Abstract
The demonstration of batch-to-batch consistency to confirm the reliability of the manufacturing process has become a mandatory step in vaccine development. This is a post-hoc analysis aimed to provide more solid evidence on the immunogenicity and consistency of 3 consecutive batches of a novel inactivated enterovirus 71 (EV71) vaccine. In total 10 245 healthy Chinese children aged 6–35 months had been recruited and randomized to receive one of 3 batches of EV71 vaccine or placebo according to a two-dose immunization schedule in a phase 3 clinical trial. Blood samples were taken just before and 28 days after vaccinations for serological tests of EV71 neutralizing antibody (NTAb) titer from the subjects. Among them, 7263 (70.9%) subjects with seronegative EV71 NTAb at baseline and the data of serological tests post-vaccination available were included for the analysis. The results showed that EV71 vaccine elicited high geometric mean titers (GMTs) of 407.0 U/mL (95% CI, 373.5–443.6) for batch 1, 468.1 U/mL (95% CI, 432.2–507.0) for batch 2, and 520.6 U/mL (95% CI, 481.2–563.3) for batch 3. The two-sided 95% confidence intervals (CIs) for the GMT ratios between each pair of vaccine batches were all within an interval of [0.67, 1.5]. Subjects who received EV71 vaccines demonstrated significant higher GMTs than those received placebos did (P < 0.001). In terms of incidence of both local and general adverse reactions, no differences were found among 3 vaccine batches and placebos. EV71 vaccine was highly immunogenic in children, and the 3 consecutive batches were well consistent.
Keywords: enterovirus 71, inactivated vaccine, batch consistency, immunogenicity, safety
Introduction
Since its first discovery in 1969, enterovirus 71 (EV71), which is the primary pathogenic agent responsible for periodic epidemics of hand, foot, and mouth disease (HFMD), has caused outbreaks of infection periodically throughout the world, particularly in Asia-Pacific region.1-6 Children, especially those younger than 3-y-old, are susceptible to the most severe forms of EV71-associated neurological disease.7
Currently, there was no effective medicine could against EV71 infection. EV71 vaccine could probably be the most promising method to prevent or control the prevalence of the EV71. So far, 5 EV71 vaccine candidates had been assessed in clinical trials, 3 of which manufactured in mainland China, and another 2 in Taiwan and Singapore.8 From January 2012 to March 2013, we completed a multicenter, double-blind, placebo-controlled phase 3 clinical trial of an inactivated EV71 vaccine, which was first reported in June 2013.9 The results suggested that the EV71 vaccine could provide significant protection against EV71-associated disease and sustained immunogenicity against EV71in Chinese healthy young children aged 6–35 mo.
However, besides the efficacy and safety, for new vaccines intending for marketing, confirming the manufacturing consistency of consecutively produced batches of vaccine by clinical evaluations is prerequisite for licensure.10 Therefore, in this phase 3 clinical trial for EV71 vaccine efficacy assessment, 3 consecutive batches of EV71 vaccines were applied. Subjects were randomly assigned to receive either 1 of the 3 batches of EV71 vaccine or placebo. Though, serological test results from a small part of subjects in the immunogenicity subset had been reported before,9 but for the most subjects who were not in the immunogenicity subset, their immunogenicity data have not been reported. Therefore, we performed this post-hoc analysis based on the immunogenicity data collected from the large cohort which derived from the phase 3 clinical trial. Corresponding data from all subjects with seronegative EV71 neutralizing antibody (NTAb) titers at baseline were used in this study, aiming to provide further evidence on the immunogenicity and consistency of 3 batches of EV71 vaccine in healthy Chinese infants and young children aged 6–35 mo.
Results
Demographic characteristics
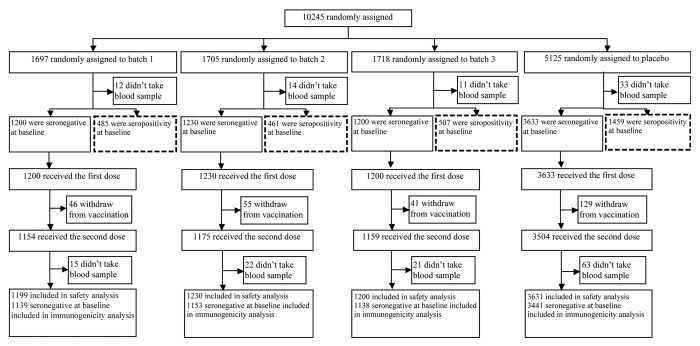
A total of 10 245 subjects aged 6–35 mo were recruited and randomized in a 1:1:1:3 ratio to receive either one of the 3 batches of EV71 vaccine or placebo in 4 study centers in this phase 3 trial, which was performed from January 2012 to March 2013. Among all the recruited subjects, 7263 (70.9%) subjects were seronegative at baseline. Except for 3 subjects had lost their diary cards, 7260 (70.9%) subjects who received at least one dose of assigned injections and had at least once safety record were included in the safety cohort of this study, and 6871 (67.1%) subjects who completed the two-dose injections and with EV71 NTAb titers in serum pre- and post-vaccination were available (1139 in batch 1, 1153 in batch 2, and 1138 in batch 3), were included in the analysis for immunogenicity and batch consistency (Fig. 1). Baseline demographic characteristics were similar across the 3 vaccine batch groups and placebo group with respect to age, height, weight, and body mass index (Table 1).
