Abstract
Background
Physical activity has been associated with a lower risk of pancreatic cancer in several studies, but the overall epidemiologic evidence is not consistent. We therefore performed a systematic review to evaluate the association between physical activity and pancreatic cancer risk.
Methods
We searched MEDLINE and EMBASE through April 2008 and examined the reference lists of the retrieved articles. We excluded studies that relied on job titles as surrogate measures for physical activity. We used a random-effects model to pool study-specific risk estimates comparing the highest versus the lowest category of physical activity.
Results
Total physical activity (occupational and leisure-time) was not significantly associated with risk of pancreatic cancer (4 prospective studies; summary relative risk (RR) = 0.76, 95% confidence interval (CI) = 0.53-1.09). A decreased risk of pancreatic cancer was observed for occupational physical activity (3 prospective studies; RR = 0.75, 95% CI = 0.58-0.96) but not for leisure-time physical activity (14 prospective studies; RR = 0.94, 95% CI = 0.83-1.05). No association was found with light physical activity (2 prospective studies; RR = 1.01, 95% CI = 0.77-1.34), moderate physical activity (6 prospective studies; RR = 0.83, 95% CI = 0.58-1.18) or vigorous physical activity (7 prospective studies; RR = 0.94, 95% CI = 0.80-1.12).
Conclusions
This systematic review does not provide strong evidence for an association between physical activity and risk of pancreatic cancer.
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer mortality in the United States (1). Due to the late presentation of symptoms and the high metastatic potential, pancreatic cancer is one of the most fatal diseases in humans and more than 75 percent of newly diagnosed patients die within a year (2). Unfortunately, little is known about the etiology of this malignancy. Over the past decade, accumulating evidence has suggested that impaired glucose tolerance and insulin resistance play important roles in the development of pancreatic cancer. The hypothesized biological mechanism involves the growth-promoting effects of excess insulin or insulin-like growth factors (3-5). Obesity and type 2 diabetes, both of which are closely linked to abnormal glucose metabolism and insulin resistance, increase the risk of pancreatic cancer in most epidemiologic studies (6, 7).
Physical activity can improve glucose tolerance and insulin sensitivity by increasing insulin-stimulated glycogen synthesis and enhancing skeletal muscle glucose transport (8, 9). Randomized clinical trials have indicated that physical activity alone or combined with dietary changes can produce weight loss and reduce risk of type 2 diabetes (10-13). Therefore, physical activity may lower pancreatic cancer risk by regulating glucose metabolism or by modifying other factors such as obesity and diabetes.
Epidemiologic findings are inconsistent regarding the association between physical activity and pancreatic cancer, perhaps due to the inadequate statistical power resulting from the limited number of pancreatic cancer cases in most studies or a weak relation. In addition, as physical activity encompasses a variety of types (occupational, leisure-time and transport) and characteristics (frequency, intensity and duration), different aspects of physical activity assessed may also contribute to the divergent results.
Epidemiologic evidence on the relation between physical activity and pancreatic cancer has not been quantitatively summarized. We therefore systematically reviewed and synthesized the available evidence to evaluate whether total physical activity or physical activity of a particular type or intensity is associated with risk of pancreatic cancer.
MATERIALS AND METHODS
Study selection
We conducted a comprehensive literature search of MEDLINE (1966 to April 2008) and EMBASE (1974 to April 2008) using both exploded index terms (Medical Subject Headings (MeSH) for MEDLINE, Excerpta Medica Tree (EMTREE) for EMBASE) and text words of physical activity (exploded index terms: physical activity or exercise or leisure activities or walking, or text words: physical activity or exercise or leisure activities or walking or sedentary) in combination with pancreatic cancer (exploded index terms: pancreatic cancer or pancreas cancer or pancreas tumor, or text words: pancreatic cancer or pancreatic neoplasms or pancreas cancer). We also searched the reference lists of relevant research reports and review articles. We limited the search to publications in English.
The inclusion criteria were predefined and the selection process comprised two steps. The first-round selection was based on a review of the identified titles or abstracts. The studies were considered potentially eligible if they were original observational studies, only involved human subjects, and investigated the association between physical activity and pancreatic cancer risk. Studies with mortality due to pancreatic cancer were included because pancreatic cancer is a rapidly fatal disease with its mortality almost identical to its incidence (1). The second-round selection was based on full-text review of the retrieved articles. The studies were included if they had individual-based measurement of physical activity, provided age-adjusted relative risks (RRs) with 95% confidence intervals (CIs), and did not involve study populations overlapping with other studies. Where multiple publications from one study were found, only the most recent report was selected. Studies that failed to provide data to allow calculation of age-adjusted RRs were excluded because age is strongly associated with both physical activity and pancreatic cancer risk. Studies that relied on job titles as surrogate measures for physical activity were also excluded because this crude assessment would have led to substantial measurement errors and therefore would have underestimated the association.
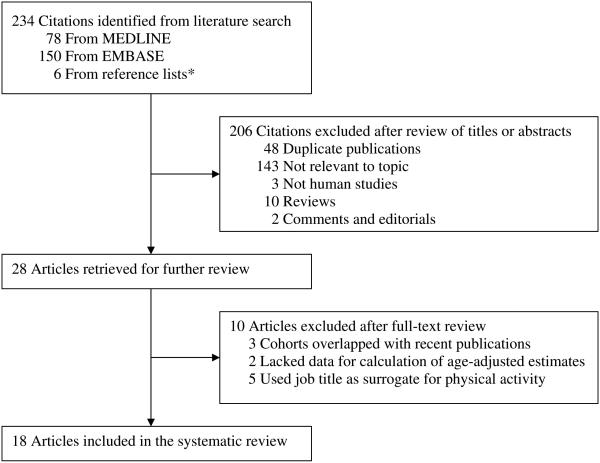
The systematic search identified 234 citations: 78 from MEDLINE, 150 from EMBASE and 6 from hand searching of the reference lists of the relevant research reports and review articles. Of these, 206 citations were excluded after a review of titles or abstracts and 10 articles were excluded based on full-text review, leaving 18 eligible reports in the systematic review (Figure 1).
