Abstract
Purpose
To investigate the feasibility of high temporal resolution quantitative perfusion imaging of bladder tumors performed simultaneously with conventional multi-phase MR urography (MRU) using a novel free-breathing continuously acquired radial MRI sequence with compressed-sensing reconstruction. Methods: 22 patients with bladder lesions underwent MRU using GRASP (Golden-angle RAdial Sparse Parallel) acquisition. Multi-phase contrast-enhanced abdominopelvic GRASP was performed during free-breathing (1.4 × 1.4 × 3.0 mm3 voxel size; 3:44 min acquisition). Two dynamic datasets were retrospectively reconstructed by combining different numbers of sequentially acquired spokes into each dynamic frame: 110 spokes per frame for 25-s temporal resolution (serving as conventional MRU for clinical interpretation) and 8 spokes per frame for 1.7-s resolution. Using 1.7-s resolution images, ROIs were placed within bladder lesions and normal bladder wall, a femoral artery arterial input function was generated, and the Generalized Kinetic Model was applied.
Results
Biopsy/cystectomy demonstrated 16 bladder tumors (13 stage ≥ T2, 3 stage ≤ T1) and 6 benign lesions. All lesions were well visualized using 25-s clinical multi-phase images. Using 1.7-s resolution images, Ktrans was significantly higher in tumors (0.38 ± 0.24) than normal bladder (0.12 ± 0.02 = 8, p b 0.001) or benign lesions (0.15 ± 0.04, p = 0.033). Ratio between Ktrans of lesions and normal bladder was nearly double for tumors than benign lesions (4.3 ± 3.4 vs. 2.2 ± 1.6), and Ktrans was nearly double in stage ≥ T2 than stage ≤ T1 tumors (0.44 ± 0.24 vs. 0.24 ± 0.24), although these did not approach significance (p = 0.180–0.209), possibly related to small sample size.
Conclusion
GRASP allows simultaneous quantitative high temporal resolution perfusion of bladder lesions during clinical MRU examinations using only one contrast injection and without additional scan time.
Keywords: MRI techniques, MR urography, Novel techniques, Bladder cancer
1. Introduction
Bladder cancer is a common disease, although it exhibits heterogeneous biologic behavior and prognosis [1]. A growing spectrum of treatment options has emerged, including various combinations of partial and radical surgical intervention, radiation, intravesical pharmacologic therapy, various systemic adjuvant and neo-adjuvant chemotherapy regimens, and systemic immunotherapy [2–8]. While histologic findings from biopsy serve as primary determinants of risk [1], biopsy is invasive, prone to sampling error, and remains an incomplete predictor of treatment response and other clinical outcomes [9,10]. Therefore, additional non-invasive biomarkers may be useful. Molecular markers of angiogenesis are altered in bladder cancer and have been shown to be associated with bladder cancer stage, lymphovascular invasion, and nodal metastases, as well as to serve as independent predictors of post-treatment recurrences and mortality [11]. These observations raise the possibility that imaging-based measures of tumor vascularity may likewise serve as useful predictors of bladder cancer aggressiveness. For instance, Tuncbilek et al. observed associations between perfusion metrics obtained using dynamic contrast-enhanced (DCE) MRI and tumor grade likelihood of recurrence [12], while Nguyen et al. observed DCE-MRI based perfusion metrics to be associated with chemotherapy response [13]. However, despite these promising preliminary studies, the available peer-reviewed literature evaluating quantitative imaging-based perfusion metrics in bladder cancer remains highly limited, and this technique is rarely applied in clinical practice.
The paucity of studies of quantitative perfusion of bladder cancer in part relates to the challenge of implementing this technique within the context of standard imaging protocols. This challenge results from inherent trade-offs in MRI between temporal resolution, spatial resolution, and anatomic coverage, as well as the limits placed on all of these factors when performing breath-hold acquisitions [14]. From a clinical perspective, bladder cancer is routinely imaged as part of a full evaluation of both the upper and lower urothelial tracts as well as a staging examination. This scheme entails dynamic contrast-enhanced large-volume high spatial-resolution imaging of both the abdomen and pelvis to allow detection of small urothelial lesions as well as of small lymph nodes or visceral metastases, with breath-holding used for the individual multi-phase time-points. Such an approach greatly limits the ability to perform high temporal resolution imaging of the bladder as a basis for robust perfusion quantification. Indeed, as multi-phase acquisitions of the abdomen and pelvis are typically obtained in an alternating fashion between the abdominal and pelvic stations, continuous imaging of the bladder is generally not possible as part of such an examination, precluding a sophisticated pharmacokinetic assessment. While an alternate, targeted scheme may be to solely perform rapid dynamic imaging of the bladder, such a method may have limited clinical usefulness given the incomplete anatomic evaluation. A final option may be to perform separate high spatial resolution large-volume abdominopelvic multi-phase acquisitions and high temporal resolution acquisitions of the bladder. However, this scheme not only would require two separate contrast injections and greater scanner time, but would be of limited effectiveness as residual excreted contrast throughout the urinary system from the initial injection would greatly obscure further evaluation following the second injection.
