Abstract
HPV vaccination is expected to reduce the incidence of cervical cancer. The greatest and the earliest health gains will be ensured by high vaccine coverage among all susceptible people. The high costs and the risk of a reduced cost/effectiveness ratio in sexually active girls still represent the main obstacles for a more widespread use of HPV vaccination in many countries. Data on the rate, risk factors, and HPV types in sexually active women could provide information for the evaluation of vaccination policies extended to broader age cohorts.
Sexually active women aged 13–26 years enrolled in an Italian cohort study were screened for cervical HPV infections; HPV-DNA positive samples were genotyped by InnoLipa HPV Genotyping Extra or by RFLP genotype analysis.
Among the 796 women meeting the inclusion criteria, 10.80% (95% CI 8.65–12.96) were HPV-DNA infected. Age >18 years, lifetime sexual partners >1, and history of STIs were associated to higher risk of HPV infection in the multivariable models adjusted for age, lifetime sexual partners, and time of sexual exposure. The global prevalence of the four HPV vaccine-types was 3.02% (95% CI 1.83–4.20) and the cumulative probability of infection from at least one vaccine-type was 12.82% in 26-years-old women and 0.78% in 18-years-old women.
Our data confirm most of the previously reported findings on the risk factors for HPV infections. The low prevalence of the HPV vaccine-types found may be useful for the evaluation of the cost/efficacy and the cost/effectiveness of broader immunization programs beyond the 12-years-old cohort.
Keywords: HPV infection, HPV vaccine, HR-HPV, sexually active girls, risk factors
Introduction
HPV is the etiological agent in virtually 100% of cervical cancer cases; HPV-16 is the most common type and, combined with HPV-18, accounts for more than 70% of all cases of cervical cancer.1-3 In 2007 two preventive vaccines directed against these two HPV types aimed to reduce incidence of HPV-associated cervical cancers were commercialized, and more than 30 countries use the HPV vaccine as part of their national immunization programs.
Because HPV infections are commonly acquired soon after sexual activity,4 and is generally accepted that the vaccine offered to girls before the first coitus results in the best cost-effective ratio, thus limiting health costs,5 the vaccination programs are proposed to 12-y-old girls. A catch up vaccination in people between 16- and 26-y-old or vaccine programs open to girls aged between 11 and 18 y are also offered in some countries.5
The cost represents the main obstacle to the implementation of a wider use of the HPV vaccination in many countries, and the need for effective cytological screening programs even in vaccinated women as prevention strategy for non-vaccine related HPV cancers adds further limits to the wider use of the primary vaccine prevention for cervical cancers. The lack of a vaccine platform for young girls, the low vaccine acceptability related to low perceived risk, and parents’ fears regarding safety and the possible promotion of increased sexual activity combine to result in the generally low coverage obtained in several countries6-9 when vaccination is restricted to a single age group.
Several decades are needed to evaluate the effectiveness of the vaccine campaign for the long interval between HPV infection and cancer development, but it is expected that the greatest and the earliest health gains are ensured by high vaccine coverage among all susceptible people.10 In mathematical models,11 school-based and clinic-based catch-up HPV immunization and a combination of catch-up immunization and delayed beginning of the screening could result in cost savings and net health gains.
The data on the vaccine coverage in Italy for 12-y-old girls included in the National Immunization Program started in 2007 demonstrated 66.0%, 64.0%, and 58.6% vaccination rates for the cohorts born in 1997, 1998, and 1999 respectively.12 These results are far from the expected coverage of 95% for the 2003 birth cohort.13
This paper describes the rate, risk factors, and type of HPV infections in sexually active teens and young women (aged 13–26 y) enrolled in an on-going, multicenter cohort study (VALHIDATE study) in the Lombardy Region (Italy) to evaluate if an extension to other age cohorts than the 12-y-old girls is feasible in this region.
Results
Among 3954 women enrolled in the VALHIDATE study between December 2010 and January 2013, 796 met the inclusion criteria for this sub-analysis. Their mean age was 20.01 (±3.71) years ranging from 13 to 26 y. The median age of women seeking gynecologic consultation was 23 y (IQR 20–25 y) and the median age of those seeking pediatric consultation was 17 (IQR 16–17 y). The mean age of first coitus was 16.31 (±1.91) y, and the mean number of years since the first coitus was 3.69 (±3.06).
The HPV-DNA test was performed for all patients (in cervical brushes or urine samples) and 86 samples (10.80%; 95% CI 8.65–12.96) were HPV-positive. In particular, HPV-DNA was detected in 4.27% (15/351, 95% CI 2.16–6.39) and 15.96% (71/445, 95% CI 12.55–19.36) of urine and cervical brush samples, respectively. A significant difference in the prevalence rate is observed when the data are stratified for age (χ2 P < 0.0001 and χ2 for trend P < 0.0001) with a peak prevalence occurring in the 19–21 y age group (Fig. 1).
Figure 1. Distribution (prevalence and 95% CI) of HPV infection by age in 796 sexually active young women.
Cervical cytology, available for 444 patients, was positive in 42 (9.46%) cases. There were 13 cases of ASC-us, 28 cases of LSIL, and 1 case of ASC-h.
HPV vaccine and risk factors for HPV infection
Among the women included in this analysis, 133 (16.71%) were recently vaccinated for HPV with a voluntary vaccine course at ages ranging from 14 to 26 y, beyond the National Immunization Program; 59 of them had completed the third dose of vaccine at time of enrollment. The median time from the first vaccine dose and enrollment in the study was 170.5 d (IQR 15–493 d). In 15 cases (11.28%), HPV-DNA was found in cervical or urine samples. In 7 cases an HPV infection was found within 1 mo from the vaccine. There were no cases of new infection from vaccine-type HPV acquired after vaccination.
