Abstract
Bark and the ambrosia beetles dig into host plants and live most of their lives in concealed tunnels. We assessed beetle community dynamics in tropical dry forest sites in early, intermediate, and late successional stages, evaluating the influence of resource availability and seasonal variations in guild structure. We collected a total of 763 beetles from 23 species, including 14 bark beetle species, and 9 ambrosia beetle species. Local richness of bark and ambrosia beetles was estimated at 31 species. Bark and ambrosia composition was similar over the successional stages gradient, and beta diversity among sites was primarily determined by species turnover, mainly in the bark beetle community. Bark beetle richness and abundance were higher at intermediate stages; availability of wood was the main spatial mechanism. Climate factors were effectively non-seasonal. Ambrosia beetles were not influenced by successional stages, however the increase in wood resulted in increased abundance. We found higher richness at the end of the dry and wet seasons, and abundance increased with air moisture and decreased with higher temperatures and greater rainfall. In summary, bark beetle species accumulation was higher at sites with better wood production, while the needs of fungi (host and air moisture), resulted in a favorable conditions for species accumulation of ambrosia. The overall biological pattern among guilds differed from tropical rain forests, showing patterns similar to dry forest areas.
Keywords: moisture, Platypodinae, Scolytinae, succession, seasonality
The Scolytinae and Platypodinae (Coleoptera, Curculionidae) are insects with two different main feeding strategies, commonly referred to as bark and ambrosia beetles. Bark beetles feed mostly on phloem tissue (i.e., phoeophagy), while ambrosia beetles feed on fungi growing on tree trunks and branches (i.e., myelophagy), or on portions of xylem and fungal tissue (i.e., xylomycetophagy) (Atkinson and Equihua 1986). The term ‘bark beetle’ is used here strictly for phloeophagous species, while the term ‘ambrosia beetle’ applies to both xylomycetophagy and myelophagy (Hulcr et al. 2007). With both strategies, larvae and adults create a system of cavities that protect them from external risks until they bore a new gallery, typically in another host tree. The cavity system resulting from the colonization process can be secondarily occupied by other beetle species such as inquilines and predators (Feller and Mathis 1997, Calderón-Cortés et al. 2011). Hence, the beetle community influences cascade effects in natural ecosystems, and investigations of bark and ambrosia beetle spatio-temporal dynamics may prove useful for future research targeting wood-associated fauna in tropical ecosystems (Wardhaugh 2014, Seibold et al. 2015).
Hulcr et al. (2008a) found low ambrosia beetle beta diversity (β) at a scale of 1,000 km in Papua New Guinea rainforests, where most species were evenly distributed over large areas in an idiosyncratic pattern. Low host specificity likely explained this broad distribution and low β-diversity (Hulcr et al. 2008b). The same pattern occurs in other generalist herbivorous insect guilds (Novotny et al. 2007). β-diversity can be decomposed into two components: 1) species turnover, which consists of replacement of species at a given site with different species from another site, and) nestedness, which describes the loss (or gain) of species such that a site with lower species richness harbors a subset of species found in a site with higher species richness (Baselga 2010). Breaking down β-diversity into these two components may improve our descriptions of bark and ambrosia beetle spatial distribution over structural habitat changes such as historical human land use (Marques and Schoereder 2014, Araujo et al. 2015).
Plant diversity has been suggested as the primary mechanism preserving the remarkable diversity of tropical herbivore species (Novotny et al., 2010; Neves et al., 2014). However, plant diversity does not seem to shape bark and ambrosia beetle diversity. These beetles are likely closely associated with the range of suitable wood availability (Grove 2002, Hulcr et al. 2008b), and wood diameter and moisture content seem better predictors of diversity. Hence, an increase in wood and plant species abundance along a gradient of ecological secondary succession should increase resource availability for them (Guariguata and Ostertag 2001; Kalacska et al. 2004; Madeira et al. 2009).
There are few studies reporting an influence of season on ambrosia beetle abundance in Brazil. Furthermore, season is rarely clearly defined, and catches among seasons not statistically compared; when these variables are controlled for some patterns emerge. Although the majority of species are active throughout the year, most species are more abundant in the rainy/warm season, and a smaller number are either more active in the dry/cold season, or show no significant differences in activity between seasons (Dall’Oglio and Peres-Filho 1997, Flechtmann et al. 2001).
In contrast with tropical rainforests, leaf primary production in tropical dry forests is concentrated in the rainy season (Pezzini et al. 2014). There is a demonstrated increase in herbivorous insect activity in this season (Neves et al. 2014), especially for Scarabaeinae beetles (Neves et al. 2010) and butterflies (Neves et al. 2013).
We investigated spatio-temporal dynamics of ambrosia beetles (Curculionidae: Platypodinae and Scolytinae) in tropical dry forests. The main goal of this study was to describe the bark and ambrosia beetle community by measuring temporal climate factors and variation in resource availability over a gradient of ecological secondary succession. We then described mechanisms that determine beetle β-diversity, addressing two questions: 1) Is nestedness more important than species turnover in this group? and 2) does species composition differ between successional stages? We expected beetle richness and abundance to increase over the course of secondary succession, and to find temporal variation in beetle richness and abundance.
Materials and Methods
Study Area
The study was carried out in the Mata Seca State Park, located in the São Francisco River Valley in Manga, northern Minas Gerais state, southeastern Brazil (14°48′36″–14°56′59″ S, 43°55′12″–44°04′12″ W). The park has an area of 15,466 ha, with predominance of seasonal deciduous forest (Madeira et al. 2009). The climate of the region is classified as semi-arid by the Köppen system, with an average temperature of 24.4°C and average annual rainfall of 818 ± 242 mm (Pezzini et al. 2014). Approximately 1,525 ha consist of abandoned pastures in different stages of succession (Madeira et al. 2009).
We categorized sampling sites according to number of years in secondary succession after disturbance, as follows: early = 10 years; intermediate = between 17 and 25 years; and late = over 50 years. The early successional stage was dominated by herbaceous and shrubby plants, with a discontinuous canopy ∼4 m in height. The intermediate stage was composed of trees reaching 10–12 m in height with some emergent trees reaching up to 15 m, with dense understory and with many lianas. The late stage was composed of trees forming a closed canopy 18–20 m in height, with sparse understory with little light penetration and low density of lianas (Madeira et al. 2009).