This challenge may be addressed through the use of a novel free-breathing MRI sequence with compressed sensing reconstruction, termed golden-angle radial sparse parallel (GRASP) [15]. With GRASP, the raw k-space data are acquired in a continuous fashion without predetermined temporal resolution, allowing for retrospective reconstruction at variable temporal resolutions of interest [14]. In particular, both high spatial resolution multi-phase data sets, analogous to those used for conventional MR urography, as well as high temporal resolution data sets to support robust quantitative perfusion assessment, may be reconstructed from the same continuous GRASP acquisition performed using a single contrast injection. This newly described technique may greatly facilitate the further evaluation and application of quantitative perfusion of bladder cancer within the context of conventional clinical MR urography examinations. Therefore, the objective of this study is to investigate the feasibility of high temporal resolution quantitative perfusion imaging of bladder tumors performed simultaneously with conventional multi-phase MR urography using the GRASP technique.
2. Methods
2.1. Patients
This retrospective study was HIPAA compliant and approved by our institutional review board, which waived the requirement for written informed consent. We searched a departmental database to identify 112 consecutive MR urograms that included DCE acquired using radial free-breathing GRASP acquisition. GRASP has been included within the routine MR urography protocol at our institution since October 2014. Of the initially identified examinations, 26 cases were identified in which a bladder lesion was present and a reference standard was available (cystoscopy or cystectomy/ cystoprostatectomy). Cases were then excluded for the following reasons: repeat patient (n = 2); lesion involved a neobladder (n =2). These exclusions left a final included cohort of 22 patients (18 men, 4 women; average age 76 ± 9 years).
2.2. MRI technique
MRI was performed using a whole-body 1.5 T or 3 T clinical system (Siemens Healthcare GmbH, Erlangen, Germany) and a pelvic phased-array coil. Our institution's standard MR urography protocol was performed, which includes multi-planar multi-sequence imaging of the upper and lower tracts. Pre-contrast sequences of the upper and lower tracts included axial 2D in-and-out-of-phase gradient-echo T1-weighted, axial echo-planar-imaging diffusion-weighted, and axial 3D fat-suppressed gradient-echo T1-weighted acquisitions. Additional sequences of the upper tract included axial and coronal 2D half-Fourier acquisition single-shot turbo-spin echo (HASTE) acquisition; additional sequences of the lower tract included axial and sagittal turbo spin-echo T2-weighted acquisition. These sequences were not further evaluated as part of this study.
Dynamic contrast-enhanced imaging was performed continuously during free-breathing using the GRASP scheme, with volumetric coverage of the kidneys, ureters, and bladder. GRASP comprises a fat-suppressed radial “stack-of-stars” 3D FLASH acquisition and was performed using the following parameters: TR/TE 3.48/1.53 ms, flip angle 12, slice thickness 3 mm, 128 slices, FOV 350 × 350 mm, matrix 256 × 256, voxel size 1.4 × 1.4 × 3.0 mm, 1000 radial spokes, total acquisition time 3:44 min. All patients received an intravenous injection of 0.1 mmol/kg of Gadavist (Gadobutrol) via power injector at a rate of 3 cm3/s, followed by a 20 cm3 saline flush at the same rate. The injection was initiated ten seconds following the start of the continuous GRASP acquisition. Following completion of the continuous GRASP acquisition, a delayed excretory-phase coronal urogram was acquired, although was also not further evaluated as part of this study. 1 mg/ml of furosemide was administered intravenously immediately prior to contrast administration in order to improve distension of the upper tracts at the time of the delayed urographic acquisition.