In Table 1 are reported the OR and 95% CI of HPV infection in the univariate and multivariate models adjusted for age and lifetime sexual partners (model 1), and adjusted for age, lifetime sexual partners, and time between first sexual intercourse and enrollment in the study (models 2): age between 19 and 26 y, being born abroad, current or past cigarette smoking, time of sexual exposure ≥4 y, lifetime sexual partners ≥2 or >1 in the last 6 mo, past or ever use of EP, and a history of STIs are related to a higher risk of HPV infection.
Table 1. Prevalence of cervical HPV infection and associations between infection and selected characteristics in the univariate and multivariate analyses.
| HPV prevalence | Univariate model | Multivariate Model 1 | Multivariate Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | HPV pos/N tested | Fisher exact test (P) | χ2 for trend | Rate (%) | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | |
| Age group | ||||||||||||
| 13–15 | 3/79 | <0.0001 | 3.80 | -0.42-8.01 | 1 | ref | 1 | ref | 1 | ref | ||
| 16–18 | 14/285 | 4.91 | 2.40–7.42 | 1.31 | 0.37–4.67 | 1.01 | 0.28–3.78 | 1.93 | 0.40–9.38 | |||
| 19–21 | 25/120 | 20.83 | 13.57–28.10 | 6.67 | 1.94–22.93 | 4.13 | 1.17–14.60 | 9.61 | 1.71–54.09 | |||
| 22–26 | 44/312 | 14.10 | 10.24-17.96 | 4.16 | 1.26–13.77 | 2.33 | 0.68–7.99 | 6.25 | 1.03–37.79 | |||
| Geographical origin | ||||||||||||
| Italian | 77/752 | 0.044 | 10.24 | 8.07–12.41 | 1 | ref | 1 | ref | 1 | ref | ||
| Foreign born | 9/44 | 20.45 | 8.54–32.37 | 2.25 | 1.04–4.87 | 1.70 | 0.79–3.67 | 1.79 | 0.81–3.96 | |||
| Cigarette smoking | ||||||||||||
| yes | 40/292 | 0.05 | 13.70 | 9.75–17.74 | 1.58 | 1.0–2.48 | 1.29 | 0.80–2.10 | 1.29 | 0.78–2.13 | ||
| never | 46/504 | 9.13 | 6.61–11.64 | 1 | ref | 1 | ref | 1 | ref | |||
| HPV vaccine | ||||||||||||
| No | 71/663 | 0.88 | 10.71 | 8.36–13.06 | 0.94 | 0.52–1.7 | 1.13 | 0.61–2.08 | 1.13 | 0.60–2.12 | ||
| Yes | 15/133 | 11.28 | 5.90–16.65 | 1 | ref | 1 | ref | 1 | ref | |||
| Age of 1st sexual intercourse | ||||||||||||
| ≤15 | 27/278 | 0.40 | 9.71 | 6.23–13.19 | 0.8 | 0.49–1.3 | 1.10 | 0.61–1.97 | 1.23 | 0.63–2.40 | ||
| >15 | 57/481 | 11.85 | 8.96–14.74 | 1 | ref | 1 | ref | 1 | ref | |||
| Δ years | ||||||||||||
| 0 | 5/104 | 0.0012 | <0.0001 | 4.81 | 0.70–8.92 | 1 | ref | 1 | ref | 1 | ref | |
| 1 | 8/151 | 5.30 | 1.73–8.87 | 1.11 | 0.35–3.49 | 0.56 | 0.16–1.93 | 0.56 | 0.16–1.93 | |||
| 2 | 7/91 | 7.69 | 2.22–13.17 | 1.65 | 0.50–5.39 | 0.52 | 0.14–2.02 | 0.52 | 0.14–2.02 | |||
| 3 | 7/65 | 10.77 | 3.23–18.31 | 2.39 | 0.72–7.88 | 0.37 | 0.08–1.65 | 0.37 | 0.08–1.65 | |||
| 4 | 10/67 | 14.93 | 6.39–23.46 | 3.47 | 1.13–10.67 | 0.33 | 0.07–1.56 | 0.33 | 0.07–1.56 | |||
| 5 | 9/65 | 13.85 | 5.45–22.24 | 3.3 | 1.05–10.35 | 0.30 | 0.06–1.45 | 0.30 | 0.06–1.45 | |||
| 6 | 13/51 | 25.49 | 13.53–37.45 | 6.77 | 2.26–20.30 | 0.62 | 0.13–2.97 | 0.62 | 0.13–2.97 | |||
| 7 | 8/52 | 15.38 | 5.58–25.19 | 3.6 | 1.11–11.63 | 0.33 | 0.06–1.80 | 0.33 | 0.06–1.80 | |||
| ≥8 | 17/111 | 15.32 | 8.62–22.02 | 3.58 | 1.27–10.10 | 0.33 | 0.07–1.67 | 0.33 | 0.07–1.67 | |||
| Total N of sexual partners | ||||||||||||
| 1 | 14/326 | <0.0001 | <0.0001 | 4.29 | 2.09–6.50 | 1 | ref | 1 | ref | 1 | ref | |
| 2 -5 | 54/390 | 13.85 | 10.42–17.27 | 3.58 | 1.95–6.58 | 2.69 | 1.42–5.09 | 3.69 | 1.71–8.00 | |||
| >5 | 17/70 | 24.29 | 14.24–34.33 | 7.15 | 3.33–15.36 | 4.71 | 2.10–10.57 | 6.44 | 2.46–16.86 | |||
| N of sexual partners (last 6 mo) | ||||||||||||
