Abstract
Background
Improved therapies for high-grade glioma (HGG) are urgently needed as the median survival for grade IV gliomas is only 15 months. Bone morphogenetic protein (BMP) signaling plays critical and complex roles in many types of cancer, including glioma, with most of the recently published work focusing on BMP-mediated regulation of glioma stem cells (GSCs). We hypothesized that BMP signaling may be an important modulator of tumorigenic properties in glioma cells outside of the GSC compartment.
Methods
We used a human HGG tissue microarray and performed immunohistochemistry for phospho-Smads1,5,8. To examine the role of BMP signaling in tumorigenic astrocytes, transgenic mice were used to delete the BMP type IA receptor (Bmpr1a) and generate astrocytes transformed with oncogenic Ras and homozygous deletion of p53. The cells were transplanted orthotopically into immunocompetent adult host mice.
Results
First we established that BMP signaling is active within the vast majority of HGG tumor cells. Mice implanted with BMPR1a-knockout transformed astrocytes showed an increase in median survival compared with mice that received BMPR1a-intact transformed astrocytes (52.5 vs 16 days). In vitro analysis showed that deletion of BMPR1a in oncogenic astrocytes resulted in decreased proliferation, decreased invasion, decreased migration, and increased expression of stemness markers. In addition, inhibition of BMP signaling in murine cells and astrocytoma cells with a small molecule BMP receptor kinase inhibitor resulted in similar tumor suppressive effects in vitro.
Conclusion
BMP inhibition may represent a viable therapeutic approach in adult HGG.
Keywords: BMP, BMPR1a, high-grade glioma, DMH1, genetically engineered mice
High-grade gliomas (HGGs) are aggressive tumors with a dismal prognosis despite treatment with surgery, radiation, and chemotherapy. Glioblastoma (GBM), a grade IV astrocytoma, is the most common malignant CNS tumor, with only a 5% five-year survival rate, underscoring our poor understanding of glioma biology and the obvious need for more effective therapies.1
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-beta (TGFβ) family. During canonical BMP signaling, BMP ligands bind to BMP type I and type II serine-threonine kinase receptor complexes. Upon ligand binding, the type I receptor phosphorylates the regulatory Smads1, 5, and 8. These regulatory Smads bind to the co-Smad (Smad4), and the complex is translocated to the nucleus. BMP signaling regulates the transcription of genes affecting critical cell processes including proliferation, differentiation, and apoptosis.2,3 Id1–4 gene transcripts are induced in most types of cells by BMP ligands.2 BMP signaling is tightly regulated by both extracellular antagonists and intracellular modulators such as the inhibitory Smad, Smad6, which acts in a negative feedback manner in response to active BMP signaling.2
In many types of cancer, BMPs play both tumor-promoting and tumor-suppressing roles, similar to TGFβ signaling.4,5 Various lines of evidence suggest that BMP signaling may be important in glioma biology, although contradictory findings appear in the literature.6–8 For example, increased expression of BMP signaling molecules has been associated with HGG.6,7 Expression of the BMP type IB receptor and the ligand BMP2 were both found to be expressed more frequently and at higher intensity in grade IV gliomas than in low-grade gliomas.6,7 Additionally, BMP type IA receptor has been implicated as a tumor driver in gliomas.9 Conversely, expression of BMP4 has been associated with low-grade gliomas and positive association with survival.8 In addition, several studies have reported that BMP signaling acts as a tumor suppressor on the subpopulation of glioma cells known as glioma stem cells (GSCs) by inhibiting proliferation and promoting differentiation10,11
Here we present evidence that BMP signaling is present and active in the vast majority of human HGG cells. Furthermore, in a novel transgenic, orthotopic model we show that BMP signaling in transformed astrocytes promotes aggressive tumor behavior via regulation of tumor cell proliferation and migration. Taken together, these findings provide evidence that there are major differences in the role of BMP signaling in the regulation of GSCs and more differentiated neoplastic cells.
Materials and Methods
Transgenic Mice
All animals were housed in the animal care facility at Vanderbilt University, and all experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. All procedures followed the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Cre/KrasG12D/p53fl/fl mice were generated and genotyped as described previously.12 Cre/KrasG12D/p53fl/fl mice were bred with conditional Bmpr1afl/fl mice.13 Cre/KrasG12D/p53fl/fl and Cre/KrasG12D/p53fl/fl/Bmpr1afl/fl mice were bred to mT/mG mice, a double-fluorescent Cre reporter mouse.14 Mice were bred on a mixed background.
Astrocyte Cell Culture
Astrocytes were harvested from neonatal (<7 days old) GFAP-Cre/KrasG12D/p53fl/fl/mT+ or GFAP-Cre/KrasG12D/p53fl/fl/Bmpr1afl/fl/mT+ pups as previously described.12 Astrocytes were harvested from 3 mice per group to establish 3 cell lines per genotype. Astrocytes were grown as monolayer cultures in T75 cell culture flasks. Recombined cells (GFP-positive, RFP-negative) were isolated using fluorescence-activated cell sorting (FACS) with a FACSAria III flow cytometer (BD). Flow cytometry experiments were performed in the Vanderbilt Medical Center Flow Cytometry Shared Resource. DNA was extracted from cultured astrocytes, and PCR was performed to detect the recombined Bmpr1a allele using the following primers:
5′¶-GGGTAGGTGTTGGGATAGCTG-3′¶
5′¶- TCCGAATTCAGTGACTACAGATGTACAGAG-3′¶.
U87 MG and T98G human GBM cells were obtained from ATCC.
GBM xenograft lines 10, 22, and 46 were obtained from the Mayo Clinic. The cells were maintained by serial transplantation in mice and were characterized as previously described.15
Orthotopic Injections
Three-month-old female, adult C57BL6 mice were purchased from Charles River Laboratories and anesthetized with a ketamine (100 mg/kg) and xylazine (10 mg/kg) mixture. Using a stereotactic frame (Kopf Instruments), 200 000 dissociated astrocytes (resuspended in 2.5 µL sterile phosphate-buffered saline were implanted into the left corpus striatum at a depth of 2.5 mm from the dural surface.12 The animals were monitored for neurological signs or weight loss for at least 75 days and euthanized if there were signs of significant neurological dysfunction or 20% weight loss.