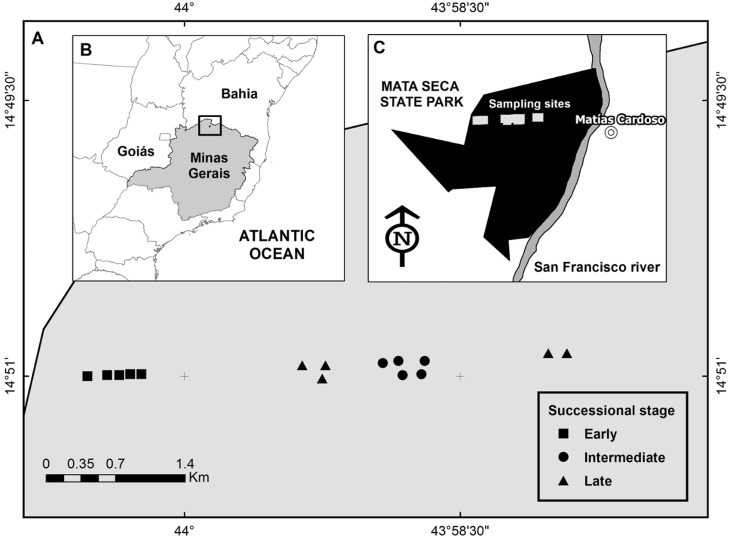
We selected fifteen 20 × 10 m plots, five in each successional stage. Plots were established along a 7 km transect. Distance between plots within each stage varied from 0.2 to 0.8 km, and the distance between plots of different successional stages varied from 0.8 to 6 km (Fig. 1). We sampled the plots ten times from 2009 to 2011, during rainy and dry seasons (Fig. 2). Sampling was carried out in December 2009, February, April, September, and October 2010, and January, April, June, September, and December 2011. The dry season occurs from May to October, at which time ∼90–95% of the tree species shed their leaves (Pezzini et al. 2014).
Fig. 1.
Sampling sites at Mata Seca State Park in southeastern Brazil. Sampling design (A) represents the distribution of successional stages, (B) indicates the location in Brazil, and (C) represents the shape of park.
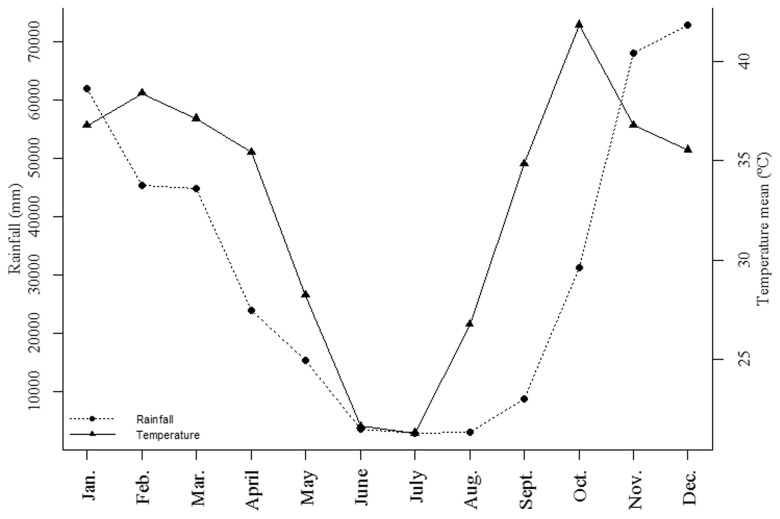
Fig. 2.
Total historical average precipitation (filled circle), and monthly average temperature (filled triangle) from 1976 to 2011. Data from Mocambinho Meteorological Station located 15 km from Mata Seca State Park.
In each site, we measured tree circumference at breast height (CBH), wood-area-index (WAI), and tree abundance (CBH ≥ 15 cm). We measured the WAI using canopy photos taken in the dry season (October 2010). We used one photo per site taken with a digital camera using an 8 mm fisheye lens (Nassar et al. 2008). The photos represented the relative contribution of trunks and branches to canopy structure, and were analyzed using Gap Light Analyzer software (Frazer et al. 1999).
e measured cumulative rainfall, average temperature, and air moisture for each of the 5 d required for each sampling period. We used a wireless net station located in the study area, which measured temperature and moisture every 30 min.
Insect Sampling
We collected beetles using combined flight Malaise/window traps (Basset 1988, Novais et al. 2016). One trap per site (15 total) holding two vial collectors with 70% ethanol were exposed for 5 d (120 h) per month in the tree canopy in the center of each site. Bark and ambrosia beetles are attracted to ethanol released by dead and dying trees. Some species tend to be more attracted than others, thus the trapping technique may be biased towards certain taxonomic group (Hulcr et al., 2008). Specimens were determined based on Wood (2007), and divided into two feeding habitat guilds: bark and ambrosia beetles. We calculated the number of species and specimens for each sampling session. Voucher specimens were deposited in the Museum of Entomology at FEIS/UNESP (MEFEIS, Ilha Solteira, São Paulo state, Brazil).
Data Analysis
We used permutational multivariate analysis of variance (PERMANOVA, Anderson 2001) to evaluate the influence of secondary succession on bark and ambrosia beetle community composition between early, intermediate, and late stages using Jaccard dissimilarity measure with 999 permutations. PERMANOVA is a permutational ANOVA that can test the simultaneous response of one or more variables to one or more factors in the analyses of variance. We perfomed the analysis using the ‘adonis’ procedure in the vegan package in R version 3.2.1.
We tested whether nestedness was the main driver of diversity by decomposing β-diversity (βbetween sites) using the Sørensen (βSOR) and the Simpson (βSIM) indices (Baselga 2010). βSOR represents the total β-diversity and includes both turnover and nestedness. βSIM does not consider differences in species richness, and therefore only represents spatial species replacement, or turnover. Hence, the total species loss due to nestedness (βNES) is given by the difference between those indices (βNES = βSOR − βSIM).
Beetle Diversity Along a Gradient of Resource Availability
The effects of a resource availability gradient on beetle diversity were determined using generalized linear models (GLMs). In these models, the richness and abundance of beetles (bark and ambrosia) were pooled by site from 2009 to 2011, whereas successional stage (early, intermediate, and late) and indicators of resource availability (CBH, WAI, and tree abundance) were used as explanatory variables. The minimal models were constructed by removing non-significant explanatory variables (P > 0.05) from the full models in the analysis. The models were submitted to residual analysis to identify the most suitable error distribution; we used the ‘rdiagnostic’ procedure in the RT4Bio package in R (Crawley 2013).
Temporal Variation
To test how climate conditions influenced temporal variation in beetle richness and abundance, we used a Generalized Linear Mixed Models analysis (lme4 package), assuming temporal pseudoreplication. The explanatory variables. Collection month, accumulated rainfall, average moisture (%), and average temperature (°C) were nested within the random effects of the sites sampled during the study (Bates et al. 2012). All analyses were carried out in the software R (R Development Core Team, 2015).
