Abstract
Background
Although many studies have been published about specific lesions characterizing von Hippel-Lindau(VHL) disease, none have dealt with the natural history of the whole disease and the consequent disabilities. We aim to define the comprehensive natural history of VHL disease and to describe the functional disabilities and their impact upon patients' quality of life, thereby tailoring the follow-up schedule accordingly.
Methods
We performed a prospective analysis on 128 VHL-affected patients beginning in 1996. For each affected organ, we defined intervals between the first and subsequent VHL-related manifestations and compared them with current VHL surveillance protocols. We looked for any association of the number of involved organs with age, sex, type of VHL gene mutation, and functional domain mutation. Ultimately, we assessed the organ-specific disabilities caused by VHL disease.
Results
Hemangioblastomas show different patterns of progression depending on their location, whereas both renal cysts and carcinomas have similar progression rates. Surgery for pheochromocytoma and CNS hemangioblastoma is performed earlier than for pancreatic or renal cancer. The number of involved organs is associated with age but not with sex, type of VHL gene mutation, or functional domain mutation. A thorough analysis of functional disabilities showed that age is related to the first-appearing functional impairment, but it is not predictive of the final number of disabilities.
Conclusions
Our study defines the disease progression and provides a comprehensive view of the syndrome over time. We analyzed for the first time the functional disability of VHL patients, assessing the progression for each function.
Keywords: disability, follow-up, functional impairment, natural history, VHL
Von Hippel-Lindau (VHL) disease (OMIM 193300) is a multiorgan neoplastic syndrome with autosomal-dominant transmission, complete penetrance, and variable expression that is caused by mutations in the tumor suppressor VHL gene.1 Affected patients may develop hemangioblastomas in the CNS, retinal angiomatosis, endolymphatic sac tumors (ELSTs), clear cell renal carcinomas, pheochromocytomas, renal and pancreatic cysts, neuroendocrine tumors, epididymal cystadenomas, and ovarian cysts (Fig. 1).2,3
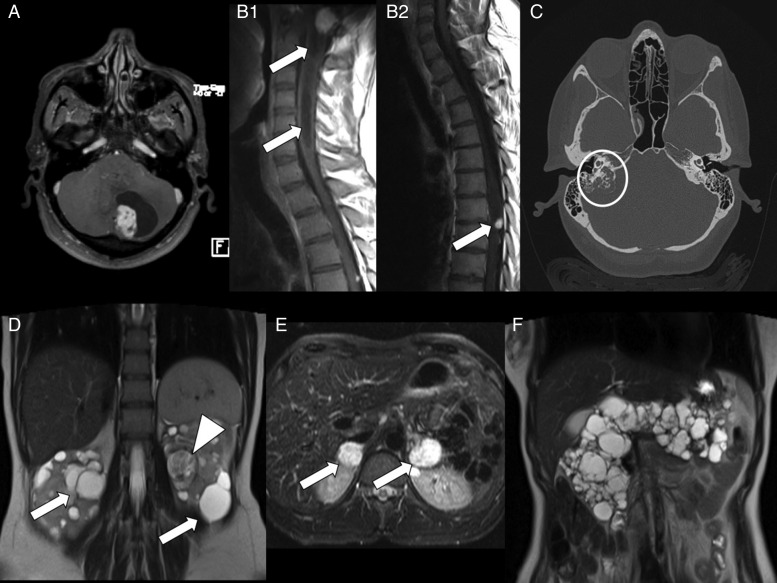
Fig. 1.
Diagnostic imaging in von Hippel-Lindau disease. (A) MR T1-weighted axial image with gadolinium showing a cystic cerebellar hemangioblastoma. (B1) MR T1-weighted sagittal image showing a brainstem hemangioblastoma with syringomyelia (arrows). (B2) MR T1-weighted sagittal image showing a thoracic spine hemangioblastoma (arrow). (C) CT scan showing a right endolymphatic sac tumor eroding the petrous bone (circle). (D) MR T2-weighted coronal image showing multiple bilateral renal cysts (arrows) and a left large clear cell carcinoma (arrowhead) characterized by inhomogeneous signal. (E) MR T2-weighted axial image showing large bilateral adrenal pheochromocytomas (arrows). (F) MR T2-weighted coronal image showing a polycystic pancreas.