| 0 | 7/57 | 0.015 | 0.06 | 12.28 | 3.76–20.80 | 1.33 | 0.58–3.06 | 2.09 | 0.86–5.09 | 2.89 | 1.13–7.42 | |
| 1 | 61/640 | 9.53 | 7.26–11.81 | 1 | ref | 1 | ref | 1 | ref | |||
| >1 | 17/86 | 19.77 | 11.35–28.18 | 2.34 | 1.29–4.23 | 1.89 | 0.92–3.91 | 2.07 | 0.97–4.40 | |||
| Current use of EP | ||||||||||||
| Yes | 31/283 | 0.59 | 10.95 | 7.32–14.59 | 1.18 | 0.70–1.98 | 0.78 | 0.45–1.35 | 0.76 | 0.43–1.35 | ||
| No | 33/350 | 9.43 | 6.37–12.49 | 1 | ref | 1 | ref | 1 | ref | |||
| Past use of EP | ||||||||||||
| Yes | 42/266 | 0.0003 | 15.79 | 11.41–20.17 | 2.62 | 1.53–4.49 | 1.35 | 0.71–2.59 | 1.61 | 0.79–3.29 | ||
| No | 23/345 | 6.67 | 4.03–9.3 | 1 | ref | 1 | ref | 1 | ref | |||
| Ever use of EP | ||||||||||||
| Yes | 51/407 | 0.023 | 12.54 | 9.31–15.75 | 2.5 | 1.1–5.65 | 1.00 | 0.41–2.44 | 1.49 | 0.54–4.01 | ||
| No | 7/129 | 5.43 | 1.52–9.34 | 1 | ref | 1 | ref | 1 | ref | |||
| Pregnancies | ||||||||||||
| Yes | 5/34 | 0.40 | 14.71 | 2.8–26.61 | 1.45 | 0.54–3.85 | 0.75 | 0.27–2.07 | 0.69 | 0.25–1.93 | ||
| No | 81/762 | 10.63 | 8.44–12.82 | 1 | ref | 1 | ref | 1 | ref | |||
| History of STIs or genital infections* | ||||||||||||
| Yes | 33/152 | <0.0001 | 21.71 | 15.16–28.26 | 3.08 | 1.91–4.96 | 1.85 | 1.09–3.15 | 1.86 | 1.07–3.24 | ||
| No | 53/641 | 8.27 | 6.14–10.40 | 1 | ref | 1 | ref | 1 | ref | |||
Model 1 is adjusted for age and lifetime sexual partners. Model 2 is adjusted for age, lifetime sexual partners, and time between first sexual intercourse and enrollment (Δ years). °Δ years, years between first sexual intercourse and enrollment in the study. *STIs or genital infections reported in the history: 115 genital mycosis, 25 genital warts, 20 bacterial vaginosis, 8 genital HSV, 6 Chlamydia, 2 Trichomoniasis. In bold are the statistically significant results.
No increased risk was found for the current use of EP drugs, for young age at first sexual intercourse (≤15 y), or for parity.
The variable “HPV vaccine” did not reach a significant preventive effect in this analysis for the very short time elapsing between vaccine and enrollment in the study.
In the multivariate models 2, older age, number of sexual partners >1, and a history of STIs were the factors strongly associated to the risk of HPV infection (Table 1).
HPV typing
Overall, 88.37% (76/86) HPV-positive samples were suitable for typing: 38 (50.0%) were infections sustained by a single HPV type and 38 (50%) by multiple HPV types (ranging from 2 to 6).
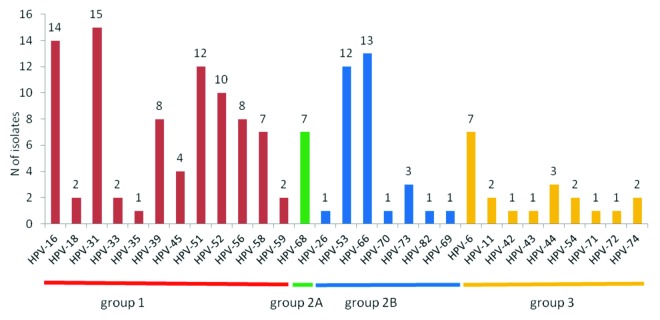
The type distribution is reported in Figure 2: HPV-31 and -16 were the most common types identified followed by HPV-66, -51, and -53. Among the other vaccine types only HPV-6 was found in several cases while we did not found a high prevalence of vaccine types HPV-11 and HPV-18.
Figure 2. HPV types identified in 76 cervical samples arranged according to IARC risk groups. Group 1, Group 2A, and Group 2B constitute the HR-clade.
Infections sustained by at least 1 HR-HPV were found in 94.74% of women with a typed HPV infection and in 9.05% of the entire cohort; infections by at least 1 of the HPV types included in the G1 of the IARC classification14 for the oncogenic risk were 78.95% of the women with a typed infection and in 7.54% of the entire cohort. The vaccine-type HPV-16 and/or HPV-18 were found in 21.05% of women with a typed infection, with an overall prevalence rate on the entire population of 2.01%.
A very low rate of infections sustained only by LR-HPV types (5.26% women with a typed infection and 0.50% of the entire cohort) was found (Table 2).