Figure 1. Trial profile.
Table 1. Demographic characteristics of the study subjects, by treatment groups.
| Batch 1 | Batch 2 | Batch 3 | Placebo | |
|---|---|---|---|---|
| Immunogenicity analysis cohort | n = 1139 | n = 1153 | n = 1138 | n = 3441 |
| Age (month) | 17.4 (8.1) | 17.5 (7.9) | 17.5 (7.9) | 17.6 (8.0) |
| Boy | 671 (58.9) | 677 (58.7) | 641 (56.3) | 1876 (54.5) |
| Height (cm) | 80.6 (8.2) | 80.7 (8.1) | 80.8 (8.1) | 80.8 (8.1) |
| Weight (kg) | 12.2 (2.2) | 12.3 (2.2) | 12.2 (2.2) | 12.2 (2.2) |
| BMI (kg/m2) | 18.8 (2.4) | 18.9 (2.6) | 18.8 (2.5) | 18.7 (2.3) |
| Safety analysis cohort | n = 1199 | n = 1230 | n = 1200 | n = 3631 |
| Age (month) | 17.4 (8.0) | 17.4 (7.9) | 17.5 (7.9) | 17.4 (8.0) |
| Boy | 704 (58.7) | 725 (58.9) | 675 (56.3) | 1990 (54.8) |
| Height (cm) | 80.5 (8.2) | 80.6 (8.0) | 80.7 (8.1) | 80.7 (8.1) |
| Weight (kg) | 12.2 (2.2) | 12.2 (2.2) | 12.2 (2.2) | 12.2 (2.2) |
| BMI (kg/m2) | 18.8 (2.4) | 18.9 (2.7) | 18.8 (2.5) | 18.7 (2.3) |
Data are mean (SD), and number (%). N, number of subjects; SD, standard deviation; BMI, body mass index.
Immunogenicity and batch consistency
The post-vaccination GMTs at day 56 were significantly higher in subjects receiving EV71 vaccines than that in those receiving placebos (P < 0.001). The NTAb GMT of Batch 1 is statistically lower than those of Batches 2 and 3 among three batches (P < 0.001) (Table 2). The GMTs of EV71 NTAb in subjects ranged from 407.0 U/mL to 520.6 U/mL for vaccine groups. The two-sided 95% confidence interval (CI) for the GMT ratio between each pair of batches was among [0.67, 1.5] interval (Table 3). At day 56, proportion of subjects with NTAb titers ≥ 8 U/mL achieved 98% or above, while proportion of subjects with NTAb titers ≥ 32 U/mL achieved 95% or above for the 3 vaccine batches.
Table 2. Immune response among different batches of EV71 vaccine or placebo in subjects who were seronegative at baseline on day 56, by treatment and age groups.