Histology and Immunohistochemistry
Mice were euthanized, and their brains were fixed, sectioned, and stained with hematoxylin and eosin. Immunohistochemical staining was performed as previously described.12 Antibodies are listed in Supplementary material, Table S1. Sections processed without primary antibody served as controls.
Tissue Microarray
The tissue-microarray was constructed from archived surgical pathology material derived from tumor resections at Vanderbilt University Medical Center. It was composed of 30 GBMs and 5 grade III gliomas. Two to four cores per specimen were represented, with 14 nontumor tissue controls. Ages of patients ranged from 18 to 77 years. The tissue-microarray was constructed with approval of the Vanderbilt Institutional Review Board, IRB number: 131389. The percentage of positive p-Smad1/5/8 tumor cells within each core was estimated based on the presence of nuclear signal. The intensity of the signal was scored as 0, 1+, 2+, or 3+.
Western Blotting Analysis
Astrocytes or brain tumor tissue were lysed in Roche Complete Lysis-M buffer. Approximately 35 µg of protein from each sample were used to perform Western blots, as previously described.12 Proteins were visualized using a chemiluminescent detection system (PerkinElmer). Actin levels were determined for each condition to verify that equal amounts of protein were loaded. Antibodies are listed in Supplementary material, Table S1.
Quantitative Real-time PCR
Astrocytes were seeded at ∼500 000 cells/well in 6-well plates. Cells were lysed using the RNeasy mini kit (Qiagen). cDNA synthesis and quantitative real-time PCR (qPCR) were performed as previously described.16 Primer sequences are listed in Supplementary material, Table S2.
Trypan Blue Exclusion
Approximately 50 000 cells were plated in 200 uL supplemented DMEM F:12. Cells were trypsinized and 0.4% trypan blue stain was added. Percent viability was determined using the Invitrogen Countess.
MTT Assay
Cell proliferation was measured using the MTT Cell Proliferation Assay (ATCC). ∼10 000 cells were plated in a 96-well plate in triplicate in 100 uL supplemented DMEM F:12. Cells were treated with DMSO, BMP4(100 ng/uL) or DMH1(3 uM–100 uM) for 48 hours at 37°C. The MTT assay was performed following the ATCC protocol.
Thymidine Incorporation
Approximately 25 000 cells were plated in a 24-well plate in 500 uL supplemented DMEM F:12 media. 24 hours after plating, cells were treated with DMSO or DMH1 (3 uM–100 uM) for 24 hours. The cells were then subjected to [3H] thymidine incorporation assay as previously described.17
Invasion Assay
25 000 cells were seeded on Matrigel invasion chambers in quadruplicate (BD BioCoat 8.0 µm) for 24 hours. Cells that had migrated to the opposing side of the filter were fixed in 10% buffered formalin overnight and were stained with hematoxylin overnight. All cells that had migrated per invasion chamber were counted and means per cell line and group were calculated.
Scratch Assay
Cells were plated in duplicate at ∼500 000 cells per well in 6 well-plates. Once cells reached >90% confluency, they were treated with 4 µg/ml of mitomycin-C (Sigma) for 2 hours in normal media. Scratch assay was performed as previously described.16 Images of the same area were captured at 0 and 24 hours after the scratch.
Results
The BMP Signaling Pathway Is Active in the Majority of Tumor Cells in Human HGG
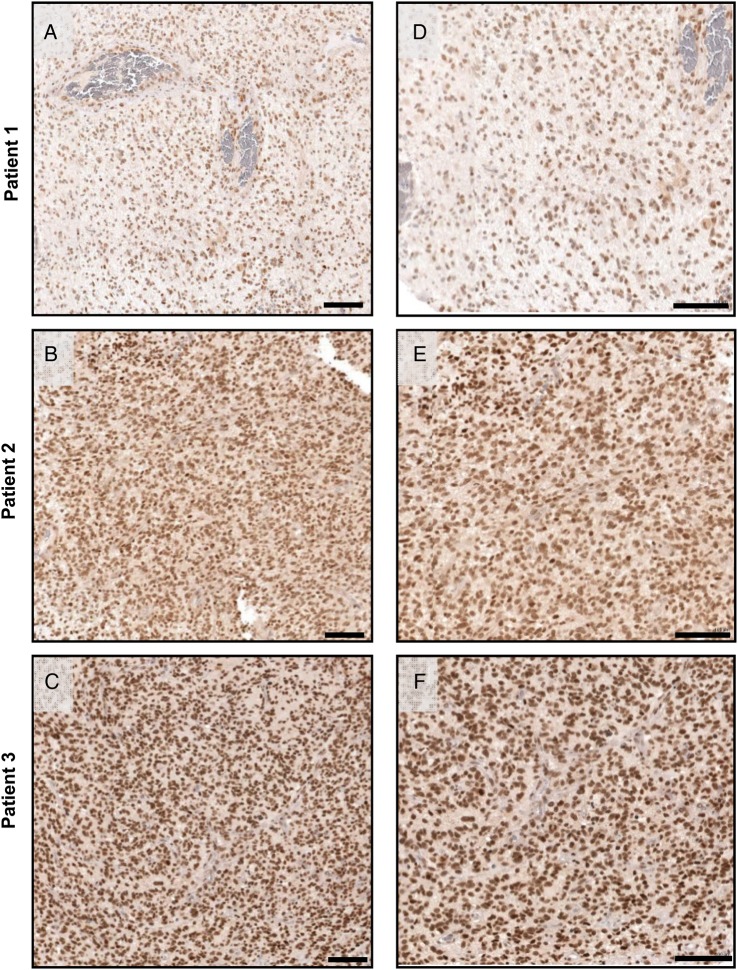
Activation of the BMP signaling pathway was assessed by immunohistochemistry for p-Smad1/5/8 using a tissue-microarray composed of 30 GBMs and 5 grade III gliomas. Signal representing p-Smad1/5/8 was present in all samples and was restricted to the cell nucleus. An average of 90% of all tumor cells stained positively within each core. However, a range of staining intensity was observed, with tumor cells staining at low, medium, or high levels of intensity (Fig. 1). The average percentage of tumor cells that stained positively ranged from 71–100% within each tumor (data not shown).
Fig. 1.