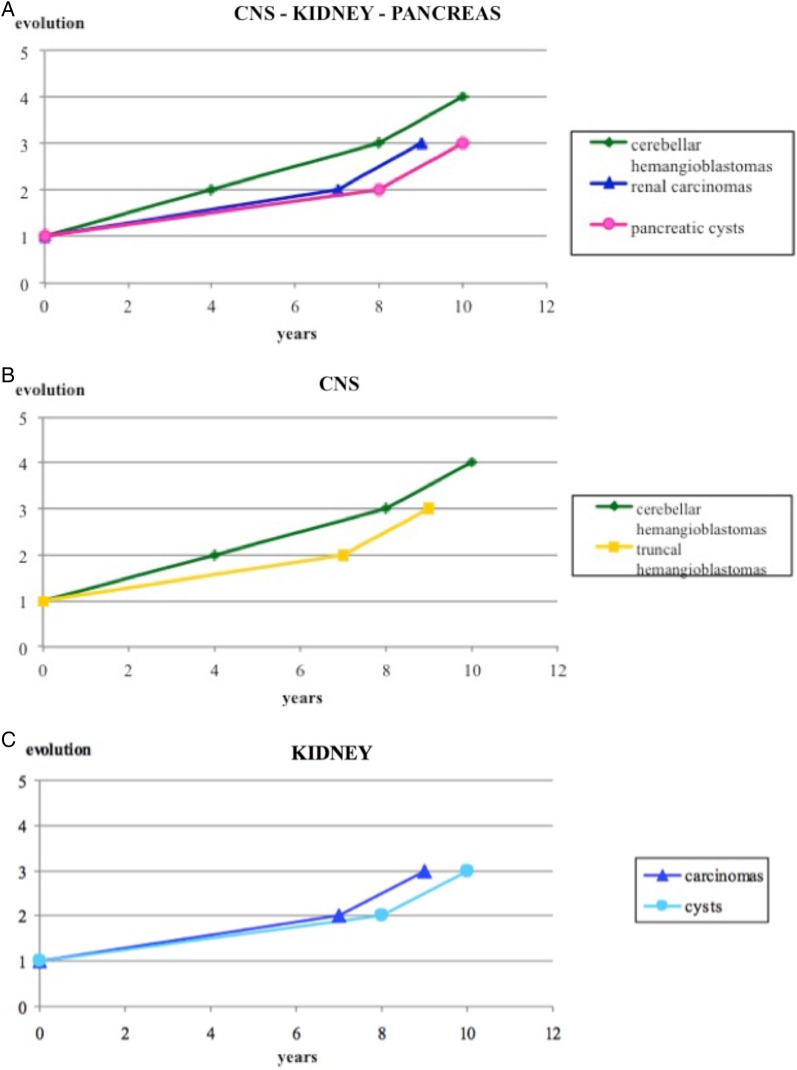
Fig. 2.
(A) Growth pattern of lesions in the main systems involved in the von Hippel-Lindau disease. (B) Different growth pattern of hemangioblastomas depending on location. (C) Parallel growth pattern of cysts and renal cell carcinoma.
The disease has a prevalence of 2–3 per 100 000 and an estimated incidence between 1 of 36 000 and 1 of 52 000 live births.1,4 All of the mutation carriers usually develop the clinical features by age 65 years.5 VHL disease was recognized as a specific syndrome in the early 1900s;6–8 however, Latif identified the VHL gene on the short arm of chromosome 3 (3p25-26) only in 1993.9 The VHL gene encodes for the VHL protein, which is primarily responsible for hypoxia-inducible factor 1, alpha (HIF1A) degradation in normoxic conditions.9 VHL protein has a fundamental role in neoangiogenesis, and VHL disease is emerging as an extraordinary model of tumor development and progression. Diagnosis is easier now than it was in the past, mainly because of genetic testing for VHL gene mutations. The spectrum of VHL alterations comprises point mutations in 80% of cases and large deletions in 20%.10,11
Several prospective and retrospective studies have been performed to clarify the natural history of specific lesions characterizing VHL disease.12–15 However, no studies about the entire disease process and comprehensive patient follow-up have been published to date. Moreover, the disabilities caused by VHL disease have never been clearly measured or discussed.
In order to better address these issues and to understand what VHL disease entails for a patient diagnosed with a positive molecular test, we conducted a prospective analysis of 128 VHL participants and followed them up for 12 years. In our attempt to define the natural history and evolution of VHL disease, we also assessed any genotype-phenotype correlation and surgical timing for each organ involved with VHL, as they both might affect the course of the disease. Moreover, we focused on the participants' disabilities, which can progressively impair their quality of life. These data are of utmost importance for tailoring the follow-up schedule, which is performed every year at our institution and includes cerebral, spinal, and abdominal nuclear MRI, audiometric testing, wide-field fundus examination with fluorescein angiography (performed in cases of suspicious lesions), and measurement of urinary metanephrine and normetanephrine levels.
