Abstract
Previous work showed the non-nutritive polyol sweetener Erythritol was toxic when ingested by Drosophila melanogaster (Meigen, 1930). This study assessed whether insect toxicity is a general property of polyols. Among tested compounds, toxicity was highest for erythritol. Adult fruit flies (D. melanogaster) fed erythritol had reduced longevity relative to controls. Other polyols did not reduce longevity; the only exception was a weaker but significant reduction of female (but not male) longevity when flies were fed D-mannitol. We conclude at least some non-nutritive polyols are not toxic to adult D. melanogaster when ingested for 17 days. The longer time course (relative to erythritol) and female specificity of D-mannitol mortality suggests different mechanisms for D-mannitol and erythritol toxicity to D. melanogaster.
Keywords: erythritol, human-safe insecticide, maltitol, D-mannitol, xylitol
Recent controlled laboratory experiments showed the polyalcohol erythritol was toxic when ingested by adult Drosophila melanogaster (Meigen, 1930) fruit flies (Baudier et al. 2014). Erythritol toxicity was dose-dependent and was not due to food avoidance/starvation: flies consumed food containing erythritol even when given free access to control food with sucrose. Erythritol was also toxic to the oriental fruit fly Bactrocera dorsalis (Tephritidae) (Zheng et al. 2015). Erythritol consumption is safe for humans (Sheet et al. 2014), demonstrating potential for use of this compound in human safe pest control applications (Tokuoka et al. 1992; Storey et al. 2007). The previous experiments of erythritol effects on D. melanogaster longevity tested a commercial sweetener mixture containing erythritol (Truvia, Cargill, Inc., Minneapolis, MN) and erythritol against sucrose (control) and against several other commercial non-nutritive sweetener mixtures (Baudier et al. 2014). The other sweeteners were commercially-produced, complex blends of sweetening agents and bulk fillers of unknown concentrations. Furthermore, the sweetening agents in the previously tested mixtures were either not polyols (saccharine, aspartame) or were halogenated polyols (sucralose).
The aim of this study was to test whether insect toxicity was a general property of non-nutritive polyols. We chose to test polyols that, like erythritol, are commercially available and approved for human consumption (Mortensen 2006; Canimoglu and Rencuzogullari 2012; Sheet et al. 2014). We fed several food- additive polyol sweeteners to adult D. melanogaster in controlled laboratory feeding trials, along with sucrose and no-sweetener control foods, and we tested whether flies on these feeding treatments differed in longevity.
Materials and Methods
Drosophila Culturing and Sample Sizes.
Standard Drosophila stock food for laboratory culturing was used for rearing the flies to adulthood and as a base food for the experimental treatments. To obtain research subjects, wild type (Canton S) D. melanogaster larvae were reared on standard Drosophila stock food (Chakraborty et al. 2011) prepared as follows: We mixed 120 g cornmeal (LabScientific, Livingston, NJ, USA: FLY-8009-10), 48 g yeast (LabScientific 8030-5), 9 g agar, 120 ml molasses (LabScientific FLY-8008-4), 24 ml Tegosept (10% w/v methyl p-hydroxybenzoate in 95% ethanol), and 9.5 ml Propionic Acid with 840 ml of water. After adult emergence 0–24-h old adult flies were transferred to tubes with treatment foods. The base of the treatment foods (to which the treatment sweeteners were added) was identical to the stock food recipe, but did not contain molasses. Cornmeal and yeast in the base food assured flies received sufficient carbohydrates and protein in addition to any effects of the treatment additives. For the experimental treatments we combined the base food with either a non-nutritive polyol or sucrose (negative control) to produce final foods that were 1 M concentration of the added sweetener (based on final volume). Foods were made in batches of 100 ml, with each treatment tube containing 10 ml of food. After heating each batch of food to set the agar, food were poured into tubes and cooled until consistency was evenly firm, moist, and permeable. Food consistency was uniform across treatments: foods did not run when tubes were placed on their sides.
The polyols tested were D-mannitol (>99%, Sigma-Aldrich, Allentown, PA), xylitol (≥99%, Sigma-Aldrich), and maltitol (≥98%, Sigma-Aldrich), with meso-erythritol (99%, Acros Organics via VWR Inc., West Chester, PA) used as a positive control for lethality. We included a second negative control treatment of base food with no added molasses, sucrose, or polyol sweeteners to test whether the base food with no added sweetener treatment was fully nutritive and sufficient for adult survival. All subjects were cultured at 25°C, 50% humidity, and under a photoperiod of 12:12 (L:D) h cycle.
