Abstract
Studies assessing the economic burden of a mumps outbreak in a highly vaccinated population are limited. The Orange County Health Department (OCHD), New York State Department of Health (NYS DOH), and the Centers for Disease Control and Prevention conducted a mumps investigation in an affected village with a highly vaccinated population. To understand the epidemiology, standardized mumps case definition and active surveillance were used to identify mumps cases. In addition, an economic assessment of a combined outbreak investigation and third dose measles-mumps-rubella (MMR) vaccine intervention conducted by OCHD and NYS DOH was performed; estimated by retrospectively evaluating public health response-related activities including use of a third dose of MMR vaccine. From September 24, 2009, through June 15, 2010, 790 mumps cases were reported—64% were male and highest attack rate was among 11–17 year age group (99.1 cases per 1000 individuals). Of the 658 cases with known vaccination history, 83.6% had documentation of 2 doses of mumps containing vaccine. No deaths were reported. The 2 major exposure settings were schools (71.8%) and households (22.5%). Approximately 7736 h of public health personnel time were expended with the total approximate cost of US $463 000, including US $34 392 for MMR vaccine—the estimated cost per household was US $827. Mumps continues to be endemic in many parts of the world, resulting in importations into the United States. Large mumps outbreaks similar to this in highly vaccinated populations may require considerable investigation and control activities.
Keywords: mumps, outbreak, economic, vaccine, highly vaccinated
Introduction
With the licensure of a live, attenuated mumps-virus vaccine in the United States in 1967 and its introduction in the immunization schedule a decline in the number of mumps cases was noted. Further decline was seen with the introduction of the measles, mumps, and rubella (MMR) vaccine (licensed in 1971 containing the Jeryl Lynn mumps strain) and the two-dose schedule (1989) in response to the measles resurgence of 1989 and for mumps control in 2006.1,2 The current schedule for mumps-containing vaccine is first dose to be given at 12 mo of age and the second dose at 4–6 y of age. However, mumps outbreaks among high two-dose vaccinated populations have been recently reported in literature, both within the United States and internationally.3-8
In New York State, school immunization laws for measles, mumps, and rubella have been implemented since 1968.9 In 1989, Public Health Law (PHL) (Section 2165) was enacted to require students attending post-secondary institutions who were born on or after January 1, 1957, to have 2 doses of measles, 1 dose of mumps and 1 dose of rubella vaccine. In 1990, PHL (Section 2164) was amended to require any Kindergartener and new enterer in grades 1–12 who was born on or after January 1, 1985, to have adequate doses of vaccine against measles, mumps, and rubella.
In 2009–2010, New York State experienced the largest mumps outbreak in the state since the implementation of school immunization laws for mumps in 1968. This mumps outbreak, a part of the Northeastern United States mumps outbreak,10 primarily affected members of an Orthodox Jewish community with high two-dose MMR vaccine coverage. One such affected area in New York State was a village in Orange County (OC), with a predominantly Orthodox Jewish population. The OC village outbreak was seeded when 7 village residents developed mumps after being exposed in Brooklyn, New York City, in September 2009. By the end of December 2009, approximately 20% of all reported Northeastern United States mumps outbreak cases were from Orange County.
In the decade prior to 2009 in New York State a median of 12 mumps cases per year (range: 3–51 cases) were reported. Two peaks were noted, one in 2005 which occurred in a summer camp (n = 32) and another in 2006 when there was a general increase in the national number of cases reported (n = 51).11-17 The National Immunization Survey (NIS), a telephone survey, conducted by the Centers for Disease Control and Prevention (CDC), monitors childhood immunization coverage, the target population being children aged 19–35 mo. Based on the NIS, the MMR vaccine coverage for 19–35 mo olds (≥1 dose) in New York State (except New York City) during the same time period was persistently over 80% (average: 85.4%; range: 81.8–95.3%) except for 2008 when it was below 80%.18 The reported MMR vaccine coverage, as per NIS, for adolescents aged 13–17 y (≥2 doses) during the years 2008 and 2009 was 92.4% and 93.6% respectively indicating a highly vaccinated population.18 In the OC village where the mumps outbreak occurred, a school immunization audit of 3 religious schools, conducted during December 2009−February 2010 as part of a third dose MMR vaccine intervention, showed the weighted average two-dose vaccine coverage to be 94.3%.19 This indicated a highly vaccinated population—98% of the children in the affected OC village attended the 3 religious schools.
In the United States, mumps is a reportable disease. State and local health departments in the United States have jurisdiction over outbreaks within their borders and take the lead in investigating outbreaks and implementing control measures to reduce spread of the disease. States may invite CDC to assist with an investigation when additional expertise, capacity, or resources are needed. As part of the public health response, an outbreak investigation was conducted to understand the epidemiology of a mumps outbreak in a highly-vaccinated population. The well-defined population as well as the collaboration among the village and county, state, and federal public health departments provided the ideal setting for investigating this outbreak. The public health response also included a third dose MMR vaccine intervention with follow-up survey. The third dose MMR vaccine intervention and follow-up, undertaken with Institutional Review Board (IRB) approval from CDC and New York State Department of Health (NYSDOH), have been described elsewhere.19 At the time of the outbreak a third dose of MMR vaccine was not a part of the standard public health response nor was it a routine recommendation. In addition to the epidemiology of the OC village mumps outbreak described herein, we retrospectively assessed the economic burden for local and state public health institutions of a combined outbreak investigation and third dose MMR vaccine intervention. Limited information is available concerning the economic burden to public health during a third dose response. The epidemiological investigation and the economic assessment of an infectious disease outbreak as well as the related interventions help understand the use of resources and aid in policy and planning.