Materials and Methods
Study Population
A group of 128 participants affected by VHL syndrome was followed up by a multispecialist team in Padova, Italy. The VHL-Padova Network, founded in 1996, is currently the Italian national reference for patients affected by VHL disease. This group includes specialists in endocrinology, neurosurgery, urology, ophthalmology, otosurgery, general surgery, and molecular genetics. All VHL-affected patients who were followed up in Padova since 1996 were included in this study. This group of 128 VHL participants has been monitored for about 12 years, with an average follow-up of 45 months.
In general, all patients with clinical suspicion of VHL disease and all relatives of VHL probands were initially referred for genetic counseling. After a careful clinical history and physical examination, DNA analysis of the VHL gene represented the first step in the diagnostic path.
All participants gave their written consent for all of the studies, including the molecular analysis and the collection and management of data. The consent was approved by the ethical committee of the I.O.V. (Istituto Oncologico Veneto of Padova), which was notified about the present study.
Molecular Analysis
Genomic DNA was extracted from peripheral blood using the QIAmp DNA Blood Mini Kit (Qiagen) according to the manufacturer's instructions. Mutation scanning of the VHL gene, for identification of point- or small-size mutations, was conducted on the entire coding sequence and intron-exon boundaries by PCR amplification, denaturing high-performance liquid chromatography, and bidirectional direct sequencing.16 Gross deletion analysis was performed using Southern blot, quantitative real-time PCR, and multiple ligation-dependent probe amplification method (MLPA p016 kit, MRC-Holland).17 Nucleotide numbering and variant naming followed nomenclature recommendations of the Human Genome Variation Society.18
Clinical Assessment
Clinical evaluation of individuals found to carry a VHL gene mutation included cerebral, spinal, and abdominal nuclear MRI (petrous bone CT scan was performed in selected cases when an ELST tumor was suspected), audiometric testing, wide-field fundus examination with fluorescein angiography in case of suspicious lesions, and measurement of urinary metanephrine and normetanephrine levels to screen for pheochromocytoma.3,12
All clinical, biochemical, genetic, and imaging data were included in a database that had been specifically developed to allow careful and systematic management of VHL patients.
Because the literature does not provide any specific measure for identifying the complex disabilities due to VHL disease, we selected some disability-descriptors for each target organ that are inversely related to the function of the organ itself. Hence, we prospectively monitored the impairment of each organ, measuring the disability descriptors over time.
We selected discrete variables because we judged them to be more suitable for assessing disability than continuous variables. For example, we initially considered the estimated glomerular filtration rate for the kidney. However, a participant with a reduced estimated glomerular filtration rate due to having undergone resective kidney surgery (and not having to undergo dialysis), did not have any specific disability affecting the quality of life. For this reason, we selected the need for dialysis as the descriptor of disability. Similarly, we think the need for adrenal or pancreatic substitutive therapy represents the best parameter for assessing the impairment of adrenal and pancreatic glands; in the same manner, blindness, hypoacusia, and the presence of neurological sensory or motor impairment can describe the relevant dysfunction of retina, endolymphatic sac, and CNS, respectively. Of course, the parameters we selected do not have the same relevance; for example, blindness obviously determines a dramatically worse disability than the need for pancreatic substitutive therapy. For this reason— and to illustrate the varying impact of organ involvement—we decided to distinguish disabilities by specific organs. This decision ruled out the option of using questionnaires to assess quality of life, which could certainly describe general well-being but not the impact of specific organ impairment on the quality of life itself. Karnofsky performance status (KPS) was used to quantify general well-being and activities of daily life.
Mortality of the group of VHL participants was compared with the general mortality rate of the Italian population in the age range of 25 and 65 years (as reported by the Italian National Institute for Statistics in 2008).
Statistical Analysis
Prevalence ratios, odds ratios, Poisson's distribution, analysis of variance, Kaplan-Meier analysis, and Wilcoxon test were used for statistical analysis. Statistical analysis was performed using R software (R Core Team, 2015, version 3.2.0).
Results
General Findings
The incidence, age at onset, and time of progression of organ lesions are shown in Table 1. The average onset intervals ranged from 25 to 44 years, while the median was 24 to 47 years.
Table 1.
