Abstract
Background
Surgical excision is the standard treatment for intracranial meningiomas. Epilepsy is a major cause of morbidity in meningioma patients, but postoperative control of epilepsy is not achieved in a substantial fraction of patients. The purpose of this study was to define risk factors for postoperative epilepsy.
Methods
Patients treated for histologically confirmed intracranial meningioma at the University Hospital Zurich between 2000 and 2013 were retrospectively analyzed. Demographic, clinical, imaging, and electroencephalographic data were assessed. A binary regression model was applied to identify risk factors for postoperative epilepsy.
Results
Of the 779 patients analyzed, epileptic seizures occurred in 244 (31.3%) patients before surgery and in 204 (26.6%) patients after surgery. Of the 244 patients with preoperative epilepsy, 144 (59.0%) became seizure-free after surgery; of the 535 patients without preoperative seizures, 104 (19.4%) suffered from epilepsy after surgery. Risk factors for postoperative epilepsy were preoperative epilepsy (odds ratio [OR]: 3.46 [95% confidence interval {CI}: 2.32–5.16]), major surgical complications including CNS infections (OR: 5.89 [95% CI: 1.53–22.61]), hydrocephalus (OR: 3.27 [95% CI: 1.35–7.95]), recraniotomy (OR: 2.91 [95% CI: 1.25–6.78]), and symptomatic intracranial hemorrhage (OR: 2.60 [95% CI: 1.17–5.76]) as well as epileptiform EEG potentials (OR: 2.52 [95% CI: 1.36–4.67]), younger age (OR: 1.74 [(95% CI: 1.18–2.58]), and tumor progression (OR: 1.92 [95% CI: 1.16–3.18]). Postoperative improvement or recovery from preoperative neurologic deficits was associated with improved seizure control (OR: 0.46 [95% CI: 0.25–0.85], P = .013).
Conclusion
We suggest prospective validation of a score (“STAMPE2”) based on clinical findings, EEG, and brain-imaging measures to estimate postoperative seizure risk and guide anticonvulsant treatment in meningioma patients.
Keywords: EEG, meningioma, postoperative epilepsy, STAMPE2
Meningiomas are the most common intracranial tumors in adults, with an annual incidence per 100 000 of 10.26 and 4.55 for females and males, respectively,1 and a prevalence of about 1.5% in autopsy and brain-imaging studies.2–4 The vast majority of clinically silent incidental meningiomas are benign, both histologically and clinically,5,6 but severe morbidity can accompany the disease course.7 Surgery is required for histological diagnosis and therefore represents the standard first-line treatment for most patients with symptomatic meningiomas.8 Complications and morbidity from meningioma surgery are, however, common.9–11
Epilepsy is a major cause of morbidity in meningioma patients, with onset before or after surgery.12–14 Selection of patients who will require anticonvulsant treatment after meningioma resection is challenging because, despite advances in neurosurgery and radiation oncology during the past decades, factors predicting the outcome of epilepsy after meningioma resection are not well-defined in recent cohorts. Here we identify risk factors for postoperative epilepsy in a cohort of 779 consecutive patients who underwent surgery for meningioma between 2000 and 2013.
Patients and Methods
Subject Selection
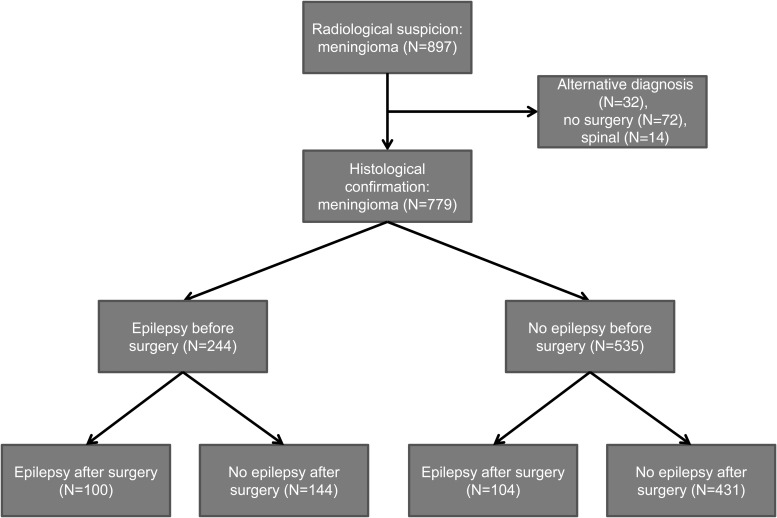
This study was approved by the local ethics committee and was performed in accordance with the Declaration of Helsinki. Figure 1 (Consort chart) details the primary analysis population. A total of 897 patients were followed or treated for meningioma at the University Hospital Zurich between 2000 and 2013. Patients were identified by an automated search of the electronic chart system; 117 patients were excluded from further analysis because of spinal tumor location (N = 13) or lack of histological confirmation (N = 104). Subjects were further classified based on the occurrence of epileptic seizures before and after surgery.
Fig. 1.
Consort chart.
Variables
Epilepsy was defined, according to criteria of the International League Against Epilepsy (ILAE), as a single seizure with a probability >60% for a second seizure within 10 years.15 The term postoperative epilepsy was confined to patients with active postoperative epilepsy (ie, patients with documented postoperative epileptic seizures at any time during follow-up). The term acute symptomatic postoperative seizures was applied to seizures that occurred within 7 days of craniotomy according to the definition issued by the ILAE.16
Demographics, histopathological data, routine electroencephalography (EEG) parameters recorded over 20–30 minutes, and clinical course including neurological deficits, seizure history, surgical complications, and medication were obtained by reviewing the medical reports. The term neurological deficit refers to any deficits attributable to a focal cerebral lesion but not headache or seizures. An ordinal scale comprising the categories better, worse, and unchanged was applied. Imaging characteristics were complemented by review of cranial computer tomography (CT) and magnetic resonance imaging (MRI) scans. Intracranial hemorrhage was defined as the detection of hyperdensity on CT scans of at least 1 cm3 diameter on 2 plains. Presence or absence of edema was defined as hypodensity on CT scans or as T2 hyperintensity on MRI scans. The term ischemic stroke was utilized according to the definition of the American Stroke Association for brain infarction attributable to ischemia and based on neuroimaging, and/or clinical evidence of permanent injury.17 Extent of resection was defined by Simpson grade18 and radiographically. Radiographic gross total resection (GTR) was defined as the absence of contrast enhancement on postoperative CT or MRI scans. Tumor progression was defined as an increase in diameter of contrast-enhancing lesions on CT or MRI scans at any time point after surgery. Interictal routine EEG measures during the entire follow-up period were analyzed. EEG recordings within 72 hours after documented seizures were considered postictal and were not included in analyses.