Figure 1.
Summary of article selection process. *Reference lists of relevant research reports and review articles.
Data extraction
Following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting meta-analyses of observational studies (14), we recorded information on study design, participant characteristics, assessment of physical activity and pancreatic cancer cases, adjustments for confounding factors, and estimates of associations. We considered studies as prospective if data on physical activity were collected before the diagnosis of pancreatic cancer and as retrospective if the data was collected after the diagnosis.
To comprehensively evaluate the association, we abstracted RRs with their 95% CIs for pancreatic cancer in relation to total physical activity (occupational and leisure-time) and various aspects of physical activity classified by type (occupational, leisure-time and transport) and by intensity (light, moderate and vigorous). Overall RRs and sex-specific RRs were extracted. We preferentially extracted the risk estimates that incorporated frequency, intensity and duration of physical activity because these components jointly determine the amount of physical activity. For studies that presented multiple levels of physical activity of interest, we utilized the RR for the highest as compared with the lowest level of physical activity to provide the most distinctive contrasts assuming monotonic relations. For studies that used the highest level of physical activity as the reference group, we derived the RR for the highest vs lowest comparison by calculating the reciprocal of the RR for the lowest vs highest level of activity. For studies that reported more than one risk estimate for the same comparison, we chose those adjusted for the greatest number of potential confounders. For studies that obtained RRs for physical activity at different time periods, we used the baseline measurements or cumulative averages, if provided, to reduce misclassification of physical activity. Although the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) were presented in the same paper (15), we extracted RRs separately for these two cohorts because the participants of the NHS and the HPFS were independently recruited. Additionally, in the preliminary analysis, we obtained similar results when we used the pooled relative risks of these two cohorts provided in the original paper (15). Although two articles (16, 17) were based on the same cohort study (the UK Whitehall Study), we treated them as two separate studies because different types of physical activity were examined and the participants in these two analyses were exclusive.
For leisure-time physical activity, it was possible to obtain a continuous measure of association because 4 prospective studies (15, 18, 19) used the same unit of MET-hrs/wk for physical activity at leisure-time. All of these studies were based on the U.S. population and captured similar activities during leisure-time. We therefore additionally extracted RRs with 95% CIs and the means of MET-hrs/wk for each category of leisure-time physical activity. Where the means of MET-hrs/wk were not reported, we calculated the midpoint values based on cutpoints.
For occupational physical activity, we excluded the study by Nilsen and Vatten (20) because they assessed physical activity at work by asking the participants how often they felt physically worn out after work, which is not a good indicator for occupational physical activity.
One author (YB) performed the literature search, selection and data extraction on three occasions. Any differences were resolved through reexamination of the original reports.
Overall, 18 articles contained 19 studies: 4 studies had information on total physical activity, 3 on occupational physical activity, 16 on leisure-time physical activity, 1 on transport physical activity, 2 on light physical activity, 7 on moderate physical activity and 8 on vigorous physical activity.
Statistical analysis
Because the selected studies differed in design, population and quality of physical activity measurement, we estimated summary RRs via the DerSimonian and Laird random-effects models to account for variations between the results of individual studies. Throughout the analyses, we used the originally reported overall RRs, if provided; otherwise, we used sex-specific RRs because they were independent estimates. Our primary analyses examined pooled RRs comparing the highest vs the lowest category of the physical activity of interest, including total physical activity, a range of types and intensities. For leisure-time physical activity, we additionally assessed linear association using GLST procedure, which first generated a continuous estimate for each of the studies and then pooled these estimates in the following step.
We assessed heterogeneity across studies by calculating the Cochrane Q statistic and the I2 statistic (21, 22). To examine sources of heterogeneity, we conducted both stratified analysis and meta-regression analysis with study region (North America/Europe/Asia/multiethnic), sex (men/women), follow-up duration (<10 years/≥10 years) and adjustment for BMI or diabetes or both (yes/no).
To evaluate the influence of study quality on the results, we performed separate meta-analyses for prospective and retrospective studies. In addition, we conducted a sensitivity analysis by removing the studies that did not exclude prevalent cancers at baseline, did not adjust for cigarette smoking, or did not adjust for smoking adequately (i.e., lacked adjustment for intensity or duration of smoking).
To determine whether or not the pooled risk estimate was affected by a single study, we omitted each study in turn and recalculated the pooled RR for the remaining studies. To assess the influence of different categorizations of exposure on the pooled risk estimates, we repeated the analyses for the studies that had at least 4 categories of physical activity with more than 15 cases in the highest and the lowest categories.
We evaluated publication bias by the Begg (23) and the Egger (24) tests and visual inspection of funnel plots. All statistical analyses were performed using STATA version 9.1 (STATACorp, College Station, Texas). All tests were two-sided; P<0.05 was considered statistically significant.
RESULTS
Study characteristics
The selected studies included 16 prospective cohort studies (15-20, 25-33), 1 nested case-control study (34) and 2 retrospective case-control studies (35, 36) (Table 1). Of the 19 studies, 9 were conducted in the United States (one of them was multiethnic), 1 in Canada, 6 in European countries and 3 in Japan. Four studies involved men only, 3 studies involved women only, and the other 12 studies included both men and women. Exclusion of baseline prevalent cancers was not explicitly specified in 4 prospective studies (16, 17, 26, 34). In all studies, information on usual physical activity was self-reported and collected after 1960. The highest and lowest levels of physical activity in each study according to the type of physical activity are provided in Figure 2-4. Four studies (15, 27-29) tested the validity of the assessment of physical activity and only one study (27) updated data on physical activity during follow-up. Most studies ascertained pancreatic cancer cases through cancer registries. For prospective studies, the follow-up was nearly complete and the duration ranged from 5 to 30 years. All risk estimates were adjusted for age and all but two studies (20, 34) adjusted for cigarette smoking. Approximately two third adjusted for diabetes and about half of the studies adjusted for BMI. The degree of the adjustment for other potential confounding factors varied across studies.