Incidence, age of onset, and progression of von Hippel-Lindau disease-related lesions
| Lesion | Number of Patients (%) | Mean Age of Appearance, y | Median Age of Appearance, y | SD of Appearance, y | Interval Appearance–1st Evolution, y |
Interval 1st–2nd Evolution, y |
Interval 2nd–3rd Evolution, y |
Interval 3rd–4th Evolution, y |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Cases | Median | Number of Cases | Median | Number of Cases | Median | Number of Cases | Median | |||||
| Cerebellar hemangioblastomas | 77 (60) | 30 | 27 | 11.77 | 49 | 3.5 | 28 | 3.5 | 14 | 2 | 9 | 3 |
| Brainstem hemangioblastomas | 21 (16) | 30 | 28 | 10.87 | 10 | 7 | 5 | 1.5 | ||||
| Spinal hemangioblastomas | 76 (59) | 32 | 29 | 12.01 | 26 | 5 | 13 | 2 | 5 | 1 | ||
| Supratentorial hemangioblastomas | 15 (12) | 32 | 30 | 9.65 | 2 | 3 | ||||||
| Cystic renal lesions | 54 (42) | 37 | 32 | 12.13 | 32 | 7 | 12 | 1.5 | 4 | 4 | ||
| Renal cell carcinomas | 79 (62) | 34 | 31 | 11.39 | 22 | 8 | 12 | 1.5 | 2 | 1 | ||
| Cystic pancreatic lesions | 74 (58) | 33 | 30 | 10.91 | 36 | 5 | 14 | 3 | 4 | 3 | ||
| Pancreatic endocrine tumors | 26 (20) | 34 | 32 | 10.29 | 6 | 9 | ||||||
| Pheochromocytomas | 39 (30) | 29 | 29 | 15.49 | 5 | 9 | ||||||
| Retinal hemangiomas | 74 (58) | 29 | 25 | 14.20 | 29 | 7 | 4 | 4 | ||||
| Hypoacusia | 14 (11) | 42 | 44 | 11.5 | 4 | 2 | ||||||
| Endolymphatic sac tumors | 3 (2) | 28 | 24 | 8.33 | 3 | 2.8 | ||||||
| Epididymal cystadenomas | 12 (9) | 26 | 24 | 10.27 | 4 | 6 | ||||||
| Ovarian cysts | 11 (8) | 28 | 27 | 8.35 | ||||||||
Abbreviations: SD, standard deviation; y, years.
Retinal capillary hemangiomas and epididymal cysts were the earliest tumors to appear. Cerebellar and brainstem hemangioblastomas, pheochromocytomas, ovarian cysts, pancreatic cysts, and spinal and supratentorial hemangioblastomas were more delayed, whereas clear cell renal carcinomas, pancreatic neuroendocrine tumors, and inner-ear lesions were usually the last.
Natural History of Each Organ Disease
We analyzed the progression of disease in each organ since the first appearance. We considered “disease progression” and “evolution” to define the manifestation of a new lesion and the growth of an already-known lesion, respectively. Therefore, we were able to perform Kaplan-Meier analysis to show the loss-of-steadiness for CNS, kidney, and pancreas. The median survival value was used to define the time to progression as time is a non-normal distributed variable (Table 1).
We studied the progression of each lesion and compared both the evolution of the same lesion (hemangioblastoma) in different locations and the natural history of different lesions (cysts and solid tumors) in the same organ (results are shown in Fig. 2).
We observed that the median and the mean ages for appearance of cerebellar hemangioblastomas were 30 and 27 years, respectively, as reported in the literature.5 They maintained a constant pattern of progression, with a median time of 3.5 years between onset and subsequent progressions. The pattern of progression was different for brainstem hemangioblastomas. Although their initial evolution occurs after about 7 years, the subsequent worsening is rather fast (median time: 1.5 y). The median progression-free survival for spinal hemangioblastomas progressively halves during their natural history. Subsequent disease progressions occur at medians of 5, 2, and 1 years after the onset, respectively. So, each progression-free survival time between two progressions lasts half time compared to the previous progression-free period. It was not possible to calculate a median evolution time for supratentorial hemangioblastomas because of the low number of cases. Therefore, the same tumor may have different growth patterns depending on its location.
Renal cystic lesions usually appear at the median age of 32 years (mean age: 37 y). They show a first increase in number or volume after approximately 7 years, but a second progression occurs after another 1 to 2 years. The median age of appearance for renal clear cell carcinoma is 31 years (mean age: 34 y); the first progression takes place after 7 to 8 years and the second after another 1 to 2 years. Despite their claimed different biological nature, renal cysts and carcinomas share an analogous progression pattern and maintain a parallel rhythm of growth.
Pancreatic cysts appear at the median age of 30 years (mean age: 33 y). They show their first worsening after a median of 5 years and their second after 3 years. Neuroendocrine tumors first emerge at the median age of 32 years (mean age: 34 y), with a progression-free survival of 9 years. It is not possible to identify another time interval, most likely because the lesions are removed after their first occurrence or because their evolution rates are consistent, and our follow up is not suitable for this purpose.
It was not possible to determine the growth pattern of pheochromocytomas because they are usually removed as soon as they are detected. Only 2 of 38 participants affected by nonsecreting pheochromocytoma were treated with alpha blockers and thus avoided surgery.