Bone morphogenetic protein (BMP) signaling is active in most tumor cells in human high-grade glioma (HGG). Immunohistochemistry with an antibody against p-Smad1/5/8 was performed on a tissue microarray consisting of 35 samples of human HGG. p-Smad1/5/8 expression was observed in all HGGs at varying levels of intensity in most of the tumor cells. Examples of low (A and D), intermediate (B and E), and high (C and F) intensity staining are shown in tumors from 3 glioblastoma patients at 40x (A–C, scale bar = 100 microns) and 100x (D–F, scale bar = 100 microns) magnification.
Astrocytes Cultured From Transgenic hGFAP-Cre/KrasG12D/p53fl/fl/Bmpr1afl/fl Mice Show Loss of the BMP Receptor and Impaired Response to BMP Ligand
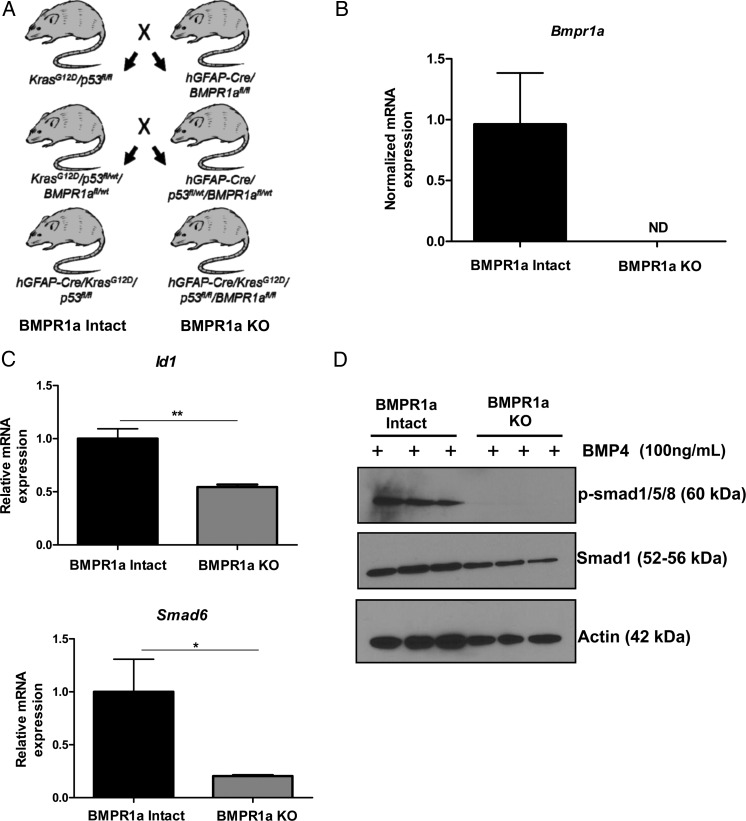
We previously established a transgenic, orthotopic transplant model in our laboratory using the human GFAP (hGFAP) promoter and Cre/lox technology to simultaneously drive oncogenic Kras (KrasG12D) expression while deleting p53 in astrocytes.12 To test the hypothesis that BMP signaling promotes gliomagenesis, mice harboring floxed BMP type IA receptor (Bmpr1a) alleles were used to establish quadragenic, hGFAP-Cre/KrasG12D/p53fl/fl/Bmpr1afl/fl mice (Fig. 2A). Both hGFAP-Cre/KrasG12D/p53fl/fl and hGFAP-Cre/KrasG12D/p53fl/fl/Bmpr1afl/fl mice were mated with mTom+ mice to introduce a Cre reporter gene to monitor recombination.
Fig. 2.
Generation and characterization of transformed astrocytes with genetic loss of BMPR1a. (A) Breeding scheme used to generate mice with constitutively active Kras (KrasG12D) and homozygous deletion of p53 (p53fl/fl) with and without homozygous deletion of the type IA BMP receptor (Bmpr1afl/fl). Mice with oncogenic Kras and homozygous deletion of p53 are termed “BMPR1a-intact.” Mice with the addition of Bmpr1afl/fl are termed “BMPR1a-KO.” (B–D) Validation of BMPR1a KO. (B) mRNA expression of Bmpr1a in 3 BMPR1a-KO transformed astrocyte cell lines was not detected (ND). (C) The mRNA expression of the downstream signaling targets of the BMP pathway Id1 and Smad6 were significantly decreased in BMPR1a-KO transformed astrocytes compared with BMPR1a-intact transformed astrocytes in response to 24-hour BMP4 treatment (n = 3 per group). A 2-tailed Student t test was performed to compare the mean mRNA expression. Bars indicate SEM. *P < .05, **P < .01. mRNA is normalized to Gapdh levels and relative to BMPR1a-intact expression. (D) As shown by Western blot, BMPR1a-KO astrocytes do not phosphorylate Smads1/5/8 in response to BMP4 treatment (1 h) showing the absence of canonical BMP signaling.
Astrocytes from the cortex of neonatal hGFAP-Cre/KrasG12D/p53fl/fl/mTom (BMPR1a-intact) or hGFAP-Cre/KrasG12D/p53fl/fl/Bmpr1afl/fl/mTom (BMPR1a-KO) pups (<7 days old) were harvested and grown under standard conditions as adherent monolayers.18 Similar culture methods resulted in cultures that were >98% astrocytes.19 Astrocytes of both genotypes grew robustly under these conditions.
Primary astrocyte cultures were subjected to FACS, gating on the mGFP+/RFP− population (Supplementary material, Fig. S1A). Sorted mGFP+RFP− astrocytes were maintained in culture, and subsequent experiments were conducted with pure populations of recombined astrocytes.
Recombination PCR performed on DNA isolated from astrocytes showed recombination of Bmpr1a in all BMPR1a-KO astrocyte cell lines (Supplementary material, Fig. S1B). In addition, qPCR analysis showed the presence of Bmpr1a mRNA transcript at varying levels in BMPR1a-intact astrocyte cell lines, while Bmpr1a mRNA transcripts were undetectable in BMPR1a-KO astrocyte lines (Fig. 2B). Loss of canonical BMP signaling was assessed by examining the mRNA expression for the primary BMP downstream targets Id1 and Smad6. In response to BMP ligand (BMP4) treatment, both Id1 and Smad6 expression was significantly lower in BMPR1a-KO astrocytes than in BMPR1a-intact cells (P < .001, P < .05, respectively, Fig. 2C). In addition, we analyzed phosphorylation of Smads1/5/8 by Western blot. Treatment of BMPR1a-intact astrocytes with BMP4 resulted in robust phosphorylation of Smads1/5/8 (Fig. 2D). However, phospho-Smads1/5/8 were undetectable by Western blot in BMP4-treated BMPR1a-KO cells, indicating loss of canonical BMP signaling (Fig. 2D).