2.3. Dual temporal resolution reconstruction
The GRASP reconstruction was performed using an in-house developed standalone application coded in the C ++ programming language and housed on a Linux server to which the raw k-space data were automatically transferred. The application reconstructed images in the DICOM format and stored reconstructed images directly on our PACS. A single set of reconstructed multi-phase images could be available for clinical use in less than one hour following triggering of the reconstruction task by the MRI technologist upon completion of the scan, due to parallel processing of multiple slices on the Linux multi-CPU server.
The GRASP reconstruction employed a radial variant of the multicoil k-t SPARSE-SENSE algorithm that makes use of spatiotemporal sparsity of the dynamic image data. This iterative reconstruction scheme uses both parallel-imaging and compressed-sensing in an integrated fashion in order to allow for potentially very rapid temporal resolution on the basis of inherent redundancies within the raw dynamic data. Two separate dynamic data sets, both with identical spatial resolution matching that of the initially acquisition, were reconstructed from the single continuous GRASP acquisition, each combining a different number of consecutive spokes into individual dynamic frames, thereby providing two separate temporal resolutions: [1] data set with approximately 25 s temporal resolution, comprising 110 spokes per frame, which was used for clinical interpretation, and [2] data set with 1.7 s temporal resolution, comprising 8 spokes per frame, to allow for high temporal resolution quantitative perfusion evaluation.
2.4. Image analysis
The 1.7-s temporal resolution data sets were evaluated using in-house software (FireVoxel;https://files.nyu.edu/hr18/public/projects.html) by a fellowship trained radiologist with two years of experience (JR), blinded to the pathologic findings. First, a small circular region-of-interest (ROI) was placed within the common femoral artery in order to obtain a kinetic curve of signal intensity (SI) vs. time within the artery across all time points to serve as an arterial input function (AIF). Then, a free-hand 3D ROI was traced around the bladder tumor on multiple slices using a single time-point (mean tumor ROI size 7.6 ± 21.1 mm3), and a free-hand 3D ROI of comparable size was also traced on benign bladder wall. A two-compartment general kinetic model (GKM) was applied using the defined AIF to compute Ktrans for tumor and benign bladder for each patient [16].
At least three months following the quantitative assessment, the above radiologist, as well as an additional board-certified radiologist with seven years of experience (AR), independently reviewed the clinical 25-s temporal resolution data sets for each patient using our institutional PACS (Phillips iSite, Foster City, CA). These readers subjectively scored the visual conspicuity of the bladder lesion on the dynamic GRASP images on a 1–5 scale (5 representing greatest conspicuity).
2.5. Statistics
Each bladder lesion was classified as benign or malignant, and when malignant, as either stage ≤ T1 (not invading the muscularis propria of the bladder wall) or stage ≥ T2 (invading the muscularis propria of the bladder wall), based on the available reference standard (combination of cystoscopy and pathology). This stage cut-off represents a critical threshold in clinical decisions whether to manage patients via radical surgery [1]. The ratio between Ktrans of the lesion and of the normal bladder wall was computed for each patient. Standard summary statistics were computed for Ktrans in normal bladder wall, benign lesions, and tumors (including tumors stratified by stage), as well as for the readers' subjective scores. Ktrans and the Ktrans ratios were compared between groups using a combination of paired and unpaired t tests. Statistical assessment was performed using MedCalc for Windows (version 9.1, MedCalc Software, Ostend, Belgium).
3. Results
16 patients had histologically confirmed urothelial carcinoma (3 stage ≤ T1, 13 stage ≥ T2), and the remaining 6 patients had benign lesions (one with no corresponding lesion identified on subsequent cystoscopy; five with biopsy at the time of cystoscopy demonstrating benign inflammatory changes or dysplasia). All lesions were well visualized using the conventional 25-s clinical multi-phase images (mean conspicuity scores of 4.42 ± 1.06 and 4.42 ± 0.83 for the two readers). The high-temporal resolution quantitative perfusion assessment was successfully conducted for the lesion and benign bladder wall in all patients using the 1.7-s temporal resolution images. Fig. 1 and 2 demonstrates a representative patient.