Retinal angiomatosis first develops at the median age of 25 years (mean age: 29 y). Nine participants presented with unilateral blindness and 3 with bilateral blindness. Multiple retinal capillary hemangiomas represent a broad group of lesions that can merge during progression. For this reason, we could only observe their mean and median ages of appearance without any precise description of their evolution through time. In the present series, all 28 participants who received laser treatment retained normal central vision. Photocoagulation was not amenable for 3 participants who underwent surgical procedures and obtained a variable level of visual function. In one participant, the presence of bilateral taut and long-standing traction retinal detachment prevented any medical or surgical approach and led to blindness.
The onset of ELSTs occurs at a median age of 24 years (mean age: 28 y). These tumors are likely to progress 2.8 years after their diagnosis, showing either an increase in volume or a worsening of auditory function. Interestingly, although we detected sensorineural hearing loss in 14 participants, imaging was positive for ELST in only 3 cases. As already reported in the literature, audiometric abnormalities in VHL patients without any MRI-detectable lesion could be due to microscopic ELST.24 For this reason, periodic clinical and MRI surveillance is required.
Ovarian cysts are common findings in the general population, while epididymal cystadenoma is a rare benign neoplasm that is associated with VHL in more than one-third of cases reported in the literature.13 Epididymal cystadenomas first appear at a median age of 24 years (mean age: 26 y). Existing data are not sufficient to describe the development of these lesions statistically in VHL patients.
Genotype-phenotype Correlation
We analyzed the influence of sex, type of VHL gene mutation, and functional domain of the mutation (once considered the effect of age) on the number of organs involved with VHL lesions. Supplementary material, Table S1 shows the VHL gene mutations and functional domains of the mutations. We modeled the number of involved organs through a Poisson log linear model (Supplementary material, Table S2). However, the effects of these variables are not significant for predicting the number of involved organs. As expected, age has a significant negative effect (Supplementary material, Table S3, P < .01).
Surgery Timing
Mean and median ages at first surgery were calculated for the main organs (Table 2). VHL patients usually undergo several surgeries during their lifetime. In particular, pheochromocytoma (median age: 25 y) and CNS hemangioblastomas (median age: 25 y) require surgery earlier than renal cancer (median age: 36.5 y; P = .014 and P = .003, respectively). Surgery for pancreatic neoplasms is usually required later (median age: 31 y) compared with pheochromocytoma (P = .043). The Wilcoxon test was used to compare median ages.
Table 2.
Timing for surgery
| Number of Cases | Mean Age, y | Median Age, y | Minimum Age, y | Maximum Age, y | 25th Percentile | 75th Percentile | Standard Deviation | |
|---|---|---|---|---|---|---|---|---|
| Adrenal gland | 30 | 27.3 | 25 | 9 | 59 | 15 | 35 | 14.87 |
| CNS | 46 | 27.8 | 25 | 9 | 51 | 21 | 33 | 10.67 |
| Kidney | 24 | 36.5 | 31.5 | 21 | 62 | 25 | 47 | 12.9 |
| Pancreas | 13 | 34.5 | 31 | 24 | 58 | 26 | 38 | 11.2 |
Abbreviation: y, years.
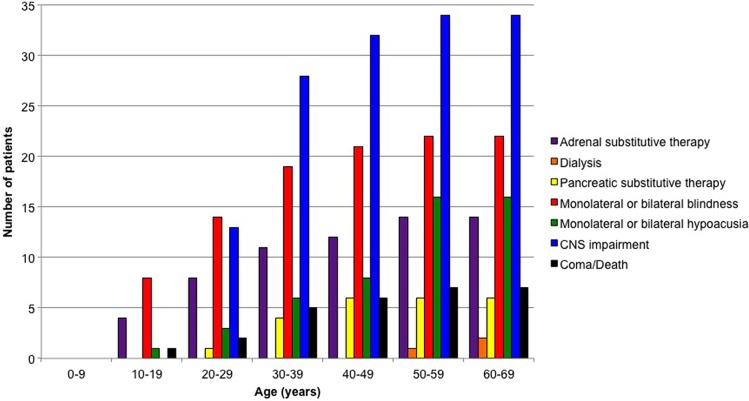
A cumulative incidence of disability was obtained (Fig. 3). The analysis showed that the major disabilities were those related to the CNS (34 participants, 26.5%), followed by impairment of visual (21 participants, 16.4%), acoustic (16 participants, 12.5%), adrenergic (14 participants, 10.9%), pancreatic (6 participants, 4.6%), and renal (2 participants, 1.5%) functions. We reported one case of vegetative status (0.7%) after an operation for cerebellar hemangioblastoma. Seven deaths occurred, due either to postoperative medical complications (5 cases) or metastases from renal cell cancer (1 case). In one case, the cause of death was not clear. Including these patients among the group with CNS disabilities increased the percentage of impaired participants to 28%. Overall, the average KPS of the 128 participants was 80% at the end of the follow-up period.