Deletion of BMPR1a Increases Survival in Immunocompetent Mice With Orthotopic Implants
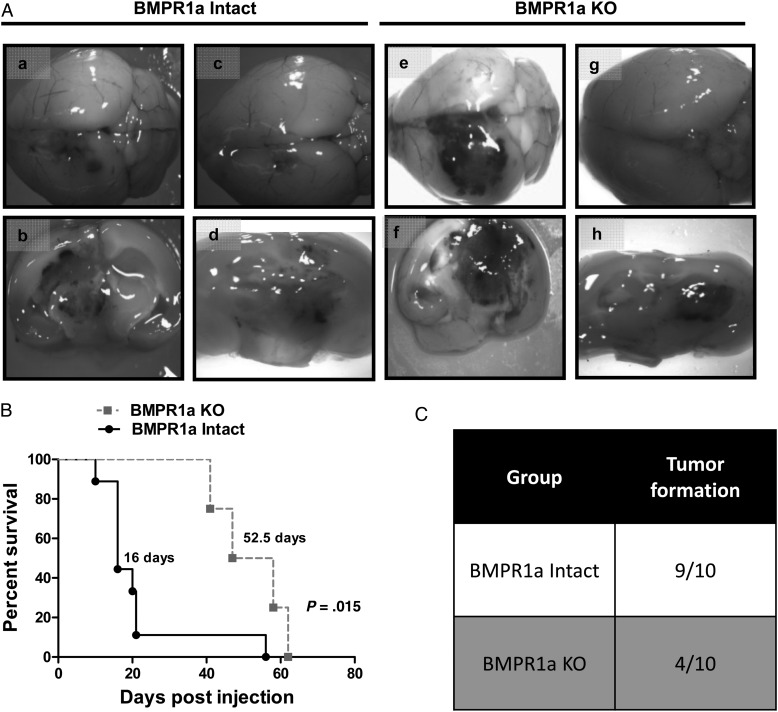
Recombined astrocytes were injected into the striata of immunocompetent adult host mice (n = 10 per group). The control group received transformed BMP-intact astrocytes, while the other group received BMPR1a-KO astrocytes. Tumors formed as a result of both BMPR1a-intact and BMPR1a-KO injections. All tumors that formed were highly invasive, with gross hemorrhage and necrosis. (Fig. 3A). However, BMPR1a-intact astrocytes were more effective in forming tumors than BMPR1a-KO astrocytes (9/10 vs 4/10, P = .06, Fig. 3C). In addition, BMPR1a-intact astrocytes formed more aggressive tumors, with a median survival of 16 days compared with 52.5 days in the BMPR1a-KO group (P = .015, Fig. 3B).
Fig. 3.
Reduced engraftment and prolonged survival in mice receiving orthotopic injections of BMPR1a-KO versus BMPR1a-intact astrocytes. (A) Mice receiving orthotopic injections of BMPR1a-intact (a–d) or KO (e–h) transformed astrocytes formed tumors (a–h), which often appeared on the surface of the brain as hemorrhagic masses (a,e). Coronal sections showed highly infiltrative tumors with multifocal hemorrhage and diffuse hemorrhage (b,d,f,h). (B) Kaplan-Meier curves showing survival of mice injected with BMPR1a-intact (black line) versus BMPR1a-KO (gray line) tumorigenic astrocytes. The median survival for mice injected with BMPR1a-intact cells was 16 days compared with 52.5 days in mice injected with BMPR1a-KO cells (P = .015). (C) Nine of ten mice injected with BMPR1a-intact cells formed tumors compared with only 4 of 10 mice injected with BMPR1a-KO cells.
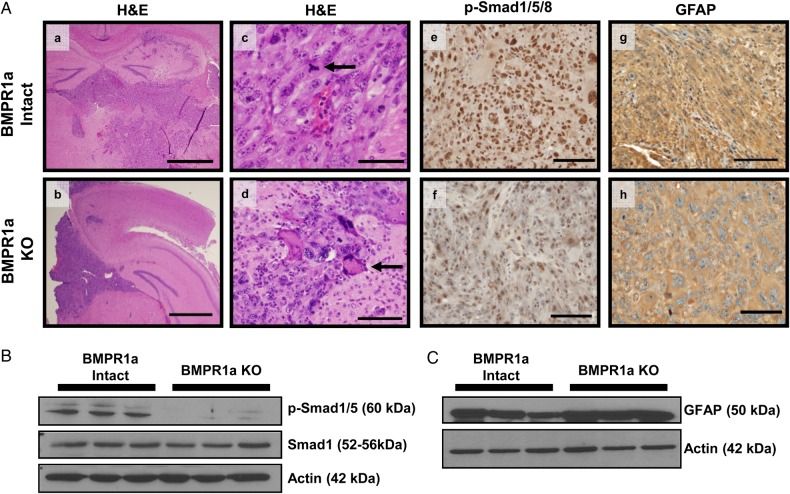
Histopathologically, the tumors that developed from BMPR1a-intact and BMPR1a-KO astrocytes were similar in appearance (Fig. 4A, a,b,c,d). Tumors showed the characteristic features of human HGG including infiltrating pleomorphic cells, (arrow Fig. 4A, d), necrosis, and many mitotic figures (arrow Fig. 4A, c). Tumors were highly infiltrative, often invading both cerebral hemispheres as well as the brainstem. Immunohistochemical analysis showed GFP expression in tumor cells of both groups of mice, consistent with recombination of the mTomato reporter (Supplementary material, Fig. S2A).
Fig. 4.
Intracranial tumors derived from BMPR1a-intact and BMPR1a-KO injections show histopathological features characteristic of human high-grade glioma). (A) Representative H&E-stained sections of BMPR1a-intact (a,c) and BMPR1a-KO tumors (b,d). Tumors are highly infiltrative, with necrosis, mitotic figures (arrow in c), and pleomorphic cells (arrow in d). The histopathology is reminiscent of a human giant cell glioblastoma. BMPR1a-intact tumors show increased p-Smad1/5/8 staining (e) compared with BMPR1a-KO tumors (f) indicating higher levels of bone morphogenetic protein signaling (e). GFAP expression is similar in BMPR1a-intact and KO tumors, indicating astrocytic differentiation (g,h). Scale bar 2 mm (a,b) Scale bar 200 µm(c-h) (B and C) Western blot analysis on tumor tissue lysates confirms the immunohistochemistry findings.