Results
Case reports
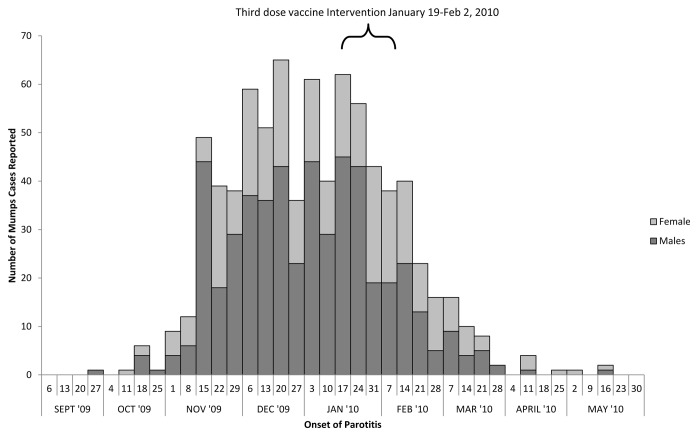
During September 24, 2009, through June 15, 2010 (the outbreak period), 790 mumps cases were reported to the OCHD (Fig. 1)—508 (64.0%) of the cases were male. The OC village outbreak was analogous to the overall Northeastern outbreak10: in that (1) the highest proportion of cases was among the 11–17 y old age group, (2) males were predominantly affected, and (3) <10% of the case-patients had documented zero doses (Table 1). In the OC village, the highest attack rate was seen in 11–17 y age group (99.1 cases per 1000 individuals) followed by 6–10 y (53.8 per 1000 individuals), 18–24 y (48.2 per 1000 individuals), and those at least 25 y of age (15.0 per 1000 individuals). The age groups of less than 1 y and those 1–5 y had the lowest attack rates—0.7 per 1000 individuals and 13.6 per 1000 individuals respectively. Males had a higher attack rate than females (48.6 cases per 1000 vs. 29.0 cases per 1000).
Figure 1. Epidemiological curve of the mumps outbreak in Orange County village by Sex, New York, 2009–2010 (n = 790) .
Table 1. Characteristics of reported mumps cases, Orange County, New York, 2009–2010 (n = 790).
| Characteristics | n (%) |
|---|---|
| Age (y) | |
| <1 | 6 (0.8) |
| 1–5 | 60 (7.6) |
| 6–10 | 186 (23.5) |
| 11–17 | 338 (42.8) |
| 18–24 | 117 (14.8) |
| 25–34 | 55 (7.0) |
| ≥35 | 28 (3.5) |
| Sex | |
|---|---|
| Female | 282 (35.7) |
| Male | 508 (64.3) |
| Documented Vaccine History (n = 658) | |
|---|---|
| 0 dose | 44 (6.7) |
| 1 dose | 64 (9.7) |
| 2 doses | 550 (83.6) |
| Clinical Presentation and Complications | |
|---|---|
| Parotitis | 790 (100) |
| Orchitis* (n = 312) | 20 (6.4) |
| Meningititis | 2 (0.3)** |
| Hospitalization | 1 |
| Orchitis* and vaccination status | |
|---|---|
| 0 dose (n = 5) | 1 (20) |
| 1 dose (n = 12) | 0 |
| 2 doses (n = 228) | 14 (6.1) |
| Unknown vaccination status (n = 67) | 5 (7.5) |
| Time from vaccination for those who received only 1 dose of MMR vaccine (n = 65) | |
|---|---|
| Median (Range) | 5.5 y (3 mo–34.3 y) |
| <1 y | 4 (6.2) |
| 1–4 y | 27 (41.5) |
| 5–9 y | 20 (30.8) |
| ≥10 y | 14 (21.5) |
| Time from last vaccination for those who received 2 doses of MMR vaccine (n = 532) | |
|---|---|
| Median (Range) | 8.7 y (3 mo–19.7 y). |
| <1 y | 9 |
| 1–4 y | 97 (18.2) |
| 5–9 y | 221 (41.5) ± |
| 10–14 y | 168 (31.6) |
| 15–19 y | 37 (7.0) |
| Reported Source of transmission (n = 599) | |
|---|---|
| School | 430 (71.8) |
| Household/Family | 135 (22.5) |
| Religious gathering | 12 (2) |
| Workplace | 11 (1.8) |
| Community | 5 (0.8) |
| Unknown | 3 (0.5) |
| Outside the community | 2 (0.3) |
| Medical | 1 (0.2) |
| Laboratory: Serology |
| IgM Positive (n = 39) Mumps Vaccination status |
|
|---|---|
| 0 dose | 7 (18.0) |
| 1 dose | 2 (5.1) |
| 2 doses | 27 (69.2) |
| Unknown | 3 (7.7) |
*In males ≥12 y of age. **One case-patient had 2 documented doses of MMR vaccine; the other had unknown vaccination status. ±Majority of mumps cases were 11–17 y of age.
The median age among the reported mumps cases in OC was 14.1 y (range: 5.0 mo to 55.0 y). As parotitis was part of the case-definition, all cases had parotitis. There were no reports of deaths. Among those with documented history of only 1 dose of MMR vaccine, the mean time since vaccination was 7.2 y (95% CI: 1.0 to 13.9). Among those with the second dose of MMR vaccine, the mean time since vaccination was 8.5 y (95% CI: 4.6 to 12.3).