Results
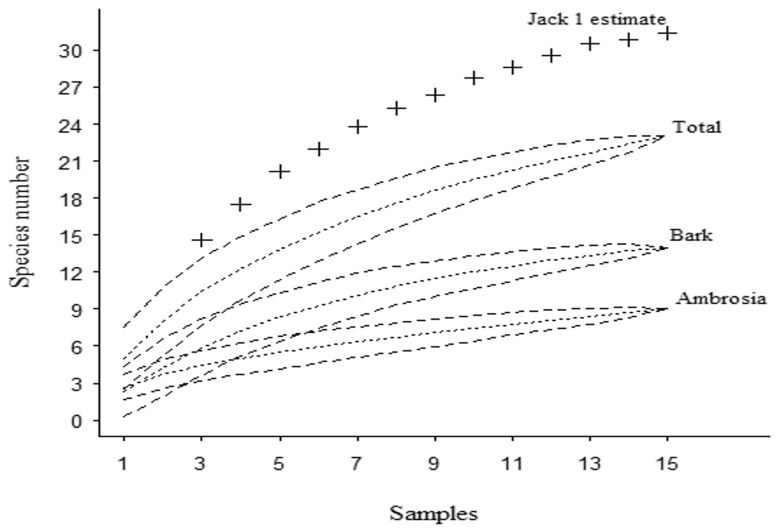
We collected 763 Scolytinae beetles from 23 species across the successional gradient, including 14 bark beetle species and nine ambrosia beetle species. Fifty-eight specimens were bark beetles and 705 specimens were ambrosia beetles. The collected bark beetles included the Cryphalini, Micracini, Phloeosinini and Scolytini tribes, and the Araptus and Cryptocarenus genera. The ambrosia beetles collected included all Xyleborini species, along with Euplatypus parallelus, Corthylus sp.1, and Tricolus affinis (Table 1). The tribe Cryphalini had the highest species richness and abundance among the bark beetle guild, and the tribe Xyleborini had the highest species richness and abundance among the ambrosia beetle guild. E. parallelus was the only species of the subfamily Platypodinae, with 12 specimens. Xyleborus affinis was the only species sampled in all sites and sampling periods, representing 87% of the total beetle abundance. In general, most species were considered rare; 15 species had fewer than 3 individuals among sites and sampling periods, and only 1 individual was found for 8 species (four species per guild) (Table 1). The species accumulation curve did not approach an asymptote for the complete data set or for guilds, indicating the need for further sampling to arrive at a reliable estimate of α diversity. However, the curve was approaching the Jackknife estimate of 31 species (Fig. 3), suggesting that a majority of local species were represented in the analysis.
Table 1.
List of ambrosia beetles collected in the Mata Seca State Park in southeastern Brazil
| Taxa | Genus/Species | FP | Early | Inter. | Late | Total |
|---|---|---|---|---|---|---|
| Scolytinae | ||||||
| Corthylini | Araptus sp.1 | 1 | 0 | 1 | 0 | 1 |
| Corthylus sp.1 | 1 | 1 | 0 | 0 | 1 | |
| Cryptocarenus diadematuEggers | 1 | 0 | 1 | 1 | 2 | |
| Cryptocarenus heveae (Hagedorn) | 2 | 0 | 1 | 1 | 2 | |
| T. affinis Eggers | 4 | 0 | 2 | 2 | 4 | |
| Cryphalini | Hypothenemus areccae Weswood | 1 | 0 | 0 | 1 | 1 |
| Hypothenemus eruditus Weswood | 2 | 0 | 2 | 0 | 2 | |
| Hypothenemus plumeriae (Nordlinger) | 3 | 2 | 3 | 0 | 5 | |
| Hypothenemus sp.1 | 2 | 0 | 2 | 0 | 2 | |
| Pityophthorus sp.1 | 6 | 0 | 3 | 3 | 6 | |
| Pityophthorus sp.2 | 6 | 0 | 16 | 3 | 19 | |
| Pityophthorus sp.3 | 3 | 0 | 1 | 2 | 3 | |
| Pityophthorus sp.4 | 1 | 0 | 0 | 1 | 1 | |
| Micracini | Hylocurus sp.1 | 3 | 0 | 2 | 4 | 6 |
| Phloeosinini | Pseudochramesus acuteclavatus (Hagedorn) | 1 | 0 | 1 | 0 | 1 |
| Scolytini | Scolytopsis sp.1 | 4 | 1 | 6 | 0 | 7 |
| Xyleborini | Cnestus retusus (Eichhoff) | 6 | 3 | 7 | 10 | 20 |
| Dryocoetoides sp.1 | 1 | 0 | 0 | 1 | 1 | |
| X. affinis Eichhoff | 10 | 152 | 223 | 288 | 663 | |
| Xyleborus ferrugineus (F.) | 1 | 0 | 0 | 1 | 1 | |
| Xyleborus spinulosus Blandford | 1 | 1 | 0 | 0 | 1 | |
| Xylosandrus curtulus (Eichhoff) | 1 | 0 | 1 | 1 | 2 | |
| Platypodinae | ||||||
| Platypodini | E. parallelus (F.) | 5 | 2 | 4 | 6 | 12 |
Frequency per sample period (FP, n = 10), and abundance per successional stage are reported for each species.
Fig. 3.
Species accumulation and an estimate of total species richness at Mata Seca State Park for the complete data set, and for bark and ambrosia beetle species separately. The dotted lines represent the analytically derived species accumulation curve, and dashed lines represent the 95% confidence interval. The symbol ‘+’ represents first order jackknife in abundance-based estimates using the ‘poolaccum’ procedure in the vegan package in R.
The sites in early successional stage had lower CBH (Poisson: Deviance [2.12] = 32.42, P < 0.001) and WAI (Quasi-Poisson: Deviance [2.12] = 0.21, P = 0.007) than did sites at intermediate and late successional stages. Tree abundance was similar between successional stages (Negative binomial: Deviance [2.12] = 2.84, P = 0.24) (Table 2).
Table 2.
Mean values (mean ± SE) of the resource CBH, WAI, and tree abundance, in 20 × 10 m plots in three successional stages in a Brazilian tropical dry forest
| Successional stage | CBH | WAI | Tree abundance |
|---|---|---|---|
| Early | 20.1 ± 2.80a | 0.246 ± 0.04a | 20.4 ± 5.58a |
| Intermediate | 40.6 ± 4.09b | 0.348 ± 0.02b | 16.0 ± 1.05a |
| Late | 47.3 ± 1.69b | 0.412 ± 0.02b | 23.2 ± 1.77a |
GLMs were used, and the different letters represent statistically different means (P < 0.05).by aggregating levels in a contrast analysis.