| Assessment variables | Vaccine | Placebo | P value# | ||||
|---|---|---|---|---|---|---|---|
| Pooled batches | Batch 1 | Batch 2 | Batch 3 | P value* | |||
| 6–11 mo | n = 998 | n = 343 | n = 330 | n = 325 | n = 997 | ||
| GMT (U/mL) | 439.1 (402.1–479.5) | 420.1 (359.8–490.5) | 413.1 (353.7–482.4) | 489.6 (422.3–567.6) | 0.24 | 3.6 (3.3–3.9) | <0.001 |
| Proportion with NTAb titer ≥ 8U/mL | 983, 98.5 (97.5–99.2) | 337, 98.3 (96.0–99.4) | 325, 98.5 (96.3–99.6) | 321, 98.8 (96.7–99.7) | 0.86 | 189, 19.0 (16.6–21.6) | <0.001 |
| Proportion with NTAb titer ≥ 32U/mL | 957, 95.9 (94.4–97.0) | 326, 95.0 (92.0–97.1) | 315, 95.5 (92.5–97.4) | 316, 97.2 (94.6–98.7) | 0.32 | 57, 5.7 (4.4–7.4) | <0.001 |
| 12–35 mo | n = 2432 | n = 796 | n = 823 | n = 813 | n = 2444 | ||
| GMT (U/mL) | 473.0 (447.3–500.2) | 401.5 (362.0–445.4) | 492.2 (448.6–540.1) | 533.6 (486.0–585.8) | <0.001 | 4.1 (3.8–4.4) | <0.001 |
| Proportion with NTAb titer ≥ 8U/mL | 2404, 98.9 (98.3–99.2) | 783, 98.4 (97.2–99.1) | 818, 99.4 (98.5–99.8) | 803, 98.8 (97.7–99.4) | 0.15 | 504, 20.6 (19.0–22.3) | <0.001 |
| Proportion with NTAb titer ≥ 32U/mL | 2342, 96.3 (95.5–97.0) | 761, 95.6 (93.9–96.9) | 794, 96.5 (94.9–97.6) | 787, 96.8 (95.3–97.9) | 0.42 | 209, 8.6 (7.5–9.8) | <0.001 |
| Total | n = 3430 | n = 1139 | n = 1153 | n = 1138 | n = 3441 | ||
| GMT (U/mL) | 462.9 (441.6–485.3) | 407.0 (373.5–443.6) | 468.1 (432.2–507.0) | 520.6 (481.2–563.3) | <0.001 | 4.0 (3.7–4.2) | <0.001 |
| Proportion with NTAb titer ≥ 8U/mL | 3387, 98.8 (98.3–99.1) | 1120, 98.3 (97.4–99.0) | 1143, 99.1 (98.4–99.6) | 1124, 98.8 (97.9–99.3) | 0.23 | 693, 20.1 (18.8–21.5) | <0.001 |
| Proportion with NTAb titer ≥ 32U/mL | 3299, 96.2 (95.5–96.8) | 1087, 95.4 (94.0–96.6) | 1109, 96.2 (94.9–97.2) | 1103, 96.9 (95.7–97.8) | 0.18 | 266, 7.7 (6.9–8.7) | <0.001 |
Data are GMT (95% CI) or n, % (95% CI). GMT, geometric mean titer; n, number of subjects. *The P values were calculated for the comparison among the 3 batches of EV71 vaccines. #The P values were calculated for the comparison between the pooled EV71 vaccine group and the placebo group.
Table 3. The GMT ratios among different batches of EV71 vaccine in subjects who were seronegative at baseline on day 56, by treatment and age groups.
| Batch number | n | GMT (95% CI) | GMT ratio (95% CI) |
|---|---|---|---|
| 6–11 mo | |||
| Batch 1 | 343 | 420.1 (359.8–490.5) | 1.02 (0.82–1.27) |
| Batch 2 | 330 | 413.1 (353.7–482.4) | |
| Batch 1 | 343 | 420.1 (359.8–490.5) | 0.89 (0.69–1.06) |
| Batch 3 | 325 | 489.6 (422.3–567.6) | |
| Batch 2 | 330 | 413.1 (353.7–482.4) | 0.84 (0.68–1.05) |
| Batch 3 | 325 | 489.6 (422.3–567.6) | |
| 12–35 mo | |||
| Batch 1 | 796 | 401.5 (362.0–445.4) | 0.82 (0.71–0.94) |
| Batch 2 | 823 | 492.2 (448.6–540.1) | |
| Batch 1 | 796 | 401.5 (362.0–445.4) | 0.75 (0.66–0.87) |
| Batch 3 | 813 | 533.6 (486.0–585.8) | |
| Batch 2 | 823 | 492.2 (448.6–540.1) | 0.92 (0.81–1.05) |
| Batch 3 | 813 | 533.6 (486.0–585.8) | |
| Total | |||
| Batch 1 | 1139 | 407.0 (373.5–443.6) | 0.87 (0.77–0.98) |
| Batch 2 | 1153 | 468.1 (432.2–507.0) | |
| Batch 1 | 1139 | 407.0 (373.5–443.6) | 0.78 (0.70–0.88) |
| Batch 3 | 1138 | 520.6 (481.2–563.3) | |
| Batch 2 | 1153 | 468.1 (432.2–507.0) | 0.90 (0.80–0.99) |
| Batch 3 | 1138 | 520.6 (481.2–563.3) | |
n, number of subjects. The equivalence margin of GMT is 0.67–1.5. The results were calculated on basis of subjects whom completed the two-dose immune schedule and had their serum detection results at day 56.
Batch consistency was further evaluated for age stratified subgroups with subjects aged 6–11 mo and 12–35 mo. Only one exception of the GMT ratio between batch 1 and batch 3 in 12–35 mo group was 0.75 (95% CI 0.66–0.87) with the low limit out of the [0.67, 1.5] interval (Table 3). Numerically higher EV71 NATb GMTs and larger proportions of subjects with NTAb titers ≥ 8 U/mL or ≥ 32 U/mL in the older group of subjects aged 12–35 mo were observed than that in the younger group of subjects aged 6–11 mo, but no statistical significant difference was found (Table 2).