Statistical Methods
SPSS V22.0 was utilized for all statistical analyses. The chi-square test was performed for analysis of nominal variables, and the Mann-Whitney U test was performed for linearly scaled variables. Binary logistic regression was performed for multivariate testing of factors associated with postoperative epilepsy. Included in this model were age, World Health Organization (WHO) grade, tumor location, radiographic extent of resection, tumor progression at any time point, and the presence of preoperative seizures.
Results
Study Population
Patients undergoing surgical resection of intracranial meningioma (N = 779) were included in subsequent analyses (Figure 1). Demographic and clinical data are summarized in Table 1. The median follow-up time after surgery was 67 (95% CI: 63–72) months, and 42 patients (5.4%) died. Simpson grade was documented in 468 patients, with Simpson grade 1/2 resection accomplished in 364 patients (77.3%). Imaging data were available for 694 patients, and among these radiographic GTR was achieved in 531 patients (76.5%). A subset of 129 patients (14.8%) underwent 2 or more meningioma resections. Co-treatments included preoperative intravascular embolization (N = 392, 47.8%), postoperative radiotherapy (N = 118, 15.1%), and systemic therapies (N = 18, 2.3%). Thirty-three patients (4.3%) were asymptomatic at diagnosis. Preoperative symptoms or signs in the other patients included headache (N = 172, 22.1%), epileptic seizures (N = 244, 31.2%), and neurological deficits (N = 379, 48.6%). In contrast, of the 72 patients with radiographic diagnosis of intracranial meningioma who did not undergo neurosurgery, 39 (54.1%) remained asymptomatic with respect to headache, epileptic seizures, and neurological deficits for a median follow-up of 71 months.
Table 1.
Demographic and clinical data
| Age at diagnosis (y) | |
|---|---|
| Median | 57 |
| Range | 18–90 |
| Sex, N (%) | |
| Male | 247 (31.8) |
| Female | 532 (68.2) |
| WHO grade, N (%) | |
| I | 638 (81.9) |
| II | 119 (15.3) |
| III | 22 (2.8) |
| Locationa, N (%) | |
| Convexity | 167 (24.8) |
| Parasagittal | 131 (20.2) |
| Skull base | 260 (39.5) |
| Posterior fossa | 81 (12.3) |
| Other | 22 (3.3) |
| Maximal diameterb, mm | |
| Median | 40.0 |
| Range | 11–119 |
| Simpson gradec, N (%) | |
| 1 | 143 (30.4) |
| 2 | 221 (46.9) |
| 3 | 41 (8.7) |
| 4 | 55 (11.7) |
| 5 | 11 (2.3) |
| Multiple meningiomas. N (%) | |
| Yes | 118 (15.1) |
| No | 661 (84.9) |
| Neurological deficit before surgeryd, N (%) | |
| Yes | 379 (48.6) |
| No | 389 (51.4) |
| Outcome of neurological deficits after surgery, N (%) | |
| Recovered | 139 (36.7) |
| Residual deficit | 124 (32.7) |
| Not improved | 116 (30.6) |
| Epilepsy, N (%) | |
| Before surgery | 244 (31.3) |
| After surgery | 204 (26.2) |
| Epileptiform EEG potentials, N (%) | |
| Before surgerye | 40 (15.8) |
| After surgeryf | 78 (22.9) |
| Tumor progression, N (%) | |
| Yes | 203 (26.1) |
| No | 576 (73.9) |
aNot including patients with multiple meningiomas (N = 661).
bDocumentation available for 694 patients (CT/MRI = 631/63).
cDocumentation available for 468 patients.
dNot including headache and seizures.
eDocumentation available for 253 patients.
fDocumentation available for 340 patients.
Clinical Correlates of Preoperative Epilepsy
The clinical characteristics of patients with preoperative epilepsy versus those without are summarized in Table 2. Localization at the cerebral convexity and epileptic discharges on EEG recordings were associated with epilepsy, whereas age, sex, WHO grade, tumor size, and the percentage of patients with multiple meningiomas were comparable in both groups. While localization at the skull base was not associated with preoperative epilepsy, further subclassification of this entity revealed an association of sphenoid wing meningiomas with epilepsy (P = .032). Preoperative epilepsy was less common among patients with petrous ridge (P = .023) or tuberculum sellae (P = .018) meningiomas, and no association with preoperative seizures was detected for cavernous sinus (P = .584), olfactory groove (P =.215), or suprasellar (P = .079) meningiomas. Among patients with preoperative epilepsy, tumors were less commonly associated with neurologic deficits, recovery from these deficits was more likely, and tumor recurrence or progression occurred less frequently. Only the secretory histological subtype was weakly associated with preoperative epilepsy (P = .045) (Supplementary material, Table S1). On brain imaging scans, edema was detected more frequently in patients with preoperative epilepsy, whereas hyperostosis was more frequent in patients without preoperative epilepsy, and no difference was apparent in the radiographic detection of tumor calcification as well as intraosseous, extracranial, or intra-axial growth (Supplementary material, Table S2). Recording of focal or background slowing on EEG was not associated with preoperative epilepsy (Supplementary material, Table S3) including frontal intermittent rhythmic delta activity (observed in 7 patients; data not shown).