Beetle Composition Over the Course of Ecological Secondary Succession
Similarity analysis of bark beetle composition within three categories could not be assessed due to absence of species at three of the five early stage sites. However, bark beetle composition was similar between the intermediate and the late stages (PERMANOVA r2 = 0.11, P = 0.35). No species were present in all three stages. The intermediate and early stages shared two species, and intermediate and late stages shared six species. There were no species found in both early and late stages. Two species were exclusive to the late stage and four to the intermediate stage, with no bark beetle found exclusively in the early stage. Ambrosia beetle composition was similar among successional stages (PERMANOVA r2 = 0.11, P = 0.64). Three ambrosia species were common to all stages: X. affinis, Xyleborus retusus, and E. parallelus. The intermediate stage had two species in common with the late stage and none in common with the early stage, despite having three species that were common in all stages. Finally, two species were exclusive to early stages and another two exclusive to late stages, with no species exclusive to intermediate stages (Table 1).
Decomposition of β-Diversity
Decomposition of β-diversity revealed species turnover between sites as the main driver of the βSOR diversity for both bark (βSIM = 0.77; βSOR = 0.90), and ambrosia (βSIM = 0.57, βSOR = 0.76) beetles, representing 85.6% of the bark beetle β diversity and 74.4% of the ambrosia beetle β diversity.
Beetle Diversity Along a Gradient of Resource Availability
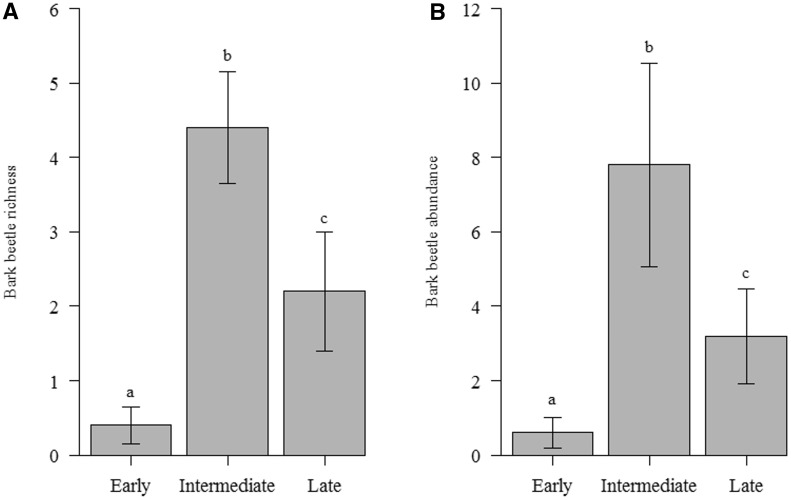
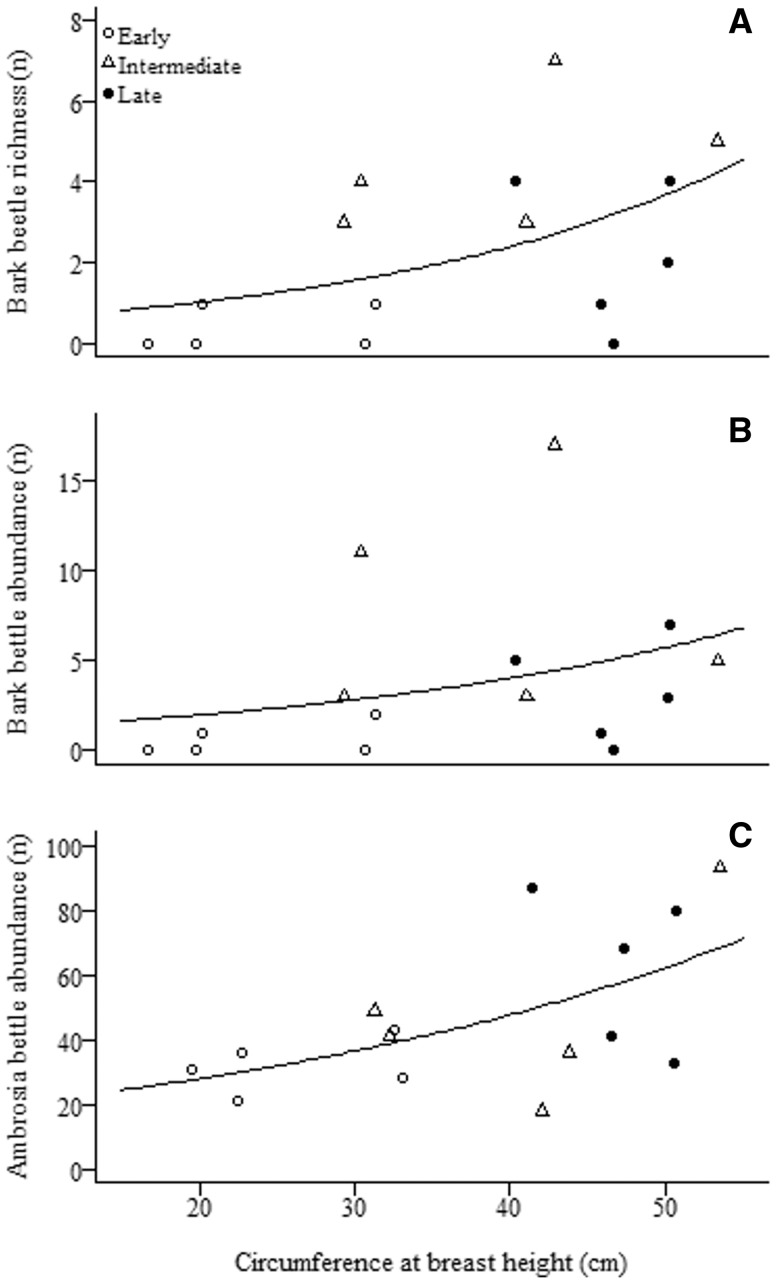
We found higher bark beetle richness and abundance at intermediate successional stages, followed by the late and the early stages, respectively (Fig. 4). We also detected a positive relationship between bark beetle richness and abundance and tree CBH (Table 3; Fig. 5A and B). Ambrosia beetles increased in abundance (but not richness) with increased tree CBH (Table 3; Fig. 5C).
Fig. 4.