We examined BMP signaling as measured by phosphorylation of Smads1/5/8. There were diminished p-Smads1/5/8 in BMPR1a-KO tumor cells compared with BMPR1a-intact tumors, which indicated decreased BMP signaling in BMPR1a-KO tumor cells (Fig. 4A, e,f). We also examined the expression of GFAP, an intermediate filament, which is the primary marker for astroglial cells and universally expressed within human astrocytic tumors. GFAP expression was equally expressed in both types of tumors (Fig. 4A, g,h). Western blot analysis from tumor lysates confirmed the immunohistochemistry findings (Fig. 4B and C).
Because BMPs play a crucial role in mediating differentiation of neural and glioma stem cells, we examined the expression of the neural stem cell markers nestin and OLIG2. We observed a subset of cells that were positive for nestin and OLIG2; however, there were no apparent differences in expression levels between the BMPR1a-intact and BMPR1a-KO tumors based on immunohistochemistry (Supplementary material, Fig. S2B and C).
BMP Signaling Promotes Proliferation and Migration of Transformed Astrocytes
As loss of BMPR1a markedly increased survival in the orthotopic transplant model, we investigated (in vitro) the effect of BMPR1a loss on 2 hallmarks of cancer: proliferation and invasion. BMPR1a-intact astrocytes proliferated at approximately twice the rate of BMPR1a-KO cells (P = .04, Fig. 5A). Similar results were obtained with the MTT assay, and cell counts showed no difference in viability between BMPR1a-intact and BMPR1a-KO cells (Supplementary material, Fig. S3A and B).
Fig. 5.
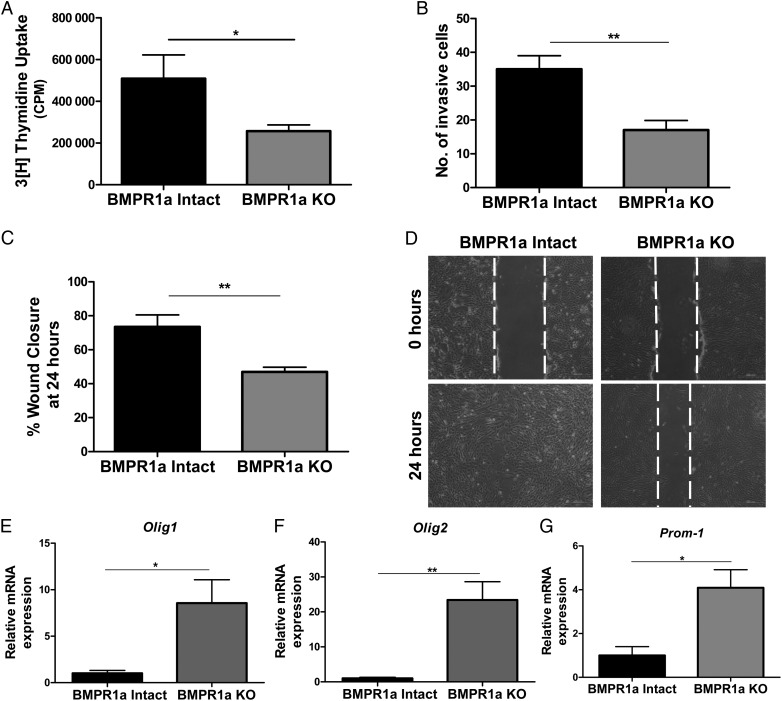
Abrogation of BMP signaling in transformed murine astrocytes inhibits proliferation, invasion, and migration and increases mRNA expression of stemness markers. (A)Tritiated thymidine incorporation assay shows that proliferation is inhibited in BMPR1a-KO astrocytes (gray bars) compared with BMPR1a-intact cells (black bars). Results are expressed as mean counts per minute (CPM) for quadruplicate samples at each time point for 3 cell lines per group. (B) In a 24-hour Matrigel transwell invasion assay, BMPR1a deletion results in a 50% reduction in the number of cells able to invade. Results are expressed as the mean number of cells able to invade with quadruplicate transwells and 3 cell lines per group. (C) Scratch assay was performed in 6-well plates with >90% confluent BMPR1a-intact and KO astrocytes. The BMPR1a-intact cells migrated into the wound, resulting in a smaller gap at 24 hours compared with the BMPR1a-KO cells. All cells were treated with mitomycin-C 2 hours prior to performing the scratch assay. (D) Representative images of BMPR1a-intact and BMPR1a-KO astrocytes at time of the original scratch (0 h) and at 24 hours (4x). (E,F,G)The mRNA expression levels of Olig-1 (E), Olig-2(F), and Prom-1 (G) were higher in BMPR1a-KO astrocytes compared with BMPR1a-intact astrocytes (n = 3 cell lines per group). A 2-tailed Student t test was performed to compare the mean mRNA expression. mRNA is normalized to Gapdh levels and relative to BMPR1a-intact expression. Error bars indicate SEM. *P < .05 **P < .01.
Next, we compared BMPR1a-intact and BMPR1a-KO astrocytes using a Matrigel invasion assay. Loss of BMPR1a inhibited the ability of transformed astrocytes to migrate and invade, with a 2-fold reduction in the number of invading BMPR1a-KO astrocytes compared with BMPR1a-intact astrocytes (P = .001, Fig. 5B). In addition, the mean wound closure in a scratch assay was 73% for BMPR1a-intact cells compared with a mean of 47% by BMPR1a-KO cells (P = .002, Fig. 5C and D). In parallel, qPCR was performed for a panel of genes known to be involved in migration. Loss of BMPR1a in transformed astrocytes resulted in diminished expression of mRNA for the integrin beta subunits 4 and 7 (Supplementary material, Fig. S3C and D).