We placed newly emerged adult flies into 80 ml cotton-capped plastic Drosophila culturing tubes with 10 ml of food placed in the bottom of the tube. For each treatment n = 30 flies were tested in groups of 10 flies per tube with three tubes per treatment. In each food treatment one tube contained only males, one contained only females, and one tube contained five flies of each sex. Flies were moved to fresh food of the same formulation twice per week. The total number of fruit flies used for the experiments was 180.
Fly Longevity Data Collection and Statistics.
During longevity observations tubes were placed on their sides to minimize risk of flies adhering to the food surface. The number of dead flies was scored daily in each tube up to 17 days adult age. In two trials in a previous study using n = 30 subject flies fed on 1 M erythritol using similar methods, all subjects were dead in 5 and 14 days, respectively (Baudier et al. 2014). D. melanogaster typically die of starvation in 2–3 days adult age when kept moist and without access to food (Chippindale et al. 1996; Goenaga et al. 2012). Therefore we expected 17 days of observation to be sufficient time to observe similar effects of other polyols at the same concentration. Analyses were conducted with SPSS software v. 23 (IBM corporation 2011). We analyzed fly adult longevity data using survival analysis. Subjects that lived to the end of the study or were lost for reasons other than death (e.g. escapees or flies found adhering to the food) were included in the analysis as right-censored values. Differences in survival distributions [Pr(flies alive) vs. fly adult age] were tested using the log-rank (Mantel-Cox) test to make all pairwise comparisons of longevity distributions among treatments.
We report mean ± SE longevities in days of adult age for some of our treatments, but these statistics are biased conservative estimates in nearly all cases because some flies lived until the end of the study (i.e. there were right-censored subjects remaining at the end of observations). Therefore these values are biased minimum estimates of longevity and are only valid for comparisons among treatments within our study.
Results
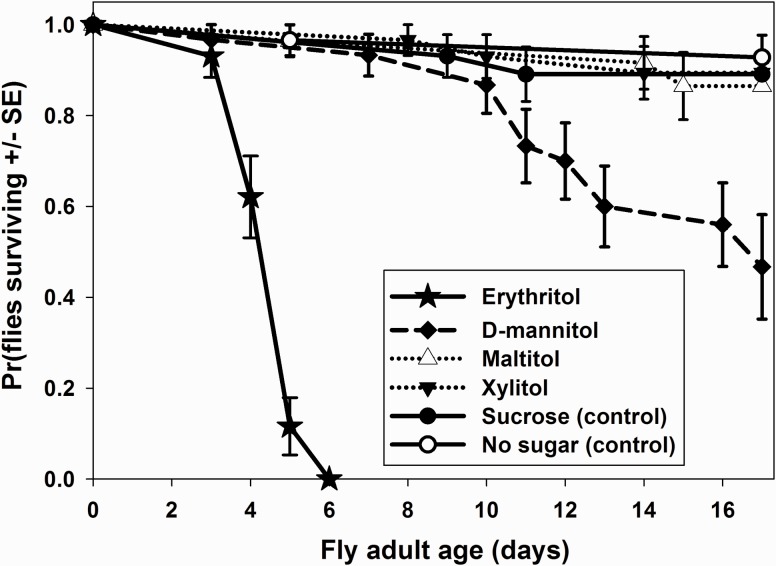
Flies fed food with added sucrose had similar longevities to the no sweetener control, demonstrating that an extra carbohydrate source in the base food is not needed to support adult fly survival (X2 = 0.24, P = 0.62). Adult flies fed erythritol-treated food had significantly shorter lifespans than flies in all other treatments (Fig. 1; all X2 > 52.5, all P < 0.001). The only other treatment that differed significantly in longevity from the controls was D-mannitol (Fig. 1; D-mannitol from sucrose: X2 = 8.1, P = 0.004; D-mannitol from no sweetener: X2 = 13.0, P < 0.001). Flies fed food with the other polyols had longevities similar to both control treatments (maltitol: vs. sucrose X2 = 0.29, P = 0.86; versus no sweetener X2 = 0.54, P = 0.46; xylitol: vs. sucrose X2 = 0.003, P = 0.96; vs. no sweetener X2 = 0.20, P = 0.65). The decrease in longevity of D-mannitol treated flies (relative to sucrose and no sweetener) was small in magnitude relative to the erythritol effect (Fig. 1). Erythritol fly mortality was significantly elevated (relative to sucrose and to no sweetener) by day 4 of adult age, while D-mannitol treated fly mortality did not differ significantly from the control flies until day 12 (Fig. 1).
Fig. 1.
Survival plots showing the probability of survival (with SEs) versus adult age for D. melanogaster fruit flies given food with sucrose and no sweetener controls, or with non-nutritive polyol sweeteners. Observations were terminated when flies reached 17 days of adult age. Total sample size n = 30 subjects for each treatment.