For cases with known reported exposure information, the 2 major settings were school (71.8%) and household (22.5%) (Table 1). Only 3 of the 790 mumps cases reported in OC occurred in non-village residents; all were employed in the village. No secondary transmission was reported from the non-village cases.
During this outbreak, of the 183 case-patients with documented 2 doses of MMR vaccine, 27 (15%) were found to be mumps IgM antibody positive while among the 17 case-patients with documented 1 dose of MMR vaccine, 2 (12%) were found to be mumps IgM antibody positive. The genotype G identified was similar to the rest of the Northeastern mumps outbreak as well as similar to the virus circulating during a concurrent mumps outbreak in the United Kingdom.10
Economic evaluation
We identified 42 public health personnel who participated in this outbreak response. Approximately 7736 h of personnel time were expended; of which 3656 h (47.3%) were by NYS DOH and 4080 h (52.7%) were by OCHD (Table 2). Among the main activities demanding personnel time were database analysis and report preparation (23.6%), case finding and isolation (16.5%), response planning and coordination (14.4%), and school based vaccination (12.4%). Overall, the total estimated cost was US$ 463 202 of which 89.0% was attributable to personnel costs including fringe benefits and overhead costs. The cost incurred by the OCHD was US$ 206 264 while the NYS DOH incurred US $256 938. A total of 1812 doses of MMR vaccine were provided during the outbreak, the cost of which was estimated to be US $34 392.19 Five-hundred and sixty households with mumps cases were contacted during the response; the estimated cost per household was US $827.
Table 2. Resource Utilization and direct costs of containing Mumps, Orange County, New York, 2009–2010.
| Orange County Health Department* | New York State Health Department* | Overall | |
|---|---|---|---|
| Resource utilization | |||
| Number of responders | 20 | 22 | 42 |
| Hours per activity† | |||
| Planning and coordination | 429 | 687 | 1116 |
| Case confirmation & isolation |
1206 | 74 | 1280 |
| Contact identification | 549 | - | 549 |
| School based vaccination | 476 | 482 | 958 |
| Specimen collection and lab testing |
- | 920 | 920 |
| Database analysis and report | 1419 | 410 | 1829 |
| Public and media communication |
1 | 206 | 207 |
| Other ‡ | - | 877 | 877 |
| Total personnel hours | 4080 | 3656 | 7736 |
| Vehicle Miles | 1759 | 6975 | 8734 |
| Lodging (days) | - | 49 | 49 |
| MMR Vaccine (doses)§ | - | 1812 | 1812 |
| IFA test kits¶ | - | 120 | 120 |
| Costs (US $) | |||
| Personnel | |||
| Salary and wages | 111 537 | 122 017 | 233 554 |
| Fringe benefits | 34 063 | 49 874 | 83 937 |
| Overhead | 59 802 | 35 054 | 94 856 |
| Miles║ | 862 | 3418 | 4280 |
| Lodging | - | 7194 | 7194 |
| MMR Vaccine§lll | - | 34 392 | 34 392 |
| IFA test kits and laboratory costs | - | 4166 | 4166 |
| Other costs** | - | 825 | 825 |
| Total Cost | $206 264 | 256 938 | 463 202 |
*Dashes indicate “not applicable.” †Activities included case-finding and interviewing case-patients and tracing contacts planning and coordinating responses, vaccinating contacts, collecting specimens, encourage isolation and quarantines, developing and reports, answering public inquiries, and working with the media, analyzing databases, developing information for the public and preparing reports. ‡Other activities included data entry, immunization program (maintaining mumps surveillance and assessment of vaccine adverse events). §MMR vaccine intervention and associated cost. ¶Specimen collection includes blood-collection kits, swabs, urine tests, and serologic tests for mumps IgM and IgG antibodies. ║Unitary mileage costs are at US $0.49 per mile. lllUnitary costs are from public sector prices of MMR vaccine at US$18.98 per dose (as September 2011) http://www.cdc.gov/vaccine/program/vfc/cdc-vac-price-list.htm. **Other costs include tolls, meals.
Discussion
Outbreaks of mumps among highly vaccinated populations have mainly affected adolescents and young adults.4-6 A concern that always arises in such outbreaks is the documentation of mumps vaccine doses. The documentation of 2 doses of MMR vaccine in this outbreak is reliable as it was obtained from the state immunization registry or from the healthcare provider’s office or immunization records.
One of the hypotheses raised in literature regarding the occurrence of such outbreaks is the effectiveness of mumps vaccines that contain the Jeryl Lynn strain.20 For a single dose the effectiveness is 78% (median, range: 49–92%) and for 2 doses it is 88% (median, range: 66–95%).21,22 All the cases were village residents except for 3 case-patients who worked in the village. There was no secondary transmission to individuals who lived outside the village indicating that population immunity provided by high two-dose coverage levels of MMR vaccine may have been more effective in limiting transmission in a less crowded setting. It is also possible that the non-village residents were less likely to be exposed.
