Abstract
Background
The proposed use of bevacizumab with radiotherapy/temozolomide for newly diagnosed glioblastoma raised potential safety concerns. Bevacizumab has been linked with stroke, bleeding events, and wound-healing complications in other tumor types; these events are of particular concern for glioblastoma (highly vascular tumors that are usually resected). Published data on the interaction of bevacizumab with radiotherapy/temozolomide are also limited. We report safety data from a phase III randomized trial (Avastin in Glioblastoma), focusing on these considerations.
Methods
Eligible patients received: radiotherapy and temozolomide plus bevacizumab/placebo, 6 cycles; a 4-week treatment break; temozolomide plus bevacizumab/placebo, 6 cycles; and bevacizumab/placebo until progression. Data on adverse events (AEs) were collected throughout.
Results
Bevacizumab-treated patients (n = 461) had a longer median safety follow-up time (12.3 vs 8.5 mo), and a higher proportion completed 6 cycles of maintenance temozolomide (64.6% vs 36.9%) versus placebo (n = 450). The incidences of relevant AEs (bevacizumab vs placebo, respectively) were: arterial thromboembolic events (5.9% vs 1.6%); cerebral hemorrhage (3.3% vs 2.0%); wound-healing complications (6.9% vs 4.7%); thrombocytopenia (34.1% vs 27.3%); radiotherapy-associated skin injury (8.2% vs 9.3%); alopecia (39.0% vs 36.0%); gastrointestinal perforation (including gastrointestinal abscesses and fistulae, 1.7% vs 0.4%); and radiotherapy-associated injury (0.4% vs 0.0%). Overall, 15.8% and 23.8% of bevacizumab- and placebo-treated patients had surgery (including biopsy) after progression. Within 30 days of postprogression surgery, AE incidence was 10.9% (bevacizumab) and 23.4% (placebo).
Conclusion
The safety profile was consistent with that expected from radiotherapy/temozolomide plus bevacizumab. The increased AE incidence with bevacizumab did not impact patients' ability to receive standard-of-care treatment or to undergo further surgery.
Keywords: adverse events, AVAglio, bevacizumab, glioblastoma, safety
Bevacizumab, an inhibitor of vascular endothelial growth factor–stimulated angiogenesis, has been approved for the treatment of 7 oncologic indications, including recurrent glioblastoma. More than 1 726 400 patients worldwide have received bevacizumab.1 Accordingly, bevacizumab has a well-characterized safety profile.2 However, investigations into the use of bevacizumab plus standard of care for newly diagnosed glioblastoma raised some specific potential safety considerations. Bevacizumab has been associated with bleeding events and stroke, both of which have potentially important consequences in highly vascular intracranial tumors such as glioblastoma. Bevacizumab has also been associated with wound-healing complications, a particular concern considering that patients with newly diagnosed glioblastoma undergo debulking surgery at diagnosis, with potentially further surgeries later in the disease course. There are limited data on the interaction of bevacizumab with surgical resection in this patient population and it was unknown whether bevacizumab might hinder or complicate life-extending surgeries.3–5 Safety considerations for the combination of bevacizumab with standard-of-care treatment for newly diagnosed glioblastoma (radiotherapy and the myelotoxic agent temozolomide6) were also an unknown quantity. When the Avastin in Glioblastoma (AVAglio) trial was initiated, bevacizumab had not been approved in conjunction with radiotherapy for any indication and there were only limited data on this combination in the literature. Similarly, the additive toxicity of temozolomide and bevacizumab had been studied in only a limited dataset, and patients receiving myelotoxic chemotherapies, such as temozolomide, are often at increased risk of infection.
With current standard-of-care therapy, the outlook for patients with glioblastoma is dismal7; the disease recurs or progresses in almost all patients.8 The addition of bevacizumab to radiotherapy and temozolomide for newly diagnosed glioblastoma has shown efficacy in 2 phase III, randomized, double-blind, placebo-controlled trials.9,10 In the AVAglio trial, bevacizumab significantly improved progression-free survival (PFS) versus placebo by 4.4 months (hazard ratio [HR]: 0.64) and maintained health-related quality of life (HRQoL), but overall survival (OS) was not significantly improved (HR: 0.88).9 Overall safety results were generally as expected,9 but this phase III study offered an opportunity to examine in detail the tolerability of this combination in a large patient population with respect to safety topics of interest, namely bleeding/stroke events, wound-healing complications, and events associated with radiotherapy, myelotoxicity, or reoperation.
Methods
Study Design
AVAglio (NCT00943826) was a randomized, double-blind, placebo-controlled phase III trial sponsored by F. Hoffmann-La Roche Ltd. Eligibility criteria have been described previously.9 Patients were randomized to receive radiotherapy and temozolomide plus either bevacizumab (Avastin) or placebo in 3 treatment phases. In the concurrent phase, patients received radiotherapy (60 Gy, administered as 2-Gy fractions 5 d/wk) and temozolomide (75 mg/m2/d, orally, for a maximum of 49 d) plus bevacizumab (10 mg/kg i.v.) or placebo (i.v.), every 2 weeks. The final concurrent doses of temozolomide plus bevacizumab or placebo were administered on the day of the last radiotherapy dose. A 28-day treatment break began at radiotherapy completion. In the maintenance phase, patients received temozolomide (150 mg/m2/d, days 1–5 during the first cycle and 200 mg/m2/d during subsequent cycles if toxicity permitted) plus bevacizumab (10 mg/kg i.v.) or placebo (i.v.), every 2 weeks, for six 4-week cycles. During the monotherapy phase, patients received single-agent bevacizumab (15 mg/kg i.v.) or placebo (i.v.) every 3 weeks until onset of progressive disease (PD) or unacceptable toxicity.9 After confirmed PD, patients were treated according to investigator choice; all further anti-neoplastic treatment, including surgery and reirradiation, was recorded. The coprimary endpoints were OS and PFS.9 Safety was a secondary endpoint. The protocol was approved by applicable independent ethics committees and institutional review boards. Real-time monitoring of safety events was overseen by an independent data and safety monitoring board. The study adhered to the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice.
Reoperation at Progressive Disease
The number of patients who underwent reoperation at PD was recorded. Investigators also recorded the type of reoperation (complete or partial resection), the number of surgeries per patient, and the date of surgery. Data on adverse events (AEs) were available for 30 days postsurgery.
Reporting of Safety Data
AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v3.0.11 AEs were predefined as any unfavorable and unintended medical occurrence or sign, symptom, or disease where the patient was administered a pharmaceutical product and which did not necessarily have a causal relationship with treatment. Preexisting conditions that worsened during the study were reported as AEs.