Bark beetle (Curculionidae) richness (A) and abundance (B) (mean ± SE) in sites at different successional stages in the Mata Seca State Park in southeastern Brazil. Different letters above the columns represent statistically different means (P < 0.05).
Table 3.
Analysis of deviance of the minimal adequate models showing the effects of successional stage and resource CBH on beetle richness and abundance in a Brazilian tropical dry forest
| Response variable | Explanatory variable | df | Deviance | P |
|---|---|---|---|---|
| Bark beetle richness | CBH | 1 | 25.99 | 0.007 |
| Successional stage | 2 | 13.16 | 0.001 | |
| Bark beetle abundance | CBH | 1 | 28.64 | 0.036 |
| Successional stage | 2 | 16.13 | 0.001 | |
| Ambrosia beetle abundance | CBH | 1 | 15.12 | 0.005 |
Fig. 5.
Effect of tree CBH on bark beetle richness (A), bark beetle abundance (B) and ambrosia beetle abundance (C) in the Mata Seca State Park in southeastern Brazil.
Temporal Variation
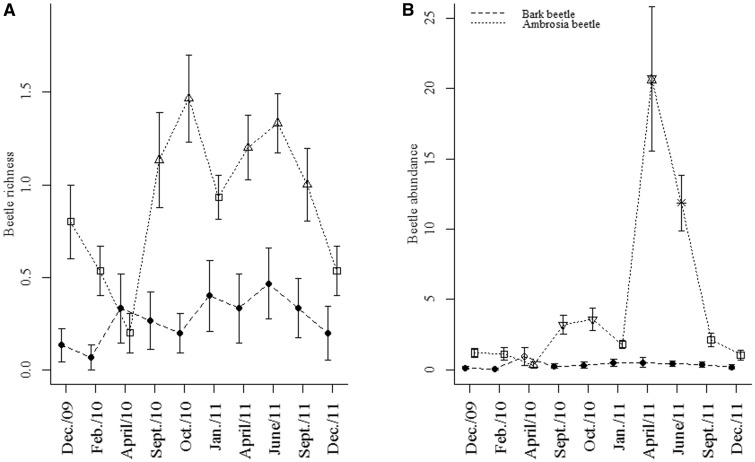
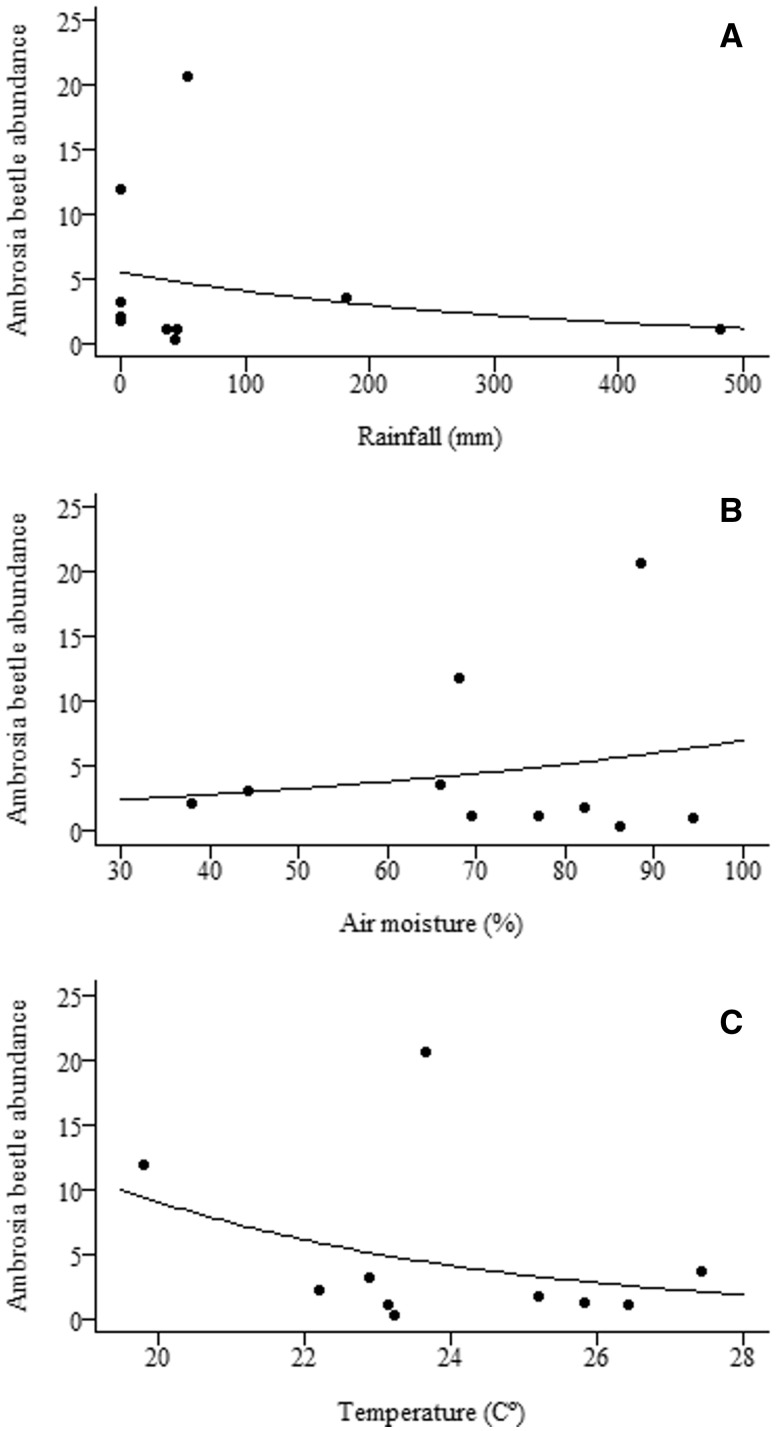
Bark beetle richness showed no variation among months, and bark beetle abundance was higher only in April 2010 (Fig. 6). Climate measures did not affect bark beetle richness and abundance (Table 4). Although moisture almost reached statistical significance, on explaining the bark beetles abundance increase (Table 4). Ambrosia beetle richness was higher in September and October of 2010, and in April, June, and September of 2011, compared with samples from December, February, and April of 2010, and January and December of 2011 (Fig. 6A). Overall the driest months were richer than the rainy months, and no relationships were found between climate measures and ambrosia beetle richness (Table 4). Ambrosia beetle abundance was higher in September and October of 2010, with peaks in activity in April and June 2011; these sample periods together yielded 69% of the total abundance (Fig. 6B). We found negative relationships between monthly variation in ambrosia beetle abundance and increased rainfall and temperature, and a positive relationship between variation in abundance and increased air moisture (Table 4; Fig. 7).