The role of waning immunity has been suggested in recent outbreaks among highly vaccinated populations.5,23 In Finland, where indigenous mumps has been declared to be eliminated, Davidkin et al. were able to follow individuals who received the MMR vaccine.24 The authors found that 20 y after the first dose, 74% were seropositive for mumps (lower than 95% for measles and 100% for rubella). The authors also noted that during the first 8 y after the receipt of the second dose of MMR vaccine, there was a significant decline in antibodies for measles (50%), mumps (69%), and rubella (58%). Decline in mumps antibodies over time, including to levels described as seronegative 10 y after the second dose in 5% of study subjects, has also been demonstrated in the United States.25 In another study by Date et al., lower levels of neutralizing antibodies were observed among individuals who had received the second dose of MMR vaccine at least 15 y before.26 However there are currently no established immunologic correlates of mumps protection. Arguing against a key role of waning immunity in this outbreak was the fact that an increase in the number of cases among the older age groups was not evident as would have been expected if waning immunity played a major role. The lack of illness among the older age groups may have also been due to possible exposure to wild-type virus previously. As serological testing was not conducted prior to and after the outbreak, we are also unable to evaluate the protective threshold level of mumps IgG antibody. In the OC village, the larger household size in general and high-density settings such as schools may have resulted in transmission of mumps.27 In previously reported studies crowded educational settings such as schools, universities, and camps, have played an important role in transmission of mumps.5,28-33 No evidence has suggested that other factors such as handling changes and vaccine lots were risk factors for vaccine failure.
In the OC village mumps outbreak, a higher proportion of our cases were male. It has been reported that males in this community spent long hours studying in a communal setting at school where they faced each other as they studied.10,34 Since the first reported case was a young male attending one of the village schools, this likely facilitated initial outbreak transmission among males. Because in this community, schools are segregated by sex, there may have been less opportunity for transmission among the females, at least in the school environment. Behavior facilitating increased transmission of mumps has been reported in previous mumps outbreaks.35
Viral testing in a highly vaccinated population is of importance. In a highly vaccinated population, laboratory-confirmation of mumps can be challenging. In a previously vaccinated case the timing of serum collection and the choice of the assay are both important as these individuals may not produce adequate levels of detectable mumps-specific IgM.36 During this outbreak, of the 183 case-patients with documented 2 doses of MMR vaccine, 27 (15%) were found to be mumps IgM antibody positive while among the 17 case-patients with documented 1 dose of MMR vaccine, 2 (12%) were found to be mumps IgM antibody positive. PCR testing was conducted initially but with the confirmation of the outbreak, it was stopped.
When routine control measures, such as isolation and vaccination of exposed contacts failed to control the outbreak, a third dose of MMR vaccine was provided to students in grades 6–12 (11–17 y of age) in the 3 religious schools in the village. The school-based intervention was conducted over a 2-wk period (from January 19–February 2, 2010). The intervention, its impact and vaccine-related adverse events is described elsewhere.19,37
Studies assessing the economic burden of a mumps outbreak are limited. Most of the cost during an outbreak is driven by contact/case-finding as these activities take the most amounts of time and resources. The only other reported study where a third dose of MMR vaccine intervention was conducted was in Guam. In 2009–2010, 505 mumps cases were reported in a mumps outbreak among highly vaccinated population in Guam.38 In the public health response, 76 public health personnel were involved and approximately 8264 h of personnel time were spent. The cost for outbreak response and control was estimated to be $256 785 overall with 93% costs spent by public health institutions. Based on the household study the estimated cost per household was US $761 compared with US $827 in our study. In 2007, Nova Scotia, Canada, experienced a mumps outbreak with 706 reported cases with at least 1 dose of mumps vaccine.39,40 The estimated cost for containment was US $2 396 295 which included vaccination costs, case management, and laboratory testing40; the cost per contact was estimated to be US $614. In comparison, the cost per contact in measles outbreaks in the United States ranges from US $120 to $546.41 However there are limitations to these estimates as they are based on outbreak location, as well as how the estimate is calculated.
Our study had several limitations. It is possible that once the outbreak was declared and that individuals understood that there was no treatment, the sick may not have sought medical care. Among many of the adult cases, vaccination status was unknown. This is probably due to lack of documentation rather than being unvaccinated. We did not assess the association between age at vaccination and protection as seroepidemiological information as well as vaccine coverage data for the village were not available. A seroepidemiology mumps study conducted in Europe (1996–2008) indicated that the one-dose MMR vaccine coverage of ≥ 90% as well as an interval of 4–8 y between doses were significantly associated with lower odds of an outbreak.42
The economic assessment in this study was not designed to distinguish costs from usual outbreak control activities from specific costs associated with the third dose intervention. The personnel time was assessed post-hoc rather than being systematically collected throughout the investigation. Also due to the active research investigation of this outbreak by NYS DOH and CDC staff it is possible that the resources allocated to the outbreak response were in excess of the actual requirement. Because our survey did not query about routine activities performed by responders, the amount of personnel hours and resources diverted for this outbreak investigation are considered a gross but close estimate of the real impact of the outbreak. Such evaluation is supported in part for the type of activities performed by responders in the investigation (e.g., active contact-tracing, screening for cases, etc.) and for the direct involvement of the NYS DOH. Recall bias related to costs incurred may have occurred but we tried to minimize this by collecting data from various time periods and specific tasks performed by responders. To emphasize the local and state perspective of the analysis we did not include costs incurred by the federal healthcare personnel. We also did not assess how the active outreach to healthcare providers affected the cost.
As long as mumps continues to be endemic in other regions of the world, importations to United States will continue to occur.43 Implementation of current vaccine policy has resulted in ≥96% decline in reported mumps cases since the pre-vaccine era. Thus maintaining high two-dose MMR vaccine coverage is essential to maintain measles and rubella elimination and mumps control in the United States. In 2013, CDC issued guidance for consideration of a third dose of MMR vaccine in outbreak settings based on certain criteria that would aid in decision-making.44 A general recommendation for a third dose was not made as data remain insufficient.44
An outbreak such as that in Orange County requires detailed investigation that can result in extensive public health effort. Studies are required to assess risk factors that can contribute to the transmission of mumps in a highly vaccinated population, the effectiveness of the current vaccine policy in different settings, the protective level of mumps antibodies, and the economic assessment of the burden on public health to provide guidance for policy.