Table 1.
Studies included in the systematic review
| Source | Country | Sex | Age, y | N cohort/controls | n cases |
|---|---|---|---|---|---|
| Prospective cohort studies | |||||
| Davey Smith et al, 2000 (17) | United Kingdom | M | 40-64 | 6702 | 30 |
| Nilsen and Vatten, 2000 (20) | Norway | M/F | ≥30 | 63374* | 166* |
| Batty et al, 2001 (16) | United Kingdom | M | 40-64 | 11663 | 80 |
| Michaud et al, 2001 (15) † | United States | M | 40-75 | 46115 | 139 |
| United States | F | 30-55 | 77559 | 110 | |
| Isaksson et al, 2002 (26) | Sweden | M/F | 36-75 | 21884* | 176* |
| Stolzenberg-Solomon et al, 2002 (32) | Finland | M | 50-69 | 29048 | 172 |
| Lee et al, 2003 (27) | United States | M/F | mean 47.1 | 32687 | 212 |
| Patel et al, 2005 (18) | United States | M/F | 50-74 | 145627 | 242 |
| Sinner et al, 2005 (31) | United States | F | 55-69 | 38002 | 209 |
| Berrington de González et al, 2006 (25) | 9 European Countries | M/F | 19-84 | 438405* | 324* |
| Lin et al, 2007 (28) | Japan | M/F | 40-79 | 100932* | 402* |
| Luo et al, 2007 (29) | Japan | M/F | 40-69 | 99670 | 224 |
| Nöthlings et al, 2007 (30) | United States (multiethnic) | M/F | 45-75 | 167430 | 472 |
| Stolzenberg-Solomon et al, 2008 (19) | United States | M/F | 50-71 | 294609 | 399 |
| Calton et al, 2008 (33) | United States | F | mean 61.2 | 33530 | 70 |
| Nested case-control studies | |||||
| Inoue et al, 2003 (34) | Japan | M/F | 30-89 | 2000 | 200 |
| Retrospective case-control studies | |||||
| Eberle et al, 2005 (35) | United States | M/F | 22-85 | 1701 | 532 |
| Hanley et al, 2001 (36) | Canada | M/F | NA | 2919 | 312 |
| Source | Self-report physical activity (year of measurement) |
Case ascertainment | Follow-up, y |
Adjustments |
|---|---|---|---|---|
| Prospective cohort studies | ||||
| Davey Smith et al, 2000 (17) |
Leisure-time physical activity (1969-1970) |
National health registry | approx. 25 | Age, smoking, BMI, employment grade, forced expiratory volume in 1 second |
| Nilsen and Vatten, 2000 (20) |
Leisure-time physical activity (1984-1986) |
National cancer registry | mean 9.8 | Age ‡ |
| Batty et al, 2001 (16) | Transport physical activity (1967-1969) |
National health registry | approx. 25 | Age, smoking, BMI, employment grade, forced expiratory volume in 1 second |
| Michaud et al, 2001 (15) |
Leisure-time physical activity, vigorous activity, moderate activity (1986) |
Self-report confirmed by medical records, pathology reports and death certificate |
approx.12 | Age, smoking, height, diabetes, cholecystectomy. moderate activity (for vigorous activity) |
| Leisure-time physical activity, vigorous activity, moderate activity (1986) |
Self-report confirmed by medical records, pathology reports and death certificate |
approx.10 | Age, smoking, height, diabetes, cholecystectomy, moderate activity (for vigorous activity) |
|
| Isaksson et al, 2002 (26) |
Occupational physical activity, leisure-time physical activity (1961) |
National cancer registry | median 16 | Age, sex, smoking |
| Stolzenberg-Solomon et al, 2002 (32) |
Total physical activity, occupational physical activity, leisure-time physical activity (1985) |
National cancer registry | median 10.2 |
Age, smoking, diabetes, bronchial asthma, high blood pressure |
| Lee et al, 2003 (27) | Leisure-time physical activity, light activity, moderate activity, vigorous activity (Baseline in 1962 or 1966 and updated later) |
University records of deceased alumni confirmed by death certificate |
approx.30 | Age, sex, smoking, diabetes; each physical activity component was adjusted for the remaining components. |
| Patel et al, 2005 (18) | Leisure-time physical activity (1992) |
National death index, state cancer registry and medical records |
approx. 7 | Age, sex, smoking, height, diabetes, education, family history of pancreatic cancer, gallbladder disease, energy intake |
| Sinner et al, 2005 (31) |
Leisure-time physical activity, moderate activity, vigorous activity (1986) |
State cancer registry | approx. 15 | Age, smoking, multivitamin |
| Berrington de González et al, 2006 (25) |
Total physical activity, leisure-time physical activity, occupational physical activity, vigorous activity (1991-2000) |
Mainly national or regional cancer registries |
mean 6 | Age, sex, smoking, diabetes, country |
| Lin et al, 2007 (28) | Leisure-time physical activity (1988-1990) |
Population-based cancer registry and death certificate |
approx. 15 | Age, smoking, BMI, diabetes, gallbladder diseases ‡ |
| Luo et al, 2007 (29) | Leisure-time physical activity (1990 or 1993-1994) |
Population-based cancer registry and death certificate |
mean 11 | Age, smoking, BMI, diabetes, study area, alcohol, history of cholelithiasis ‡ |
| Nöthlings et al, 2007 (30) |
Total physical activity, moderate activity (1993-1996) |
State and regional cancer registries |
mean 8.3 | Age, smoking, BMI, diabetes, ethnicity, family history of pancreatic cancer, energy intake, meat ‡ |
| Stolzenberg-Solomon et al, 2008 (19) |
Leisure-time physical activity, light activity, vigorous activity (1995-1996) |
State cancer registries and national death index |
approx. 5 | Age, sex, smoking, BMI, diabetes, race |
| Calton et al, 2008 (33) |
Total physical activity, moderate activity, vigorous activity (1987) |
National death index, state cancer registry and pathology reports |
approx. 11 | Age, smoking, BMI, height, diabetes, race; moderate activity and vigorous activity were mutually adjusted. |
| Nested case-control studies | ||||
| Inoue et al, 2003 (34) | Leisure-time physical activity (1988-1999) |
Hospital-based and population-based cancer registries |
1 to 13 | Age, sex, diabetes, family history of pancreatic cancer, bowel habits, vegetable intake, alcohol |
| Retrospective case-control studies | ||||
| Eberle et al, 2005 (35) |
Leisure-time physical activity (1995-1999) |
State cancer registry and medical records |
NA | Men: age, smoking, education; women: age |
| Hanley et al, 2001 (36) |
Leisure-time physical activity, moderate activity, vigorous activity (1994-1997) |
Provincial cancer registries | NA | Men: age, maximum BMI, percent change in weight, province, caloric intake, leisure-time physical activity (for moderate and vigorous activity); women: age, smoking, province, age at menarche |
Total cohort/case numbers. Do not equal cohort/case numbers for physical activity due to missing data.