Specific AEs related to bevacizumab were identified from previous studies where higher incidences were observed in the bevacizumab arm relative to the comparator.2 These were protocol defined: hypertension, proteinuria, gastrointestinal (GI) perforation, wound-healing complications (craniotomy/noncraniotomy), thromboembolic events, bleeding, congestive heart failure, abscesses and fistulae, or reversible posterior leukoencephalopathy syndrome. Post-hoc analyses were conducted to further evaluate thrombocytopenia and infections.
Overall AEs and bevacizumab-related AEs had data collected throughout all treatment phases and were monitored for up to 90 days and up to 6 months, respectively, after last treatment dose. Related serious AEs were to be reported indefinitely.
Analysis of Safety Data
Preferred terms were assigned using the Medical Dictionary for Regulatory Activities (MedDRA) v16.0. AEs were summarized by seriousness/relationship to trial treatment. AEs leading to death, study withdrawal, or dose modification/interruption were recorded. Dose modification due to bevacizumab toxicity did not affect temozolomide dose, to allow the patient to receive standard of care even if bevacizumab modification was required. Summarized by treatment arm were incidence of AEs by age (<65 or ≥65 y) and in subgroups of patients with risk factors for arterial thromboembolic events (ATEs). Patients who experienced the same event more than once were counted once by worst severity. Analysis of AEs related to bevacizumab was based on grouping AE terms by specific MedDRA baskets or AE Group Terms and Standardized MedDRA Queries.
The radiation dose and the dose/duration of temozolomide treatment were recorded. Time to onset of selected AEs was evaluated using Kaplan–Meier methodology. The day of death was recorded, making it possible to categorize deaths as early (randomization to study day 70), late (study day 71–90 d after the last dose), or during follow-up.
A benefit-risk profile for bevacizumab was evaluated graphically using a qualitative approach employing a descriptive framework (the Benefit-Risk Action Team Framework).12 The context of the benefit-risk decision was based on the indication for bevacizumab and the current standard-of-care treatment.2 Benefit elements were specified efficacy outcomes, and risk elements were standard safety parameters. Benefit and risk elements were organized hierarchically and used to construct a “value tree.” Nonessential elements and elements considered unlikely to drive clinical decision making were removed. Clinically relevant benefits for the patient population included in the final value tree were PFS, OS, Karnofsky Performance Score (KPS), corticosteroid use, and time to definitive deterioration in 5 prespecified HRQoL domains (global health status, physical functioning, social functioning, motor dysfunction, and communication deficit). Clinically significant risks for the patient population included in the final value tree were grade 5 AEs, grade ≥3 AEs related to bevacizumab, grade ≥3 infection AEs, and serious AEs. No weights were assigned to the outcomes in this descriptive framework.
Results
Patients
From June 2009 to March 2011, a total of 921 patients (120 centers/23 countries) were enrolled (intent-to-treat [ITT] population). Of these, 458 were randomized to receive bevacizumab and 463 to receive placebo; 452 and 459, respectively, received study treatment in each arm. Due to a small number of protocol violations (patients in the placebo arm receiving bevacizumab), the safety population was 461 and 450 patients (bevacizumab arm and placebo arm, respectively). Baseline characteristics of the safety population were generally balanced between arms (Table 1) and were comparable to the ITT population.9 At baseline, more patients in the bevacizumab versus the placebo arm had hypertension (38.6% vs 11.3%) or hypercholesterolemia (1.1% vs 0.2%), but there were similar incidences of diabetes mellitus (1.5% vs 1.3%), and a similar proportion of patients were aged ≥65 years (21.7% vs 21.8%). Most patients underwent complete or partial tumor resection in both study arms.
Table 1.
Baseline characteristics of the safety population in the AVAglio study
| Characteristic | BEV + RT/TMZ (n = 461) | Plb + RT/TMZ (n = 450) |
|---|---|---|
| Median age, y (range) | 57.0 (20–84) | 56.0 (18–79) |
| Age, y, n (%) | ||
| <50 | 120 (26.0) | 108 (24.0) |
| 50–59 | 156 (33.8) | 161 (35.8) |
| 60–69 | 146 (31.7) | 148 (32.9) |
| ≥70 | 39 (8.5) | 33 (7.3) |
| Gender, n (%) | ||
| Male | 285 (61.8) | 290 (64.4) |
| Female | 176 (38.2) | 160 (35.6) |
| RPA class, n (%) | ||
| III | 79 (17.1) | 72 (16.0) |
| IV | 260 (56.4) | 275 (61.1) |
| V | 122 (26.5) | 103 (22.9) |
| KPS at baseline, n (%)a | ||
| 50–80 | 147 (32.0) | 138 (30.7) |
| 90–100 | 313 (68.0) | 312 (69.3) |
| MMSE score, n (%)b | ||
| <27 | 106 (23.4) | 103 (23.0) |
| ≥27 | 347 (76.6) | 344 (77.0) |
| WHO performance status, n (%) | ||
| 0 | 231 (50.1) | 231 (51.3) |
| 1–2 | 230 (49.9) | 219 (48.7) |
| MGMT gene promoter status, n (%) | ||
| Methylated | 121 (26.2) | 115 (25.6) |
| Nonmethylated | 226 (49.0) | 230 (51.1) |
| Missing | 114 (24.7) | 105 (23.3) |
| Surgical status, n (%) | ||
| Biopsy only | 61 (13.2) | 42 (9.3) |
| Partial resection | 208 (45.1) | 221 (49.1) |
| Complete resection | 192 (41.6) | 187 (41.6) |
| Corticosteroid use at baseline, n (%) | ||
| On | 189 (41.0) | 204 (45.3) |
| Off | 270 (58.6) | 244 (54.2) |
| Missing | 2 (0.4) | 2 (0.4) |
| Time between surgery and first treatment, n (%) | ||
| <4 wk | 3 (0.7) | 2 (0.4) |
| 4–7 wk | 444 (96.3) | 429 (95.3) |
| >7 wk | 14 (3.0) | 19 (4.2) |
Abbreviations: BEV, bevacizumab; MGMT, methylguanine methyltransferase; MMSE, Mini Mental State Examination; Plb, placebo; RPA, recursive partitioning analysis; RT, radiotherapy; TMZ, temozolomide; WHO, World Health Organization.
an = 460 for BEV + RT/TMZ and n = 450 for Plb + RT/TMZ.
bn = 453 for BEV + RT/TMZ and n = 447 for Plb + RT/TMZ.