Methods
Population
The affected village in OC, New York, had an estimated population of 20 175 in 2010 with a predominantly Orthodox Jewish population; the median age was 13.2 y and males comprised 51.8% of the population.45 The US Census reported an average household size of 5.7, twice the national average of 2.6.45
Mumps case reports
Reporting of communicable diseases, including mumps, is mandated under New York State sanitary code.46 Physicians, nurses, laboratory directors, infection control practitioners, health care facilities, state institutions, and schools are required to report suspected and confirmed cases. In Orange County mumps cases were reported to the Orange County Health Department (OCHD). Active surveillance was instituted. Healthcare is provided mainly by 4 healthcare practices. In addition to healthcare provider reporting, active outreach to providers was conducted to identify new cases. Telephone follow-up to assess any other cases in households was also conducted. The cases, confirmed and probable, were then reported to the NYS DOH through the Communicable Disease Electronic Surveillance System (CDESS). The CDC received case reports through the National Notifiable Diseases Surveillance System (NNDSS).47 The vaccination status of the cases was verified with health-care providers, New York State Immunization Information System (NYSIIS) or child immunization records provided by the parents.
Mumps cases were classified according to the 2008 mumps definition of the Council of State and Territorial Epidemiologists (CSTE).48 A clinical case was defined as an illness with acute onset of the parotitis, lasting at least 2 d, and without other apparent cause. Clinically compatible illness was defined as that which may present as aseptic meningitis, encephalitis, hearing loss, orchitis, oophoritis, parotitis, or other salivary gland swelling, mastitis or pancreatitis. Laboratory confirmation included isolation of mumps virus from clinical specimen, or detection of mumps nucleic acid, or detection of mumps IgM antibody, or demonstration of a 4-fold increase in mumps IgG antibody titer. Mumps cases were then classified epidemiologically.48 A probable case was defined as that which meets the clinical case definition without laboratory confirmation and is epidemiologically linked to a clinically compatible case. A confirmed case was defined as a case that met the clinical case definition or had clinically compatible illness, and was either laboratory confirmed or was epidemiologically linked to a confirmed case. During the OC village mumps outbreak, laboratory testing ceased shortly after the outbreak was confirmed; cases were classified based on their epidemiological linkage. To identify the site of exposure for the cases, during the phone call, the respondents were queried about the most likely site. Hence only one site was entered in the line list. We also compared the characteristics of cases between OC village mumps outbreak to the overall Northeast mumps outbreak.10 Attempts were made to identify probable cases exposed to the case during his/her infectious period.
Age groups were determined based upon required receipt of mumps containing vaccine and school and social structure. To calculate the mumps attack rate for each age group, the village population as indicated by the 2010 US census was used as the denominator. We estimated the number of individuals in each age group. The number of reported cases were divided by the estimated number of individuals in that age group.
Laboratory testing
Testing was conducted by Wadsworth Center, NYS DOH, and CDC laboratories. Detection of IgM antibody to mumps virus was conducted at the Wadsworth Center, NYS DOH, using an in-house immunofluorescence assay (IFA) assay, a modification of the Scimedx Corporation Mumps IFA slides.49 This assay was validated by comparison against the IgM ELISA to mumps virus nucleoprotein as performed at the CDC. The detection of mumps RNA by real-time reverse transcription-polymerase chain reaction (rRT-PCR)50,51 and isolation of mumps virus in cell culture was conducted at the CDC Measles, mumps, rubella, and varicella laboratory. To identify the genotype, genetic analysis of mumps viruses was performed at CDC using standard method.52,53
Economic assessment
Using data from the county and state public health departments, we assessed the direct economic impact of response activities for this mumps outbreak, including the cost of the vaccine intervention. For this purpose, we retrospectively collected and analyzed information on response-related resources used and associated costs based on specific outbreak response activities. Activities required for the containment of the mumps outbreak included active surveillance, case confirmation, contact tracing, communication with other affected states, issuance of press releases to the relevant media, issuance of health advisories to all providers in Orange County, public health education of the local population, specimen collection and laboratory testing by the State and local health departments. Quarantine was strongly encouraged. An activity specific to this outbreak was the school-based third dose vaccination (2 wk, January 19–February 2, 2010) which included preparatory and follow-up activities such as baseline and follow-up surveys.11
Specifically we estimated the cost of personnel time and materials allocated to response activities including number of MMR vaccine doses administered and laboratory testing, and other direct costs related to logistics incurred by the NYS DOH and OCHD separately. The overall outbreak cost includes the cost of evaluation of use of a third dose of MMR vaccine for selected cohorts with a high two-dose vaccination history.
All costs are reported in 2010 US dollars. The study period was defined as October 1, 2009 (when active surveillance was instituted) through June 15, 2010 (when the outbreak ended). A standardized questionnaire was self-administered to personnel who participated in the outbreak response to assess time allotted to specific outbreak response activities.
Efforts were made to allocate personnel costs to specific activities. Once the number of personnel hours allocated to the response was calculated, we estimated the associated costs by using the reported gross hourly earnings for each individual, plus fringe benefits when available. Overhead costs were also estimated based on the number of person-hours and the overhead cost accounting method followed by each institution. Unitary mileage costs are at US $0.49 per mile. Unitary costs are from public sector prices of MMR vaccine at US $18.98 per dose.