Table 2.
Clinical characteristics of patients with versus without epilepsy before surgery
| Epilepsy before Surgery |
P | ||
|---|---|---|---|
| Yes | No | ||
| N = 244 (31.3%) | N = 535 (68.7%) | ||
| Age at diagnosis, y | |||
| Median | 56 | 58 | .265 |
| Range | 18–88 | 19–87 | |
| Sex, N (%) | |||
| Male | 85 (34.8) | 162 (30.3) | .214 |
| Female | 159 (65.2) | 373 (69.7) | |
| WHO grade, N (%) | |||
| I | 200 (82.0) | 438 (81.9) | .765 |
| II | 38 (15.6) | 81 (15.1) | |
| III | 6 (2.5) | 16 (3) | |
| Locationa, N (%) | |||
| Convexity | 77 (35.9) | 90 (19.1) | <.001 |
| Parasagittal | 39 (18.7) | 92 (20.7) | .555 |
| Skull base | 81 (38.8) | 179 (40.2)1 | .733 |
| Posterior fossa | 9 (4.3) | 72 (16.2) | <.001 |
| Other | 5 (1.9) | 17 (3.8) | .347 |
| Maximal diameterb, mm | |||
| Median | 42 | 40 | .088 |
| Range | 11–89 | 12–100 | |
| Simpson gradec, N (%) | |||
| 1 | 51 (35.9) | 92 (28.2) | .295 |
| 2 | 57 (40.1) | 162 (49.7) | |
| 3 | 15 (10.6) | 26 (8.0) | |
| 4 | 15 (10.6) | 39 (12.0) | |
| 5 | 4 (2.8) | 7 (2.1) | |
| Multiple meningiomas, N (%) | |||
| Yes | 35 (14.3) | 90 (16.8) | .402 |
| No | 209 (85.7) | 445 (83.2) | |
| Neurological deficit before surgeryd, N (%) | |||
| Yes | 104 (42.6) | 275 (51.4) | .023 |
| No | 140 (57.4) | 269 (48.6) | |
| Outcome of neurological deficits after surgery, N (%) | |||
| Recovered | 57 (54.8) | 82 (29.8) | <.001 |
| Residual deficit | 19 (18.3) | 105 (38.2) | |
| Not improved | 28 (26.9) | 88 (32.0) | |
| Epileptiform EEG potentialsf, N (%) | |||
| Yes | 25 (21.9) | 15 (10.8) | .016 |
| No | 89 (78.1) | 124 (89.2) | |
| Tumor progression, N (%) | |||
| Yes | 51 (20.9) | 152 (28.4) | .027 |
| No | 193 (79.1) | 383 (71.6) | |
aN = 661, not including patients with multiple meningiomas.
bDocumentation available for 694 patients (CT/MRI = 631/63).
cDocumentation available for 468 patients.
dNot including headache and seizures.
ePatients with perioperative-onset neurologic deficits and a minimum follow-up of 1 year (N = 338).
fDocumentation of preoperative EEG recordings available for 253 patients.
Clinical Correlates of Postoperative Epilepsy
Of all patients undergoing meningioma resection (N = 779), 204 (26.3%) suffered from epilepsy after surgery (Figure 1). Acute symptomatic postoperative seizures, defined as seizures occurring within 7 days of craniotomy,16 were associated with nonacute postoperative seizures in 29 (63%) of 46 cases, thus fulfilling the epilepsy-defining criteria of the ILAE.15 Clinical characteristics of patients with postoperative epilepsy are summarized in Table 3. Younger age, higher WHO grade, tumor location at the cerebral convexity, multiple meningiomas, lack of postoperative improvement of preoperatively apparent neurological deficits, epileptic discharges on EEG recordings, and tumor progression were associated with postoperative epilepsy. Further subclassification of skull base meningiomas into sphenoid wing, petrous ridge, tuberculum sellae, cavernous sinus, olfactory groove, and suprasellar meningiomas revealed no association between any of these tumor locations and postoperative epilepsy (data not shown).
Table 3.