Fig. 6.
Bark and ambrosia beetle (Curculionidae) richness (A) and abundance (B) (mean ± SE) during two years of sampling in the Mata Seca State Park in southeastern Brazil. Different symbols represent statistically different means (P < 0.05).
Table 4.
Results of linear mixed effects model, showing the temporal variation and effects of the rainfall, moisture, and temperature on bark and ambrosia beetle richness and abundance
| Response variable | Explanatory variable | d.f. | AIC (H1) | AIC (H0) | P |
|---|---|---|---|---|---|
| Bark beetle richness | Month | 9 | 205.76 | 196.14 | 0.497 |
| Rainfall | 1 | 198.58 | 197.87 | 0.254 | |
| Moisture | 1 | 200.73 | 198.58 | 1.000 | |
| Temperature | 1 | 199.41 | 200.73 | 0.068 | |
| Bark beetle abundance | Month | 9 | 252.93 | 256.75 | 0.009 |
| Rainfall | 1 | 256.88 | 256.75 | 0.172 | |
| Moisture | 1 | 255.12 | 256.88 | 0.052 | |
| Temperature | 1 | 253.92 | 255.12 | 0.073 | |
| Ambrosia beetle richness | Month | 9 | 343.11 | 352.07 | 0.001 |
| Rainfall | 1 | 352.82 | 352.07 | 0.264 | |
| Moisture | 1 | 352.14 | 352.82 | 0.101 | |
| Temperature | 1 | 354.13 | 352.14 | 0.931 | |
| Ambrosia beetle abundance | Month | 9 | 778.19 | 1668.59 | <0.001 |
| Rainfall | 1 | 746.58 | 750.41 | 0.015 | |
| Moisture | 1 | 746.58 | 754.54 | 0.001 | |
| Temperature | 1 | 746.58 | 752.97 | 0.003 |
Significance was estimated by ANOVA comparing complete (H1) and null models (H0). The Akaike’s information criterion (AIC) represents the uncertainty of the model whereby lower AIC values represent the more parsimonious models.
Fig. 7.
Effect of rainfall (A), air moisture (B), and temperature (C) on ambrosia beetle abundance in the Mata Seca State Park in southeastern Brazil.
Discussion
Studies of variation in composition, richness and abundance of beetles in relation to spatial factors and climatic conditions are unprecedented in Brazilian dry forests. This study revealed a total species richness lower than has been found in to other dry forests in Mexico (Atkinson and Equihua 1986), and equal to some other Brazilian forest biomes (Amazon and Atlantic forest) (Müller and Andreiv 2004, Abreu et al. 2012). Bark beetle richness (14 species) was greater than ambrosia beetle richness (9 species); this result may have been affected by the low release of ethanol during sampling because ethanol tends to attract a greater number of bark beetles (Hulcr et al. 2008b). However, the dominance of phloeophagous species is a pattern more common in Mexican dry forest than in Brazilian rainforests (Atkinson and Equihua 1986, Müller and Andreiv 2004, Abreu et al. 2012). Phloeophagy as a dominant feeding habit also occurs in temperate areas, while tropical rainy areas show the opposite trend, with a greater proportion of ambrosia species typically found (Beaver 1979, Hulcr 2007, Abreu et al. 2012). Atkinson and Equihua (1986) reported that the relative low importance of ambrosia beetles in dry forests may be associated with the long dry season, which may limit growth of fungi on wood; this suggestion is supported in this study. Although other factors like species interactions are surely involved (Beaver 1979).
We found similar species compositions with the advancement of secondary succession, confirming the independence of bark and ambrosia beetles with respect to local tree community composition (Beaver 1979, Hulcr et al. 2007). However, we found that turnover is the primary driver of beta diversity for both guilds, and turnover was higher in bark beetle communities. These results suggest that despite low host-specificity, site factors may determine the local species pool (mainly in the bark beetle community).
Bark beetle richness and abundance was higher in intermediate stage sites, followed by late and early stages, respectively. We found no between-stage differences in ambrosia beetle richness or abundance, likely due to the different strategies of obtaining and using food resources among feeding guilds (Hulcr et al. 2007). Overall, the degree of host specificity is higher in bark beetle species, which favor monophagy (i.e., restricted to one plant genus) rather than ambrosia species, which tend to favor polyphagy (i.e., several host families) (Atickson and Equihua 1986). Our results are analogous to the pattern observed among free-living herbivorous guilds, where the guilds more closely associated with their host plant traits (e.g., as in sap-sucking insects) were sensitive to successional stage while the more generalist (chewer) insects were not (Neves et al. 2014).
Among the structural differences between stages, an increase in tree CBH was the main mechanism leading to higher diversity, richness and abundance of bark beetles, and increased abundance of ambrosia beetles. These results suggest that size of wood affects mainly the bark beetle guild. Large diameter is an important factor for wood inhabitants due to increased probability of finding resource in a suitable state for colonization along temporal shifts in spatial parameters (Grove 2002). Wood availability is thus important for rare bark beetle species, and it is not surprising that ambrosia species do not follow these patterns because their foraging strategy is closer to detritivory than to herbivory (Hulcr et al. 2007). For ambrosia beetles, Grove’s (2002) statement with respect to saproxylic beetles that ‘all wood is good, but bigger is better’ seems to fit our results.
X. affinis was the dominant species in the study area, a consistent pattern among ambrosia beetles in other studies (Flechtmann et al. 1995, Abreu 2012). This species has circumtropical distribution, and has been reported to feed on more than 300 plant species. It is considered of high importance among the ambrosia species due to attacks on Eucalyptus and Pine stands in Brazil and other tropical regions (Flechtmann 1995, Beaver 1988). We demonstrated in this study that X. affinis also has a strong preference for the Mata Seca State Park, a result in concordance with its high tolerance for dry and disturbed areas (Hulcr et al 2008b).
Temporal factors did not affect bark beetles richness, and abundance was only higher in April 2010. Climate factors did not affect guild richness and abundance. Most of the bark beetle species were trapped in very low numbers, and even the groups of species reported to occupy dry material such as Hypothenemus (Hulcr et al. 2008b) were found in low abundance. This could be a sampling artifact due to trap bias; however, occurrence was unaffected by seasonality in other studies as well (Hulcr et al. 2008b, Abreu et al. 2012). Our results suggest that the effect of climate seasonality on bark beetle communities is lower than that of spatial differences between sites.