With active surveillance in place, we tried to estimate the number of households contacted during this outbreak. We then estimated the cost per household.
Glossary
Abbreviations:
- ACIP
Advisory Committee for Immunization Practices
- CDC
Centers for Disease Control and Prevention
- MMR vaccine
Measles-mumps-rubella vaccine
- NYS DOH
New York State Department of Health
- OC
Orange County
- OCHD
Orange County Health Department
- PHL
Public Health Law
- US
United States
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Acknowledgments
The authors would like to thank the leadership and school administrations of the village in Orange County, New York, the physicians who serve that village, the public health professionals from Orange County Health Department and New York State Department of Health who participated in the outbreak investigation, assisted with data collection, and conducted the laboratory testing. We would also like to thank Drs Jane Seward and Gregory Wallace from the Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, for scientific advice. The primary author (PKK) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Presented in part at the 48th Annual Meeting of the Infectious Diseases Society of America (IDSA), Vancouver, Canada, October 21–24, 2010.
References
- 1.Centers for Disease Control (CDC). . Measles prevention. MMWR Morb Mortal Wkly Rep 1989; 38:Suppl 9 1 - 18; PMID: 2513473 [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). . Notice to readers: updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the control and elimination of mumps. MMWR Morb Mortal Wkly Rep 2006; 55:629 - 30; PMID: 16761359 [PubMed] [Google Scholar]
- 3.Vandermeulen C, Leroux-Roels G, Hoppenbrouwers K. . Mumps outbreaks in highly vaccinated populations: What makes good even better?. Hum Vaccin 2009; 5:494 - 6; PMID: 19279405 [DOI] [PubMed] [Google Scholar]
- 4.Brockhoff HJ, Mollema L, Sonder GJ, Postema CA, van Binnendijk RS, Kohl RH, de Melker HE, Hahné SJ. . Mumps outbreak in a highly vaccinated student population, The Netherlands, 2004. Vaccine 2010; 28:2932 - 6; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.020; PMID: 20188683 [DOI] [PubMed] [Google Scholar]
- 5.Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O’Keefe AL, et al. . Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580 - 9; http://dx.doi.org/ 10.1056/NEJMoa0706589; PMID: 18403766 [DOI] [PubMed] [Google Scholar]
- 6.Whelan J, van Binnendijk R, Greenland K, Fanoy E, Khargi M, Yap K, Boot H, Veltman N, Swaan C, van der Bij A, et al.. . Ongoing mumps outbreak in a student population with high vaccination coverage, Netherlands, 2010. Euro Surveill 2010; 15:19554; PMID: 20460086 [DOI] [PubMed] [Google Scholar]
- 7.Bangor-Jones RD, Dowse GK, Giele CM, van Buynder PG, Hodge MM, Whitty MM. . A prolonged mumps outbreak among highly vaccinated Aboriginal people in the Kimberley region of Western Australia. Med J Aust 2009; 191:398 - 401; PMID: 19807634 [DOI] [PubMed] [Google Scholar]
- 8.Anis E, Grotto I, Moerman L, Warshavsky B, Slater PE, Lev B. . Mumps outbreak in Israel’s highly vaccinated society: are two doses enough?. Epidemiol Infect 2012; 140:439 - 46; http://dx.doi.org/ 10.1017/S095026881100063X; PMID: 21554780 [DOI] [PubMed] [Google Scholar]
- 9.New York State Public Health Law. PHL 2164. [cited 2014 Feb 17]. Available from: http://www.health.ny.gov/regulations/public_health_law/.
- 10.Barskey AE, Schulte C, Rosen JB, Handschur EF, Rausch-Phung E, Doll MK, Cummings KP, Alleyne EO, High P, Lawler J, et al. . Mumps outbreak in Orthodox Jewish communities in the United States. N Engl J Med 2012; 367:1704 - 13; http://dx.doi.org/ 10.1056/NEJMoa1202865; PMID: 23113481 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). . Summary of notifiable diseases: United States, 2000. MMWR Morb Mortal Wkly Rep 2002; 49:i - xxii, 1-100; PMID: 12083162 [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). . Summary of notifiable diseases: United States, 2001. MMWR Morb Mortal Wkly Rep 2003; 50:i - xxiv, 1-108; PMID: 12892074 [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). . Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep 2011; 58:1 - 100; PMID: 21566560 [PubMed] [Google Scholar]
- 14.Jajosky RA, Hall PA, Adams DA, Dawkins FJ, Sharp P, Anderson WJ, Aponte JJ, Jones GF, Nitschke DA, Worsham CA, et al. , Centers for Disease Control and Prevention (CDC). . Summary of notifiable diseases: United States, 2004. MMWR Morb Mortal Wkly Rep 2006; 53:1 - 79; PMID: 16775578 [PubMed] [Google Scholar]
- 15.McNabb SJ, Jajosky RA, Hall-Baker PA, Adams DA, Sharp P, Worshams C, Anderson WJ, Javier AJ, Jones GJ, Nitschke DA, et al. , Centers for Disease Control and Prevention (CDC). . Summary of notifiable diseases: United States, 2006. MMWR Morb Mortal Wkly Rep 2008; 55:1 - 92; PMID: 18354375 [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. . Summary of Notifiable Diseases: United States, 2007. MMWR CDC Surveill Summ 2009; 56:1 - 94 [Google Scholar]
- 17.Centers for Disease Control and Prevention. . Summary of Notifiable Diseases: United States, 2008. MMWR CDC Surveill Summ 2010; 57:1 - 94 [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Immunization Survey (NIS). Vaccination Coverage Reported in the U.S. [cited 2014 Feb 17]. Available from: http://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm.