Table 2. Prevalence rate (and the related 95% CI) of HPV infections sustained by vaccine, G1 or HR-types, by single HPV-type or multiple HPV-types, in the HPV-typed women and in the entire cohort.
| N positive women | Prevalence rate (among 76 women with a typed HPV infection) | 95% CI | Prevalence rate (on the entire cohort, 796) | 95% CI | |
|---|---|---|---|---|---|
| HPV-6/11/16/18 | 24 | 31.58 | 22.13–42.03 | 3.02 | 1.83–4.20 |
| HPV-16/18 | 16 | 21.05 | 11.89–30.22 | 2.01 | 1.04–2.99 |
| HPV-HR G1* | 60 | 78.95 | 69.78–88.11 | 7.54 | 5.70–9.37 |
| any HR-HPV^ | 72 | 94.74 | 89.72–99.76 | 9.05 | 7.05–11.04 |
| Only LR-HPV | 4 | 5.26 | 0.24–10.28 | 0.50 | 0.01–0.99 |
| Single HPV | 38 | 50.00 | 46.53–53.47 | 4.77 | 3.29–6.26 |
| Multiple HPV | 38 | 50.00 | 46.53–53.47 | 4.77 | 3.29–6.26 |
*G1 types (carcinogenic to humans) include: HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -59. ^Any HR types include G1 (carcinogenic to humans); G2A (probably carcinogenic to humans): HPV-68; G2B (possibly carcinogenic to humans): HPV-26, -30, -34, -53, -66, -67, 69, -70, -73, -82, -- 85, -97.
Probability of infection by age
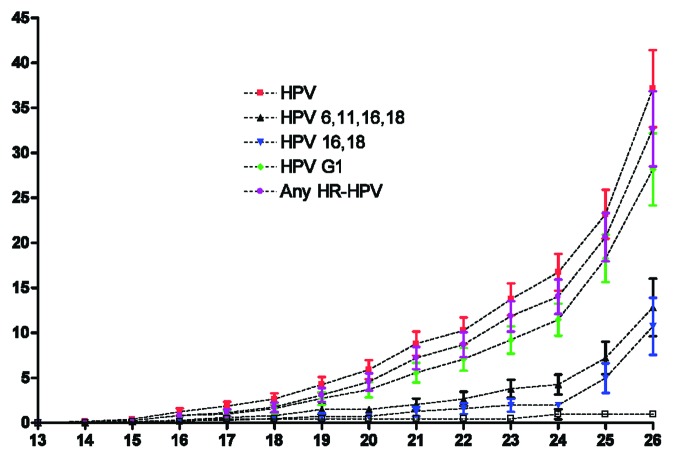
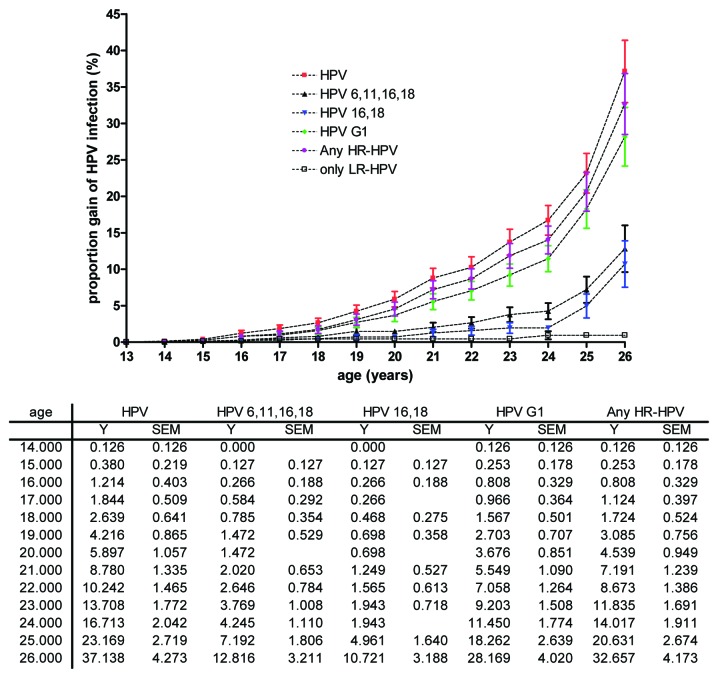
The Kaplan–Meier plot of the estimated cumulative proportion of women with an HPV infection is represented in Figure 3. Women aged 26 y have a cumulative risk of being infected with HPV (any HPV type), with an HR-HPV type, or with at least 1 of the HPV types included in the G1 of 37.13%, 32.66%, and 28.17%, respectively; the cumulative proportion of women with an infection caused by the vaccine-types HPV-16/18 or HPV-6/11/16/18 was 10.72% and 12.82 respectively, with a sharper increase in older ages. At 18 y of age, the risk of having an HPV infection (any type) was 2.64%, and the risk of having a vaccine-types infection was 0.47% for HPV-16/18 and 0.78% for HPV-6/11/16/18.
Figure 3. Cumulative proportion (mean and SEM) of infections from selected HPV types or HPV groups in young sexually active women (age 13–26 y).
Discussion
An association between young age and cervical HPV infections15-19 is generally reported with a higher susceptibility to the infection at the beginning of sexual activity, with peak prevalence in younger women and progressive decline with increasing age. Winer et al.4 reported a cumulative 24-mo incidence of HPV infection in female students aged 18–20 y of 38.8% (95% CI 33.3–45.0) for the students who were sexually active at time of enrollment and of 38.9% (95% CI 29.4–50.3) for the students who become sexually active during the study.
This paper analyses a sample of sexually active teens and young women enrolled in an on-going prospective cohort study to evaluate the prevalence rate, the risk factors, and the type specific distribution of the early HPV infections.
The age of the first coitus found in this cohort was 16.31 y (±1.91) which is consistent with other recent studies on sexual behavior of young people in Italy.20
The prevalence of infection found in our study (10.80%; 95% CI 8.65–12.96) is lower than that reported by other Authors for the young women cohort.
A recent cross sectional study21 in women undergoing routine cervical cancer screening in Spain found a prevalence of HPV infection ranging from 26% to 29.1% in the age group 18–25 y and observed that young age is one of the strongest independent risk factors for HPV infection compared with the risk of older women. Howell-Jones et al.22 found a prevalence of HR-HPV of 34.6% (95% CI 32.6–36.7) and 18.2% (95% CI 16.1–20.5) in 2 groups of young women (16- to 24-y-old) recruited among participants to Chlamydia screening programs and 22.6% (95% CI 17.6–28.6) in younger women (13- to 15-y-old) recruited into the same programs. Giambi et al.23 found a point prevalence of HR-HPV infection in a sample of Italian women aged 18–26 y of 18.7%, which is very close to that found in our study for the same age group.