Median safety follow-up time (from randomization) was longer in the bevacizumab arm than the placebo arm (12.3 mo [range, 3.1–44.4] vs 8.5 mo [range, 3.0–40.9]).
Treatment Exposure
Radiotherapy
Similar proportions of patients in the bevacizumab and placebo arms completed ≥90% of the planned doses of radiotherapy (95.9% and 95.6%, respectively).
Temozolomide
The same proportion of patients in the bevacizumab and placebo arms completed ≥90% of the planned doses of temozolomide during the concurrent phase (89.8% and 89.8%). More patients in the bevacizumab than placebo arm completed the 6 cycles of maintenance therapy with temozolomide (64.6% vs 36.9%). Relatively few patients discontinued temozolomide in either arm (5.2% and 6.0%, bevacizumab and placebo arms, respectively).
Bevacizumab or placebo
More patients in the bevacizumab than placebo arm completed the 6 cycles of maintenance therapy with bevacizumab/placebo (67.2% vs 39.6%). Throughout all phases of the study, patients in the bevacizumab arm received more doses of bevacizumab (median 19 [range, 1–59]) than patients in the placebo arm received of placebo (median 12 [range, 1–63]), which is reflective of the longer time to progression in the bevacizumab arm.9
Overall Adverse Events
AEs (any grade) were reported in 454 bevacizumab-treated patients and 432 placebo-treated patients (Table 2).9 More patients in the bevacizumab versus the placebo arm experienced serious AEs, grade ≥3 AEs, and AEs related to bevacizumab. The most commonly reported AEs of any grade (in ≥10% of patients) in both arms were nausea, fatigue, and alopecia (Table 3). Fatigue occurred at a similar frequency in the 2 arms. AEs that occurred more frequently with bevacizumab than with placebo (≥10% difference) were epistaxis, hypertension, and proteinuria.
Table 2.
Summary of adverse events9
| Adverse Event, n (%) | BEV + RT/TMZ (n = 461) | Plb + RT/TMZ (n = 450) |
|---|---|---|
| Any adverse event | 454 (98.5) | 432 (96.0) |
| Serious adverse event | 179 (38.8) | 115 (25.6) |
| Grade ≥3 adverse event | 308 (66.8) | 231 (51.3) |
| Grade ≥3 adverse event related to bevacizumab | 150 (32.5) | 71 (15.8) |
| Grade 5 adverse event | 20 (4.3) | 12 (2.7) |
| Discontinued any treatment due to adverse event | 122 (26.5) | 61 (13.6) |
| Discontinued BEV/Plb due to adverse event | 114 (24.7) | 46 (10.2) |
Abbreviations: BEV, bevacizumab; Plb, placebo; RT, radiotherapy; TMZ, temozolomide.
Table 3.
Summary of adverse events (any grade) by body system (at an incidence of ≥10%)
| Body System/Adverse Event, n (%) | BEV + RT/TMZ (n = 461) | Plb + RT/TMZ (n = 450) |
|---|---|---|
| GI disorders | ||
| Nausea | 223 (48.4) | 191 (42.4) |
| Constipation | 178 (38.6) | 137 (30.4) |
| Vomiting | 149 (32.3) | 102 (22.7) |
| Diarrhea | 96 (20.8) | 70 (15.6) |
| General disorders and administration site conditions | ||
| Fatigue | 191 (41.4) | 178 (39.6) |
| Asthenia | 86 (18.7) | 63 (14.0) |
| Pyrexia | 47 (10.2) | 28 (6.2) |
| Skin and subcutaneous tissue disorders | ||
| Alopecia | 180 (39.0) | 162 (36.0) |
| Rash | 77 (16.7) | 61 (13.6) |
| Pruritus | 54 (11.7) | 37 (8.2) |
| Blood and lymphatic system disorders | ||
| Thrombocytopenia | 157 (34.1) | 123 (27.3) |
| Neutropenia | 66 (14.3) | 55 (12.2) |
| Leukopenia | 56 (12.1) | 41 (9.1) |
| Nervous system disorders | ||
| Headache | 174 (37.7) | 130 (28.9) |
| Dizziness | 46 (10.0) | 54 (12.0) |
| Vascular disorders | ||
| Hypertension | 178 (38.6) | 51 (11.3) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Epistaxis | 98 (21.3) | 22 (4.9) |
| Cough | 55 (11.9) | 46 (10.2) |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 116 (25.2) | 76 (16.9) |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 71 (15.4) | 30 (6.7) |
| Pain in extremity | 48 (10.4) | 22 (4.9) |
| Infections | ||
| Nasopharyngitis | 63 (13.7) | 26 (5.8) |
| Urinary tract infection | 50 (10.8) | 29 (6.4) |
| Psychiatric disorders | ||
| Insomnia | 53 (11.5) | 42 (9.3) |
| Renal and urinary disorders | ||
| Proteinuria | 72 (15.6) | 19 (4.2) |
Abbreviations: BEV, bevacizumab; Plb, placebo; RT, radiotherapy; TMZ, temozolomide; GI, gastrointestinal.
All-Cause Deaths
The number of all-cause deaths was balanced between arms. At the time of the data cutoff, 72.7% versus 74.9% of patients in the bevacizumab and placebo arms, respectively, had died. Deaths in both arms were predominantly due to PD (66.2% and 67.8%). A comparable number of non-PD deaths occurred in the bevacizumab and placebo arms (6.5% vs 7.1%) (note: non-PD deaths were not necessarily related to AEs).
There was an imbalance of deaths between arms in the early treatment period. More non-PD deaths occurred during the early treatment phase in the bevacizumab versus the placebo arm (patients, 1.5% vs 0.9%), mostly as a result of potential AEs related to bevacizumab in the bevacizumab arm (pulmonary embolism: n = 2; GI perforation: n = 1; bleeding: n = 1). There was no common pattern or risk factor (such as age, medical history, KPS, recursive partitioning analysis class) for patients who experienced an AE leading to death in the early treatment phase. A similar number of patients died from non-PD events during the late phase in the bevacizumab and placebo arms (3.2% vs 2.8%), despite the longer treatment period being associated with longer progression-free time with bevacizumab. Fewer patients died during the follow-up period in the bevacizumab versus the placebo arm (3.0% vs 5.3%).