Included two studies: Health Professionals Follow-up Study (Male) and Nurses’ Health Study (Female).
Men and women were analyzed separately.
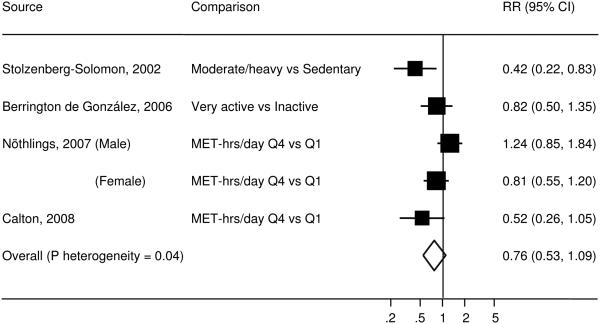
Figure 2.
Study-specific and summary RRs (95% CIs) of pancreatic cancer for total physical activity among prospective studies. Box area proportional to weight, with horizontal lines showing 95% CIs.
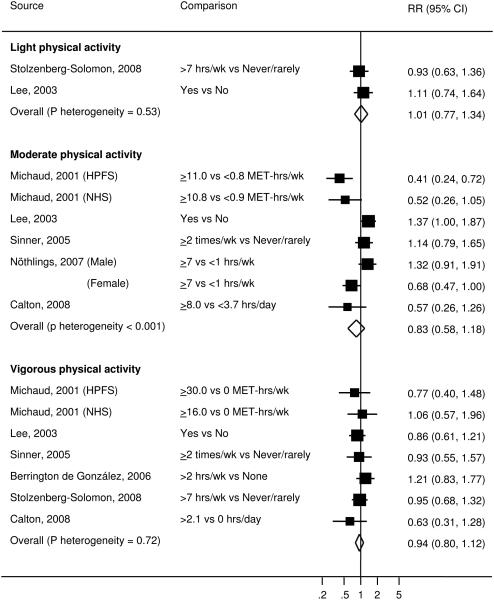
Figure 4.
Study-specific and summary RRs (95% CIs) of pancreatic cancer for physical activity of different intensity among prospective studies. Box area proportional to weight, with horizontal lines showing 95% CIs.
Total physical activity and pancreatic cancer
Four prospective studies (25, 30, 32, 33) examined the association between total physical activity and pancreatic cancer risk. The individual RRs ranged from 0.42 to 1.24 and the pooled RR was 0.76 (95% CI=0.53-1.09) (Figure 2). Heterogeneity between studies was statistically significant (P=0.04), explaining 60% of the total variations in results. The study by Stolzenberg-Solomon et al (32) and the study by Nöthlings et al (30) contributed substantially to heterogeneity. After exclusion of the study by Stolzenberg-Solomon et al, the P value for heterogeneity was 0.14 (% of variation explained=45%) and the pooled RR was 0.87 (95% CI=0.63-1.19). After exclusion of the study by Nöthlings et al, the P value for heterogeneity was 0.25 (% of variation explained=28%) and the pooled RR was 0.60 (95% CI=0.39-0.90). Exclusion of any other study did not materially change the P value for heterogeneity or the result of no association. Subgroup analyses were not conducted due to small number of studies.
Types of physical activity and pancreatic cancer
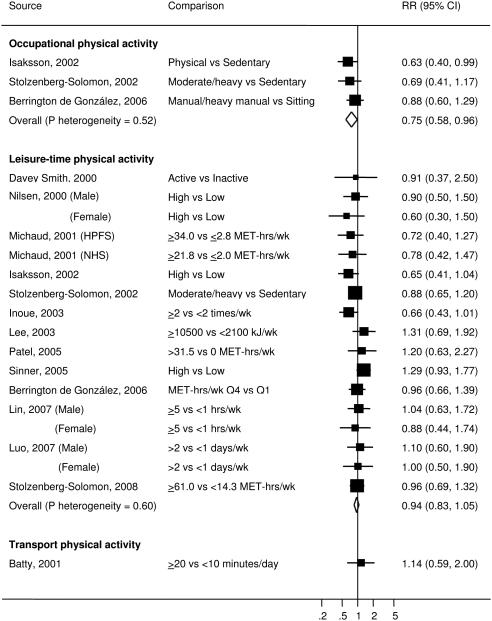
Occupational physical activity was reported in 3 prospective studies (25, 26, 32). The individual RRs ranged from 0.63 to 0.88 and the pooled RR was 0.75 (95% CI=0.58-0.96, P for heterogeneity=0.52) (Figure 3). Subgroup and sensitivity analyses were not conducted due to small number of studies.