Clinical characteristics of patients with versus without epilepsy after surgery
| Epilepsy before Surgery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes |
No |
||||||||
|
N = 244 (31.3%) |
N = 535 (68.7%) | ||||||||
| Epilepsy after Surgery |
Epilepsy after Surgery |
||||||||
| Yes | No | P | Yes | No | P | Yes | No | P | |
| N = 204 (26.2%) | N = 575 (73.8%) | N = 100 (41.0%) | N = 144 (59.0%) | N = 104 (19.4%) | N = 431 (80.6%) | ||||
| Age at diagnosis, y | |||||||||
| Median | 54 | 58 | <.001 | 53.5 | 58 | .001 | 56.5 | 59 | .025 |
| Range | 18-80 | 18-88 | 18–76 | 18–88 | 18–80 | 18–87 | |||
| Sex, N (%) | |||||||||
| Male | 129 (36.8) | 172 (29.9) | .071 | 35 (35.0) | 50 (34.7) | .964 | 40 (38.5) | 122 (28.3) | .043 |
| Female | 75 (63.2) | 403 (70.1) | 65 (65.0) | 94 (75.3) | 64 (61.5) | 309 (71.7) | |||
| WHO grade, N (%) | |||||||||
| I | 158 (77.5) | 480 (83.5) | <.001 | 82 (82.0) | 118 (81.9) | .386 | 76 (73.1) | 362 (84.0) | <.001 |
| II | 32 (15.7) | 78 (15.1) | 14 (14.0) | 24 (16.7) | 18 (17.3) | 63 (14.6) | |||
| III | 14 (6.9) | 8 (1.4) | 4 (4.0) | 2 (1.4) | 10 (9.6) | 6 (1.4) | |||
| Locationa, N (%) | |||||||||
| Convexity | 54 (31.6) | 113 (22.2) | .007 | 28 (33.7) | 49 (38.3) | .503 | 26 (32.9) | 64 (17.3) | .013 |
| Parasagittal | 34 (21.5) | 97 (19.6) | .667 | 17 (20.5) | 22 (17.2) | .547 | 17 (21.5) | 75 (20.2) | .798 |
| Skull base | 67 (42.4) | 193 (38.9) | .544 | 34 (41.0) | 47 (36.7) | .536 | 33 (41.8) | 146 (39.4) | .677 |
| Posterior fossa | 6 (3.8) | 75 (15.1) | <.001 | 3 (3.6) | 6 (4.7) | .706 | 3 (3.8) | 69 (18.6) | <.001 |
| Other | 1 (0.6) | 21 (4.2) | .027 | 1 (1.2) | 4 (3.1) | .65 | 0 (0.0) | 17 (4.6) | .040 |
| Maximal diameter, mm | |||||||||
| Median | 41 | 40 | .057 | 40 | 42 | .427 | 40 | 41 | .107 |
| Range | 10–96 | 11–100 | 15–90 | 10–90 | 10–96 | 10–100 | |||
| Simpson gradeb, N (%) | |||||||||
| 1 | 30 (29.4) | 113 (30.9) | .065 | 17 (30.4) | 34 (39.5) | .456 | 13 (28.3) | 79 (28.2) | .001 |
| 2 | 43 (42.2) | 176 (48.1) | 24 (42.9) | 33 (38.4) | 19 (41.3) | 143 (51.1) | |||
| 3 | 8 (7.8) | 33 (9.0) | 5 (8.9) | 10 (11.6) | 3 (6.5) | 23 (8.2) | |||
| 4 | 15 (14.7) | 39 (10.7) | 7 (12.5) | 8 (9.3) | 8 (17.4) | 31 (11.1) | |||
| 5 | 6 (5.6) | 5 (1.4) | 3 (5.4) | 1 (1.2) | 3 (6.5) | 4 (1.4) | |||
| Multiple meningiomas, N (%) | |||||||||
| Yes | 46 (22.5) | 79 (13.7) | .003 | 17 (17.0) | 18 (12.5) | .324 | 25 (24.0) | 60 (13.9) | .011 |
| No | 158 (77.5) | 496 (86.3) | 83 (83.0) | 126 (87.5) | 79 (76.0) | 371 (86.1) | |||
| Neurological deficits before surgeryc, N (%) | |||||||||
| Yes | 94 (46.1) | 285 (49.6) | .392 | 44 (44.0) | 60 (41.7) | .717 | 50 (48.1) | 225 (52.2) | .450 |
| No | 110 (53.9) | 290 (50.4) | 56 (56.0) | 84 (58.3) | 42 (51.9) | 206 (47.8) | |||
| Postsurgical outcome of neurological deficits: N (%) | |||||||||
| Recovered | 31 (32.9) | 108 (37.9) | .011 | 19 (43.2) | 38 (63.3) | .100 | 13 (26.0) | 92 (40.9) | .010 |
| Residual deficit | 22 (23.4) | 102 (35.8) | 9 (20.5) | 10 (16.7) | 12 (24.0) | 70 (31.1) | |||
| Not improved | 41 (43.7) | 75 (26.3) | 16 (36.3) | 12 (20.0) | 25 (50.0) | 63 (28.0) | |||
| Epileptiform EEG potentialse, N (%) | |||||||||
| Yes | 48 (29.6) | 30 (16.9) | .005 | 25 (30.5) | 13 (15.3) | .019 | 23 (27.8) | 17 (18.3) | .096 |
| No | 114 (70.4) | 148 (83.1) | 57 (69.5) | 72 (84.7) | 57 (72.2) | 76 (81.7) | |||
| Tumor progression, N (%) | |||||||||
| Yes | 74 (36.3) | 129 (22.4) | <.001 | 29 (29.0) | 22 (15.3) | .01 | 45 (43.3) | 107 (24.8) | <.001 |
| No | 130 (63.7) | 446 (77.6) | 71 (71.0) | 122 (84.7) | 59 (56.7) | 324 (75.2) | |||
aN = 661, not including patients with multiple meningiomas.
bDocumentation available for 468 patients.
cNot including headache and seizures.
dPatients with perioperative-onset neurologic deficits and a minimum follow-up of 1 year (N = 338).
eDocumentation of postoperative EEG recordings available for 340 patients.
Tumor diameter and Simpson grade did not differ in patients with or without postoperative epilepsy, although larger tumor diameter (P = .057) and higher Simpson grade (P = .065) favored postoperative epilepsy. The duration of surgery was not associated with postoperative epilepsy (P = .111) including acute symptomatic seizures (P = .678). We next evaluated surgical complications associated with postoperative epilepsy. Sensorimotor deficits with perioperative onset, CNS infections, clinically symptomatic intracranial hemorrhage, hydrocephalus, or recraniotomy for any reason were associated with postoperative epilepsy, whereas the frequencies of cranial nerve palsies, trigeminal neuralgias, and visual deficits were comparable in both groups (Table 4). Perioperative cardiovascular events including deep vein thrombosis, pulmonary embolism, myocardial infarction, and cardiac arrest were comparable between patients with and without postoperative epilepsy (data not shown).
Table 4.