Fig. 3.
Cumulative incidence of disability related to each involved organ.
None of the variables (age, type of VHL mutation, functional domain mutation) was related to the number and type of disability. Conversely, only age was found to be significantly associated with the appearance of the first disability (P < .01), while sex (P = .73), type of mutation (P = .34), and functional domain mutation (P = .33) were not predictive.
Mortality
Six of our 128 participants died during the follow-up period. In order to assess the influence of VHL disease on life expectancy, we compared the mortality rates of the affected participants between the ages of 25 and 64 years (MR25-64) with those of the corresponding general population. The MR25-64 of our VHL population (55.36 × 103) was significantly higher than the MR25-64 of the Italian population in the year 2008, which was 1.469 × 103 (P = .0001).
Discussion
General Findings and Natural History of the Disease in Each Organ
In general, the frequencies of the lesions and the age of onset are consistent with other published studies;19–24 however, the present study adds data on disease progression. In particular, cerebellar hemangioblastomas progress constantly with a 3.5-year median evolution time. Conversely, brainstem hemangioblastomas first progress after 7 years and subsequently tend to evolve quickly with a median time of 1.5 years. The median time for subsequent evolutions of spinal hemangioblastomas decreases progressively over time. Both renal cysts and carcinomas have similar progression rates, with the first progression after approximately 7 years and following evolutions after about 1 to 2 years.
Follow up Plan for von Hippel-Lindau Patients
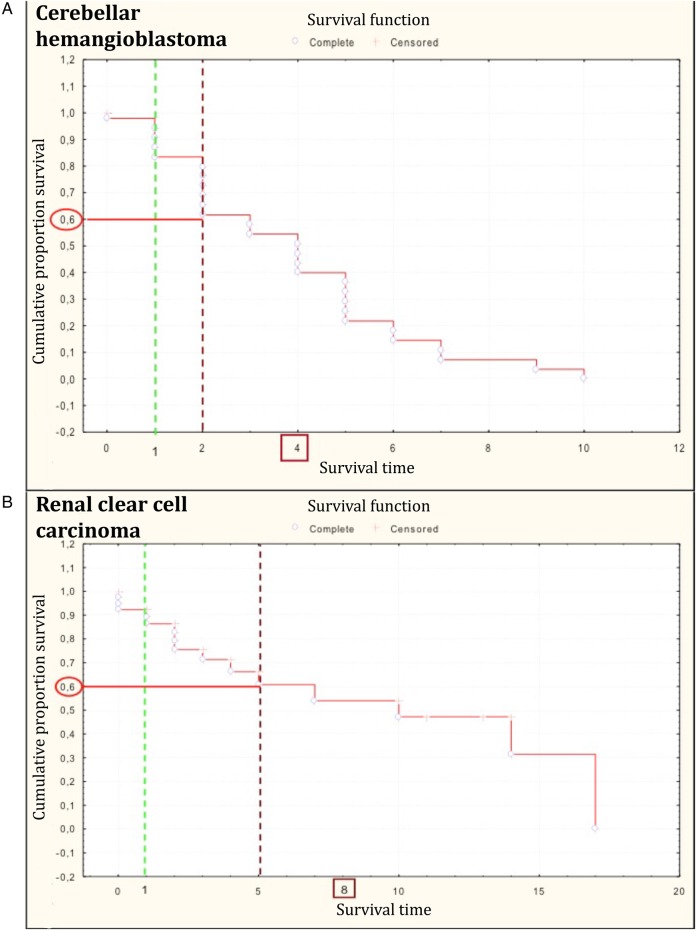
VHL disease has a variable expression, and onset times of the lesions overlap. As a consequence, designing a proper follow-up program is challenging. To this purpose, we compared the median evolution time of lesions with the annual follow-up schedule adopted at our institution (Fig. 4).
Fig. 4.
Kaplan-Meier graphs showing the stability-loss of (A) cerebellar hemangioblastomas and (B) renal cell carcinomas due to an increase in both volume and number. Green dotted line: first time point used in current follow-up schedule. Red dotted line: delayed first time point for follow up.
An evolution of a cerebellar hemangioblastoma occurs in 4 years in 50% of the VHL-patient population. This interval is much longer than the annual deadline established by the current follow-up protocol.25 However, if we hypothesized postponement of the first time point follow-up 2 years after the appearance of the hemangioblastoma, we would delay the diagnosis of evolution for almost 20% of patients.
