) supported this work.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
References Cited
- Antúnez K., Martín-Hernández R., Prieto L., Meana A., Zunino P., Higes M. 2009. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 11: 2284–2290. [DOI] [PubMed] [Google Scholar]
- Bale J. S., Hayward S.A.L. 2010. Insect overwintering in a changing climate. J. Exp. Biol. 213: 980–994. [DOI] [PubMed] [Google Scholar]
- Cohen A. 2001. Formalizing insect rearing and artificial diet technology. Am. Entomol. 49: 154–164. (http://www.ingentaconnect.com/content/esa/ae/2001/00000047/00000004/art00003). [Google Scholar]
- Cohen A. C. 2000. A review of feeding studies of lygus spp. with emphasis on artificial diets. SW. Entomol. 23: 111–119. [Google Scholar]
- Conner W. E. (ed.). 2008. Tiger Moths and Woolly Bears—Behavior, Ecology, and Evolution of the Arctiidae. Oxford University Press, New-York. [Google Scholar]
- Coustau C., Chevillon C., Ffrench-Constant R. 2000. Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol. Evol. 15: 378–383. [DOI] [PubMed] [Google Scholar]
- Daghrir R., Drogui P. 2013. Tetracycline antibiotics in the environment: a review. Environ. Chem. Lett. 11: 209–227. [Google Scholar]
- Dimou I., Rempoulakis P., Economopoulos A. P. 2010. Olive fruit fly [Bactrocera (Dacus) oleae (Rossi) (Diptera: Tephritidae)] adult rearing diet without antibiotic. J. Appl. Entomol. 134: 72–79. [Google Scholar]
- Fallon J. P., Reeves E. P., Kavanagh K. 2011. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology 157: 1481–1488. [DOI] [PubMed] [Google Scholar]
- Freitak D., Heckel D. G., Vogel H. 2009. Dietary-dependent trans-generational immune priming in an insect herbivore. Proc. Biol. Sci. R. Soc. 276: 2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friman V. P., Lindstedt C., Hiltunen T., Laakso J., Mappes J. 2009. Predation on multiple trophic levels shapes the evolution of pathogen virulence. PLoS One 4: e6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. P., Kanost M. R., Trenczek T. 1997. Biological mediators of insect immunity. Annu. Rev. Entomol. 42: 611–643. [DOI] [PubMed] [Google Scholar]
- Hegna R. H., Galarza J. A., Mappes J. 2015. Global phylogeography and geographical variation in warning coloration of the wood tiger moth (Parasemia plantaginis). J. Biogeogr. 42: 1469–1481. [Google Scholar]
- Higes M., García-Palencia P., Martín-Hernández R., Meana A. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94: 211–217. [DOI] [PubMed] [Google Scholar]
- Huang W. F., Solter L. F., Yau P. M., Imai B. S. 2013. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 9: e1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L. K., Meunier J. 2014. Mother and offspring fitness in an insect with maternal care: phenotypic trade-offs between egg number, egg mass and egg care. BMC Evol. Biol. 14: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyneb A., Toft S. 2006. Effects of maternal diet quality on offspring performance in the rove beetle Tachyporus hypnorum. Ecol. Entomol. 31: 322–330. [Google Scholar]
- Lehman R. M., Lundgren J. G., Petzke L. M. 2009. Bacterial communities associated with the digestive tract of the predatory ground beetle, poecilus chalcites, and their modification by laboratory rearing and antibiotic treatment. Microb. Ecol. 57: 349–358. [DOI] [PubMed] [Google Scholar]
- Leppla N. C. 2009. Rearing of insects, pp. 866–869. In J. L. Capinera (eds.), Encyclopedia of Insects. IAEA Springer, Netherlands. doi:10.1016/B978-0-12-374144-8.00227-7 [Google Scholar]
- Lin J. 2011. Effect of antibiotic growth promoters on intestinal microbiota in food animals: a novel model for studying the relationship between gut microbiota and human obesity? Front. Microbiol. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt C., Lindström L., Mappes J. 2009. Thermoregulation constrains effective warning signal expression. Evolution 63: 469–478. [DOI] [PubMed] [Google Scholar]
- McLean-Cooper N., Achee N., Foggie T., Grieco J., Williams J. 2008. Space optimizing methods for laboratory rearing of Aedes aegypti. J. Am. Mosq. Control Assoc. 24: 460–462. [DOI] [PubMed] [Google Scholar]
- Moret Y., Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290: 1166–1168. [DOI] [PubMed] [Google Scholar]
- Mousseau T., Effects M., Insect I. N., Histories L. 1991. Maternal effects in insect life histories. Annu. Rev. Entomol. 36: 511–534. [Google Scholar]