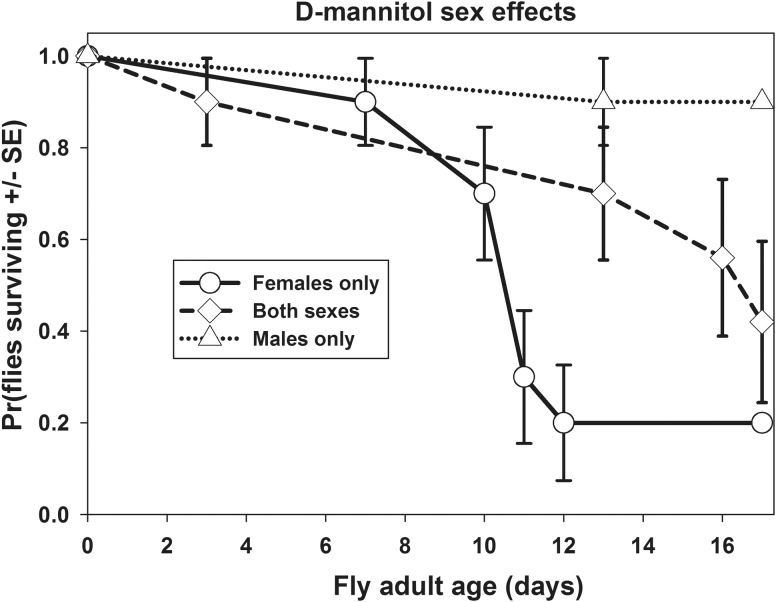
Examination of the data from the sex-specific tubes suggested the elevated D-mannitol mortality was elevated only for female flies. Separate tests on sex-effects within the D-mannitol treatment showed female flies had shorter lifespan distributions than D-mannitol fed males (X2 = 11.1, P = 0.001), and trended toward having shorter lifespans than the flies in the mixed-sex D-mannitol fed tube; the mixed sex tube had longevity intermediate between male-only and female-only tubes (Fig. 2). The sex-specific negative effect of D-mannitol on female flies was weaker than the erythritol effect on females. The female-only tube of erythritol fed flies had shorter lifespans than the female-only D-mannitol fed tube (X2 = 17.1, P < 0.001; mean ± SE female tube longevities: erythritol = 4.7 ± 0.15 days, D-mannitol = 11.7 ± 0.93 days). We did not record the sex of dead flies in the mixed-sex tubes, so we could not determine whether the reduced longevity in the D-mannitol fed mixed-sex tube was due mainly to female fly mortality. Erythritol-fed male and female flies had similar lifespans (X2 = 2.2, P = 0.14; mean ± SE female tube longevity: 4.7 ± 0.15 days; male tube longevity: 5.14 ± 0.25 days), and no other sex differences in longevity within treatments were significant.
Fig. 2.
Survival plots showing the probability of survival (with SEs) versus adult age for D. melanogaster fruit flies given food containing 1 M D-mannitol. Observations were terminated when flies reached 17 days of adult age. Total sample size n = 10 subjects for each treatment.
Discussion
None of the compounds we tested were as strongly insecticidal to D. melanogaster upon ingestion as erythritol. Two of the other polyols (maltitol and xylitol) were indistinguishable from sucrose and no sweetener controls in their effects on longevity when added to base fly food. These findings show that at least some food-safe non-nutritive polyols are not toxic at adult D. melanogaster when ingested, at least over the 17 day time course of our study and at the concentration we tested (1 M). Because the other polyols were non-nutritive (like erythritol), and were added to an identical food base, these results also suggest erythritol-induced mortaility was not associated with food-avoidance starvation, confirming earlier findings (Baudier et al. 2014). Furthermore, base food with no added sweetener did not differ from sucrose control. Added sweeteners are not necessary for adult fly nutrition for the first 17 days of adult life, and the toxic effects of erythritol cannot be explained by insufficient nutrition in the food. Erythritol may interfere with nutrient uptake through the midgut lining (Silva and Terra 1995), but the mechanism(s) of erythritol toxicity are unknown.
Erythritol is readily ingested by D. melanogaster when added to food, although it may not be perceived as sweet by the flies (Fujii et al. 2015). Several polyols including erythritol, mannitol, and xylitol, failed to induce feeding activity relative to water controls in imported red fire ant workers (Solenopsis invicta) (Vander Meer et al. 1995).