Safety
Both solicited adverse events (AEs) and unsolicited AEs were reported with similar frequency among 3 vaccine batches (Table 4). The number of subjects undergoing solicited adverse reactions within 7 d after vaccination ranged from 595 (49.6%, 95% CI 46.8–52.5) to 641 (52.1%, 95% CI 49.3–54.9), as to unsolicited AEs reported within 28 d after vaccination, the number ranged from 576 (48.0%, 95% CI 45.1–50.9) to 635 (51.6%, 95% CI 48.8–54.5). Most of AEs were mild or moderate and resolved within 3 d. Grade 3 adverse events occurred in 8 subjects who reported injection-site adverse reactions and 126 subjects who reported systemic adverse reactions, respectively. The frequencies of grade 3 adverse events in subjects receiving EV71 vaccines and placebos were not significantly different. In total 28 subjects (0.4%) experienced serious adverse events (SAEs): 8 (0.2%) in vaccine group vs. 20 (0.6%) in placebo group (P = 0.02). All of the SAEs were determined irrelevant to vaccinations (Table 4).
Table 4. Summary of solicited adverse reactions, unsolicited adverse events and serious adverse event in the safety analysis cohort.
| Reaction severity | Vaccine (n = 3629) | Placebo (n = 3631) | P value# | |||
|---|---|---|---|---|---|---|
| Batch 1 (n = 1199) | Batch 2 (n = 1230) | Batch 3 (n = 1200) | P value* | |||
| Solicited adverse reactions within 0–7 d | ||||||
| Any | 595, 49.6 (46.8–52.5) | 641, 52.1 (49.3–54.9) | 600, 50.0 (47.1–52.9) | 0.42 | 1786, 49.2 (47.6–50.8) | 0.23 |
| Grade 3 * | 30, 2.5 (1.7–3.6) | 17, 1.4 (0.8–2.3) | 28, 2.3 (1.6–3.4) | 0.11 | 59, 1.6 (1.3–2.1) | 0.16 |
| Injection-site adverse reactions | ||||||
| Any | 138, 11.5 (9.8–13.5) | 146, 11.9 (10.1–13.8) | 139, 11.6 (9.9–13.6) | 0.96 | 397, 10.9 (10.0–12.0) | 0.33 |
| Grade 3 | 3, 0.3 (0.1–0.8) | 0, 0.0 (0.0–0.4) | 2, 0.2 (0.0–0.7) | 0.18 | 3, 0.1 (0.0–0.3) | 0.51 |
| Systemic adverse reactions | ||||||
| Any | 534, 44.5 (41.7–47.4) | 578, 47.0 (44.2–49.8) | 539, 44.9 (42.1–47.8) | 0.42 | 1621, 44.6 (43.0–46.3) | 0.47 |
| Grade 3 | 27, 2.3 (1.5–3.3) | 17, 1.4 (0.8–2.3) | 26, 2.2 (1.5–3.2) | 0.23 | 56, 1.5 (1.2–2.0) | 0.21 |
| Unsolicited adverse events within 0–28 d | 591, 49.3 (46.4–52.2) | 635, 51.6 (48.8–54.5) | 576, 48.0 (45.1–50.9) | 0.19 | 1761, 48.5 (46.9–50.1) | 0.32 |
| Serious adverse events | 1, 0.1 (0.0–0.5) | 3, 0.2 (0.1–0.8) | 4, 0.3 (0.1–0.9) | 0.46 | 20, 0.6 (0.4–0.9) | 0.02 |
| Infections and infestations | 0, 0.0 (0.0–0.4) | 2, 0.2 (0.0–0.7) | 4, 0.3 (0.1–0.9) | 0.13 | 16, 0.4 (0.3–0.7) | 0.03 |
| Injury, poisoning, and procedural complications | 0, 0.0 (0.0–0.4) | 0, 0.0 (0.0–0.4) | 0, 0.0 (0.0–0.4) | - | 3, 0.1 (0.0–0.3) | 0.25 |
| General disorders and administration site conditions | 0, 0.0 (0.0–0.4) | 0, 0.0 (0.0–0.4) | 0, 0.0 (0.0–0.4) | - | 1, 0.0 (0.0–0.2) | 1.00 |
| Cardiac disorders | 1, 0.1 (0.0–0.5) | 0, 0.0 (0.0–0.4) | 0, 0.0 (0.0–0.4) | 0.33 | 0, 0.0 (0.0–0.1) | 0.50 |
| Gastrointestinal disorders | 0, 0.0 (0.0–0.4) | 1, 0.1 (0.0–0.5) | 0, 0.0 (0.0–0.4) | 1.00 | 0, 0.0 (0.0–0.1) | 0.50 |
Data are n, % (95% CI). n, number of subjects; Any, all the subjects with certain adverse reactions. *Grade 3 events were severe (i.e., prevented activity). *The P values were calculated for the comparison among the 3 batches of EV71 vaccines. #The P values were calculated for the comparison between the pooled EV71 vaccine group and the placebo group.