Safety Considerations Related to Bevacizumab Treatment
The overall incidence of AEs related to bevacizumab (all grades and grade ≥3) was higher with bevacizumab versus placebo (Table 4). The most frequent AEs related to bevacizumab were hypertension and proteinuria. Hypertension was resolved in the majority of cases and only 4 patients required discontinuation of bevacizumab. Proteinuria resolved without specific treatment in most cases; however, 17 patients discontinued bevacizumab treatment due to proteinuria.
Table 4.
Overall incidences of adverse events of special interest for bevacizumab (all grades and grade ≥3)
| AESI/Adverse Event, n (%) | BEV + RT/TMZ (n = 461) |
Plb + RT/TMZ (n = 450) |
||
|---|---|---|---|---|
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| Total patients with at least one AESI | 349 (75.7) | 150 (32.5) | 204 (45.3) | 71 (15.8) |
| Bleeding (cerebral hemorrhage) | 15 (3.3) | 9 (2.0) | 9 (2.0) | 4 (0.9) |
| Resolved | 11 (73.3) | 8 (88.9) | 5 (55.6) | 1 (25.0) |
| Unresolved | 4 (26.7) | 1 (11.1) | 3 (33.3) | 2 (50.0) |
| Death | 0 | 0 | 1a (11.1) | 1a (25.0) |
| Other bleeding (including mucocutaneous bleeding) | 171 (37.1) | 6 (1.3) | 88 (19.6) | 4 (0.9) |
| Resolved | 145 (84.8) | 4 (66.7) | 69 (78.4) | 2 (50.0) |
| Unresolved | 43 (25.1) | 1 (16.7) | 22 (25.0) | 1 (25.0) |
| Death | 1b (0.6) | 1b (16.7) | 1c (1.1) | 1c (25.0) |
| Wound-healing complications | 32 (6.9) | 15 (3.3) | 21 (4.7) | 7 (1.6) |
| Resolved | 27 (84.4) | 11 (73.3) | 19 (90.5) | 7 (100.0) |
| Unresolved | 7 (21.9) | 3 (20.0) | 2 (9.5) | 0 |
| Death | 1 (3.1) | 1 (6.7) | 0 | 0 |
| ATEsd | 27 (5.9) | 23 (5.0) | 7 (1.6) | 6 (1.3) |
| Resolved with sequelae | 11 (40.7) | 11 (47.8) | 2 (28.6) | 2 (33.3) |
| Resolved without sequelae | 9 (33.3) | 6 (26.1) | 1 (14.3) | 1 (16.7) |
| Unresolved | 7 (25.9) | 6 (26.1) | 3 (42.9) | 2 (33.3) |
| Death | 1 (3.7) | 1 (4.3) | 1 (14.3) | 1 (16.7) |
| Venous thromboembolic events | 38 (8.2) | 35 (7.6) | 43 (9.6) | 36 (8.0) |
| Resolved | 26 (68.4) | 23 (65.7) | 22 (51.2) | 17 (47.2) |
| Unresolved | 11 (28.9) | 11 (31.4) | 21 (48.8) | 19 (52.8) |
| Death | 3e (7.9) | 3e (8.6) | 1 (2.3) | 1 (2.8) |
| Hypertension | 181 (39.3) | 52 (11.3) | 57 (12.7) | 10 (2.2) |
| Resolved | 131 (72.4) | 35 (67.3) | 46 (80.7) | 7 (70.0) |
| Unresolved | 62 (34.3) | 19 (36.5) | 13 (22.8) | 3 (30.0) |
| Death | 0 | 0 | 0 | 0 |
| Proteinuria | 72 (15.6) | 25 (5.4) | 19 (4.2) | 0 |
| Resolved | 49 (68.1) | 14 (56.0) | 16 (84.2) | 0 |
| Unresolved | 38 (52.8) | 12 (48.0) | 4 (21.1) | 0 |
| Death | 0 | 0 | 0 | 0 |
| GI perforation (including GI fistula/abscess) | 8 (1.7) | 5 (1.1) | 2 (0.4) | 1 (0.2) |
| Resolved | 6 (75.0) | 4 (80.0) | 0 | 0 |
| Unresolved | 1 (12.5) | 0 | 2 (100.0) | 1 (100.0) |
| Death | 1 (12.5) | 1 (20.0) | 0 | 0 |
| Abscesses and fistulae (non GI) | 2 (0.4) | 2 (0.4) | 3 (0.7) | 3 (0.7) |
| Resolved | 2 (100.0) | 2 (100.0) | 3 (100.0) | 3 (100.0) |
| Unresolved | 0 | 0 | 0 | 0 |
| Death | 0 | 0 | 0 | 0 |
| Congestive heart failure | 2 (0.4) | 2 (0.4) | 1 (0.2) | 0 |
| Resolved | 1 (50.0) | 1 (50.0) | 1 (100.0) | 0 |
| Unresolved | 1 (50.0) | 1 (50.0) | 0 | 0 |
| Death | 0 | 0 | 0 | 0 |
Abbreviations: AESI, adverse event of special interest; BEV, bevacizumab; Plb, placebo; RT, radiotherapy; TMZ, temozolomide; ATE, arterial thromboembolic event; GI, gastrointestinal; MedDRA, Medical Dictionary for Regulatory Activities.
In cases resolved/unresolved, multiple occurrences of the same AE in one patient are included. There were no reports of posterior reversible encephalopathy syndrome.
aCerebrovascular accident, which codes to the standard MedDRA query of “cerebral hemorrhage.” However, medical review identified this event to be of ischemic origin.
bTumor hemorrhage.
cGI hemorrhage.
d21/27 patients (78%) in the bevacizumab arm experienced a serious ATE and 5/7 patients (71%) in the placebo arm experienced a serious ATE.
eOne patient stopped trial treatment on day 49 of the concurrent phase, before receiving off-protocol anticancer treatment with 2 cycles of BEV from day 78 to day 120 and 2 cycles of TMZ from day 78 to day 134. The death occurred in the follow-up period (>90 d from the last dose but within 6 mo of the last dose).
Arterial thromboembolic events
The occurrence of ATEs (all grades and grade ≥3) was higher with bevacizumab compared with placebo (Table 4). Of patients who experienced grade ≥3 ATEs, more patients in the bevacizumab versus the placebo arm were aged ≥65 years (39.1% vs 0.0%) and a greater proportion had hypertension at baseline (60.9% vs 16.7%). Most ATEs in both arms were stroke events (determined by medical review; all grades, 4.1% vs 1.3%; grade ≥3, 3.7% vs 1.3%; Supplementary Table S1). The majority of stroke events in the bevacizumab arm were ischemic; only 1 was hemorrhagic. For 1 patient, stroke occurred more than 2 months after confirmed PD, and for 2 patients (both asymptomatic for stroke) diagnosis of a stroke event was made incidentally on radiological scans.