Loss of BMP Signaling Increases mRNA Expression for Stemness Markers in Transformed Astrocytes
BMP signaling is known to regulate neural and glioma stem and progenitor cell differentiation. Therefore, we analyzed transcripts for several established neural stem cell markers. Expression levels of Prom-1 (CD133), Olig1, and Olig2 mRNA were significantly greater in BMPR1a-KO astrocytes. Olig1 and Olig2 gene expression were increased ∼8- and 23-fold respectively (Fig. 5E and F). Prom-1 mRNA levels were 4-fold higher in BMPR1a-KO cells (Fig. 5G).
DMH1, a Small Molecule Inhibitor of BMP Signaling, Inhibits Astrocytic Proliferation and Migration in Vitro
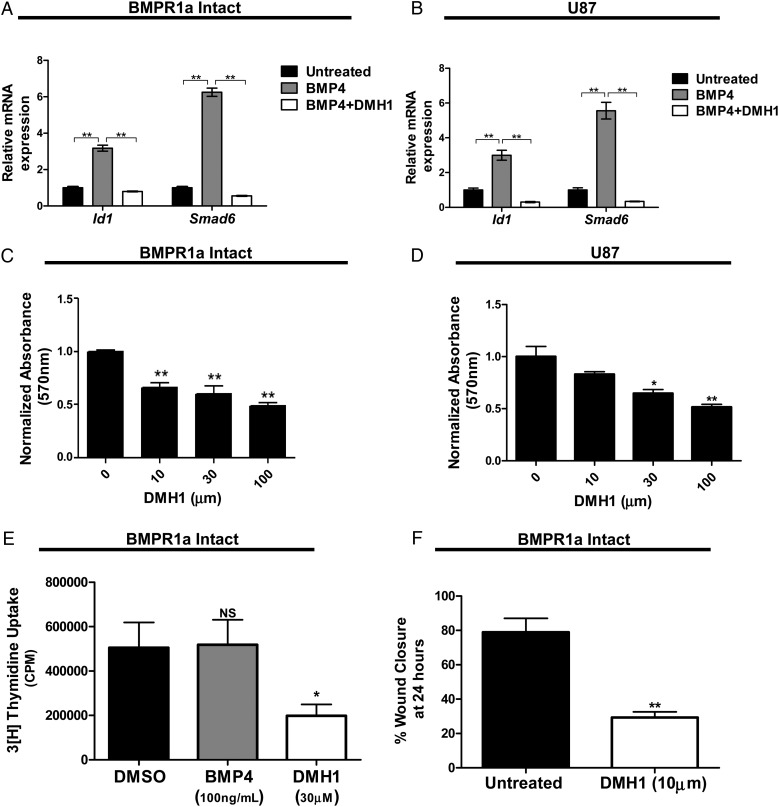
To complement the genetic approach to inhibition of BMP signaling, cells were treated with dorsomorphin homologue 1 (DMH1), a highly selective BMP type I receptor small molecule inhibitor.20 BMPR1a-intact transformed murine astrocytes and a human glioma cell line (U87) were treated with either vehicle (dimethylsulfoxide [DMSO]) or DMH1 for 24 hours. In all cell lines, DMH1 treatment (10 μm) decreased the BMP ligand-induced expression of BMP-target genes Id1 and Smad6 (Fig. 6A and B Supplementary material, Fig. S4A). In addition, DMH1, in a dose-dependent manner, inhibited proliferation in tumorigenic murine cell lines and 2 human GBM cell lines as measured by MTT assay (Fig. 6C and D Supplementary material, Fig. S4B and S5A). To ensure that these effects were due to decreased proliferation and not drug toxicity, we confirmed the decrease in proliferation by 3H-thymidine incorporation in the 3 transformed murine astrocyte lines (Fig. 6E). No effects on cell viability were observed in U87 cells treated with DMH1 (Supplementary material, Fig. S4C). As DMH1 inhibits all BMP type I receptors, we also observed decreased proliferation of BMPR1a-KO astrocytes upon DMH1 treatment as measured by MTT assay (Supplementary material, Fig S5B). However, we treated 3 human GBM cell lines grown as neurospheres with varying concentrations of DMH1and saw no effect on proliferation (Supplementary material, Fig. S5C).
Fig. 6.
Pharmacological inhibition of decreases proliferation and migration of oncogenic astrocytes. (A and B) DMH1 treatment (10 µM) decreases bone morphogenetic protein-induced Id1 and Smad6 expression in murine BMPR1a-intact transformed astrocytes and human glioblastoma (GBM) astrocytes (U87) as measured by quantitative real-time PCR. A 2-tailed Student t test was performed to compare the mean mRNA expression. mRNA is normalized to Gapdh levels and relative to untreated expression. (C and D) Transformed astrocytes were treated with various concentrations of DMH1. DMH1 inhibited proliferation, in a dose-dependent manner, in both transformed, murine astrocytes and human GBM cells. Significance was determined by ANOVA followed by post hoc t tests. (E) Treatment with DMH1 (30 µM) decreased proliferation in transformed murine astrocytes as measured by tritiated thymidine incorporation. Results are expressed as mean counts per minute for quadruplicate samples for 3 cell lines. Significance was determined by ANOVA followed by post hoc t tests. (F) Treatment with DMH1 (10 µM) inhibited migration of transformed astrocytes as determined by a scratch assay. All cells were treated with mitomycin-C 2 hours prior to performing the scratch assay. Cells were treated with DMH1 at the time of the original scratch. Significance was determined by a 2-tailed Student t test. Bars indicate SEM. *P < .05, **P < .01.
Finally, the effect of DMH1 on cell migration was tested in a scratch assay. DMH1 treatment significantly decreased the migratory ability of BMPR1a-intact cells compared with untreated controls. We observed an average of 30% closure in DMH1-treated cells compared with a mean wound closure of 79% in untreated controls (P < .0001, Fig. 6F, Supplementary material, Fig. S4D).
Discussion
BMP signaling is critical for neural development and the regulation of neural progenitor cells.3 Several lines of evidence suggest an important role for this pathway in gliomagenesis as well, although the details are poorly understood. Previous studies have indicated that BMP receptors are present on human glioma cells and that BMP receptor quantity correlates with tumor grade.6 The majority of studies regarding BMP signaling in gliomas focus on human GSCs in orthotopic transplant models, implicating BMP signaling in the differentiation of GSCs and hence as a tumor suppressor in this paradigm.10,11 However, like other members of the TGF-β superfamily, pro- or antitumorigenic effects of BMP may depend on the cellular context in which the pathway is active.5 Here, we provide evidence that BMP pathway activity extends beyond the GSC compartment and that BMP signaling fosters tumorigenesis in neoplastic astrocytes through promotion of proliferation and invasion. These data suggest that BMPs may differentially regulate the GSC and ‘bulk tumor’ compartments in HGG.