Discussion
The study which was based on a large-scale phase 3 clinical trial with an inactivated EV71 vaccine was performed to evaluate the immunogenicity and consistency of 3 consecutive batches of EV71 vaccine. A large sample can usually reduce sampling error, increase the statistical power, and make the study population more representative.
In this study, only subjects with seronegative EV71 NTAb titers at baseline were involved in analysis. Because, seropositivity subjects who were infected by EV71 virus before may generate an immune recall response with high EV71 NTAb titers after vaccination.11 By excluding the subjects with positive EV71 NTAb titers before vaccination, potential confounding factors on the EV71 NTAb response post-vaccination could be reduced. Besides, the NTAb titers against CoxsackievirusA16 (CVA16), which is another common enterovirus for HFMDs, before the vaccination were not considered in this study, because there was no evidence that EV71 vaccine could induce cross-reactivity between EV71 and CVA16.9
Demonstration of batch-to-batch consistency is critical in the development of a vaccine.10,12 Antigen content, which generally derived from detection of semi-finished products, could be interfered by many factors, such as complex molecular structure of the antigens, different manufacturing processes, and the interaction between vaccines and agents that used during manufacturing and/or presented in the final product, and thus result in a different immune effect in the vaccinees. Therefore, batch consistency analysis for the final vaccine product is vital, a vaccine manufacturer who wants to license his vaccine must demonstrate that the manufacturing process is stabile which means that consistent batches can be produced.13
For batch consistency analysis, one issue should be noticed that vaccine batch consistency trial is equivalence study, which aims to demonstrate that 2 treatments are more or less similar, but not they are different.14 Therefore, the common statistical hypothesis and tests applied in a superiority trial which aims to show 2 treatments are different could not work in the batch consistency analysis. According to FDA/CBER, vaccine batch consistency can be achieved if all of the two-sided confidence intervals for each GMT ratio comparing groups receiving different vaccine batches were within the pre-defined consistency interval, in a vaccine consistency trial with GMT as endpoint.14 But some previous reported vaccine consistency trial had adopted statistical methods used in a superiority trial, which is inappropriate.15-17 In that case, a non-significant test result could be misinterpret, especially when the sample size was too small to gain enough power to detect the difference in the trial.18
For a vaccine consistency trial design, it is very essential to choose a consistency interval previously. An interval that is too strict will require an excessively large sample size, while an interval is too wide will not be clinically significant. The range [0.67, 1.5] is generally considered to be a reasonable one, neither too wide nor too strict.14 This interval has been applied in many articles.19,20 But sometimes, a wider interval, such as [0.5, 2.0] could also be used in vaccine consistency trials.14 Consistency analysis based on 95% CIs are widely used, but in some studies the 90% CIs could also be choosen.20 Though, in this study, the NTAb GMT of Batch 1 is statistically lower than those of Batches 2 and 3, the GMT ratios between each pair of 2 batches were within the equivalence range of [0.67, 1.5]. The results indicated that immunogenicity of EV71 vaccines could be varied from batch to batch, but the overall immunogenicity is not different.
Safety of the EV71 vaccine was also evaluated among 3 batches of EV71 vaccine in this study. The results indicated that there was no significant difference in the occurrence of adverse events among the subjects receiving different batches of vaccines and those receiving placebos. The safety profile of the EV71 vaccines was acceptable and was in line with that of other EV71 vaccines reported previously.21,22
The immunogenicity and batch consistency analysis in this study were all calculated based on the standardized NTAb titers. In this study, the standardization method of NTAb titers was applied for original NTAb titers before the statistical analysis,9,23 because the original detected results of NTAb titers were from 2 different laboratories. A lot of reasons might contribute in the deviation in the process of detection, such as system error, quality control, especially in 2 different laboratories and performed by different staffs, often result in the systematic bias.3 So we converted NTAb titers into standardized NTAb titers (U/mL) to make the results more comparable. The standardized NTAb titers applied in this study was the first established national standard for EV71 NTAb assay in mainland China, which had been used for more than 20 000 serum samples. Currently there is no international standardized NTAb titers was available, making the comparison of detected EV71 NTAb titer in various countries impractical. To accelerating the development of a stable and reliable international standard serum for EV71 NTAb assay may need an international cooperation of the laboratories.