A similar analysis was performed for the renal cell carcinoma evolution pattern. Also in this case, any attempt to postpone the first follow-up to better match the clinical control schedule with the natural evolution of the lesion resulted in the unacceptable risk of missing the evolution in a high percentage of patients. Moreover, a diagnostic delay for kidney carcinoma is particularly hazardous. In actuality, surgical treatment is usually planned depending on tumor size and not on clinical symptoms (as is the case for CNS hemangioblastomas).
According to our data, an annual check-up ensures correct, effective, and complete follow up of the disease.
Genotype-phenotype Correlation
The only known genotype-phenotype correlation in VHL disease is the association of pheochromocytoma (type 2 families) with missense mutations.26 Conversely, most type 1 families (not affected by pheochromocytoma) have deletions or premature termination mutations.27 Recently, Ong et al. pointed out the impact of DNA mutations on folding of the VHL protein and introduced the concept of functional domain.28 In the attempt to further explore the correlation between genotype and phenotype in VHL disease, we investigated the association between some variables (ie, age, sex, DNA mutation or deletion, and functional domain alteration), the number of affected organs, and the order of organ involvement. The number of involved organs is associated with age. However, it does not depend on sex, type of VHL gene mutation, or functional domain mutation. These data confirm the complexity of VHL disease, which is scarcely defined through a genotype-phenotype association.
Surgery Timing and Surgical Strategies
Minimally invasive resections (eg, adrenal sparing) are now preferred, especially for VHL patients who are likely to undergo recurring surgeries. CNS interventions are usually reserved only for symptomatic patients and are performed with microsurgical techniques.29 Therefore, it is possible to reduce both intraoperative risks and surgery- related disabilities without affecting the value of treatment. Early identification of small retinal capillary hemangiomas is mandatory for providing successful laser treatment and preventing retinal exudation and detachment. In cases in which the diagnosis of retinal lesions is delayed and laser photocoagulation is not technically feasible, more aggressive approaches (eg, vitrectomy and retinotomy) may eventually be required. If not treated, these retinal lesions lead to irreversible legal blindness.
Disability
There is a complete lack of information in the scientific literature about the impact of VHL disease on patients' everyday life. Several progressive diseases usually affect patients with VHL disease, who consequently undergo frequent surgeries. As a consequence of both disease and surgical interventions, the function of involved organs might be impaired and worsen the quality of life for these patients. Our aim was not to provide only a generic quantification of disability due to the disease itself but also to establish the impact of specific organ impairments on patient quality of life. Therefore, we prospectively focused on the disabilities affecting our study population during the follow-up period.
Although monocular and binocular blindness and impairment of adrenergic function may occur early in the life of a patient with VHL disease, the CNS is the most challenging system to deal with. In almost all cases, disabilities are most likely to occur during the third and the fourth decades of life, consistent with the onset of the disease. It is worth noting that dialysis was very uncommon in our population, probably because early diagnosis allowed performance of less-extensive resections.
Seven deaths and one case of vegetative status occurred during the follow-up period. Medical complications after surgical or neurosurgical procedures (5) and metastatic progression of renal cancer (1) were the recognizable causes of death. Notably, after an average follow-up period of 45 months, the median KPS of our participants was 90%, which demonstrated that VHL-affected patients can still carry on normal activities with only minor signs or symptoms despite the progression of disease.
Analysis of variance showed that DNA mutation and functional domain alterations influenced neither the number of disabilities nor their sequence. Moreover, although age is related to the first-appearing functional impairment, it is not predictive of the final number of disabilities. This is an important message to deliver to VHL-affected patients, who can expect a very likely evolution of their disease with age but not necessarily a worsening of their quality of life.
Mortality Rate
The comparison of the mortality rates between 25 and 64 years of age of affected patients and general population showed that, despite the outstanding improvements in diagnostic techniques, strict follow up, and advancements in medical and surgical treatments, VHL disease still significantly increases the mortality of the affected population.30
Conclusion
VHL is a multifaceted and challenging disease that requires strict follow-up. Besides the evolution of lesions in each organ, it is mandatory to consider the complexity of the whole disease. Patient's quality of life is affected by the interaction of all phenotypes of the disease. Functional impairment should be used to summarize the VHL syndrome's impact on the participant. Therefore, we suggest considering disability status as the measure for assessing outcome in this population.
Further studies are necessary to better address the natural history and functional implications of the most rare VHL lesions such as ELSTs and epididymal cystadenomas.
Supplementary Material
Funding
None declared.
Supplementary Material
Acknowledgments
Conflict of interest statement. The authors have declared no conflicts of interest.