ATEs (all grades and grade ≥3) resolved in a higher proportion of patients in the bevacizumab versus the placebo arm (Table 4). There was 1 fatal ATE in each arm: myocardial infarction (bevacizumab; during the treatment break) and cerebrovascular accident (placebo; also described under bleeding events below).
A time-to-onset analysis showed that patients in the bevacizumab arm had a higher risk of experiencing grade ≥3 ATEs versus the placebo arm. The majority of events occurred in patients who had tolerated treatment reasonably well and had been on study treatment without PD for 1.2–1.7 years.
Of the patients who experienced ATEs, an exploratory analysis showed that median PFS and OS were numerically longer in patients treated with bevacizumab (n = 27) than those who received placebo (n = 7); median PFS was 14.9 versus 8.5 months (HR: 0.50; 95% CI: 0.21–1.19; P = .1064), and median OS was 25.0 versus 11.2 months (HR: 0.39; 95% CI: 0.15–1.02; P = .0467).
Bleeding events
Cerebral hemorrhage rates were increased with bevacizumab versus placebo (all grades, 3.3% vs 2.0%; grade ≥3, 2.0% vs 0.9%). These events resolved in a higher proportion of patients in the bevacizumab than the placebo arm (Table 4). No fatal cerebral hemorrhages were reported with bevacizumab, and only 1 fatal cerebrovascular accident occurred in the placebo arm during the treatment break.
The incidence of other bleeding events, including GI/pulmonary hemorrhage (all grades), was higher with bevacizumab versus placebo; however, grade ≥3 event rates were similar between arms (Table 4). All grade and grade ≥3 events resolved in the majority of patients in both arms. Two fatal bleeding events were reported: a tumor hemorrhage (bevacizumab; on the last day of treatment) and a GI hemorrhage (placebo; during the treatment break).
Wound-healing complications
Wound-healing complications, including craniotomy complications, were more frequent in patients treated with bevacizumab than placebo (all grades, 6.9% vs 4.7%; grade ≥3, 3.3% vs 1.6%; Table 4 and Supplementary Table S2). All of the grade ≥3 wound-healing complications were related to the craniotomy site and most resolved with medical management in both arms (73.3% and 100%, bevacizumab and placebo, respectively). One fatal wound-healing complication (wound infection) was reported in the bevacizumab arm during the treatment break.
Safety Considerations Related to Radiotherapy
As noted previously, almost all patients in both arms were able to complete the full course of radiotherapy. Overall, AEs were generally comparable between arms during the 6 weeks of radiotherapy and the 4-week treatment break and were in line with trends observed in the overall study (Supplementary Table S3). During this time, a similar number of patients in the bevacizumab and placebo arms experienced AEs of any grade (91.1% vs 88.9%), serious AEs (15.4% vs 12.0%), and grade ≥3 AEs (31.5% vs 29.1%). As expected, there was a higher incidence of bevacizumab-related grade ≥3 AEs in the bevacizumab than the placebo arm (9.3% vs 6.0%) during this early stage of the study. Deaths during this time were also increased in the bevacizumab versus the placebo arm (8 patients, 1.7% vs 4 patients, 0.9%).
There was no evidence of exacerbation of specific radiotherapy-associated AEs by bevacizumab. The bevacizumab and placebo arms had similar occurrences of radiotherapy-associated skin injury (8.2% vs 9.3%) and permanent or nonpermanent alopecia (39.0% vs 36.0%). Two patients in the bevacizumab arm experienced radiotherapy-associated injury; 1 patient had late radiation injury, and the other had a left ear treatment reaction to radiotherapy. Radiotherapy-associated pain was reported for 2 patients (1 bevacizumab and 1 placebo).
Safety Considerations Related to Myelotoxicity
Thrombocytopenia
A higher incidence of thrombocytopenia was reported with bevacizumab versus placebo (all grades, 34.1% vs 27.3%; grade ≥3, 15.0% vs 9.8%). However, grade ≥3 thrombocytopenia was not associated with clinically significant (grade ≥3) bleeding. More patients had dose modification, interruption, or delay due to thrombocytopenia with bevacizumab than placebo (24.5% vs 17.8%). However, treatment discontinuation due to thrombocytopenia was similar in the bevacizumab and placebo arms (3.0% vs 3.3%).
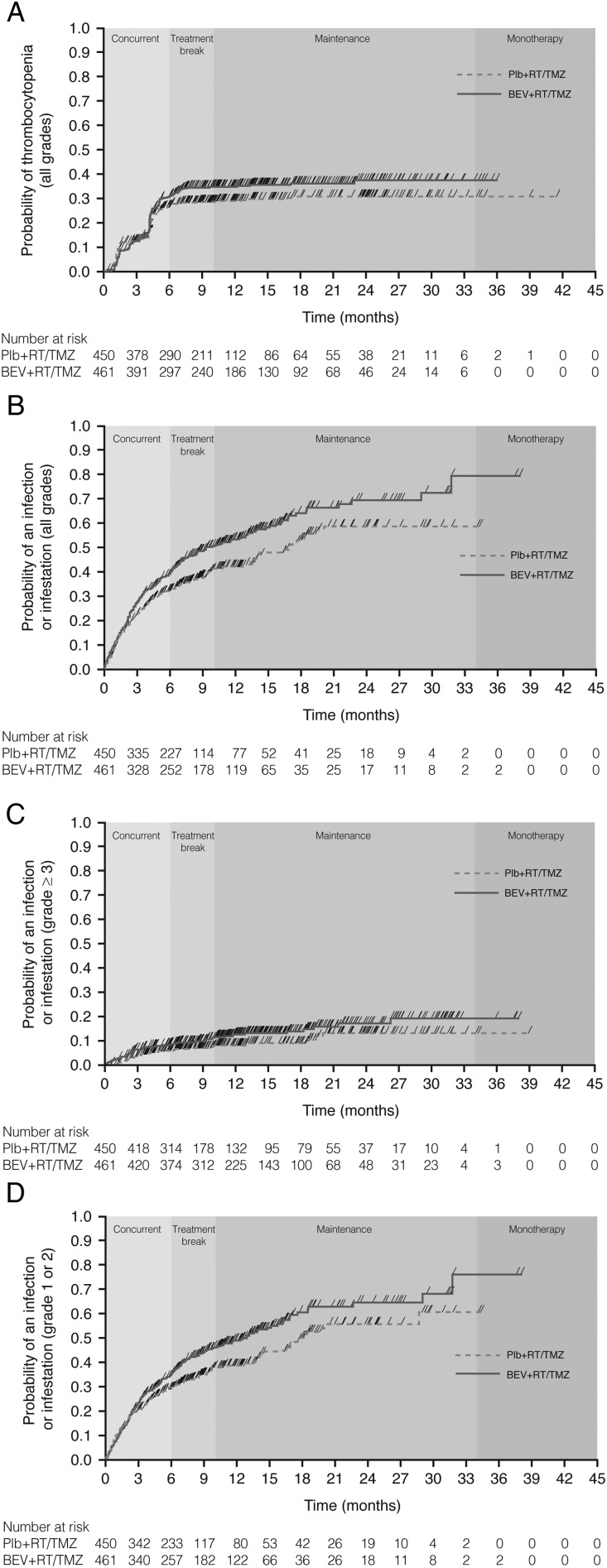
There was a similar, but steep, rate of onset of thrombocytopenia AEs in both arms over the first 3 months of the study (Fig. 1A). A sharp increase in onset rate in both arms was again observed between months 4 and 5 (the period of dose escalation of temozolomide, and when more patients in the bevacizumab arm were exposed to temozolomide). The Kaplan–Meier curve then reached a plateau in the placebo arm, while some additional events occurred in the bevacizumab arm. The incidence of thrombocytopenia, a known dose-limiting toxicity of temozolomide, appeared to be associated with the period of temozolomide treatment; after 8 months (the end of temozolomide therapy), in the maintenance phase, the curve for the bevacizumab arm also reached a plateau.