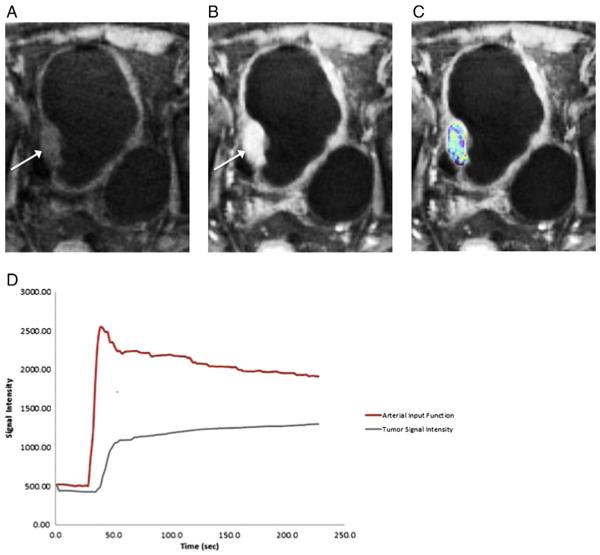
Fig. 1.
79 year old male with high-grade urothelial carcinoma of the bladder based on histologic assessment from cystoscopy. (A) Pre-contrast and (B) post-contrast images retrospectively reconstructed at 1.7-s temporal resolution from the continuously acquired radial sequence using parallel imaging and compressed sensing (GRASP) show an enhancing mass along the right lateral bladder wall (arrow). (C) A color-coded Ktrans map was generated using the high temporal-resolution data and overlaid on the post-contrast image. (D) Time–activity curves (TACs) of the individual arterial input function generated from the common femoral artery and of a region-of-interest encompassing the bladder mass used for pharmacokinetic modeling.
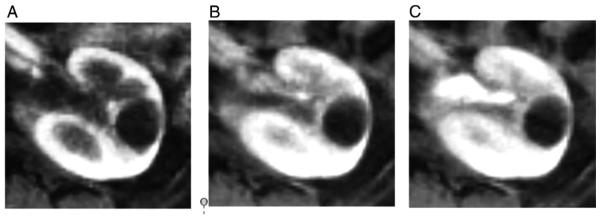
Fig. 2.
Same patient as in Fig. 1. (A) Corticomedullary, (B) nephrographic, and (C) early excretory phase images that were reconstructed prospectively at 25-s temporal resolution for purposes of clinical interpretation using the continuously acquired abdominopelvic GRASP acquisition.
Table 1 summarizes the values of Ktrans in the various study groups. Ktrans was significantly higher in bladder tumors than in both normal bladder wall (p b 0.001) as well as in benign bladder lesions (p =0.033). Ktrans was not significantly different between normal bladder wall and benign lesions (p = 0.096). The ratio between Ktrans of the lesion and of normal bladder wall in each patient was nearly double in tumors than in benign lesions (4.3 ± 3.4 vs. 2.2 ± 1.6), and Ktrans was nearly double in stage ≥ T2 tumors than in stage ≤ T1 tumors, although these differences did not approach statistical significance (p = 0.180–0.209), possibly related to small sample size.
Table 1.
Summary of ktrans in study subgroups (expressed as mean ± SD).
| Group | ktrans |
|---|---|
| Normal bladder wall | 0.13 ± 0.10 |
| Benign bladder lesions | 0.15 ± 0.04 |
| Bladder cancer | 0.38 ± 0.24 |
| Stage ≤ T1 tumors | 0.24 ± 0.24 |
| Stage ≤ T2 tumors | 0.44 ± 0.24 |
4. Discussion
In this study, we used a continuously acquired free-breathing large-FOV radial acquisition in conjunction with retrospective sparse reconstruction at flexible temporal resolution in order to perform quantitative bladder perfusion within the context of clinical MR urography. The bladder lesions were well visualized using multiphase data sets reconstructed at a conventional temporal resolution. Moreover, Ktrans determined from a separately reconstructed rapid temporal resolution data set was significantly different between benign and malignant lesions, and tended to be higher in higherstage tumors. These observations suggest a potential role for the described novel acquisition scheme to facilitate the application of quantitative perfusion for assessing bladder lesions in patients undergoing clinical MR examinations.
Scant prior literature describes quantitative perfusion MRI of bladder cancer. Moreover, among such studies, the reported acquisition protocol highlights the limitations of conventional imaging and potential added value of our free-breathing continuous acquisition scheme with flexible retrospective temporal resolution. For instance, the study by Tuncbilek et al. achieved a temporal resolution of only 30 s [12]. In comparison, the study by Nguyen et al. imaged patients at 3 T using a 2-channel parallel transmission system and a 32-channel phased-array receive coil [13]. Although achieving a relatively faster temporal resolution of 8.3 s, even this resolution does not approach the 1.7 s resolution in our study. Moreover, based on their described technique (19 slices with a slice thickness of 5.0 mm), it does not appear that concomitant imaging of the upper tracts was performed. Such differences underscore the challenges encountered when trying to perform quantitative perfusion of bladder cancer in patients also warranting a standard clinical MR evaluation.