In our study, the range of ages evaluated are wider, and the lower rates found could be due to difference in the ages included in the term “young women,” and to differences in demographic/behavioral variables. The high concordance rate of HPV DNA detection (Cohen’s unweighted k = 0.96; 95% CI: 0.90–1.0) observed in paired urine and cervical samples recently described by our group24 with the newly designed primers (ELSI-f and ELSI-r) suggest that the lower rates found in this study are not due to under estimates for the sample used. Our data show a bell-shaped curve of HPV prevalence with lower rates for the 13–15 and 16–18 y groups (prevalence 3.80% and 4.91% respectively), a peak in the 19–21 y group (rate 20.83%), and a reduction in the 22–26 y group (14.10%); the prevalence proved to be independent from the overall years of sexual exposure in the multivariate models, as already reported by Howell et al.,22 and linked mainly to sexual behavioral characteristics. Lifetime or recent sexual partners >1 and a history of other STIs are the strongest predictors of infection, as already reported by several studies.21-23,25-27
We did not observe an association between a young age at the first sexual intercourse (≤15 y) and the risk of HPV infection, which had been reported by other authors.21-23,25-28 The correlation between the years of sexual exposure to infection that we found in the univariate analysis was not confirmed by the multivariate model adjusted for age and lifetime sexual partners.
Past or ever use of EP was found to be significantly related to the risk of HPV infection in the crude analysis, but this result was not confirmed in the multivariate model. No increased risk was found for the current use of EP drugs. Cigarette smoking and being born abroad proved to be independent risk factors for HPV infection in the univariate analysis but not in the adjusted models.
Early infections are mainly due to HR-HPV types (94.74%) and mixed infections are very common and account for half of the cases. Our findings are in agreement with other epidemiological studies regarding the type distribution for the high prevalence of infections from HPV-16 and the low prevalence of infections from HPV-18.16 The vaccine-types HPV-16/18 were found in 21.05% and the vaccine-types HPV-6/11/16/18 in 31.58% of women with an HPV-typed infection.
The majority of cervical abnormalities, detected in 9.46% of Pap test smears, will be cleared within 24 mo and are not expected to progress to high grade or invasive cervical diseases, which are only rarely diagnosed in women aged less than 25 y.29 For this reason the widespread implementation of Pap screening in young women is generally considered unnecessary or even harmful because of the risk of over treatment of otherwise transitory conditions.30-32
If screening is not indicated for young women, the HPV prophylactic commercially available vaccines represent an adjunctive tool for the prevention of cervical cancer, which is still the third most common female cancer worldwide with approximately 34 000 new cases and causes more than 16 000 deaths annually in the European Union.33-35
The effects of the vaccine on the epidemiology of cervical cancers are expected within several years from the beginning of the vaccine primary prevention and the protective effect is related to the extent of vaccine coverage of susceptible people.36,37
In this study we found that the overall prevalence of infection from vaccine-type HPVs in sexually active women is low in the age group of 13–26 y (2.01% for HPV-16/18 and 3.02% for HPV-6/11/16/18 of the entire population studied) and that the cumulative risk of being already infected from any of the vaccine-HPV types is less than 1% prior the age of 18 y; a wide margin of interventions with vaccine primary prophylaxis beyond the pre-adolescent girls aged 12 y, who are actually the target for vaccine in Italy, could be expected.
Potential limitation of this study is the recruitment of women seeking gynecologic or pediatric consultation which could be not representative of the general young female population. However data on HPV infection in very young population are scarce or absent due to the difficulties of recruitment for HPV screening in otherwise healthy individuals.
Our data confirm the majority of the previously reported findings on the risk factors and type specific epidemiology of cervical HPV infections acquired soon after the beginning of sexual activity; the data show that there are significant differences in the prevalence of infection by ages within the ”young age” group, and we provide estimates of the prevalence of vaccine-type HPVs in young women before their inclusion in national screening programs which starts at the age of 26 y. These results could provide basic information regarding the prevalence of the vaccine-type and other HR-HPV types before the widespread introduction of the vaccine in the Lombardy Region, could allow for evaluation and monitoring of the cross protection and the type replacement phenomena in the next future, and could be used for evaluating the cost/efficacy and cost/effectiveness of future immunization programs in young women beyond the 12 y-old cohort.
Patients and Methods
The VALHIDATE study is a 5-y multicenter open prospective cohort study, approved by the local ethics committees of all the participating centers, aimed to provide information regarding bio-molecular epidemiology of HPV infection and cervical diseases in high-risk women in the Lombardy Region. The complete VALHIDATE study design, methods, and end points have been described elsewhere.38 Briefly, the patients are recruited at 10 clinical centers located in 3 cities in Lombardy (Milan, Brescia, Lodi). The control group is made up of women aged 26–64 y attending a spontaneous Pap screening program; the testing groups of at-risk women are recruited among HIV infected women, among recent migrant women (arrived in Italy less than 1 y previously), and among young women aged 13–26 y asking for a gynecologic or pediatric consultation.
In this analysis, we evaluated sexually active women aged less than 26 y enrolled in the main study from November 2010 to January 2013.
At baseline, all the patients underwent a medical assessment by a gynecologist, an infectious diseases specialist, or a pediatrician, as appropriate; demographic, socio-economic, behavioral data, and medical history were collected and recorded on a specially developed secure eCRF by each participating center.