Figure 3.
Study-specific and summary RRs (95% CIs) of pancreatic cancer for different types of physical activity among prospective studies. Box area proportional to weight, with horizontal lines showing 95% CIs.
Leisure-time physical activity was assessed in 16 studies (14 prospective studies and 2 retrospective studies). For the 14 prospective studies (15, 17-20, 25-29, 31, 32, 34), the individual RRs of pancreatic cancer comparing the highest vs the lowest category of leisure-time physical activity ranged from 0.60 to 1.31 and the pooled RR was 0.94 (95% CI=0.83-1.05, P for heterogeneity=0.60) (Figure 3). For the 4 prospective studies that used the same unit of MET-hrs/wk for leisure-time physical activity (15, 18, 19), the pooled RR was 0.99 (0.95-1.02) for 10 MET-hrs/wk increase. For the 2 retrospective studies (35, 36), the individual RRs comparing the highest vs lowest category ranged from 0.53 to 0.84 and the pooled RR was 0.74 (95% CI=0.58-0.94, P for heterogeneity=0.58). Because of the discrepancy in the pooled estimates between prospective and retrospective studies, the subsequent analyses were conducted only among prospective studies. In subgroup analyses, no significant difference was found between men and women (P=0.79) or between the three study regions (P=0.08 and P=0.23) (Table 2). The association of leisure-time physical activity with pancreatic cancer was also similar for studies with 10 or more years of follow-up and those with less than 10 years of follow-up (P=0.71) and for studies that adjusted for BMI or diabetes or both and those that adjusted for neither of them (P=0.84). In a sensitivity analysis, exclusion of any single study from the analysis had little impact on the overall finding. The results remained almost the same after restricting to the studies that measured frequency, intensity and duration of physical activity (15, 18-20, 25, 27), or restricting to the studies that validated the measurement of physical activity (15, 27-29), or restricting to the studies that had at least 4 categories of exposure with more than 15 cases in the highest and the lowest categories (15, 18, 19, 25, 27, 28, 32), or removing the studies that included baseline prevalent cancers, did not adjust for cigarette smoking (20, 34) or did not adjust for smoking adequately (17, 26, 31) (data not shown).
Table 2.
Summary RRs (95% CIs) of pancreatic cancer with leisure-time, moderate and vigorous physical activity in all prospective studies and in subgroups according to study characteristics
| N studies/ n estimates |
RR (95% CI) | P Heterogeneity | P Interaction | |
|---|---|---|---|---|
| Leisure-time physical activity | ||||
| All prospective studies | 14/17 | 0.94 (0.84, 1.05) | 0.6 | |
| Region | ||||
| North America | 6/6 | 1.06 (0.88, 1.28) | 0.39 | |
| Europe | 5/6 | 0.84 (0.70, 1.02) | 0.78 | 0.08 |
| Asia | 3/5 | 0.88 (0.69, 1.13) | 0.58 | 0.23 |
| Sex* | ||||
| Men | 9/9 | 0.92 (0.78, 1.09) | 0.96 | 0.79 |
| Women | 8/8 | 0.90 (0.70, 1.16) | 0.22 | |
| Follow-up | ||||
| <10 years | 4/4 | 0.90 (0.74, 1.11) | 0.38 | 0.71 |
| ≥10 years | 10/13 | 0.95 (0.83, 1.09) | 0.55 | |
| Adjustment for BMI or diabetes | ||||
| Yes | 11/13 | 0.93 (0.81, 1.06) | 0.88 | 0.84 |
| No | 3/4 | 0.88 (0.59, 1.29) | 0.07 | |
| Moderate physical activity | ||||
| All prospective studies | 6/7 | 0.83 (0.58, 1.18) | <0.001 | |
| Region | ||||
| North America | 5/5 | 0.76 (0.47, 1.24) | 0.001 | 0.62 |
| Multiethnic | 1/2 | 0.95 (0.50, 1.82) | 0.014 | |
| Sex* | ||||
| Men | 2/2 | 0.75 (0.24, 2.36) | 0.001 | 0.89 |
| Women | 4/4 | 0.75 (0.51, 1.09) | 0.09 | |
| Follow-up | ||||
| <10 years | 1/2 | 0.95 (0.50, 1.82) | 0.014 | 0.62 |
| ≥10 years | 5/5 | 0.76 (0.47, 1.24) | 0.001 | |
| Adjustment for BMI or diabetes | ||||
| Yes | 5/6 | 0.77 (0.50, 1.17) | <0.001 | 0.46 |
| No | 1/1 | 1.14 (0.79, 1.65) | NA | |
| Vigorous physical activity | ||||
| All prospective studies | 7/7 | 0.94 (0.80, 1.12) | 0.717 | |
| Region | ||||
| North America | 6/6 | 0.89 (0.73, 1.07) | 0.899 | 0.15 |
| Europe | 1/1 | 1.21 (0.83, 1.77) | NA | |
| Sex* | ||||
| Men | 2/2 | 1.04 (0.68, 1.60) | 0.247 | 0.20 |
| Women | 4/4 | 0.79 (0.59,1.06) | 0.46 | |
| Follow-up | ||||
| <10 years | 2/2 | 1.06 0.82, 1.35) | 0.346 | 0.23 |
| ≥10 years | 5/5 | 0.86 (0.68, 1.08) | 0.848 | |
| Adjustment for BMI or diabetes | ||||
| Yes | 6/6 | 0.95 (0.79, 1.13) | 0.594 | 0.96 |
| No | 1/1 | 0.93 (0.55, 1.57) | NA |
Included only studies that reported sex-specific estimates.