Association of epilepsy with neurologic complications from surgery
| Epilepsy before Surgery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes |
No |
||||||||
| N = 244 (31.3%) | N = 535 (68.7%) | ||||||||
| Epilepsy after Surgery |
Epilepsy after Surgery |
||||||||
| Yes | No | P | Yes | No | P | Yes | No | P | |
| N = 204 (26.2%) | N = 575 (83.8%) | N = 100 (41.0%) | N = 144 (59.0%) | N = 104 (19.4%) | N = 431 (80.6%) | ||||
| Cranial nerve palsy, N (%) | |||||||||
| Yes | 19 (9.3) | 62 (10.8) | .555 | 10 (10.0) | 9 (6.3) | .282 | 9 (8.7) | 53 (12.3) | .393 |
| No | 185 (90.7) | 513 (89.2) | 90 (90.0) | 135 (93.7) | 95 (91.3) | 378 (87.7) | |||
| Trigeminal neuralgia, N (%) | |||||||||
| Yes | 0 (0.0) | 9 (1.6) | .122 | 0 (0.0) | 1 (0.7) | N.A. | 0 (0.0) | 9 (2.1) | .217 |
| No | 204 (100.0) | 566 (98.4) | 100 (100.0) | 143 (99.3) | 104 (100.0) | 422 (97.9) | |||
| Sensorimotor deficit, N (%) | |||||||||
| Yes | 40 (19.6) | 66 (11.5) | .004 | 19 (19.0) | 21 (14.6) | .359 | 21 (20.2) | 45 (10.4) | .007 |
| No | 164 (80.4) | 509 (88.5) | 81 (81.0) | 123 (85.4) | 83 (79.8) | 386 (89.6) | |||
| Visual deficit, N (%) | |||||||||
| Yes | 11 (5.4) | 51 (8.9) | .115 | 7 (7.0) | 7 (4.9) | .480 | 4 (3.8) | 44 (10.2) | .054 |
| No | 193 (94.6) | 524 (91.1) | 93 (93.0) | 137 (95.1) | 100 (96.2) | 387 (89.8) | |||
| Stroke, N (%) | |||||||||
| Yes | 13 (6.4) | 23 (4.0) | .166 | 4 (4.0) | 7 (4.9) | .750 | 9 (8.7) | 16 (3.7) | .032 |
| No | 191 (93.6) | 552 (96.0) | 96 (96.0) | 137 (95.1) | 95 (91.3) | 415 (96.3) | |||
| CNS infection, N (%) | |||||||||
| Yes | 10 (4.9) | 6 (1.0) | .002 | 6 (6.0) | 1 (0.7) | .015 | 4 (3.8) | 5 (1.2) | .077 |
| No | 194 (95.1) | 569 (99.0) | 94 (94.0) | 143 (99.3) | 100 (96.2) | 426 (98.8) | |||
| Hemorrhage, N (%) | |||||||||
| Yes | 63 (30.9) | 153 (26.6) | .241 | 38 (38.0) | 37 (25.7) | .040 | 25 (24.0) | 116 (26.9) | .550 |
| No | 141 (69.1) | 422 (73.4) | 62 (62.0) | 107 (74.3) | 79 (76.0) | 315 (73.1) | |||
| Clinically symptomatic, N (%) | |||||||||
| Yes | 40 (46.8) | 74 (40.1) | .043 | 18 (47.4) | 21 (56.8) | .416 | 22 (88.0) | 53 (45.7) | <.001 |
| No | 23 (53.2) | 79 (59.9) | 20 (52.6) | 16 (43.2) | 3 (12.0) | 63 (54.3) | |||
| Hydrocephalus, N (%) | |||||||||
| Yes | 13 (6.4) | 14 | .008 | 3 (3.0) | 4 (2.8) | 1.000 | 10 (9.6) | 10 (2.3) | <.001 |
| No | 191 (93.6) | 561 | 97 (97.0) | 140 (97.2) | 94 (90.4) | 421 (97.7) | |||
| Recraniotomy, N (%) | |||||||||
| Yes | 16 (7.8) | 21 (3.7) | .016 | 7 (7.0) | 4 (2.8) | .130 | 9 (8.7) | 17 (3.9) | .045 |
| No | 188 (92.2) | 554 (96.3) | 93 (93.0) | 140 (97.2) | 95 (91.3) | 414 (96.1) | |||
We next assessed anticonvulsant drug therapies between patients with and without postoperative epilepsy (Supplementary material, Table S4). To allow for appropriate comparison of prescribed drugs, analysis was restricted to the first year of postoperative follow-up. The percentage of patients on anticonvulsants and the fraction with anticonvulsant polytherapy were larger among the cohort with postoperative epilepsy. Phenytoin was the most frequently prescribed anticonvulsant drug. More patients with postoperative epilepsy were on carbamazepine, but intake of all other evaluated drugs was balanced between the 2 groups.
Postoperative Control of Preoperative Onset Epilepsy
Among the 244 patients with preoperative epilepsy, 144 (59.1%) were seizure-free after surgery (Figure 1). Younger age, epileptic discharges on postoperative EEG recordings, and tumor progression were associated with persistent epilepsy after surgery, whereas there was no association with sex, WHO grade, tumor location (including further subclassification of skull base meningiomas; data not shown), tumor diameter, Simpson grade, multiple meningiomas, or preoperative neurologic deficits (Table 3). Postoperative complications associated with persistent epilepsy are summarized in Table 4. CNS infections and perioperative intracranial hemorrhages were associated with inferior postoperative control of preoperative-onset epilepsy. In addition, the association of hemorrhages with postoperative epilepsy was independent of neurologic symptoms arising from intracranial hemorrhage. Other postoperative complications including cranial nerve palsy, sensorimotor or visual deficits, ischemic stroke, hydrocephalus, and recraniotomy for any reason were comparable between patients with and without postoperative persistence of preoperative-onset epilepsy. Only fibrous meningioma was associated with postoperative control of preoperative epilepsy (Supplementary material, Table S1). Of note, radiographic GTR was achieved in 48 (90.6%) of 53 patients with fibrous meningioma as compared with 359 (74.5%) of 482 patients with other histological meningioma subtypes (P = .009), and fibrous meningiomas versus other subtypes were more frequently located in the posterior fossa (30.2% vs 10.8%, P< .001) including the subgroup of patients with preoperative epilepsy (26.7% vs 2.4%, P < .001). Tumor characteristics on preoperative brain imaging scans were comparable between patients with and without postoperative persistence of epilepsy including radiographic extent of resection, presence of edema, calcification, and hyperostosis as well as intraosseous, extracranial or intraaxial growth (Supplementary material, Table S2). On postoperative EEG recordings, focal slowing, but not slowing of the background rhythm, was associated with persistence of preoperative epilepsy (Supplementary material, Table S3). Preoperative seizure semiology was not associated with postoperative epilepsy (Supplementary material, Table S5).