- 19.Ogbuanu IU, Kutty PK, Hudson JM, et al. . Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics 2012; 130:e1567 74; http://dx.doi.org/ 10.1542/peds.2012-0177; PMID: 23129075 [DOI] [PubMed] [Google Scholar]
- 20.Barskey AE, Glasser JW, LeBaron CW. . Mumps resurgences in the United States: A historical perspective on unexpected elements. Vaccine 2009; 27:6186 - 95; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.109; PMID: 19815120 [DOI] [PubMed] [Google Scholar]
- 21.Mclean HQHC, Seward JF. The immunological basis for immunization series: Module 16: Mumps 2010. [cited 2014 Feb 17]. Available from: http://whqlibdoc.who.int/publications/2010/9789241500661_eng.pdf.
- 22.Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. . An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ 2011; 183:1014 - 20; http://dx.doi.org/ 10.1503/cmaj.101371; PMID: 21576295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortese MM, Jordan HT, Curns AT, Quinlan PA, Ens KA, Denning PM, Dayan GH. . Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis 2008; 46:1172 - 80; http://dx.doi.org/ 10.1086/529141; PMID: 18444852 [DOI] [PubMed] [Google Scholar]
- 24.Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. . Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis 2008; 197:950 - 6; http://dx.doi.org/ 10.1086/528993; PMID: 18419470 [DOI] [PubMed] [Google Scholar]
- 25.LeBaron CW, Forghani B, Beck C, Brown C, Bi D, Cossen C, Sullivan BJ. . Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis 2009; 199:552 - 60; http://dx.doi.org/ 10.1086/596207; PMID: 19113988 [DOI] [PubMed] [Google Scholar]
- 26.Date AA, Kyaw MH, Rue AM, Klahn J, Obrecht L, Krohn T, Rowland J, Rubin S, Safranek TJ, Bellini WJ, et al. . Long-term persistence of mumps antibody after receipt of 2 measles-mumps-rubella (MMR) vaccinations and antibody response after a third MMR vaccination among a university population. J Infect Dis 2008; 197:1662 - 8; http://dx.doi.org/ 10.1086/588197; PMID: 18419346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutty PK, McLean HQ, Lawler J, et al.. . Risk Factors for Transmission of Mumps in a Highly Vaccinated Population in Orange County, New York, 2009-2010. Pediatr Infect Dis J 2014; 33:121 5; http://dx.doi.org/ 10.1097/INF.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 28.Sullivan KM, Halpin TJ, Marks JS, Kim-Farley R. . Effectiveness of mumps vaccine in a school outbreak. Am J Dis Child 1985; 139:909 - 12; PMID: 4036925 [DOI] [PubMed] [Google Scholar]
- 29.Wharton M, Cochi SL, Hutcheson RH, Schaffner W. . Mumps transmission in hospitals. Arch Intern Med 1990; 150:47 - 9; http://dx.doi.org/ 10.1001/archinte.1990.00390130063006; PMID: 2297298 [DOI] [PubMed] [Google Scholar]
- 30.Chaiken BP, Williams NM, Preblud SR, Parkin W, Altman R. . The effect of a school entry law on mumps activity in a school district. JAMA 1987; 257:2455 - 8; http://dx.doi.org/ 10.1001/jama.1987.03390180073026; PMID: 3573244 [DOI] [PubMed] [Google Scholar]
- 31.Hersh BS, Fine PE, Kent WK, Cochi SL, Kahn LH, Zell ER, Hays PL, Wood CL. . Mumps outbreak in a highly vaccinated population. J Pediatr 1991; 119:187 - 93; http://dx.doi.org/ 10.1016/S0022-3476(05)80726-7; PMID: 1861205 [DOI] [PubMed] [Google Scholar]
- 32.Briss PA, Fehrs LJ, Parker RA, Wright PF, Sannella EC, Hutcheson RH, Schaffner W. . Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. J Infect Dis 1994; 169:77 - 82; http://dx.doi.org/ 10.1093/infdis/169.1.77; PMID: 8277201 [DOI] [PubMed] [Google Scholar]
- 33.Ruijs WL, Hautvast JL, Akkermans RP, Hulscher ME, van der Velden K. . The role of schools in the spread of mumps among unvaccinated children: a retrospective cohort study. BMC Infect Dis 2011; 11:227; http://dx.doi.org/ 10.1186/1471-2334-11-227; PMID: 21864363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker Fiebelkorn A, Rosen JB, Brown C, Zimmerman CM, Renshowitz H, D’Andrea C, Gallagher KM, Harpaz R, Zucker JR. . Environmental factors potentially associated with mumps transmission in yeshivas during a mumps outbreak among highly vaccinated students: Brooklyn, New York, 2009-2010. Hum Vaccin Immunother 2013; 9:189 - 94; http://dx.doi.org/ 10.4161/hv.22415; PMID: 23442590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheek JE, Baron R, Atlas H, Wilson DL, Crider RD Jr.. . Mumps outbreak in a highly vaccinated school population. Evidence for large-scale vaccination failure. Arch Pediatr Adolesc Med 1995; 149:774 - 8; http://dx.doi.org/ 10.1001/archpedi.1995.02170200064010; PMID: 7795768 [DOI] [PubMed] [Google Scholar]