In summary, all of those 3 consecutive batches of EV71 vaccine were found to be highly immunogenic in healthy infants and young children and a vaccine batch consistency was well demonstrated.
Material and Methods
Study design and subjects
The study was based on a multicenter, double-blind, and placebo-controlled phase 3 clinical trial which had been reported.9 The experimental EV71 vaccine (Vero Cell), containing 320 U of antigen and 0.18 mg of aluminum hydroxide, was developed and manufactured by Beijing Vigoo Biological Co. Ltd. with a seed virus of EV71 strain FY7VP5/AH/CHN/2008 (GenBank accession number JX025561). Cell factory was built for the virus culturing and the concentrated suspension was purified by gel filtration chromatography followed by ion exchange chromatography, and then inactivation by formaldehyde and adsorption on aluminum hydroxide. Each dose of placebo contained 0.18 mg aluminum hydroxide without EV71 antigen.
In this phase 3 clinical trial, a total of 10 245 healthy infants and young children aged 6–35 mo were enrolled from 4 centers, i.e., Donghai, Pizhou, and Baoying counties in Jiangsu province, and Chaoyang district in Beijing, and then randomly assigned in a ratio of 1:1:1:3 to receive either one of the 3 batches of EV71 vaccine (batch 1–3: 201108003, 201108002, and 201108004) or placebo (batch number: 201108001). Vaccine was administrated intramuscularly into the anterolateral side of thigh (6–11 mo in age) or the deltoid muscle (12–35 mo in age) according to a 0 and 28-d schedule. Among 10 245 subjects, only 1283 (12.5%) subjects who were selected as immunogenicity subset from 4 villages/towns of 3 centers in Jiangsu had their immunogenicity data pre- and post- vaccinations already been reported.9 In this study, we did a post-hoc analysis to involve all subjects with seronegative EV71 NTAb titers at baseline in this trial, including who were recruited in immunogenicity subset.
Immunogenicity assessment
Serum samples of 3 mL were collected from all subjects on day 0 (immediately before first dose) and day 56 (day 28 after second dose) for immunogenicity analysis. Serum samples from immunogenicity subset were detected by National Institutes for Food and Drug Control (NIFDC) while other serum samples were detected by Beijing Vigoo Biological Ltd. All blood samples were centrifuged and the harvested serums were stored at a temperature of –20 °C or below until shipped to the Beijing Vigoo Biological Ltd.
A modified cytopathic effect method (CPE method) was used to analyze EV71 NTAb titer.3 In brief, samples were 2-fold serially diluted from 1:8 to 1:16384 and NTAb titers were defined as the highest dilution capable of inhibiting 50% of the CPEs. In order to make the results detected by different organizations were comparable, reference serum (N12L:1000U), which was provided by the NIFDC, were applied in both laboratories and set in at least 4 wells in each plates.3 Besides, the tested original NTAb titer from 2 laboratories were standardized by divided it to the NTAb titer of the reference serum and multiplied by the assigned potency of the reference serum. The standardization method could convert the tested NTAb titer from different laboratories into standardized NTAb titers (U/mL). This method was adapted from the calibration methods applied for polio.24
Safety assessment
A diary card was used for recording solicited adverse reactions within 7 d and unsolicited adverse events within 28 d after each dose. Solicited local reactions included redness, pain, swelling, induration, rash, and pruritus. Solicited systemic reactions included fever, nausea/vomiting, diarrhea, decreased appetite, restlessness/irritability, fatigue, and allergy. Unsolicited adverse events included solicited symptoms occurred after 7 d and unsolicited symptoms occurred within 28 d after vaccination. Hospitalized cases during the study period were considered as SAEs and corresponding data was also collected and reported according to prescribed procedures. All AEs were assessed for severity according to grading scale issued by China Food and Drug Administration.25
Statistical analysis
All the subjects who received 2 doses of vaccine correctly, providing effective serum samples at relevant time points (day 0 and day 56), being seronegative on day 0, and having no major protocol deviations were included for the immunogenicity analysis. Safety analysis was performed based on subjects who were seronegative at baseline, received at least one dose and had at least once safety record.
The primary endpoint was the GMTs of EV71 NTAb on day 56. The standardized NTAb titers ranged from 4 to 16 000, while the value below 4 was assigned a value of 2 for calculation. Batch-to-batch consistency was to be claimed if all the two-sided 95% CIs of pairwise ratios of post-vaccination GMTs at day 56 were within the pre-defined [0.67, 1.5] interval. One of the secondary endpoint was the proportion of subjects with NTAb titers ≥ 8U/mL or ≥ 32U/mL. The other secondary endpoints for safety were occurrence of solicited adverse events within 7 d of injection, any AEs within 28 d of injection, and all SAEs during the study period.