Postoperative New-onset Epilepsy
Of 535 patients without preoperative epilepsy, 104 patients (19.4%) suffered from epilepsy after meningioma resection (Figure 1). Younger age, male sex, higher WHO grade, tumor location at the cerebral convexity, higher Simpson grade, multiple meningiomas, and tumor progression were associated with new-onset epilepsy after meningioma surgery but not tumor diameter and recording of epileptic discharges on postoperative EEG (Table 3). Further subclassification of skull base meningiomas revealed a weak association of petrous ridge meningiomas with postoperative new-onset epilepsy (P = .018). Of note, petrous ridge meningiomas were associated with a lower rate of radiographic GTR (P = .002) and Simpson grade 1 or 2 resection (P = .037), whereas surgical complications and recurrence rates, age, sex, and WHO grade were not associated with petrous ridge meningiomas (data not shown). Surgical complications associated with postoperative-onset epilepsy included sensorimotor deficits, ischemic stroke, and symptomatic intracranial hemorrhage as well as hydrocephalus and recraniotomy but not cranial nerve palsies, trigeminal neuralgia, visual deficits, or CNS infections (Table 4). Anaplastic meningioma was the only histopathological subtype associated with new-onset epilepsy after surgery (Supplementary material, Table S1). On brain imaging scans, edema and intra-axial growth were more frequent, and hyperostosis was less frequent in patients with onset of epilepsy after surgery, but GTR, calcification, and intraosseous or extracranial growth were balanced between patients with and without onset of epilepsy after surgery (Supplementary material, Table S2). Focal or background slowing on EEG recordings occurred equally in patients with and without postoperative new-onset epilepsy (Supplementary material, Table S3).
Prophylactic Perioperative Anticonvulsant Therapy
Prophylactic perioperative anticonvulsant therapy was administered to 244 (45.6%) of 535 patients without preoperative epilepsy. The preferred drug utilized for prophylaxis was phenytoin (N = 186, 76.3%) followed by carbamazepine (N = 15, 6.2%), levetiracetam (N = 14, 5.7%), and valproate (N = 11, 4.5%). Prophylactic perioperative anticonvulsant intake was balanced between patients with versus without postoperative epilepsy (P = .361), but prophylactic anticonvulsant intake was associated with a higher rate of acute symptomatic postoperative seizures within 7 days after surgery (P = .063). Key clinical characteristics of patients receiving prophylactic anticonvulsants versus patients who did not receive seizure prophylaxis were not balanced. More patients receiving prophylactic anticonvulsant therapy suffered from WHO grade II or III meningiomas (23.8% vs 13.4%, P = .002), had documented epileptiform potentials on preoperative EEG (4.9% vs 1.0%, P = .007), or had a complicated postoperative course (30.3% vs 20.6%, P = .010) due to new-onset sensorimotor deficits, CNS infection, hydrocephalus, recraniotomy for any reason, or symptomatic intracranial hemorrhage. In contrast, among patients who did not receive prophylactic anticonvulsants, versus patients receiving prophylactic anticonvulsants more patients underwent Simpson grade 3–5 resection (26.9% vs 17.5%, P = .046), whereas the rate of patients with radiographic GTR (P = .200), edema on preoperative imaging scans (P = .241), younger versus older age (P = .861), multiple meningiomas (P = .722), and tumor location at the convexity (P = .289) were balanced between both groups.
Multivariate Modeling of Predictors of Postoperative Seizure Control
We applied a binary logistic regression model to identify predictors of postoperative seizure control. Univariate analyses of the association of variables included in this model with postoperative epilepsy are summarized in Supplementary material, Table S6. On multivariate analyses (Table 5), younger age, tumor progression, and preoperative epilepsy were predictive for postoperative epilepsy. Such an association was not identified for WHO grade, tumor location, or extent of resection. When tested as an additional single variable in this model, epileptic discharges on postoperative EEG recordings and edema on preoperative imaging scans were identified as a risk factor for postoperative epilepsy, and postoperative improvement or recovery from preoperative neurologic deficits was predictive for better seizure control. Surgical complications identified as risk factors for postoperative epilepsy, when tested as additional single variables, included sensorimotor deficits, CNS infections, hydrocephalus, and recraniotomy for any reason. Radiographic detection of intracranial hemorrhage was not predictive for poor postoperative seizure control, though favoring epilepsy. However, intracranial hemorrhage that was associated with neurological symptoms was a risk factor for epilepsy. No predictive role for postoperative seizure control was identified for preoperative neurologic deficits (P = .736), multiple meningiomas (P = .151), sex (P = .741), intra-axial growth (P = .247), or hyperostosis (P = .438) when tested as additional single variables in this model. Simpson grade was also not predictive (P = .434) when substituting for radiographic extent of resection.
Table 5.