One additional polyol we tested, D-mannitol, showed evidence of toxic effects compared with control foods, albeit the D-mannitol effects on longevity were weak relative to erythritol effects. The suggestion of sex-specific effects of D-mannitol on adult fly longevity raises questions about mechanisms underlying this effect. Conspecific male and female insects including D. melanogaster can differ in nutritional requirements, although genetic correlations between the sexes can limit the evolution of sex divergence in feeding behavior (Reddiex et al. 2013). Our study did not distinguish between female food avoidance (see Meunier et al. 2000) versus female-specific toxicity of D-mannitol. However, erythritol toxicity was not caused by reduced food ingestion (Baudier et al. 2014). Furthermore, adult D. melanogaster typically die of starvation in 2–3 days of adult age in the lab when kept moist but without access to food (Chippindale et al. 1996; Goenaga et al. 2012), suggesting the female mortality in the D-mannitol treatment was likely not caused by food avoidance. The longer time course of onset of D-mannitol mortality, and the fact D-mannitol reduced female longevity specifically (erythritol influenced both sexes equally), together suggest the mechanisms for D-mannitol negative effects on D. melanogaster differed from erythritol effects.
Acknowledgements
We thank two anonymous reviewers who made helpful comments on an earlier version of the article. Financial support was provided by (NSF grants 1209072 to S.O'D., and 1256114 to D.R.M.), and an Eppley Foundation for Research grant to (S. O’D. and D.R.M.)
References Cited
- Baudier K. M., Kaschock-Marenda S. D., Patel N., Diangelus K. L., O’Donnell S., Marenda D. R. 2014. Erythritol, a non-nutritive sugar alcohol sweetener and the main component of Truvia®, is a palatable ingested insecticide. PLoS One 9: e98949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canimoglu S., Rencuzogullari E., 2012. The genotoxic and teratogenic effects of maltitol in rats. Toxicol. Indus. Health 29: 935–943. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Vepuri V., Mhatre S. D., Paddock V. E., Miller S., Michelson S. J., Delvadia R., Desai A., Vinokur A., Melicharek D. J., et al. 2011. Characterization of a Drosophila Alzheimer's disease model: pharmacological rescue of cognitive defects. PLoS One 6: e20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale A. K., Chu T. J., Rose M. R. 1996. Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50: 753–766. [DOI] [PubMed] [Google Scholar]
- Fujii S., Yavuz A., Slone J., Jagge C., Song X., Amrein H. 2015. Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr. Biol., 25: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenaga J., Mensch J., Fanara J. J., Hasson E. 2012. The effect of mating on starvation resistance in natural populations of Drosophila melanogaster . Evol. Ecol., 26: 813–823. [Google Scholar]
- Meunier N., Ferveur J. F., Marion-Poll F. 2000. Sex-specific non-pheromonal taste receptors in Drosophila. Curr. Biol. 10: 1583–1586. [DOI] [PubMed] [Google Scholar]
- Mortensen A. 2006. Sweeteners permitted in the European Union: safety aspects. Scand. J. Food nd Nutr. 50: 104–116. [Google Scholar]
- Reddiex A. J., Gosden T. P., Bonduriansky R., Chenoweth S. F. 2013. Sex-specific fitness consequences of nutrient intake and the evolvability of diet preferences. Am. Nat., 182: 91–102. [DOI] [PubMed] [Google Scholar]
- Sheet B. S., Artik N., Ayed M. A., Abdulaziz O. F. 2014. Some alternative sweeteners (Xylitol, Sorbitol, Sucralose and Stevia): review. Karaelmas Sci. Eng. J., 4: 63–70. [Google Scholar]
- Silva C. P., Terra W. R. 1995. An a-glucosidase from perimicrovillar membranes of Dysdercus peruvianus (Hemiptera: Pyrrhocoridae) midgut cells. Purification and properties. Insect Biochem. Mol. Biol. 25: 487–494. [Google Scholar]
- Storey D., Lee A., Bornet. F., Brouns F. 2007. Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur. J. Clin. Nutr. 61: 349–354. [DOI] [PubMed] [Google Scholar]
- Tokuoka K., Ishizuka H., Wako. K., Taniguchi H. 1992. Comparison of three forms of erythrose reductase from an Aureobasidium sp. mutant. J. Gen. Appl. Microbiol. 38: 145–155. [Google Scholar]
- Vander Meer R. K., Lofgren C. S., Seawright J. A. 1995. Specificity of the red imported fire ant (Hymenoptera: Formicidae) phagostimulant response to carbohydrates. Fl. Entomol., 78: 144–154. [Google Scholar]
- Zheng C., Zeng L., Xu Y. 2015. Effect of sweeteners on the survival and behaviour of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest Manage. Sci. 72: 990–996. [DOI] [PubMed] [Google Scholar]