- 36.Rota JS, Rosen JB, Doll MK, McNall RJ, McGrew M, Williams N, Lopareva EN, Barskey AE, Punsalang A Jr., Rota PA, et al. . Comparison of the sensitivity of laboratory diagnostic methods from a well-characterized outbreak of mumps in New York city in 2009. Clin Vaccine Immunol 2013; 20:391 - 6; http://dx.doi.org/ 10.1128/CVI.00660-12; PMID: 23324519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abedi GR, Mutuc JD, Lawler J, Leroy ZC, Hudson JM, Blog DS, Schulte CR, Rausch-Phung E, Ogbuanu IU, Gallagher K, et al. . Adverse events following a third dose of measles, mumps, and rubella vaccine in a mumps outbreak. Vaccine 2012; 30:7052 - 8; http://dx.doi.org/ 10.1016/j.vaccine.2012.09.053; PMID: 23041123 [DOI] [PubMed] [Google Scholar]
- 38.Mahamud A, Fiebelkorn AP, Nelson G, Aguon A, McKenna J, Villarruel G, Gallagher K, Ortega-Sánchez IR. . Economic impact of the 2009-2010 Guam mumps outbreak on the public health sector and affected families. Vaccine 2012; 30:6444 - 8; http://dx.doi.org/ 10.1016/j.vaccine.2012.08.001; PMID: 22902678 [DOI] [PubMed] [Google Scholar]
- 39.Watson-Creed G, Saunders A, Scott J, Lowe L, Pettipas J, Hatchette TF. . Two successive outbreaks of mumps in Nova Scotia among vaccinated adolescents and young adults. CMAJ 2006; 175:483 - 8; http://dx.doi.org/ 10.1503/cmaj.060660; PMID: 16940266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janes A. Economic burden of the Nova Scotia mumps outbreak. In: Master of development economics. Halifax, Nova Scotia: Dalhousie University; 2010. [Google Scholar]
- 41.Ortega-Sanchez IR, Vijayaraghavan M, Barskey AE, Wallace GS. . The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine 2014; 32:1311 7; http://dx.doi.org/ 10.1016/j.vaccine.2013.10.012; PMID: 24135574 [DOI] [PubMed] [Google Scholar]
- 42.Eriksen J, Davidkin I, Kafatos G, Andrews N, Barbara C, Cohen D, Duks A, Griskevicius A, Johansen K, Bartha K, et al. . Seroepidemiology of mumps in Europe (1996-2008): why do outbreaks occur in highly vaccinated populations?. Epidemiol Infect 2012; 12:1 - 16; http://dx.doi.org/ 10.1097/01.ede.0000417196.70302.1d; PMID: 22687578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Mumps. [cited 2014 Feb 17]. Available from: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/mumps/en/index.html.
- 44.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Centers for Disease Control and Prevention. . Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:RR-04 1 - 34; PMID: 23760231 [PubMed] [Google Scholar]
- 45.United States Census Bureau. 2010 Population Estimates. [cited 2014 Feb 17]. Available from: http://www.census.gov.
- 46.New York State Department of Health. Communicable Disease Reporting Requirements. [cited 2014 Feb 17]. Available from: http://www.health.ny.gov/forms/instructions/doh-389_instructions.pdf.
- 47.Centers for Disease Conrol and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Accessible at http://wwwn.cdc.gov/nndss/default.aspx. Date accessed: 2/17/2014.
- 48.Council of State and Territorial Epidemiologists. Revision of the Surveillance Case Definition for Mumps. 2009. [cited 2014 Feb 17]. Available from: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/09-ID-50.pdf.
- 49.Diamedix Corporation. SCIMEDIX Indirect Fluorescence Assay (IFA) Mumps. [cited 2014 Feb 17]. Available from: http://www.diamedix.com/en/technical-information/package-inserts/infectious-ifa/Mumps-IgG-S-I-MUV01G.pdf.
- 50.Boddicker JD, Rota PA, Kreman T, Wangeman A, Lowe L, Hummel KB, Thompson R, Bellini WJ, Pentella M, Desjardin LE. . Real-time reverse transcription-PCR assay for detection of mumps virus RNA in clinical specimens. J Clin Microbiol 2007; 45:2902 - 8; http://dx.doi.org/ 10.1128/JCM.00614-07; PMID: 17652480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Real-time (TaqMan®) RT-PCR assay for the detection of mumps virus RNA in clinical samples. [cited 2014 Feb 17]. Available from: http://www.cdc.gov/mumps/downloads/lab-rt-pcr-assay-detect.doc.
- 52.Rota JS, Turner JC, Yost-Daljev MK, Freeman M, Toney DM, Meisel E, Williams N, Sowers SB, Lowe L, Rota PA, et al. . Investigation of a mumps outbreak among university students with two measles-mumps-rubella (MMR) vaccinations, Virginia, September-December 2006. J Med Virol 2009; 81:1819 - 25; http://dx.doi.org/ 10.1002/jmv.21557; PMID: 19697404 [DOI] [PubMed] [Google Scholar]
- 53.Jin L, Rima B, Brown D, Orvell C, Tecle T, Afzal M, Uchida K, Nakayama T, Song JW, Kang C, et al. . Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch Virol 2005; 150:1903 - 9; http://dx.doi.org/ 10.1007/s00705-005-0563-4; PMID: 15959834 [DOI] [PubMed] [Google Scholar]