To assess active BMP signaling in human HGG tissue, we used immunohistochemistry for p-Smad1/5/8 on a series of HGGs. Our results showed the presence of nuclear phospho-Smads1/5/8, at varying levels of intensity in all tumors. Although others have shown the presence of BMP pathway-signaling components, including BMP ligands and receptors, the presence of these components is not directly related to pathway activity.6–8 Our data suggest that active BMP signaling is present in the majority of human HGG.
In addition, our data show that BMP signaling is active in ∼90% of the tumor cells within a given tumor. The proportion of glioma cells with stem cell-like properties is estimated to range from <1% to 30%.21,22 Therefore, it follows that many of the phospho-Smad1/5/8-expressing cells in the tumor samples we analyzed reside in the non-GSC or bulk tumor compartment. These data underscore the importance of understanding the regulation by BMP of glioma biology in the more differentiated glioma compartment.
To address the functional role of BMP signaling in tumorigenic astrocytes, we used a transgenic model highly relevant to human HGG. While KRAS mutations are not frequent in human GBMs, Ras pathway activation, by several mechanisms including copy number gains and/or mutation and upregulation of upstream receptor tyrosine kinases, is known to occur in >80% of human GBMs.23,24 In addition, alterations in cell cycle regulation are common, with p53 alterations in up to 87% of human GBMs.23 Using various methodologies for activating Ras and interfering with cell cycle regulation in neural cells, we and others have developed transgenic murine models of glioma that faithfully recapitulate key clinical and histopathological features of human glioma, allowing us to study BMP signaling in a highly relevant HGG model system.12,25–27
Previously, we showed that fatal HGG forms when astrocytes from hGFAP-Cre/KrasG12D/p53fl/f mice are harvested, maintained in short-term culture, and injected orthotopically into immunocompetent mice. In the present study, we incorporated BMPR1afl/fl transgenic mice into a breeding strategy to generate quadragenic hGFAP-Cre/KrasG12D/p53fl/fl/BMPR1afl/fl mice (BMPR1a-KO mice). We then harvested astrocytes from these animals for further experiments, comparing them with transgenic hGFAP-Cre/KrasG12D/p53fl/fl/BMPR1awt/wt (BMPR1a-intact) cells. We targeted BMPR1a specifically as it is a critical and necessary receptor during CNS development, whereas other BMP type I receptors, such as BMPR1b, are not.28 In addition, BMPR1a has been shown to promote tumor growth in multiple systems including gliomas9,16
As expected, BMPR1a-intact astrocytes formed aggressive gliomas when injected orthotopically into immunocompetent hosts. In contrast, BMPR1a-KO astrocytes engrafted at a lower rate, and survival was prolonged in the host mice. The median survival for mice receiving BMPR1a-KO astrocytes was 52.5 days, which is more than a 3-fold increase compared with the mice that received BMPR1a-intact astrocytes. The data strongly suggest that BMP signaling via the BMPR1a receptor promotes tumorigenesis in transformed astrocytes.
To investigate the functional role of BMPR1a in transformed astrocytes, we conducted a series of in vitro experiments with transgenic astrocytes and 2 human GBM cell lines. Both genetic deletion of BMPR1a and pharmacologic inhibition of BMP signaling with DMH1 inhibited the proliferation of transformed murine astrocytes in vitro. In addition, DMH1 inhibited proliferation in U87 and T98G cells. Because DMH1 inhibits all BMP type I receptors, we observed its effects on BMPR1A-KO cells and found that inhibition of all BMP type I receptors further suppresses proliferation.
Similarly, both genetic deletion of BMPR1a and DMH1 treatment impaired the ability of transformed astrocytes to migrate and invade. Taken together, using both genetic and pharmacological inhibition of BMP signaling in mouse and human cells, our findings suggest that BMP signaling regulates 3 elements of tumor cell behavior that are essential components in astrocytoma formation and progression: proliferation, invasion, and migration. Similar results were recently published in which reduction of BMPR1a by microRNA-656 resulted in decreased tumor growth, proliferation, and migration.9
HGGs are characterized by a high proliferation index and a rapidly growing tumor mass.29 Our data strongly suggest that BMP signaling promotes proliferation in tumorigenic astrocytes, including U87 cells. This contrasts with previous work with GSCs, in which BMP signaling decreased proliferation and enhanced differentiation in this population of tumor cells.10,11 Our findings are consistent, however, with studies in lung and breast cancer in which BMPs promote proliferation, while inhibition of BMP signaling reduces cell proliferation.30,31 Moreover, these findings are in line with published data showing differential effects, depending on developmental context, of signaling through pathways that are implicated in development and cancer.4,12
Malignant gliomas are highly invasive neoplasms, and infiltration of surrounding brain tissue contributes to tumor recurrence. Here we show that BMP signaling increases cell motility and the ability of astrocytoma cells to invade. These findings are consistent with studies of pancreatic and breast cancer cells.31,32 In astrocytes, BMP signaling may be driving this effect through regulation of integrins as the reduction in cell mobility we observed in BMPR1a-KO cells was associated with a marked reduction in integrins beta 4 and 7 gene expression. Integrin beta 4 has been shown to be inversely correlated with survival in GBM.33
BMP signaling is a well-known driver of astrocytic differentiation in normal neural stem/progenitor cells.3 In the present study, abrogation of BMP signaling in transformed astrocytes was associated with increased gene expression of stem cell markers including Olig1, Olig2, and Prom-1(CD133). In concordance with our findings, studies of neural progenitor cells have shown that BMP signaling suppresses the expression and activity of Olig1 and Olig2.31 CD133, a cell surface antigen, has been touted as the primary marker of glioma stem cells, and BMP treatment has been shown to reduce the number of CD133-positive cells in glioma.10
In addition, CD133-positive glioma cells may be resistant to radiotherapy, which highlights the importance of understanding the biology of CD133-expressing cells in glioma.34 Our data suggest that the BMP pathway may be a mediator of CD133 expression in the more differentiated glioma compartment. The changes in stemness markers were not observed in the tumors, which may be a result of the type of cells that engrafted, the effects of the tumor microenvironment, or the differences in protein and gene expression.