We envision that this pilot study may encourage more wide-spread integration of quantitative perfusion into MRI protocols performed in bladder cancer. Using the described approach, such examinations can continue to offer complete abdominopelvic staging of known cancers as well as screening of the upper tracts for synchronous urothelial tumors, thereby satisfying the primary needs of these examinations. However, the additional quantitative information obtained through the high temporal resolution reconstruction may provide additional prognostic data that can potentially impact clinical management, for instance decisions regarding the need for repeat biopsy, selection from among treatment options, or prediction of neo-adjuvant therapy response. The quantitative information may also be explored to differentiate benign post-treatment changes of the bladder wall from residual or recurrent tumor, which traditionally presents a challenge given mural inflammation and fibrosis resulting from interventions [17,18]. Perhaps more importantly from an immediate standpoint, the continuous acquisition scheme may greatly facilitate future investigations of perfusion metrics as potential biomarkers in bladder cancer, in essence providing a previously lacking framework for allowing formal study of bladder cancer perfusion within a practical manner.
GRASP takes advantage of compressed sensing to allow for marked data undersampling [14]. Recent reports have demonstrated the value of compressed sensing for robust rapid temporal resolution free-breathing acquisitions for a variety of purposes within abdominopelvic imaging, such as estimating liver perfusion [14], evaluating kidney glomerular filtration rate [19], and visualizing potentially subtle prostate tumors [20]. Of particular importance in such applications has been the absence of a predetermined temporal resolution for the initial acquisition and the corresponding ability to retrospectively reconstruct the raw data at any number of temporal resolutions of interest, based on the given clinical or research context. This retrospective tailoring, achieved by grouping a flexible number of consecutive spokes within each dynamic frame, provides a basis for a greater degree of sophistication in future research of clinical applications of perfusion imaging. For instance, investigations of the optimal temporal resolution for a given diagnostic setting or the impact of changes in temporal resolution upon quantitative perfusion metrics can now be conducted using only a single contrast injection.
The primary limitation of this pilot study is its small sample size. Nonetheless, we were able to demonstrate the feasibility of high temporal resolution perfusion MRI of bladder cancer using the GRASP acquisition scheme as a foundation for future larger studies. In addition, only a single rapid temporal resolution was evaluated. Indeed, the GRASP acquisition scheme allows for direct comparison of a large number of temporal resolutions, if desired. Also, other perfusion models, aside from the conventional GKM model used in this study, were not evaluated.
In conclusion, high temporal resolution quantitative perfusion MRI of bladder cancer can be performed within the context of conventional multi-phase MR urography using a novel free-breathing continuous radial acquisition with a flexible compressed sensing reconstruction. Ktrans derived from a 1.7-s temporal resolution reconstruction showed associations with pathologic findings in our preliminary cohort. The scheme may facilitate more extensive future investigations into the role of quantitative perfusion of bladder tumors without extra injection of intravenous contrast or need for additional scan time.
Footnotes
Support: The Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at New York University School of Medicine is supported by NIH/NIBIB grant number P41 EB017183.
Disclosures: Two authors (KB, HC) are listed as inventors on a provisional patent that has been filed for the GRASP acquisition scheme. Otherwise, the authors have no other disclosures.