Multivariate analyses of predictors for postoperative seizure controla
| OR and 95% CI | P | |
|---|---|---|
| Multivariate model | ||
| Age: 18 y–54 y vs ≥55 y | 1.74 (1.18–2.58) | .005 |
| WHO grade: I vs II/III | 1.01 (0.58–1.74) | .978 |
| Tumor location convexity: yes vs no | 1.40 (0.89–2.21) | .143 |
| Radiographic extent of resection: gross total vs incomplete | 0.89 (0.55–1.45) | .639 |
| Tumor progression: yes vs no | 1.92 (1.16–3.18) | .012 |
| Preoperative epilepsy: yes vs no | 3.46 (2.32–5.16) | <.001 |
| Variables tested as additional single variablesb | ||
| Improvement of preoperative neurologic deficits: yes vs no | 0.46 (0.25–0.85) | .013 |
| Epileptic dischargesc: yes vs no | 2.52 (1.36–4.67) | .003 |
| Edema before surgeryd: yes vs no | 1.63 (1.02–2.61) | .039 |
| Surgical complications | ||
| Sensorimotor deficits: yes vs no | 1.80 (1.05–3.09) | .033 |
| CNS infections: yes vs no | 5.89 (1.53–22.61) | .010 |
| Hydrocephalus: yes vs no | 3.27 (1.35–7.95) | .009 |
| Recraniotomy for any reason: yes vs no | 2.91 (1.25–6.78) | .013 |
| Radiographic intracranial hemorrhage: yes vs no | 1.51 (0.97–2.31) | .058 |
| Symptomatic intracranial hemorrhage: yes vs no | 2.60 (1.17–5.76) | .018 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aN = 623 patients with complete datasets.
bFurther variables are mentioned in the text.
cPostoperative EEG recordings (N = 340).
dDocumentation available for 690 patients (CT/MRI = 629/61).
We next applied this binary logistic regression model to detect independent risk factors for acute symptomatic perioperative seizures and included prophylactic anticonvulsant therapy and surgical complications (new-onset sensorimotor deficits, CNS infection, hydrocephalus, recraniotomy for any reason, or symptomatic intracranial hemorrhage) as additional covariates. Prophylactic anticonvulsant therapy in patients without preoperative epilepsy was not associated with perioperative seizure control in this model (odds ratio [OR]: 1.31 [95% confidence interval {CI}: 0.53–3.24]), P = .560]. Risk factors for acute symptomatic seizures were radiographic GTR (ie, more radical surgery [OR: 4.23{95% CI: 1.40–12.77}], P = .011) and surgical complications (OR: 3.18 [95% CI: 1.59–6.35], P = .001). Preoperative epilepsy was not predictive for perioperative seizure control, although it favored acute symptomatic seizures (OR: 2.32 [95% CI: 0.99–5.42], P = .051). Age (P = .144), WHO grade (P = .408), or tumor location at the convexity (P = .273) were not risk factors for acute symptomatic perioperative seizures. Edema was also not predictive for acute symptomatic seizures when included as an additional covariate in this model (P = .084).
Discussion
The efficacy of surgery on seizure control in meningioma patients is not well-defined. This retrospective analysis of the postoperative course of 779 consecutive meningioma patients indicates that predictors of poor postoperative seizure control include preoperative epilepsy, epileptiform potentials on postoperative EEG recordings, severe surgical complications including CNS infections, hydrocephalus, recraniotomy, and symptomatic intracranial hemorrhage as well as younger age and tumor progression at any time after initial surgery. In contrast, postoperative improvement of preoperative neurologic deficits predicted better seizure control.
Although the issue of poor postoperative seizure control in meningioma patients dates back to the onset of modern neurosurgery during the first half of the 20th century,19 no prospective controlled trials have been conducted to evaluate the effects of surgery on seizure control. In retrospective series of meningioma patients dating from the introduction of microsurgical techniques, the reported frequencies of patients with postoperative epilepsy ranged from 20% to 26%.12–14 These data compare well to our series (Table 1) and reflect both postoperative persistence and new onset of epilepsy. Of 244 patients with preoperative epilepsy, 100 (41.0%) suffered epilepsy after surgery, and 104 (19.4%) of 535 patients without preoperative epilepsy suffered new-onset postoperative epilepsy (Table 3).
EEG is a widely available technique and, epileptiform discharges on EEG recordings predict seizure recurrence upon withdrawal of antiepileptic drugs.20 The utility of postoperative EEG recordings in meningioma patients has been questioned because a single retrospective study of 102 patients reported no association between EEG changes and epilepsy after meningioma resection.21 However, postoperative epileptiform EEG potentials were recorded for only one patient in that series. In our series, postoperative epileptiform EEG potentials were recorded for 78 patients (Table 3) and were identified as an independent risk factor (OR: 2.52 [95% CI: 1.36–4.67], P = .003). We therefore suggest that EEG should be part of the routine clinical evaluation during the postoperative follow-up of meningioma patients, although an inherent limitation of our study was the lower frequency of patients receiving postoperative EEG (N = 340, Supplementary material, Table S2). Furthermore, only a small fraction of patients received both preoperative and postoperative EEG (N = 116), thus precluding comparisons of preoperative and postoperative EEG parameters.
Previous reports have emphasized a predictive role of tumor location for postoperative seizure control.13,14 In support of these reports, we noted an association of convexity meningiomas with postoperative epilepsy (Table 3), but this was not an independent risk factor in the binary regression model (Table 5). Edema was reported to contribute to preoperative and postoperative epilepsy in 2 cohort studies of meningioma patients,12,14 and our data support these reports (Supplementary material, Table S2). However, an inherent limitation of our study and of the above-mentioned reports is the assessment of edema mostly by CT. The lower resolution and sensitivity for detection of edema on CT scans compared with MRI precluded volumetric analyses and may have yielded an underestimation of the association of edema with epilepsy.
Measures to estimate postoperative seizure risk are required to guide anticonvulsant treatment and provide direct impact on the diagnosis of epilepsy according to the ILAE definition, which emphasizes the risk of seizure recurrence as an epilepsy-defining criterion.15 Based on the analyses reported here, we suggest a simplified score to guide anticonvulsant treatment derived by arbitrarily assigning 1 point to predictors with an OR <2 and 2 points to an OR >2 and defining 2 points as an indication for postoperative anticonvulsant treatment (Table 6). The retrospective design of our study, however, precludes estimates regarding the efficacy of anticonvulsant treatments. In fact, postoperative epilepsy became apparent under more intense anticonvulsant treatment compared with patients without postoperative epilepsy (Supplementary material, Table S4). Therefore, prospective validation of the simplified score suggested in this document is required. Such prospective studies should include MRI-based preoperative and postoperative imaging for detailed analysis of tumor location, extent of resection, gliosis, peritumoral edema, and surgical complications.