The cervical brush (Cytobrush Plus MedscandW Medical AB, cod. C0121) collected during the gynecologic visit was used to perform a conventional Pap smear and then immersed and stored into a PreservCyt solution (ThinPrep Pap Test, Hologic, cod. 70136-001) to be analyzed for HPV-DNA test and HPV genotyping using INNO-LiPA HPV Genotyping Extra (Innogenetics NV, cod. INX44948), a line probe assay based on the principle of reverse hybridization. The Pap tests were evaluated according to the 2001 Bethesda System terminology39 by expert cytopathologists of the participating centers.
For the young girls attending pediatric units, cervical cytology was not performed for ethical reasons, and a urine sample was collected and analyzed for the HPV-DNA and HPV genotyping using an in house PCR based assay and a Restriction Fragment Length Polymorphism (RFLP) method proved to be highly sensitive and specific for HPV-DNA detection and genotyping in urine samples.38
DNA extraction, HPV detection, and genotyping
Pre-treatment of cervical and urine samples, DNA extraction, HPV-DNA detection, and HPV genotyping were performed as previously described.24,38 Briefly, DNA was extracted using a commercial method (NucliSENS® EasyMAG®, bioMérieux, cod. 200111), and the HPV-DNA was detected through PCR amplification of a 450 bp segment of ORF L1 using the degenerate primer pair ELSI-f and ELSI-r in the reference centralized laboratory. HPV genotyping was performed in HPV-DNA positive cervical brushes by the commercially available Inno-LiPA® HPV Genotyping Extra (Innogenetics NV, cod. INX44948) in the microbiology laboratories of the participating centers. This test allows for the identification of 25 HPV types. All the HPV-DNA positive urine samples and the cervical samples resulted as non-typeable by the InnoLipa test (Inno-LiPA® HPV-X) were subjected to RFLP genotype analysis capable of identifying all genotypes in the high-risk clade (HR-clade) and low-risk (LR) genotypes of the α genus according to the 2011 IARC classification.14
Statistical analysis
The patient selection for this analysis was performed in the coordination center using the data collected and recorded on the eCRF by the participating centers and anonymously extracted by the data-storage center. Descriptive statistics (means ± SD or median and IQR) are applied to describe the entire study sample with regard to demographics, behavioral, clinical, and laboratory characteristics. The differences between the groups were evaluated with the Mann Whitney U test for continuous variables and the Fisher exact test or the Chi square test for categorical variables.
Univariate and multivariate logistic regression analyses were performed to calculate the OR and 95% CI of the association between the selected variables and HPV infection. The dependent variable was the HPV infection detected by positive HPV-DNA on cervical or urine samples at the time of enrollment in the VALHIDATE study. The potential associated variables evaluated in this work were age, geographical origin of the patient, cigarette smoking, HPV vaccine, age at first sexual intercourse, years from first coitus, number of sexual partners (lifetime and in the last 6 mo), current, past, or ever use of EP, parity, and history of STIs.
Two multivariate models were evaluated. In the first equation, the age and lifetime sexual partners were included as adjustment variables, and in the second equation, the time between the first sexual intercourse and enrollment in the study (Δ years) was added; all the other variables with univariate P value < 0.5 were tested in the multivariate model. A P value < 0.05 was considered statistically significant.
Estimates of the cumulative proportion of HPV infections (any HPV type or selected HPV types/groups) were analyzed using Kaplan–Meier plots of ages (from 13 to 26 y).
All the reported P values are two sided; the data were analyzed using SAS/STAT version 9.1 software.
Glossary
Abbreviations:
- HPV
Human Papillomavirus
- VALHIDATE
eVALuation and monitoring of HPV infections and relATEd cervical diseases in high-risk women
- HR
high-risk
- EP
oestroprogestinic
- STIs
sexually transmitted infections
- G1
Group 1
- IARC
International Agency for Research on Cancer
- LR
low-risk
- eCRF
electronic case report form
- LSIL
low grade squamous intraepithelial lesion
- HSIL
high grade squamous intraepithelial lesion
- ASC-us
atypical squamous cells of undetermined significance
- ASC-h
atypical squamous cells that cannot exclude HSIL
- CA
carcinoma
- RFLP
restriction fragment length polymorphism
- SD
standard deviation
- CI
confidence intervals
- IQR
interquartile range
- OR
odd ratios
- SEM
standard error of the mean
- HPV-X
non-typeable HPV
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was funded by the Health General Direction, Regione Lombardia, Italy (DGR 10813/2009). The authors thank Emanuele Castelli who developed and provided technical support for the eCRF, Liliane Chatenoud, and Paola Bertuccio for the optimisation and data quality control of the database.
Author Contributions
G.O. conceived, coordinated, and supervised the study. M.F., S.E., I.C., and G.V.Z. contributed to the recruitment of the participants contributing substantially to the acquisition of the clinical and epidemiological data. M.F., E.T., and E.F. coordinated and contributed to the laboratory testing for HPV-DNA and HPV genotyping. G.O. and M.F. conducted the first statistical analyses. Ricci E refined and performed the final statistical analysis. G.O., E.T., F.M., and M.F. evaluated the results and wrote the manuscript. M.G. contributed to the conception and design of the study, secured study funding, and critically revised the manuscript. All the authors read, revised, and approved the final manuscript.