References
- [1].Vikram R, Sandler CM, Ng CS. Imaging and staging of transitional cell carcinoma: part 1, lower urinary tract. AJR Am J Roentgenol. 2009;192(6):1481–7. doi: 10.2214/AJR.08.1318. [DOI] [PubMed] [Google Scholar]
- [2].Biagioli MC, Fernandez DC, Spiess PE, Wilder RB. Primary bladder preservation treatment for urothelial bladder cancer. Cancer Control. 2013;20(3):188–99. doi: 10.1177/107327481302000307. [DOI] [PubMed] [Google Scholar]
- [3].Bos MK, Marmolejo RO, Rasch CR, Pieters BR. Bladder preservation with brachytherapy compared to cystectomy for T1-T3 muscle-invasive bladder cancer: a systematic review. J Contemp Brachyther. 2014;6(2):191–9. doi: 10.5114/jcb.2014.43777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zagouri F, Peroukidis S, Tzannis K, Kouloulias V, Bamias A, Hellenic Genito-Urinary Cancer G Current clinical practice guidelines on chemotherapy and radiotherapy for the treatment of non-metastatic muscle-invasive urothelial cancer: a systematic review and critical evaluation by the Hellenic Genito- Urinary Cancer Group (HGUCG) Crit Rev Oncol Hematol. 2015;93(1):36–49. doi: 10.1016/j.critrevonc.2014.08.005. [DOI] [PubMed] [Google Scholar]
- [5].Vashistha V, Quinn DI, Dorff TB, Daneshmand S. Current and recent clinical trials for perioperative systemic therapy for muscle invasive bladder cancer: a systematic review. BMC Cancer. 2014;14:966. doi: 10.1186/1471-2407-14-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Teply BA, Kim JJ. Systemic therapy for bladder cancer—a medical oncologist's perspective. J Solid Tumors. 2014;4(2):25–35. doi: 10.5430/jst.v4n2p25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle- invasive bladder cancer. Eur Urol. 2012;62(3):523–33. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- [8].Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/ PD-L1, and HLA-G. Eur Urol. 2015;68(2):267–79. doi: 10.1016/j.eururo.2015.02.032. [DOI] [PubMed] [Google Scholar]
- [9].Mitropoulos D, Kiroudi-Voulgari A, Nikolopoulos P, Manousakas T, Zervas A. Accuracy of cystoscopy in predicting histologic features of bladder lesions. J Endourol. 2005;19(7):861–4. doi: 10.1089/end.2005.19.861. [DOI] [PubMed] [Google Scholar]
- [10].Kaplan AL, Litwin MS, Chamie K. The future of bladder cancer care in the USA. Nat Rev Urol. 2014;11(1):59–62. doi: 10.1038/nrurol.2013.180. [DOI] [PubMed] [Google Scholar]
- [11].Shariat SF, Youssef RF, Gupta A, Chade DC, Karakiewicz PI, Isbarn H, et al. Association of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J Urol. 2010;183(5):1744–50. doi: 10.1016/j.juro.2010.01.018. [DOI] [PubMed] [Google Scholar]
- [12].Tuncbilek N, Kaplan M, Altaner S, Atakan IH, Süt N, Inci O, et al. Value of dynamic contrast-enhanced MRI and correlation with tumor angiogenesis in bladder cancer. AJR Am J Roentgenol. 2009;192(4):949–55. doi: 10.2214/AJR.08.1332. [DOI] [PubMed] [Google Scholar]
- [13].Nguyen Huyen T, Guang Jia, Shah Zarine K, Pohar Kamal, Mortazavi Amir, Zynger Debra L, et al. Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J Magn Reson Imaging. 2015;41(5):1374–82. doi: 10.1002/jmri.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chandarana H, Block TK, Ream J, Mikheev A, Sigal SH, Otazo R, et al. Estimating liver perfusion from free-breathing continuously acquired dynamic gadolinium- ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced acquisition with compressed sensing reconstruction. Investigative radiology. 2015;50(2):88–94. doi: 10.1097/RLI.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014;72(3):707–17. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383–96. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- [17].Wang HJ, Pui MH, Guo Y, Yang D, Pan BT, Zhou XH. Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdom Imaging. 2014;39(1):135–41. doi: 10.1007/s00261-013-0038-0. [DOI] [PubMed] [Google Scholar]
- [18].El-Assmy A, Abou-El-Ghar ME, Refaie HF, Mosbah A, El-Diasty T. Diffusion-weighted magnetic resonance imaging in follow-up of superficial urinary bladder carcinoma after transurethral resection: initial experience. BJU Int. 2012;110(11):E622–7. doi: 10.1111/j.1464-410X.2012.11345.x. [DOI] [PubMed] [Google Scholar]
- [19].Rosenkrantz AB, Sigmund EE, Johnson G, Babb JS, Mussi TC, Melamed J, et al. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology. 2012;264(1):126–35. doi: 10.1148/radiol.12112290. [DOI] [PubMed] [Google Scholar]
- [20].Rosenkrantz AB, Geppert C, Grimm R, Block TK, Glielmi C, Feng L, et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. Journal of magnetic resonance imaging : JMRI. 2015;41(5):1365–73. doi: 10.1002/jmri.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]