- Nokelainen O., Lindstedt C., Mappes J. 2013. Environment-mediated morph-linked immune and life-history responses in the aposematic wood tiger moth. J. Anim. Ecol. 82: 653–662. [DOI] [PubMed] [Google Scholar]
- Ojala K., Lindström L., Mappes J. 2007. Life-history constraints and warning signal expression in an arctiid moth. Funct. Ecol. 21: 1162–1167. [Google Scholar]
- Ojala K., Julkunen-Tiitto R., Lindström L., Mappes J. 2005. Diet affects the immune defence and life-history traits of an Arctiid moth Parasemia plantaginis. Evol. Ecol. Res. 7: 1153–1170. [Google Scholar]
- Parker A. G., Dyck V. A., Hendrichs J., Robinson A. S. 2005. Mass-rearing for sterile insect release, pp. 209–232. In Dyck V. A., Hendrichs J., Robinson A. S. (eds.), Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, IAEA Springer, Netherlands. [Google Scholar]
- Rull J., Brunel O., Mendez M. E. 2005. Mass rearing history negatively affects mating success of male Anastrepha ludens (Diptera: Tephritidae) reared for sterile insect technique programs. J. Econ. Entomol. 98: 1510–1516. [DOI] [PubMed] [Google Scholar]
- Rutledge L. C. 1970. Some effects of fumagillin on Anopheles stephensi. Mosq. News 30: 118–121. [Google Scholar]
- Schmid-Hempel P. 2003. Variation in immune defence as a question of evolutionary ecology. Proc. Biol. Sci. R. Soc. 270:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50: 529–551. [DOI] [PubMed] [Google Scholar]
- Sikorowski P. P., Lawrence A. M. 1994. Microbial contamination and insect rearing. Am. Entomol. 40: 240–253. [Google Scholar]
- Sinclair B. J. 2014. Linking energetics and overwintering in temperate insects. J. Therm. Biol. 54: 5–11. [DOI] [PubMed] [Google Scholar]
- Singh P. 1982. The rearing of beneficial insects. N. Z. Entomol. 7: 304–311. doi:10.1080/00779962.1982.9722404 [Google Scholar]
- Sørensen J. G., Addison M. F., Terblanche J. S. 2012. Mass-rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop Prot. 38: 87–94. doi:10.1016/j.cropro.2012.03.023 [Google Scholar]
- Spurgeon D. W. 2012. Physiological consequences of laboratory rearing of Lygus hesperus (Hemiptera: Miridae). Environ. Entomol. 41: 415–419. [DOI] [PubMed] [Google Scholar]
- Thomson H. M. 1958. The effect of a microsporidian parasite on the development, reproduction, and mortality of the spruce budworm, Choristoneura fumiferana (clem.). Can. J. Zool. 36: 499–511. [Google Scholar]
- Upadhya R., Zhang H. S., Weiss L. M. 2006. System for expression of microsporidian methionine amino peptidase type 2 (MetAP2) in the yeast Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 50: 3389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heever J. P., Thompson T. S., Curtis J. M., Ibrahim A., Pernal S. F. 2014. Fumagillin: an overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 62: 2728–2737. [DOI] [PubMed] [Google Scholar]
- Van Der Hoeven R., Betrabet G., Forst S. 2008. Characterization of the gut bacterial community in Manduca sexta and effect of antibiotics on bacterial diversity and nematode reproduction. FEMS Microbiol. Lett. 286: 249–256. [DOI] [PubMed] [Google Scholar]
- Vilmos P., Kurucz É. 1998. Insect immunity: evolutionary roots of the mammalian innate immune system. Immunol. Lett. 62: 59–66. [DOI] [PubMed] [Google Scholar]
- Webster T. C. 1994. Fumagillin affects Nosema apis and honey bees (Hypenopterai apidae). Apiculture Soc. Insects. 93: 601–604. [Google Scholar]
- Whittington R., Winston M. L. 2003. Effects of Nosema bombi and its treatment fumagillin on bumble bee (Bombus occidentalis) colonies. J. Invertebr. Pathol. 84: 54–58. [DOI] [PubMed] [Google Scholar]
- Wilkinson T. L. 1998. The elimination of intracellular microorganisms from insects: an analysis of antibiotic-treatment in the pea aphid (Acyrthosiphon pisum). Comp. Biochem. Physiol.Mol. Integr. Physiol. 119: 871–881. [Google Scholar]
- Xu S., Wang M. L., Ding N., Ma W. H., Li Y. N., Lei C. L., Wang X. P. 2011. Relationships between body weight of overwintering larvae and supercooling capacity; diapause intensity and post-diapause reproductive potential in Chilo suppressalis Walker. J. Insect Physiol. 57: 653–659. [DOI] [PubMed] [Google Scholar]
- Zhang J., Friman V. P., Laakso J., Mappes J. 2012. Interactive effects between diet and genotypes of host and pathogen define the severity of infection. Ecol. Evol. 2: 2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]