VALHIDATE study group
The VALHIDATE study group contributes substantially to the acquisition of clinical and epidemiological data and evaluated cervical and urine samples according to the study protocol. Members of this group are listed as follows: Maria Concetta Antonacci, Irene Arcidiacono, Silvia Bianchi, Veronica Boero, Giuseppe Cambiè, Elena Casolati, Valentina Montinaro, Marcella Falchetti, Marianna Martinelli, Carlo Galli, Emanuela Bertazzoli, Giovanna Lunghi, Alberto Matteelli, Giancarlo Tisi, Anna Maria Villa, Nadia Zanchetta, Laura Pogliani.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJLM, Muñoz N. . Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 9; http://dx.doi.org/; PMID: 10451482 [DOI] [PubMed] [Google Scholar]
- 2.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. . Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 2011; 128:927 - 35; http://dx.doi.org/ 10.1002/ijc.25396; PMID: 20473886 [DOI] [PubMed] [Google Scholar]
- 3.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. , Retrospective International Survey and HPV Time Trends Study Group. . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048 - 56; http://dx.doi.org/ 10.1016/S1470-2045(10)70230-8; PMID: 20952254 [DOI] [PubMed] [Google Scholar]
- 4.Winer RL, Lee S-K, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. . Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003; 157:218 - 26; http://dx.doi.org/ 10.1093/aje/kwf180; PMID: 12543621 [DOI] [PubMed] [Google Scholar]
- 5.Dorleans F, Giambi C, Dematte L, Cotter S, Stefanoff P, Mereckiene J, O’Flanagan D, Lopalco PL, D’Ancona F, Levy-Bruhl D, VENICE 2 project gatekeepers group. . The current state of introduction of human papillomavirus vaccination into national immunisation schedules in Europe: first results of the VENICE2 2010 survey. Euro Surveill 2010; 15:19730; PMID: 21144444 [DOI] [PubMed] [Google Scholar]
- 6.Dempsey AF, Zimet GD, Davis RL, Koutsky L. . Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics 2006; 117:1486 - 93; http://dx.doi.org/ 10.1542/peds.2005-1381; PMID: 16651301 [DOI] [PubMed] [Google Scholar]
- 7.Friedman AL, Shepeard H. . Exploring the knowledge, attitudes, beliefs, and communication preferences of the general public regarding HPV: findings from CDC focus group research and implications for practice. Health Educ Behav 2007; 34:471 - 85; http://dx.doi.org/ 10.1177/1090198106292022; PMID: 17000622 [DOI] [PubMed] [Google Scholar]
- 8.Hopkins TG, Wood N. . Female human papillomavirus (HPV) vaccination: global uptake and the impact of attitudes. Vaccine 2013; 31:1673 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2013.01.028; PMID: 23375978 [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et al. . Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013; 105:175 - 201; http://dx.doi.org/ 10.1093/jnci/djs491; PMID: 23297039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gefenaite G, Smit M, Nijman HW, Tami A, Drijfhout IH, Pascal A, Postma MJ, Wolters BA, van Delden JJM, Wilschut JC, et al. . Comparatively low attendance during Human Papillomavirus catch-up vaccination among teenage girls in the Netherlands: Insights from a behavioral survey among parents. BMC Public Health 2012; 12:498; http://dx.doi.org/ 10.1186/1471-2458-12-498; PMID: 22748022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tully SP, Anonychuk AM, Sanchez DM, Galvani AP, Bauch CT. . Time for change? An economic evaluation of integrated cervical screening and HPV immunization programs in Canada. Vaccine 2012; 30:425 - 35; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.067; PMID: 22075091 [DOI] [PubMed] [Google Scholar]
- 12.Giambi C. Stato di avanzamento della campagna vaccinale per l’HPV: dati di copertura vaccinale al 30/06/2012 – Rapporto Semestrale [Internet]. Available from: http://www.epicentro.iss.it/problemi/hpv/pdf/Aggiornamento_HPV_30062012_validato.pdf
- 13.Piano Nazionale Prevenzione Vaccinale (PNPV) 2012-2014 – Ministero della salute [Internet]. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf
- 14.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks To Humans. A Review of Human Carcinogens. 100, part B. Geneva, Switzerland: WHO; 2012. Human papillomaviruses; pp. 255–314. [Google Scholar]
- 15.Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Anh PTH, Bosch FX, Ferreccio C, Hieu NT, Lazcano-Ponce E, et al. . Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer 2006; 119:2677 - 84; http://dx.doi.org/ 10.1002/ijc.22241; PMID: 16991121 [DOI] [PubMed] [Google Scholar]
- 16.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. . Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7:453 - 9; http://dx.doi.org/ 10.1016/S1473-3099(07)70158-5; PMID: 17597569 [DOI] [PubMed] [Google Scholar]
- 17.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. . Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202:1789 - 99; http://dx.doi.org/ 10.1086/657321; PMID: 21067372 [DOI] [PubMed] [Google Scholar]
- 18.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Muñoz N. . Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26:Suppl 10 K1 - 16; http://dx.doi.org/ 10.1016/j.vaccine.2008.05.064; PMID: 18847553 [DOI] [PubMed] [Google Scholar]
- 19.Agarossi A, Ferrazzi E, Parazzini F, Perno CF, Ghisoni L. . Prevalence and type distribution of high-risk human papillomavirus infection in women undergoing voluntary cervical cancer screening in Italy. J Med Virol 2009; 81:529 - 35; http://dx.doi.org/ 10.1002/jmv.21347; PMID: 19152401 [DOI] [PubMed] [Google Scholar]