Table 6.
STAMPE2: A clinical score to guide the indication for postoperative anticonvulsant therapya
| Sensorimotor Deficit | 1 Point |
| Tumor progression | 1 Point |
| Age <55 y | 1 Point |
| Major surgical complicationb | 2 Point |
| Preoperative epilepsy | 2 Point |
| Epileptiform potentials on postoperative EEG | 2 Point |
| Edema | 1 Point |
aAnticonvulsant treatment should be implemented at a score of 2 points or higher.
bHydrocephalus, recraniotomy for any reason and CNS infection.
Acute symptomatic perioperative seizures occur frequently after craniotomy, and therefore anticonvulsant drugs are commonly administered perioperatively as a means of prophylaxis, despite a lack of evidence supporting this practice.22 In our series, a substantial fraction of 45.6% of the patients without preoperative epilepsy received perioperative anticonvulsant prophylaxis, but no association with the occurrence of acute symptomatic perioperative seizures was detected, thus further questioning the utility of anticonvulsant prophylaxis.
Considering that severe surgical complications occur in a substantial fraction of patients, even in the age of microsurgery (Table 4),10,11,23 and that spontaneous growth arrest and stable clinical course are observed in some untreated meningiomas,5,6 the question arises if prospective controlled trials should evaluate watchful waiting or radiotherapy as alternatives to surgery in selected subgroups of patients. Meningioma patients presenting with epilepsy as the only apparent symptom are good candidates for such a trial because modern anticonvulsant therapy is usually effective and well-tolerated. However, despite technical advances in brain imaging, concerns persist regarding the inclusion of patients in clinical trials without histological confirmation of the radiological diagnosis.
Supplementary Material
Funding
None declared.
Supplementary Material
Acknowledgments
None declared.
Conflict of interest statement. The authors declare no conflict of interest.
References
- 1.Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16 (suppl 4):iv1–i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rausing A, Ybo W, Stenflo J. Intracranial meningioma--a population study of ten years. Acta Neurol Scand. 1970;46(1):102–110. [DOI] [PubMed] [Google Scholar]
- 3.Vernooij MW, Ikram MA, Tanghe HL et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. [DOI] [PubMed] [Google Scholar]
- 4.Annegers JF, Schoenberg BS, Okazaki H, Kurland LT. Epidemiologic study of primary intracranial neoplasms. Arch Neurol. 1981;38(4):217–219. [DOI] [PubMed] [Google Scholar]
- 5.Kuratsu J, Kochi M, Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92(5):766–770. [DOI] [PubMed] [Google Scholar]
- 6.Olivero WC, Lister JR, Elwood PW. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg. 1995;83(2):222–224. [DOI] [PubMed] [Google Scholar]
- 7.Meixensberger J, Meister T, Janka M, Haubitz B, Bushe KA, Roosen K. Factors influencing morbidity and mortality after cranial meningioma surgery--a multivariate analysis. Acta Neurochir Suppl. 1996;65:99–101. [DOI] [PubMed] [Google Scholar]
- 8.Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 9.Reinert M, Babey M, Curschmann J, Vajtai I, Seiler RW, Mariani L. Morbidity in 201 patients with small sized meningioma treated by microsurgery. Acta Neurochir. 2006;148(12):1257–1265; discussion 1266. [DOI] [PubMed] [Google Scholar]
- 10.Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112(1):1–9. [DOI] [PubMed] [Google Scholar]
- 11.Sanai N, Sughrue ME, Shangari G, Chung K, Berger MS, McDermott MW. Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg. 2010;112(5):913–919. [DOI] [PubMed] [Google Scholar]
- 12.Lieu AS, Howng SL. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors. Epilepsy Res. 2000;38(1):45–52. [DOI] [PubMed] [Google Scholar]
- 13.Chozick BS, Reinert SE, Greenblatt SH. Incidence of seizures after surgery for supratentorial meningiomas: a modern analysis. J Neurosurg. 1996;84(3):382–386. [DOI] [PubMed] [Google Scholar]
- 14.Chaichana KL, Pendleton C, Zaidi H et al. Seizure control for patients undergoing meningioma surgery. World Neurosurg. 2013;79(3–4):515–524. [DOI] [PubMed] [Google Scholar]
- 15.Fisher RS, Acevedo C, Arzimanoglou A et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. [DOI] [PubMed] [Google Scholar]
- 16.Beghi E, Carpio A, Forsgren L et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–675. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Kasner SE, Broderick JP et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White JC, Liu CT, Mixter WJ. Focal epilepsy; a statistical study of its causes and the results of surgical treatment; epilepsy secondary to intracranial tumors. N Engl J Med. 1948;238(26):891–899. [DOI] [PubMed] [Google Scholar]
- 20.Callaghan N, Garrett A, Goggin T. Withdrawal of anticonvulsant drugs in patients free of seizures for two years. A prospective study. N Engl J Med. 1988;318(15):942–946. [DOI] [PubMed] [Google Scholar]
- 21.Rothoerl RD, Bernreuther D, Woertgen C, Brawanski A. The value of routine electroencephalographic recordings in predicting postoperative seizures associated with meningioma surgery. Neurosurg Rev. 2003;26(2):108–112. [DOI] [PubMed] [Google Scholar]
- 22.Pulman J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post-craniotomy seizures. Cochrane Database Syst Rev. 2013;2:CD007286. [DOI] [PubMed] [Google Scholar]
- 23.Zeng L, Wang L, Ye F, Chen J, Lei T, Chen J. Clinical characteristics of patients with asymptomatic intracranial meningiomas and results of their surgical management. Neurosurg Rev. 2015;38(3):481–488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.