Fig. 1.
Kaplan–Meier plots of time to onset of (A) any grade thrombocytopenia, (B) any grade infection, (C) grade ≥3 infection, and (D) grade 1–2 infection. Abbreviations: BEV, bevacizumab; Plb, placebo; RT, radiotherapy; TMZ, temozolomide. Note: The timing of the treatment phases as shown on each graph are as defined in the study protocol; the actual timing of the treatment phases may have varied for each individual patient.
Infections
Infection rates were higher in the bevacizumab than the placebo arm (all grades, 54.4% vs 39.1%; grade ≥3, 12.8% vs 7.8%). Most common were nasopharyngitis (all grades, bevacizumab: 13.7%; placebo: 5.8%) and urinary tract infection (all grades, bevacizumab: 10.8%; placebo: 6.4%) (Table 3). The incidence of fatal infections was similar in the bevacizumab (1.7%) and placebo arms (1.3%). The most common causes of fatal infection were pneumonia and sepsis.
There was a similar rate of onset of all-grade infections between arms for the first 3 months of the study (Fig. 1B). After this time, the Kaplan–Meier curves separated, with a more rapid onset of events seen in the bevacizumab than the placebo arm. However, this pattern was not observed for grade ≥3 infections (Fig. 1C), which appeared to have an onset in the late phase of the study (beyond 6 mo). Indeed, the difference in rate of all-grade infections between the 2 treatment arms over the first 6 months was largely driven by grade 1–2 infections (Fig. 1D) occurring during the maintenance phase.
Safety Considerations Related to Reoperation after Progressive Disease
A total of 73/461 patients (15.8%) in the bevacizumab arm and 107/450 patients (23.8%) in the placebo arm underwent reoperation after PD in this study. Baseline characteristics of these patients were well balanced between arms and 70% of patients in each arm received additional therapy after reoperation. The median time from last study dose to reoperation was 2.1 months (95% CI: 1.8–2.8) in the bevacizumab arm and 1.5 months (95% CI: 1.3–1.9) in the placebo arm. The extent of resection was similar between bevacizumab and placebo arms (complete resection 52.1% vs 48.6%, respectively; partial resection 41.1% vs 47.7%; Supplementary Table S4).
Within 30 days of the first post-PD reoperation, fewer patients in the bevacizumab versus the placebo arm experienced AEs (10.9% vs 23.4%), grade 3–4 AEs (1.4% vs 10.2%), and grade 3–4 AEs of special interest for bevacizumab (1.4% vs 7.4%) (Supplementary Table S5). No patients died during this period. ATEs (all grade/grade ≥3) were experienced within 30 days of first post-PD reoperation by a similar number of patients in each arm. However, a greater number of patients in the placebo arm than the bevacizumab arm experienced all-grade and grade ≥3 events of hemorrhage, cerebral hemorrhage, venous thromboembolic events, wound-healing complications, and abscesses and fistulae.
In the 73 and 107 patients who underwent reoperation after PD, median OS was similar for bevacizumab versus placebo (22.6 vs 21.3 mo), and longer than that seen in the ITT population (16.8 vs 16.7 mo).9
Benefit-Risk Assessment
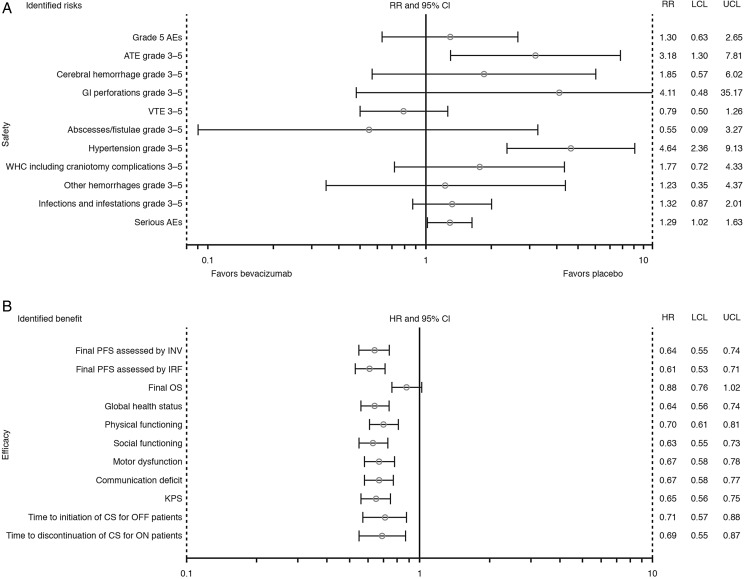
In the benefit-risk assessment (Fig. 2) all point estimates for the benefit parameters favored the bevacizumab arm. Most of the risk parameters favored the placebo arm. However, for those safety parameters for which the 95% CIs did not cross the reference line (grade ≥3 ATEs, grade ≥3 hypertension, and serious AEs), the rate per 100 patient-years was significantly higher in the bevacizumab arm versus the placebo arm (P < .05).