- 20.Panatto D, Amicizia D, Trucchi C, Casabona F, Lai PL, Bonanni P, Boccalini S, Bechini A, Tiscione E, Zotti CM, et al. . Sexual behaviour and risk factors for the acquisition of human papillomavirus infections in young people in Italy: suggestions for future vaccination policies. BMC Public Health 2012; 12:623; http://dx.doi.org/ 10.1186/1471-2458-12-623; PMID: 22871132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roura E, Iftner T, Vidart JA, Kjaer SK, Bosch FX, Muñoz N, Palacios S, Rodriguez MSM, Morillo C, Serradell L, et al. , CLEOPATRE Spain Study Group. . Predictors of human papillomavirus infection in women undergoing routine cervical cancer screening in Spain: the CLEOPATRE study. BMC Infect Dis 2012; 12:145; http://dx.doi.org/ 10.1186/1471-2334-12-145; PMID: 22734435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell-Jones R, de Silva N, Akpan M, Oakeshott P, Carder C, Coupland L, Sillis M, Mallinson H, Ellis V, Frodsham D, et al. . Prevalence of human papillomavirus (HPV) infections in sexually active adolescents and young women in England, prior to widespread HPV immunisation. Vaccine 2012; 30:3867 - 75; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.006; PMID: 22516212 [DOI] [PubMed] [Google Scholar]
- 23.Giambi C, Donati S, Carozzi F, Salmaso S, Declich S, Atti ML, Ronco G, Alibrandi MP, Brezzi S, Collina N, et al. . A cross-sectional study to estimate high-risk human papillomavirus prevalence and type distribution in Italian women aged 18-26 years. BMC Infect Dis 2013; 13:74; http://dx.doi.org/ 10.1186/1471-2334-13-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanzi E, Bianchi S, Fasolo MM, Frati ER, Mazza F, Martinelli M, Colzani D, Beretta R, Zappa A, Orlando G. . High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J Med Virol 2013; 85:91 - 8; http://dx.doi.org/ 10.1002/jmv.23434; PMID: 23097252 [DOI] [PubMed] [Google Scholar]
- 25.Monsonego J, Zerat L, Syrjänen K, Zerat JC, Smith JS, Halfon P. . Prevalence of type-specific human papillomavirus infection among women in France: Implications for screening, vaccination, and a future generation of multivalent HPV vaccines. Vaccine 2012; 30:5215 - 21; http://dx.doi.org/ 10.1016/j.vaccine.2012.06.013; PMID: 22713720 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen A, Kjaer SK, Munk C, Iftner T. . Type-specific HPV infection and multiple HPV types: prevalence and risk factor profile in nearly 12,000 younger and older Danish women. Sex Transm Dis 2008; 35:276 - 82; http://dx.doi.org/ 10.1097/OLQ.0b013e31815ac5c7; PMID: 18091564 [DOI] [PubMed] [Google Scholar]
- 27.Heard I, Cubie HA, Mesher D, Sasieni P, MACH-1 Study Group. . Characteristics of HPV infection over time in European women who are HIV-1 positive. BJOG 2013; 120:41 - 9; http://dx.doi.org/ 10.1111/1471-0528.12015; PMID: 23121095 [DOI] [PubMed] [Google Scholar]
- 28.Confortini M, Carozzi F, Zappa M, Ventura L, Iossa A, Cariaggi P, Brandigi L, Franchini M, Mirri F, Viacava P, et al. . Human papillomavirus infection and risk factors in a cohort of Tuscan women aged 18-24: results at recruitment. BMC Infect Dis 2010; 10:157; http://dx.doi.org/ 10.1186/1471-2334-10-157; PMID: 20529280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benard VB, Watson M, Castle PE, Saraiya M. . Cervical carcinoma rates among young females in the United States. Obstet Gynecol 2012; 120:1117 - 23; PMID: 23090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, Cheung LC, Raine-Bennett T, Gage JC, Kinney WK. . Five-year risks of CIN 3+ and cervical cancer among women with HPV testing of ASC-US Pap results. J Low Genit Tract Dis 2013; 17:Suppl 1 S36 - 42; http://dx.doi.org/ 10.1097/LGT.0b013e3182854253; PMID: 23519303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FAR, Moriarty AT, Waxman AG, Wilbur DC, et al. , American Cancer Society, American Society for Colposcopy and Cervical Pathology, American Society for Clinical Pathology. . American cancer society, american society for colposcopy and cervical pathology, and american society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516 - 42; http://dx.doi.org/ 10.1309/AJCPTGD94EVRSJCG; PMID: 22431528 [DOI] [PubMed] [Google Scholar]
- 32.Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L. . European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition--summary document. Ann Oncol 2010; 21:448 - 58; http://dx.doi.org/ 10.1093/annonc/mdp471; PMID: 20176693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr
- 34.Arbyn M, Raifu AO, Autier P, Ferlay J. . Burden of cervical cancer in Europe: estimates for 2004. Ann Oncol 2007; 18:1708 - 15; http://dx.doi.org/ 10.1093/annonc/mdm079; PMID: 17369600 [DOI] [PubMed] [Google Scholar]
- 35.Arbyn M, Autier P, Ferlay J. . Burden of cervical cancer in the 27 member states of the European Union: estimates for 2004. Ann Oncol 2007; 18:1423 - 5; http://dx.doi.org/ 10.1093/annonc/mdm377; PMID: 17693658 [DOI] [PubMed] [Google Scholar]
- 36.Chesson HW, Flagg EW, Koutsky L, Hsu K, Unger ER, Shlay JC, Kerndt P, Ghanem KG, Zenilman JM, Hagensee M, et al. . Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States. Vaccine 2013; 31:3019 - 24; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.051; PMID: 23664991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J. . Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer 2013; 13:10; http://dx.doi.org/ 10.1186/1471-2407-13-10; PMID: 23298365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlando G, Tanzi E, Chatenoud L, Gramegna M, Rizzardini G, VALHIDATE Study Group. . Rationale and design of a multicenter prospective cohort study for the eVALuation and monitoring of HPV infections and relATEd cervical diseases in high-risk women (VALHIDATE study). BMC Cancer 2012; 12:204; http://dx.doi.org/ 10.1186/1471-2407-12-204; PMID: 22646512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr., et al. , Forum Group Members, Bethesda 2001 Workshop. . The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114 - 9; http://dx.doi.org/ 10.1001/jama.287.16.2114; PMID: 11966386 [DOI] [PubMed] [Google Scholar]