Transport physical activity was examined in 1 prospective study (16). Those who walked or bicycled to work more than 20 minutes per day did not have a lower risk of pancreatic cancer compared to those who walked or bicycled to work less than 10 minutes per day (RR=1.14, 95% CI=0.59-2.00).
Intensity of physical activity and pancreatic cancer
Light physical activity was reported in 2 prospective studies (19, 27). The pooled RR was 1.01 (95% CI=0.77-1.34, P for heterogeneity=0.53) (Figure 4). Subgroup and sensitivity analyses were not conducted due to small number of studies.
Moderate physical activity was presented in 7 studies (6 prospective and 1 retrospective). For the 6 prospective studies (15, 27, 30, 31, 33), the individual RRs ranged from 0.41 to 1.37 and the pooled RR was 0.83 (95% CI=0.58-1.18) (Figure 4). The case-control study (36) showed no association of moderate physical activity with pancreatic cancer risk among men (RR=1.21, 95% CI=0.50-2.91) or among women (RR=1.00, 95% CI=0.53-1.91). Given the inherent biases and weaknesses associated with retrospective designs, the subsequent analyses were conducted only among prospective studies. Heterogeneity across the prospective studies was evident (P<0.001). To further explore the heterogeneity, we examined whether results differed according to participant and design characteristics. No effect modifications were found by study region (P=0.62), sex (P=0.89), follow-up duration (P=0.62) or adjustment for BMI or diabetes (P=0.46) (Table 2). Furthermore, exclusion of any single study did not markedly influence the P value for heterogeneity or the pooled estimate. The results did not change after restricting to the studies that measured frequency, intensity and duration of physical activity (15, 27, 30, 33), or restricting to the studies that validated the assessment of physical activity (15, 27), or when pooling over the studies that had at least 4 categories of exposure with more than 15 cases in the highest and the lowest categories (15, 27, 30) (data not shown).
Vigorous physical activity was examined in 8 studies (7 prospective and 1 retrospective). For the 7 prospective studies (15, 19, 25, 27, 31, 33), the individual RRs ranged from 0.63 to 1.21 and the pooled RR was 0.94 (95% CI=0.80-1.12, P for heterogeneity=0.72) (Figure 4). The case-control study (36) showed no significant association among men (RR=0.61, 95% CI=0.28-1.30) or among women (RR=0.68, 95% CI=0.35-1.32). The subgroup and sensitivity analyses were conducted only among prospective studies. No effect modifications were found by study region (P=0.15), sex (P=0.20), follow-up duration (P=0.23) or adjustment for BMI or diabetes (P=0.96) (Table 2). Exclusion of any single study from the analysis did not change the overall findings. Similar results were yielded after restricting to the studies that measured frequency, intensity and duration of physical activity (15, 19, 25, 27, 33), or restricting to the studies that validated the assessment of physical activity (15, 27), or when pooling over the studies that had at least 4 categories of exposure with more than 15 cases in the highest and the lowest categories (15, 19, 25, 27) (data not shown).
Assessment of publication bias
The funnel plots appeared slightly asymmetrical for total physical activity and moderate physical activity. However, neither the Egger nor the Begg test provided evidence for significant publication bias for the analyses of any exposure.
DISCUSSION
This meta-analysis did not support a significant association between total physical activity and risk of pancreatic cancer. The risk decreased with high physical activity at work but did not change with physical activity at leisure time. No clear benefit against pancreatic cancer was found with light, moderate or vigorous physical activity.
Quality of the included studies
Meta-analyses of observational studies are prone to biases that occurred in the original studies (14). We therefore focused on the results from prospective cohort studies and the case–control studies nested within them rather than traditional case-control studies to avoid systematic bias due to differential recall. Prospective design and low loss to follow-up in most included studies also minimized the likelihood of selection bias.
The precise assessment of physical activity presents a great challenge in observational research and the quality of activity measurement varied substantially from study to study. To reduce potential misclassification, we excluded studies that used job titles exclusively as surrogate indicators for physical activity level. We also conducted a sensitivity analysis restricted to the studies that measured activity components including frequency, duration and intensity in order to assess the influence of the measurement quality on the pooled risk estimates. The results were virtually unchanged from the results of all studies included. However, as physical activity is a complex behavior which accumulates many short, unstructured activities which occur in varying contexts, self-reported physical activity measurements, even those with estimation of all types and all parameters of activity, can contain a substantial degree of measurement error which may attenuate the magnitude of the associations between physical activity and health outcomes (37). We therefore performed a sensitivity analysis, pooling studies with validated assessment of physical activity. Among those studies, the study-specific risk estimates varied greatly and the pooled relative risks were also near the null. However, the validation instruments used in some studies were self-reported physical activity record (15, 29) or energy expenditure estimated from the same questionnaire (28), which may share the same sources of errors as questionnaire measurements and thus lead to overestimating the validity of the physical activity assessment (37). Therefore, we cannot completely rule out the possibility that the lack of associations in this meta-analysis were due to measurement error in physical activity.
Although physical activity was assessed only once in most studies, changes in physical activity were not likely to substantially attenuate the association given relatively short follow-up duration for many of the cohort studies. Additionally, in the study by Lee et al (27), the only study that updated information on physical activity during follow-up, physical activity was not associated with decreased risk of pancreatic cancer.