In contrast, the ambrosia guild showed higher richness in the drier month (June) and at the end of the dry and wet seasons, and no relationships between community traits and climate factors. The periods of higher flight activity reflect the emergence of adults to colonize new, suitable hosts (Wood 1982). According to Wood (1982), these hosts usually bear senescent leaves that produce attractive substances. The months with increased percentage of senescent leaves in the study area are the same months with higher richness of ambrosia beetles (Pezzini et al. 2014). Thus it is likely that host attractiveness determines ambrosia richness fluctuations. Further studies are necessary to verify the consistency of the described pattern.
The relatively constant environmental conditions of tropical rainforests allow year-round beetle activity, and thus there is no clear dispersal pattern for these species (Hulcr et al. 2008a, Abreu et al. 2012). In tropical dry forests, the abrupt changes among seasons may lead ambrosia beetles to higher colonization success due to better conditions for fungal growth.
The fluctuations in species abundance decrease with higher rainfall and in warmer months, and increase with higher air moisture. Dispersal from the parental gallery to a new host plant/gallery imposes high energy costs for ambrosia beetles, as the cultivation of symbiotic fungi frequently fails (Biedermann et al. 2011, Iidzuka et al. 2014). Our results show that peaks in abundance are closely followed by an additional peak, as in April and June 2011. This pattern of emergence has been observed in X. affinis (Biedermann et al. 2011), the most abundant species in this study. Those peaks in dispersal occurred on days with lower rainfall and higher air moisture, with temperature varying from 20 to 23°C. Other studies showed that the flight activity of Scolytinae beetles is stimulated by light and high temperature, varying from 20 to 40°C (Wood 1982), and that a temperature around 30°C seems to be optimal for ontogenetic stages of development (Walgama and Zalucki 2007). Rainfall usually negatively affects Scolytinae flight (McMullen and Atkins 1962, Moser and Dell 1979), whereas the decreased rain in the end of the dry season sufficiently maintains air moisture to avoid heat exposure and desiccation (Wardhaugh 2014). Hence, the sampling periods characterized by hot days, but high enough humidity to avoid desiccation represent favorable abiotic conditions for beetle flight and selection of new hosts.
Conclusions
The data presented earlier describe a diverse array of spatio-temporal distributions among bark and ambrosia beetle guilds. The spatial and the temporal factors have mixed contributions to shaping the observed patterns, and the main differences among guilds reflect their use of resources. In summary, bark beetles are more sensitive to wood content along the secondary succession than are ambrosia beetles, and they have higher density at sites with greater availability of wood. Further, climate factors were effectively non-seasonal for bark beetles and seasonal for ambrosia beetles. Species accumulation was higher in bark beetle species over time, while factors favorable for fungal growth, such as host and air moisture. Resulted in a broad spatial distribution of ambrosia species and a greater degree of species redundancy over time. Detecting spatial and temporal patterns in beetles and insects is not an easy task, and we highlight interspecific interactions of these beetles as a focus for further investigations. The overall biological pattern among guilds differed from that of tropical rainforests, showing patterns similar to other dry forest areas.
Acknowledgments
We are grateful to the staff of the Instituto Estadual de Florestas (IEF-MG) and ICMBIO for allowing us to work at the Mata Seca State Park (MSSP), and for logistical support. This work was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), and the Inter-American Institute for Global Change Research (IAI-CRN II-021). This study was in partial fulfillment of requirements for the PhD degree at Universidade Federal de Minas Gerais.
References Cited
- Abreu R.L.S., Ribeiro G. A., Vianez B. F., Sales-Campos C. 2012. Insects of the subfamily scolytinae (Insecta: Coleoptera, Curculionidae) collected with pitfall and ethanol traps in primary forests of central amazonia. Psyche A J. Entomol. 2012: 1–8. [Google Scholar]
- Anderson M. J. 2001. A new method for non parametric multivariate analysis of variance. Austral Ecol. 26: 32–46. [Google Scholar]
- Araujo L. S., Komonen A., Lopes-Andrade C. 2015. Influences of landscape structure on diversity of beetles associated with bracket fungi in Brazilian Atlantic Forest. Biol. Conserv. 191: 659–666. [Google Scholar]
- Atkinson T. H., Equihua A. 1986. Biology of the Scolytidae and Platypodidae (Coleoptera) in a tropical decuduous forest at Chamela, Jalisco, Mexico. Fla. Entomol 69: 303–309. [Google Scholar]
- Beaver R. A. 1988. Biological studies on ambrosia beetles of the Seychelles (Col., Scolytidae and Platypodidae). J. Appl. Ent. 105: 62–73. [Google Scholar]
- Beaver R. A. 1979. Host specificity of temperate and tropical animals. Nature 281:139–141 [Google Scholar]
- Baselga A. 2010. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19: 134–143. [Google Scholar]
- Basset Y. 1988. A composite interception trap for sampling arthropods in tree canopies. Aust. J. Entomol. 27: 213–219. [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. [Google Scholar]
- Biedermann P.H.W., Klepzig K. D., Taborsky M. 2011. Costs of delayed dispersal and alloparental care in the fungus-cultivating ambrosia beetle Xyleborus affinis Eichhoff (Scolytinae: Curculionidae). Behav. Ecol. Sociobiol. 65: 1753–1761. [Google Scholar]
- Calderón-Cortés N., Quesada M., Escalera-Vázquez L. H. 2011. Insects as stem engineers: Interactions mediated by the twig-girdler oncideres albomarginata chamela enhance arthropod diversity. PLoS One 6: e19083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley M. J. 2013. The R book. Edited by Crawley Michael J. 2nd ed. John Wiley & Sons, Chichester. [Google Scholar]
- Dall’Oglio O. T., Peres-Filho O. 1997. Survey and populational dinamic of borers in homogeneous plantations of rubber trees in Itiquira - MT, Brazil. Sci. For. 51: 49–58. [Google Scholar]