Fig. 2.
Benefit-risk assessment for bevacizumab plus radiotherapy and temozolomide for newly diagnosed glioblastoma: (A) ratio of rates per 100 patient-years and 95% CI, and (B) hazard ratios and 95% CI of efficacy parameters. Abbreviations: AE, adverse event; ATE, arterial thromboembolic event; CI, confidence interval; CS, corticosteroid; HR, hazard ratio; INV, investigator; IRF, independent review facility; KPS, Karnofsky Performance Score; LCL, lower confidence limit; OS, overall survival; PFS, progression-free survival; RR, rate ratio of rates per 100 patient-years; UCL, upper confidence limit; VTE, venous thromboembolic event; WHC, wound-healing complication. Safety (A) and final OS results (B) were derived from the dataset used for the OS cutoff for AVAglio (February 2013); all other data were derived from the dataset used for the final PFS cutoff for AVAglio (March 2012).
Discussion
Bevacizumab has been used clinically for more than 10 years, and its safety profile is well known. However, its use in the treatment of newly diagnosed glioblastoma brings unique considerations, such as its association with bleeding events (glioblastomas are highly vascular tumors), stroke, and wound-healing complications (most patients with glioblastoma undergo craniotomy).10,13,14 There are also very limited data on the use of bevacizumab in combination with radiotherapy or temozolomide, or before reoperation.
Although no new safety signals were observed compared with previous studies,2,10,13,14 overall there were more serious AEs, grade ≥3 AEs, and AEs related to bevacizumab in the bevacizumab arm compared with placebo. This was expected with the addition of an extra component to a standard regimen. The higher incidence of events in the bevacizumab arm can be partially explained by higher cumulative exposure to study treatment and longer duration of study treatment in that arm. There was also a greater exposure to temozolomide therapy in the bevacizumab arm compared with the placebo arm—therefore, patients in the bevacizumab arm may have experienced more events associated with temozolomide treatment. Also, patients in the bevacizumab arm had longer progression-free time and, consequently, a longer period of treatment and safety follow-up time. AEs related to bevacizumab were also followed up for 6 months after the last study dose, compared with 30 days for all other AEs. The increased toxicity with bevacizumab did not impact patients' ability to receive the standard-of-care treatment; in fact, fatigue occurred similarly in both treatment arms, and more patients in the bevacizumab than the placebo arm were able to enter the maintenance and monotherapy phases of the study.
Overall, all-cause mortality was balanced between arms, with the most common cause being PD, which was similar to results obtained for bevacizumab treatment of patients with recurrent glioblastoma.15 AEs leading to death were experienced by more patients in the bevacizumab versus the placebo arm, driven by events in the early treatment phase. There was no common pattern of risk factors associated with these early AEs leading to death. Importantly, the imbalance in early non-PD deaths in the bevacizumab arm did not appear to adversely impact median OS compared with placebo. Late-phase deaths were reported in a similar proportion of patients in each treatment arm; this phase was the longest treatment period of the study and included both maintenance and monotherapy. Although the number of AEs leading to death was higher in the bevacizumab arm, overall exposure during the late period was longer in the bevacizumab than the placebo arm, hence more patients in the bevacizumab arm were at risk of experiencing a safety event.
The higher incidence of AEs with bevacizumab versus placebo was mainly attributable to the increased occurrence of protocol-defined AEs related to bevacizumab. The most frequent AEs related to bevacizumab were hypertension and proteinuria. Clear guidance on the management of these events is provided in current labeling information. In this study, hypertension was manageable and resolved in the majority of cases. Approximately one-third of patients in the bevacizumab arm whose proteinuria did not resolve discontinued bevacizumab therapy, indicating that this may be a major factor in bevacizumab discontinuation, as has been previously reported.13 Revision of the threshold urinary protein:creatinine ratio at which to withhold bevacizumab therapy could improve resolution of proteinuria and reduce discontinuation rates.13
The rate of ATEs in the current study was similar to that seen in a study of recurrent glioblastoma.15 The increased incidence of ATEs in the bevacizumab arm manifested in the late stage of the study and may have been due to increased treatment exposure. Additionally, imbalances in baseline comorbidities and risk factors for ATEs, such as hypertension, may have contributed to the increased incidence of ATEs for bevacizumab versus placebo. Most ATE events were classified as “stroke,” the majority of which were ischemic, which is in line with observations in patients with recurrent glioblastoma.15 Reassuringly, most grade ≥3 ATEs resolved, and the higher incidence of ATEs with bevacizumab did not appear to adversely impact either PFS or OS versus placebo, although these results were obtained from a very small patient subset. Overall, the incidence levels of ATEs were in line with those seen in previous bevacizumab studies.1,2
Although clinically important, cerebral hemorrhage rates were low (but slightly higher with bevacizumab vs placebo), and most resolved in the bevacizumab arm. The increased rate of other bleeding events with bevacizumab versus placebo was primarily driven by grade 1–2 events, the majority of which were manageable and resolved without specific intervention. AEs related to bevacizumab were in line with the trends reported in prior bevacizumab trials for other tumor types, except for an increased incidence of ATEs.2
As best practice, patients with newly diagnosed glioblastoma undergo surgical resection of their tumors where feasible. In AVAglio, 87% of patients in the bevacizumab arm underwent complete or partial resection 4–7 weeks before the start of study treatment. The remaining patients had biopsies. Wound-healing complications, including craniotomy complications, were more frequent in patients treated with bevacizumab versus placebo; however, most resolved with medical management. The rates were not higher than expected from previous studies, suggesting that healing of patients' surgical incisions was not adversely affected. There was no evidence to suggest a contraindication of bevacizumab with recent surgical resection when an interval of 4 weeks between surgery and bevacizumab treatment was respected.
There have been limited data on the use of bevacizumab with radiotherapy, particularly as none of the approved regimens in other tumor types incorporate radiotherapy. As the combination of radiotherapy and temozolomide is the standard of care for first-line treatment of glioblastoma, it was critical that bevacizumab not prevent patients from receiving radiotherapy. Here, a similar proportion of patients in the bevacizumab and placebo arms were able to complete ≥90% of the planned doses of radiotherapy. During the 6 weeks of radiotherapy and the 4-week treatment break, AEs were comparable between arms and there was no evidence of exacerbation of specific radiotherapy-associated AEs by bevacizumab. These data confirm, in a large patient population, that bevacizumab does not appear to add to radiotherapy toxicity.