Statistical analysis was performed using SAS version 9.1 and SPSS version 18.0. Student’s t test was used for the paired-wise comparison of the log-transferred NTAb titers and the calculation of 95% CIs of the GMT ratios between 2 batches.14 Pearson’s chi-square or Fisher’s exact test was used for analyzing categorical data with a two-sided significance level of 0.05.
Glossary
Abbreviations:
- EV71
enterovirus 71
- GMT
geometric mean titer
- HFMD
hand, foot, and mouth disease
- NTAb
neutralizing antibody
- 95% CI
95% confidence interval
- AE
adverse event
- SAE
serious adverse event
- SD
standard deviation
- BMI
body mass index
- NIFDC
National Institutes for Food and Drug Control
- CPE
cytopathic effect
- CVA16
CoxsackievirusA16
Disclosure of Potential Conflicts of Interest
X.-L.L., Y.-T.Z., Q.-H.C., and H.-J.G. are employees of National Engineering Research Center of Innovative Vaccine of Beijing Vigoo Biological Co., Ltd. Other authors claimed that they have no conflicts of interest.
The study was co-funded by China 12–5 National Major Infectious Disease Programs (2012ZX10004-703 and 2012ZX10002-001), and Beijing Vigoo Biological Co., Ltd.
Acknowledgments
We thank all the investigators from Jiangsu Provincial Center for Disease Control and Prevention, National Institute for Food and Drug Control, Donghai County Center for Disease Control and Prevention, Pizhou County Center for Disease Control and Prevention, Baoying County Center for Disease Control and Prevention, and Chaoyang District Center for Disease Control and Prevention.
References
- 1.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. . Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778 - 90; http://dx.doi.org/ 10.1016/S1473-3099(10)70194-8; PMID: 20961813 [DOI] [PubMed] [Google Scholar]
- 2.Lee MS, Chiang PS, Luo ST, Huang ML, Liou GY, Tsao KC, Lin TY. . Incidence rates of enterovirus 71 infections in young children during a nationwide epidemic in Taiwan, 2008-09. PLoS Negl Trop Dis 2012; 6:e1476; http://dx.doi.org/ 10.1371/journal.pntd.0001476; PMID: 22348156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Z, Mao Q, Gao Q, Li X, Dong C, Yu X, Yao X, Li F, Yin W, Li Q, et al. . Establishing China’s national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine 2011; 29:9668 - 74; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.018; PMID: 22015395 [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health of the People’s Republic of China. National notifiable diseases report. Available from: http://www.chinacdc.cn/tjsj/fdcrbbg
- 5.Chua KB, Kasri AR. . Hand foot and mouth disease due to enterovirus 71 in Malaysia. Virol Sin 2011; 26:221 - 8; http://dx.doi.org/ 10.1007/s12250-011-3195-8; PMID: 21847753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR, Taiwan Enterovirus Epidemic Working Group. . An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med 1999; 341:929 - 35; http://dx.doi.org/ 10.1056/NEJM199909233411301; PMID: 10498487 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Feng Z, Yang Y, Self S, Gao Y, Longini IM, Wakefield J, Zhang J, Wang L, Chen X, et al. . Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology 2011; 22:781 - 92; http://dx.doi.org/ 10.1097/EDE.0b013e318231d67a; PMID: 21968769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang ZL, Mao QY, Wang YP, Zhu FC, Li JX, Yao X, Gao F, Wu X, Xu M, Wang JZ. . Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum Vaccin Immunother 2013; 9:1701 - 5; http://dx.doi.org/ 10.4161/hv.24949; PMID: 23744508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al. . Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381:2024 - 32; http://dx.doi.org/ 10.1016/S0140-6736(13)61049-1; PMID: 23726161 [DOI] [PubMed] [Google Scholar]
- 10.WHO. Clinical considerations for evaluation of vaccines for prequalification. Points to consider for manufacturers of human vaccines. 2010 [cited 2013 September 12]. Available from:http://www.who.int/immunization_standards/vaccine_quality/clinical_considerations_oct10.pdf