- Feller I. C., Mathis W. N. 1997. Primary herbivory by wood-boring insects along an architectural gradient of rhizophora mangle. Biotropica 29: 440–451. [Google Scholar]
- Flechtmann C.A.H., Couto H.T.Z, Gaspareto C.L., Berti Filho E. 1995. Scolytidae em reflorestamento com pinheiros tropicais. IPEF, Piracicaba. [Google Scholar]
- Flechtmann C.A.H., Ottati A.L.T., Berisford C. W. 2001. Ambrosia and bark beetles (Scolytidae: Coleoptera) in pine and eucalypt stands in southern Brazil. For. Ecol. Manage. 142: 183–191. [Google Scholar]
- Frazer G., Canham C., Lertzman K. 1999. Gap Light Analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Program. Copyright © 1999: Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York. doi:citeulike-article-id:4887176. [Google Scholar]
- Grove S. J. 2002. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Syst. 33: 1–23. [Google Scholar]
- Guariguata M R, Ostertag R. 2001. Neotropical secondary forest succession: changes in structural and functional characteristics. For. Ecol. Manage. 148: 185–206. [Google Scholar]
- Hulcr J., Novotny V., Maurer B. A., Cognato A. I. 2008a. Low beta diversity of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) in lowland rainforests of Papua New Guinea. Oikos 117: 214–222. [Google Scholar]
- Hulcr J., Beaver R. A, Puranasakul W., Dole S. A, Sonthichai S. 2008b. A comparison of bark and ambrosia beetle communities in two forest types in northern Thailand (Coleoptera: Curculionidae: Scolytinae and Platypodinae). Environ. Entomol. 37: 1461–1470. [DOI] [PubMed] [Google Scholar]
- Hulcr J., Mogia M., Isua B., Novotny V. 2007. Host specificity of ambrosia and bark beetles (Col., Curculionidae: Scolytinae and Platypodinae) in a New Guinea rainforest. Ecol. Entomol. 32: 762–772. [Google Scholar]
- Iidzuka H., Goto H., Yamasaki M., Osawa N. 2014. Ambrosia beetles (Curculionidae: Scolytinae and Platypodinae) on Fagus crenataBlume: community structure, seasonal population trends and resource utilization patterns. Entomol. Sci. 17: 167–180. [Google Scholar]
- Kalacska M., Sanchez-azofeifa G. A., Calvo-alvarado J. C., Quesada M. 2004. Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manage. 200: 227–247. [Google Scholar]
- Madeira B. G., Espírito-Santo M. M., Neto S. D., Nunes Y.R.F., Sanchez-Azofeifa G.A., Fernandes G. W., Quesada M. 2009. Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecol. 2: 291–304. [Google Scholar]
- Marques T., Schoereder J. H. 2014. Ant diversity partitioning across spatial scales: Ecological processes and implications for conserving Tropical Dry Forests. Aust. Ecol. 39: 72–82. [Google Scholar]
- McMullen L. H., Atkins M. D. 1962. The Life History and Habits of Scolytus unispinosus Leconte (Coleoptera: Scolytidae) in the Interior of British Columbia. Can. Entomol. 94: 17–25. [Google Scholar]
- Moser J. C., Dell T. R. 1979. Predictors of Southern pine beetle flight activity. For. Sci. 25: 217–222. [Google Scholar]
- Müller J. A., Andreiv J. 2004. Caracterização da família scolytidae (insecta: coleoptera) em três ambientes florestais. CERNE 10: 39–45. [Google Scholar]
- Nassar J.M., Rodríguez J.P., Sánchez-Azofeifa A., Garvin T., Quesada M. 2008. Manual of methods: human, ecological and biophysical dimensions of tropical dry forests. Caracas, Venezuela. [Google Scholar]
- Neves F. S., Silva J. O., Espírito-Santo M. M., Fernandes G. W. 2014. Insect herbivores and leaf damage along successional and vertical gradients in a tropical dry forest. Biotropica 46: 14–24. [Google Scholar]
- Neves F. S., Silva J. O., Marques T., Mota-Souza J., Madeira B., Espírito-Santo M. M., Fernandes G. W. 2013. Spatiotemporal dynamics of insects in a brazilian tropical dry forest, pp. 225–239. In Sánchez-Azofeifa A., Powers J. S., Fernandes G. W., Quesada M. (eds.), Tropical dry forests in the Americas. CRC Press, New York. [Google Scholar]
- Neves F. S., Oliveira V.H.F., do Espírito-Santo M. M., Vaz-de-Mello F. Z., Louzada J., Sanchez-Azofeifa A., Fernandes G. W. 2010. Successional and seasonal changes in a community of dung beetles (Coleoptera: Scarabaeinae) in a Brazilian tropical dry forest. Nat. Conserv. 8: 160–164. [Google Scholar]
- Novais S.M.A., Macedo-Reis L. E., DaRocha W. D., Neves F. S. 2016. Effects of habitat management on different feeding guilds of herbivorous insects in cacao agroforestry systems. Int. J. Trop. Biol. Conserv. 64(1). [DOI] [PubMed] [Google Scholar]
- Novotny V., Miller S. E., Baje L., Balagawi S., Basset Y., Cizek L., Craft K. J., et al. 2010. Guild-specific patterns of species richness and host specialization in plant-herbivore food webs from a tropical forest. J. Anim. Ecol. 79: 1193–1203. [DOI] [PubMed] [Google Scholar]
- Novotny V., Miller S. E, Hulcr J., Drew R.A.I., Basset Y., Janda M., Setliff G. P, et al. 2007. Low beta diversity of herbivorous insects in tropical forests. Nature 448: 692–5. [DOI] [PubMed] [Google Scholar]
- Pezzini F. F., Ranieri B. D., Brandão D. O., Fernandes G. W., Quesada M., Espírito-Santo M. M., Jacobi C. M. 2014. Changes in tree phenology along natural regeneration in a seasonally dry tropical forest. Plant Biosyst. 148: 1–10. [Google Scholar]
- R Development Core Team. 2015. R: A language and environment for statistical computing. Version 3.1.1. User’s guide and application published: http://www.R-project.org.
- Seibold S., Bässler C., Brandl R., Gossner M. M., Thorn S., Ulyshen M. D, Müller J. 2015. Experimental studies of dead-wood biodiversity — a review identifying global gaps in knowledge. Biol. Conserv. 191: 139–149. [Google Scholar]
- Walgama R. S., Zalucki M. P. 2007. Temperature-dependent development of Xyleborus fornicatus (Coleoptera: Scolytidae), the shot-hole borer of tea in Sri Lanka: Implications for distribution and abundance. Insect Sci. 14: 301–308. [Google Scholar]
- Wardhaugh C. W. 2014. The spatial and temporal distributions of arthropods in forest canopies: uniting disparate patterns with hypotheses for specialisation. Biol. Rev. 89: 1021–1041. [DOI] [PubMed] [Google Scholar]
- Wood Stephen L. 1982. The bark and Ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. 6th ed. Harvard University, Cambridge. [Google Scholar]
- Wood S.L. 2007. Bark and Ambrosia beetles of South America (Coleoptera: Scolytidae). Monte L. Bean Life Science Museum, Brigham Young University. [Google Scholar]