Another concern of the present meta-analysis is confounding bias. To reduce confounding by age, we only included the studies that provided age-adjusted estimates. To determine the extent of confounding by comorbidity, we performed a sensitivity analysis by removing the studies that included baseline prevalent cancer; these results were similar. Subgroup analysis also showed that the pooled risk estimates were not quite different according to adjustment for smoking, BMI or diabetes. Other possible confounding factors, including family history of pancreatic cancer, gallbladder disease, social economic status and diet, were not likely to influence the main findings to a great extent because the risk estimates were similar for studies adjusted for these variables and studies that did not adjust for these variables.
Although the level of physical activity might change due to subclinical pancreatic cancer, exclusion of the first few years of follow-up did not substantially change the results in all studies that performed this type of analysis (15-19, 25, 32, 33), which reduced this possibility.
Methodological issues
In the primary analyses, we compared the highest vs the lowest category of physical activity assuming that the relation between physical activity and pancreatic cancer risk is monotonic. This assumption is reasonable based on the results of the included studies. As studies categorized physical activity differently and therefore the activity level of the highest category in one study might correspond to the activity level of the middle category in another study, this approach of combining studies may underestimate the association. However, when we restricted the analyses to the studies that had at least 4 categories of exposure with more than 15 cases in the highest and the lowest categories (15, 18, 19, 25, 27, 28, 30, 32), the results were not materially different. In addition, we were able to assess a dose-response relation for leisure-time physical activity and no linear association was found, similar to the null result from the highest vs lowest comparison.
For total physical activity and moderate physical activity, relative risks were highly heterogeneous across studies. We therefore utilized random effect models which aimed to account for variability between studies. For total physical activity, one source of heterogeneity could be the difference in study populations because the two studies that contributed substantially to the variability were conducted among multiethnic cohort (30) or male current smokers (32), whereas the other two studies measured total physical activity mainly comprised Caucasians with current smokers less than 25%. However, we were limited in our ability to confirm the source of heterogeneity as too few studies examined total physical activity. For moderate physical activity, tests for interaction indicated that study region, sex and follow-up duration were not likely to account for a great deal of variations across studies. However, these tests were underpowered and heterogeneity by a variety of characteristics could not be fully investigated as not all studies provided relevant information.
Eleven prospective studies investigated the interaction by BMI for the association between physical activity and pancreatic cancer. The paper by Michaud et al (15) reported significant effect modification by BMI when pooling the data from the Health Professionals Follow-up Study and the Nurses’ Health Study. The health benefit of physical activity against pancreatic cancer was apparent among overweight participants (RR=0.59, 95% CI=0.37-0.94) while no association was found among normal weight population. The other nine prospective studies (18, 19, 25, 27, 29-33) did not find significant difference between subgroups defined by BMI. These subgroup analyses were based upon very few cases and thus lacked statistical power. Unfortunately, we were unable to estimate the pooled relative risks in obese individuals and in normal weight individuals as most studies did not provide stratum-specific relative risks. Stratified analysis by the percentage of participants who were obese was not feasible either because the percentage was not uniformly given across studies.
Available data also precluded exploration of critical periods in which physical activity may influence risk of pancreatic cancer. Publication bias is not likely to be serious in this meta-analysis based on the Egger and the Begg tests.
Interpretations of the findings
The inverse association between occupational physical activity and pancreatic cancer should be interpreted with caution as it was based on only 3 studies. Additionally, the observed association could be due to unmeasured confounding. However, the confounding may exist in both directions: on one hand, individuals who have medical conditions such as diabetes are ordinarily excluded from employment as manual laborers; on the other hand, physically demanding occupations are usually associated with harmful occupational exposures, lower social economic status and unhealthy lifestyles such as smoking and drinking. Studies that were excluded because of the use of job titles for the exposures had inconsistent findings; a significantly inverse association (38), no significant association (39-41), and a significantly elevated risk (42) were reported with occupations that require more physical activity. However, these conflicting findings were probably resulted from imprecise measurements of occupational physical activity.
Alternatively, the observed benefit of occupational physical activity might be real. Unlike leisure-time physical activity which is usually unstructured, of shorter duration and varies over time, occupational physical activity is likely to be continuous physical activity and last for many years. Therefore physical activity at work may have a long-term and more pronounced effect on improving insulin sensitivity, which may protect against pancreatic cancer. In addition, occupational physical activity may be better recalled due to its routine nature and being part of daily life, which decreases misclassification and thus makes the association more likely to be detected.
In the present meta-analysis, high level of total physical activity was associated with a 24% lower risk of pancreatic cancer, but the association was not statistically significant. Although all studies were conducted in developed countries where lifestyle was relatively sedentary, because occupational physical activity likely represents long-term exposure to physical activity and is more likely to be accurately reported, it may contribute a greater proportion to the total estimation of physical activity, which could explain the reduced risk with total physical activity.
Potential biological mechanisms
Hyperglycemia, hyperinsulinemia and insulin resistance have been hypothesized to be important in the development of pancreatic cancer (4, 43). Physical activity can enhance insulin-stimulated glycogen synthesis in skeletal muscles by increasing intramuscular glucose-6-phosphate concentration (8). Exercise can also increase the expression of GLUT4 glucose transporters and thus activate muscle glucose transport (9). Both of these effects improve insulin responsiveness and lower serum levels of glucose and insulin. Therefore physical activity may reduce the risk of pancreatic cancer through regulation of glucose metabolism and insulin sensitivity.
Conclusions and recommendations
In conclusion, the current data do not support an overall association between physical activity and pancreatic cancer risk. However, because occupational physical activity was inversely associated with the risk in our analysis and it may be critical to the total estimation of physical activity, we cannot exclude the possibility that with more data, an inverse association between total physical activity and pancreatic cancer will emerge.