Our in vivo model and in vitro studies suggest that pharmacological inhibition of BMP signaling could be a promising new therapeutic modality in HGG for the non-GSC component of the tumor by reducing proliferation and invasion. Conceivably, the reduction in invasive properties of glioma cells would increase the effectiveness of standard therapies such as surgery and radiotherapy. Our studies also highlight the concept that the stem/progenitor-like glioma compartment may respond differently than the bulk tumor to BMP-based and other therapies.10,11 For example, when we treat human GBM cells grown as neurospheres with DMH1, we see no effect on proliferation supporting this hypothesis. This suggests that for effective treatment, multiple therapies may be required to target the differentiated and progenitor populations separately. Indeed, an understanding of the mechanisms that maintain glioma cells in more or less differentiated populations, while governing transitions from one compartment to the other, may lead to progress in glioma therapies.
Supplementary Material
Funding
NIH/NINDS 5K08NS062107 (T.W.A.).
This work was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Supplementary Material
Acknowledgments
The authors thank Dr. Jann Sarkaria for generously providing the GBM xenograft lines. The authors thank Agnieszka Gorska and Anna Chytil for assistance with PCR, immunohistochemistry, and Western blots; Dr. Bojana Jovanovic and Dr. Sergey Novitskiy for assistance with the manuscript and figures; Jennifer L. Harvey and Heather E. Russell for expert histological service; and Dr. Michael K. Cooper for helpful comments on the manuscript.
Conflict of interest statement. The authors have no conflicts of interest to declare.
References
- 1.Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16 (suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. [DOI] [PubMed] [Google Scholar]
- 3.Chen H-L, Panchision DM. Concise review: bone morphogenetic protein pleiotropism in neural stem cells and their derivatives-alternative pathways, convergent signals. Stem Cells. 2007;25(1):63–68. [DOI] [PubMed] [Google Scholar]
- 4.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehata S, Yokoyama Y, Takahashi K et al. Bi-directional roles of bone morphogenetic proteins in cancer: Another molecular Jekyll and Hyde? Pathol Int. 2013;63(6):287–296. [DOI] [PubMed] [Google Scholar]
- 6.Yamada N, Kato M, Dijkel P et al. Bone morphogenetic protein type IB receptor is progressively expressed in malignant glioma tumours. Br J Cancer. 1996;73(5):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Tian G, Tu Y et al. Expression pattern and clinical prognostic relevance of bone morphogenetic protein-2 in human gliomas. Jpn J Clin Oncol. 2009;39(10):625–631. [DOI] [PubMed] [Google Scholar]
- 8.Bao Z, Zhang C, Yan W et al. BMP4, a strong better prognosis predictor, has a subtype preference and cell development association in gliomas. J Transl Med. 2013;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo M, Jiang Z, Zhang X et al. miR-656 inhibits glioma tumorigenesis through repression of BMPR1A. Carcinogenesis. 2014;35(8):1698–1706. [DOI] [PubMed] [Google Scholar]
- 10.Piccirillo SGM, Reynolds BA, Zanetti N et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–765. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Son MJ, Woolard K et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein developmental pathway inhibits differentiation of human glioblastoma tumor initiating cells. Cancer Cell. 2008;13(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghazi SO, Stark M, Zhao Z et al. Cell of origin determines tumor phenotype in an oncogenic Ras/p53 knockout transgenic model of high-grade glioma. J Neuropathol Exp Neurol. 2012;71(8):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishina Y, Hanks MC, Miura S et al. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 2002;32(2):69–72. [DOI] [PubMed] [Google Scholar]
- 14.Muzumdar MD, Tasic B, Miyamichi K et al. A global double-fluorescent cre reporter mouse. Genesis. 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 15.Carlson BL, Pokorny JL, Schroeder MA et al. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr Protoc Pharmacol. 2012;52(14):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickup MW, Hover LD, Guo Y et al. Deletion of the BMP receptor BMPR1a impairs mammary tumor formation and metastasis. Oncotarget. 2015;6(26):22890–22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierie B, Chung CH, Parker JS et al. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119(6):1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 19.Schildge S, Bohrer C, Beck K et al. Isolation and culture of mouse cortical astrocytes. J Vis Exp. 2013;19(71):50079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao J, Ho JN, Lewis JA et al. In vivo structure activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh SK, Hawkins CC, Clarke ID et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–400. [DOI] [PubMed] [Google Scholar]
- 22.Clément V, Dutoit V, Marino D et al. Limits of CD133 as a marker of glioma self-renewing cells. Int J Cancer 2009;125(1):244–248. [DOI] [PubMed] [Google Scholar]
- 23.McLendon R, Friedman A, Bigner D et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeuken J, van den Broecke C, Gijsen S et al. RAS/RAF pathway activation in gliomas: The result of copy number gains rather than activating mutations. Acta Neuropathol. 2007;114(2):121–133. [DOI] [PubMed] [Google Scholar]
- 25.Abel TW, Clark C, Bierie B et al. GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol Cancer Res 2009;7(5):645–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland EC, Celestino J, Dai C et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–57. [DOI] [PubMed] [Google Scholar]
- 27.Ding H, Roncari L, Shannon P et al. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse. Cancer Res. 2001;61(9):3826–3836. [PubMed] [Google Scholar]
- 28.Samanta J, Burke GM, McGuire T et al. BMPR1a signaling determines numbers of oligodendrocytes and calbindin-expressing interneurons in the cortex. J Neurosci. 2007;27(28):7397–7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao J, Lee R, Chang A et al. DMH1, a small molecule inhibitor of BMP type i receptors, suppresses growth and invasion of lung cancer. PLoS One. 2014;9(6):e90748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens P, Pickup MW, Novitskiy SV et al. Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene. 2015;34(19):2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virtanen S, Alarmo E-L, Sandström S et al. Bone morphogenetic protein -4 and -5 in pancreatic cancer–novel bidirectional players. Exp Cell Res. 2011;317(15):2136–2146. [DOI] [PubMed] [Google Scholar]
- 33.Hu Y, Ylivinkka I, Chen P et al. Netrin-4 promotes glioblastoma cell proliferation through integrin β4 signaling. Neoplasia. 2012;14(3):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao S, Wu Q, McLendon R et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.