There are also limited data on the use of bevacizumab with temozolomide. We noted a higher incidence of thrombocytopenia and infections with bevacizumab versus placebo plus temozolomide, which mainly occurred after 3 months from the start of treatment, and not during the concomitant phase of treatment with radiotherapy plus temozolomide. Patients receiving myelotoxic chemotherapies are often at increased risk of infection, but this is the first report of increased infections in patients with newly diagnosed glioblastoma treated with bevacizumab plus temozolomide (as the first large study to investigate this combination). Increased infection rates have previously been reported for bevacizumab, but mostly following treatment with platinum or taxane-based therapies.2 As temozolomide is associated with myelotoxicity,16–18 in turn linked with risk of infections, the higher incidence of thrombocytopenic events and infections in the bevacizumab arm may be due to the greater exposure to temozolomide versus the placebo arm. Indeed, the incidence of thrombocytopenia, a known dose-limiting toxicity of temozolomide, appeared to be associated with the period of concurrent radiotherapy and temozolomide and then again with the period of temozolomide dose escalation. However, the imbalance of infections between treatment arms was primarily driven by uncomplicated grade 1–2 infections, which are also possible in the presence of normal blood counts. Urinary tract infection, reported at higher rates in the bevacizumab arm, is also a known AE associated with bevacizumab treatment. In clinical practice, thrombocytopenic events and infections are proactively and closely monitored.
For patients with glioblastoma, it is important to maintain therapeutic options after PD, including further resection.6,19 Here, similar proportions of patients in both arms were able to undergo a second surgery. In the following month, there were no indicators that patients who had received bevacizumab fared worse in terms of AEs; in fact, more patients in the placebo arm experienced AEs. Patients who underwent reoperation after PD had longer median OS in both arms, compared with the ITT population. There were limitations to this post-hoc analysis: the sample size was small, there may be some selection bias (ie, healthier patients selected for reoperation), and there was the potential for underreporting after discontinuation of study treatment.
Addition of any agent to standard-of-care treatment brings safety concerns, and the increased occurrence of AEs needs to be considered in the context of benefit from bevacizumab, notably the delayed time until tumor progression versus placebo. Prolonging the time to first PD means prolonging the time that patients experience their relative “best” health during their disease course, as HRQoL and neurologic function decline once the tumor grows again. The benefit-risk assessment identified a number of clinical outcomes that were significantly improved with bevacizumab versus placebo, including PFS, HRQoL, KPS, and corticosteroid use. This indicated that during the extended progression-free time in the bevacizumab arm, baseline quality of life was maintained, and patients had functional independence and reduced corticosteroid use, despite the increased toxicity.9 However, despite the fact that more than twice as many patients in the bevacizumab group than the placebo group completed 6 cycles of adjuvant temozolomide, OS was identical between arms.9
In summary, the safety profile observed in the AVAglio trial was consistent with that expected from standard-of-care radiotherapy and temozolomide treatment in patients with newly diagnosed glioblastoma, with the addition of the well-established safety profile of bevacizumab. AEs were as expected, with no new safety signals observed. The increased incidence of AEs with bevacizumab did not impact patients' ability to receive standard-of-care treatment or to undergo further surgery at disease progression.
Supplementary Material
Funding
This study was funded by F. Hoffmann-La Roche Ltd.
Supplementary Material
Acknowledgments
This study was sponsored by F. Hoffmann-La Roche Ltd. We would like to thank the patients and their families. We thank the AVAglio study investigators and the AVAglio study coordinators and nurses. We also thank Barbara Mueller for her advice and support with the preparation of this manuscript. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Conflict of interest statement. F.S. has received personal fees and nonfinancial support from F. Hoffmann-La Roche Ltd.
O.L.C. has received personal fees and nonfinancial support from F. Hoffmann-La Roche Ltd and personal fees from AstraZeneca and has a patent issued related to a plasmatic biomarker of bevacizumab efficacy (Europe 12305565.9). R.H. and W.M. have received personal fees from F. Hoffmann-La Roche Ltd. W.W. has received research grant support and personal fees from F. Hoffmann-La Roche Ltd, research grant support from Merck Sharp & Dohme, personal fees from Prime Oncology, and nonfinancial support from Eli Lilly and Apogenix. T.C. has received research grant support and personal fees from F. Hoffmann-La Roche Ltd and Genentech. S.D., E.P., and J.G. are employed by F. Hoffmann-La Roche Ltd. R.N. has received personal fees from Eisai, Merck Sharp & Dohme, F. Hoffmann-La Roche Ltd, and Chugai.
Trial identification number and URL: NCT00943826 (https://clinicaltrials.gov/ct2/show/NCT00943826).
References
- 1.Avastin Periodic Safety Update Report (PSUR) (Data on file).
- 2.Avastin® SPC. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf Accessed April 16, 2015.
- 3.Hervey-Jumper SL, Berger MS. Reoperation for recurrent high-grade glioma: a current perspective of the literature. Neurosurgery. 2014;75(5):491–499. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL, Zadnik P, Weingart JD et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118(4):812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bonis P, Fiorentino A, Anile C et al. The impact of repeated surgery and adjuvant therapy on survival for patients with recurrent glioblastoma. Clin Neurol Neurosurg. 2013;115(7):883–836. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi E, Tosoni A, Girardi F et al. Bevacizumab in brain tumors: ready for primetime? Future Oncol. 2009;5(8):1183–1184. [DOI] [PubMed] [Google Scholar]
- 9.Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Cancer Therapy Evaluation Program. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed April 16, 2015.
- 12.Coplan PM, Noel RA, Levitan BS et al. Development of a framework for enhancing the transparency, reproducibility and communication of the benefit-risk balance of medicines. Clin Pharmacol Ther. 2011;89(2):312–315. [DOI] [PubMed] [Google Scholar]
- 13.Lai A, Tran A, Nghiemphu PL et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vredenburgh JJ, Desjardins A, Herndon JE et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 15.Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong TS, Cao YM, Scheurer ME et al. Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neuro Oncol. 2009;11(6):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lombardi G, Rumiato E, Farina P et al. Clinical and genetic factors associated with severe myelotoxicity during radiation therapy (RI) plus temozolomide (TMZ) in glioblastoma (GBM) patients: a prospective observational study [abstract]. Eur J Cancer. 2013;49(suppl 2):S790. [Google Scholar]
- 18.Temodal® SPC. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000229/WC500035621.pdf Accessed April 13, 2015.
- 19.Bloch O, Han SJ, Cha S et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032–1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.