- 11.Ahmed R, Gray D. . Immunological memory and protective immunity: understanding their relation. Science 1996; 272:54 - 60; http://dx.doi.org/ 10.1126/science.272.5258.54; PMID: 8600537 [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency. Note for guidance on the clinical evaluation of vaccines. 2005 [cited 2013 September 12] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003875.pdf
- 13.Hendriksen C, Arciniega JL, Bruckner L, Chevalier M, Coppens E, Descamps J, Duchêne M, Dusek DM, Halder M, Kreeftenberg H, et al. . The consistency approach for the quality control of vaccines. Biologicals 2008; 36:73 - 7; http://dx.doi.org/ 10.1016/j.biologicals.2007.05.002; PMID: 17892948 [DOI] [PubMed] [Google Scholar]
- 14.Statistics JN. in Clinical Vaccine Trials. Springer, 2010. [Google Scholar]
- 15.Talbot HK, Keitel W, Cate TR, Treanor J, Campbell J, Brady RC, Graham I, Dekker CL, Ho D, Winokur P, et al. . Immunogenicity, safety and consistency of new trivalent inactivated influenza vaccine. Vaccine 2008; 26:4057 - 61; http://dx.doi.org/ 10.1016/j.vaccine.2008.05.024; PMID: 18602726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RL, Froeschle JE, Atmar RL, Matthews JS, Sanders R, Pardalos J, Moeller L, Chin JE, Famula M, Briggs DJ, et al. . Immunogenicity, safety and lot consistency in adults of a chromatographically purified Vero-cell rabies vaccine: a randomized, double-blind trial with human diploid cell rabies vaccine. Vaccine 2001; 19:4635 - 43; http://dx.doi.org/ 10.1016/S0264-410X(01)00238-9; PMID: 11535311 [DOI] [PubMed] [Google Scholar]
- 17.Jiang WP, Chen JT, Wang X, Wang YL, Liu Y, Chen WY, Xu WG, Qiu YZ, Yin WD. . Immunogenicity and safety of three consecutive lots of a new preservative-free inactivated hepatitis A vaccine (Healive): a double-blind, randomized and controlled trial. Vaccine 2008; 26:2297 - 301; http://dx.doi.org/ 10.1016/j.vaccine.2007.11.008; PMID: 18395305 [DOI] [PubMed] [Google Scholar]
- 18.Ganju J, Izu A, Anemona A. . Sample size for equivalence trials: a case study from a vaccine lot consistency trial. Stat Med 2008; 27:3743 - 54; http://dx.doi.org/ 10.1002/sim.3273; PMID: 18416439 [DOI] [PubMed] [Google Scholar]
- 19.Hu YM, Wang X, Wang JZ, Wang L, Zhang YJ, Chang L, Liang ZL, Xia JL, Dai QG, Hu YL, et al. . Immunogenicity, safety, and lot consistency of a novel inactivated enterovirus 71 vaccine in Chinese children aged 6 to 59 months. Clin Vaccine Immunol 2013; 20:1805 - 11; http://dx.doi.org/ 10.1128/CVI.00491-13; PMID: 24108780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroux-Roels G, Dobson S, Bernstein DI, Fowler S, Romanowski B, Leroux-Roels I, et al. . Clinical evaluation to confirm the manufacturing consistency of three lots of an adjuvanted glycoprotein D genital herpes vaccine in healthy seronegative pre-teen and adolescent girls: A phase III multi-center double-blind randomized trial. Trials in Vaccinology 2013; 2:10 - 8; http://dx.doi.org/ 10.1016/j.trivac.2013.03.001 [DOI] [Google Scholar]
- 21.Cheng A, Fung CP, Liu CC, Lin YT, Tsai HY, Chang SC, Chou AH, Chang JY, Jiang RH, Hsieh YC, et al. . A Phase I, randomized, open-label study to evaluate the safety and immunogenicity of an enterovirus 71 vaccine. Vaccine 2013; 31:2471 - 6; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.015; PMID: 23541623 [DOI] [PubMed] [Google Scholar]
- 22.Li YP, Liang ZL, Xia JL, Wu JY, Wang L, Song LF, Mao QY, Wen SQ, Huang RG, Hu YS, et al. . Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 vaccine: a phase II, Randomized, double-blind, placebo-controlled Trial. J Infect Dis 2014; 209:46 - 55; http://dx.doi.org/ 10.1093/infdis/jit429; PMID: 23922377 [DOI] [PubMed] [Google Scholar]
- 23.Zhu FC, Liang ZL, Li XL, Ge HM, Meng FY, Mao QY, Zhang YT, Hu YM, Zhang ZY, Li JX, et al. . Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2013; 381:1037 - 45; http://dx.doi.org/ 10.1016/S0140-6736(12)61764-4; PMID: 23352749 [DOI] [PubMed] [Google Scholar]
- 24.ERADICATION P. Manual for the virological investigation of poliomyelitis. 1997 [cited 2013 September 12] Available from: http://whqlibdoc.who.int/Hq/1990/WHO_EPI_CDS_POLIO_90.1.pdf
- 25.State Food and Drug Administration. The standard guidelines for adverse reactions grading of vaccine clinical trials. 2005 [cited 2013 September 12] Available from: http://www.sda.gov.cn/WS01/CL0844/9350_5html