In addition to incorporating occupational physical activity for the estimation of the benefits of physical activity on pancreatic cancer risk, future research should make great efforts to improve the accuracy of physical activity measurement. More adequately powered studies are needed to assess the relevant time period of physical activity (e.g., physical activity in early life) and whether the association varies by BMI.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004;59:99–111. [PubMed] [Google Scholar]
- 3.Gapstur SM, Gann P. Is pancreatic cancer a preventable disease? Jama. 2001;286:967–8. doi: 10.1001/jama.286.8.967. [DOI] [PubMed] [Google Scholar]
- 4.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. Jama. 2005;294:2872–8. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 5.Wolpin BM, Michaud DS, Giovannucci EL, et al. Circulating insulin-like growth factor binding protein-1 and the risk of pancreatic cancer. Cancer Res. 2007;67:7923–8. doi: 10.1158/0008-5472.CAN-07-0373. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–8. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 7.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. Jama. 1995;273:1605–9. [PubMed] [Google Scholar]
- 8.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–62. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 9.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–61. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 10.Shaw K, Gennat H, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. Journal of the American Medical Association. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 16.Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men: Further evidence from the Whitehall study. European Journal of Epidemiology. 2001;17:863–869. doi: 10.1023/a:1015609909969. [DOI] [PubMed] [Google Scholar]
- 17.Davey Smith G, Shipley MJ, Batty GD, Morris JN, Marmot M. Physical activity and cause-specific mortality in the Whitehall study. Public Health. 2000;114:308–15. doi: 10.1038/sj.ph.1900675. [DOI] [PubMed] [Google Scholar]
- 18.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. cohort. Cancer Epidemiology Biomarkers and Prevention. 2005;14:459–466. doi: 10.1158/1055-9965.EPI-04-0583. [DOI] [PubMed] [Google Scholar]
- 19.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, et al. Adiposity, Physical Activity, and Pancreatic Cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008 doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 20.Nilsen TI, Vatten LJ. A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trondelag, Norway. Cancer Causes Control. 2000;11:645–52. doi: 10.1023/a:1008916123357. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berrington De Gonzalez A, Spencer EA, Bueno-De-Mesquita HB, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiology Biomarkers and Prevention. 2006;15:879–885. doi: 10.1158/1055-9965.EPI-05-0800. [DOI] [PubMed] [Google Scholar]
- 26.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: A cohort study from the Swedish Twin Registry. International Journal of Cancer. 2002;98:480–482. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 27.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS., Jr Physical activity, body weight, and pancreatic cancer mortality. British Journal of Cancer. 2003;88:679–683. doi: 10.1038/sj.bjc.6600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Kikuchi S, Tamakoshi A, et al. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. International Journal of Cancer. 2007;120:2665–2671. doi: 10.1002/ijc.22614. [DOI] [PubMed] [Google Scholar]
- 29.Luo J, Iwasaki M, Inoue M, et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: A large-scale population-based cohort study in Japan - The JPHC study. Cancer Causes and Control. 2007;18:603–612. doi: 10.1007/s10552-007-9002-z. [DOI] [PubMed] [Google Scholar]
- 30.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: The Multiethnic Cohort Study. Cancer Causes and Control. 2007;18:165–175. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 31.Sinner PJ, Schmitz KH, Anderson KE, Folsom AR. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women. Cancer Epidemiol Biomarkers Prev. 2005;14:1571–3. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 32.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland) Cancer Causes and Control. 2002;13:417–426. doi: 10.1023/a:1015729615148. [DOI] [PubMed] [Google Scholar]
- 33.Calton BA, Stolzenberg-Solomon RZ, Moore SC, et al. A prospective study of physical activity and the risk of pancreatic cancer among women (United States) BMC Cancer. 2008;8:63. doi: 10.1186/1471-2407-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue M, Tajima K, Takezaki T, et al. Epidemiology of pancreatic cancer in Japan: A nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) International Journal of Epidemiology. 2003;32:257–262. doi: 10.1093/ije/dyg062. [DOI] [PubMed] [Google Scholar]
- 35.Eberle CA, Bracci PM, Holly EA. Anthropometric factors and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes and Control. 2005;16:1235–1244. doi: 10.1007/s10552-005-0354-y. [DOI] [PubMed] [Google Scholar]
- 36.Hanley AJG, Johnson KC, Villeneuve PJ, Mao Y. Physical activity, anthropometric factors and risk of pancreatic cancer: Results from the Canadian enhanced cancer surveillance system. International Journal of Cancer. 2001;94:140–147. doi: 10.1002/ijc.1446. [DOI] [PubMed] [Google Scholar]
- 37.Ferrari P, Friedenreich C, Matthews CE. The role of measurement error in estimating levels of physical activity. Am J Epidemiol. 2007;166:832–40. doi: 10.1093/aje/kwm148. [DOI] [PubMed] [Google Scholar]
- 38.Weiderpass E, Vainio H, Kauppinen T, Vasama-Neuvonen K, Partanen T, Pukkala E. Occupational exposures and gastrointestinal cancers among Finnish women. J Occup Environ Med. 2003;45:305–15. doi: 10.1097/01.jom.0000052963.43131.44. [DOI] [PubMed] [Google Scholar]
- 39.Brownson RC, Chang JC, Davis JR, Smith CA. Physical activity on the job and cancer in Missouri. Am J Public Health. 1991;81:639–42. doi: 10.2105/ajph.81.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alguacil J, Kauppinen T, Porta M, et al. Risk of pancreatic cancer and occupational exposures in Spain. Annals of Occupational Hygiene. 2000;44:391–403. [PubMed] [Google Scholar]
- 41.Waterbor J, Cole P, Delzell E, Andjelkovich D. The mortality experience of major-league baseball players. N Engl J Med. 1988;318:1278–80. doi: 10.1056/NEJM198805123181917. [DOI] [PubMed] [Google Scholar]
- 42.Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005;20:237–42. doi: 10.1007/s10654-004-6879-7. [DOI] [PubMed] [Google Scholar]
- 43.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. Jama. 2000;283:2552–8. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]