References
- 1.Maher ER, Iselius L, Yates JR et al. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28(7):443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedrich CA. Genotype-phenotype correlation in von Hippel-Lindau syndrome. Hum Mol Genet. 2001;10(7):763–767. [DOI] [PubMed] [Google Scholar]
- 3.Lonser RR, Glenn G, Walther M et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. [DOI] [PubMed] [Google Scholar]
- 4.Neumann HP, Wiestler OD. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337(8749):1052–1054. [DOI] [PubMed] [Google Scholar]
- 5.Maher ER, Yates JR, Harries R et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77(283):1151–1163. [DOI] [PubMed] [Google Scholar]
- 6.Von Hippel E. Die anatomische Grundlage der von mir beschriebenen “sehr seltenen Erkrankung der Netzhaut”. Arch Ophtalmol. 1911;79:350–377. [Google Scholar]
- 7.Lindau A. Studien über Kleinhirncysten. APMIS. 1926;3(suppl I):1–128. [Google Scholar]
- 8.Lindau A. Zur Frage der Angiomatosis Retinae und ihrer Hirnkomplikationen. Acta Ophthalmol Scand. 1927;4:193–226. [Google Scholar]
- 9.Latif F, Tory K, Gnarra J et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. [DOI] [PubMed] [Google Scholar]
- 10.Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Cancer. 2002;2(9):673–681. [DOI] [PubMed] [Google Scholar]
- 11.Pugh CW, Ratcliffe PJ. The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis. Semin Cancer Biol. 2003;13(1):83–89. [DOI] [PubMed] [Google Scholar]
- 12.Hes FJ, van der Luijt RB, Lips CJM. Clinical management of Von Hippel-Lindau (VHL) disease. Neth J Med 2001;59(5):225–234. [DOI] [PubMed] [Google Scholar]
- 13.Odrzywolski KJ, Mukhopadhyay S. Papillary cystadenoma of the epididymis. Arch Pathol Lab Med. 2010;134(4):630–633. [DOI] [PubMed] [Google Scholar]
- 14.Dollfus H, Massin P, Taupin P et al. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Invest Ophthalmol Vis Sci. 2002;43(9):3067–3074. [PubMed] [Google Scholar]
- 15.Hammel PR, Vilgrain V, Terris B et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119(4):1087–1095. [DOI] [PubMed] [Google Scholar]
- 16.Martella M, Salviati L, Casarin A et al. Molecular analysis of two uncharacterized sequence variants of the VHL gene. J Hum Genet. 2006;51(11):964–968. [DOI] [PubMed] [Google Scholar]
- 17.Casarin A, Martella M, Polli R et al. Molecular characterization of large deletions in the von Hippel-Lindau gene by quantitative real-time PCR. Mol Diag Ther. 2006;10(4):243–249. [DOI] [PubMed] [Google Scholar]
- 18.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15(1):7–12. [DOI] [PubMed] [Google Scholar]
- 19.Park D, Zhuang Z, Chen L et al. Von Hippel-Lindau disease associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4(2):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomè F, Colombeau P, Fermeaux V et al. Renal lesions in von Hippel-Lindau disease: the benign, the malignant, the unknown. Eur Urol. 1998;34(5):383–392. [DOI] [PubMed] [Google Scholar]
- 21.Libutti SK, Choyke PL, Richard A et al. Clinical and genetic analysis of patients with pancreatic neuroendocrine tumors associated with von Hippel-Lindau disease. Surgery. 2000;128(6):1023–1031. [DOI] [PubMed] [Google Scholar]
- 22.Opocher G, Conton P, Schiavi F et al. Pheochromocytoma in von Hippel-Lindau disease and neurofibromatosis type 1. Fam Cancer. 2005;4(1):13–16. [DOI] [PubMed] [Google Scholar]
- 23.Wong WT, Agròn E, Coleman HR et al. Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel-Lindau disease. Ophthalmology. 2008;115(1):181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulsen ML, Gimsing S, Kosteljanetz M et al. von Hippel-Lindau disease: surveillance strategy for endolymphatic sac tumors. Genet Med. 2011;13(12):1032–1041. [DOI] [PubMed] [Google Scholar]
- 25.Ammerman JM, Lonser RR, Dambrosia J et al. Long-term natural history of hemangioblastomas in von Hippel-Lindau disease implications for treatment. J Neurosurg. 2006;105(2):248–255. [DOI] [PubMed] [Google Scholar]
- 26.Gimenez-Roqueplo A, Lehnert H, Mannelli M et al. Phaeochromocytoma, new genes and screening strategies. Clin Endocrinol (Oxf). 2006;65(6):699–705. [DOI] [PubMed] [Google Scholar]
- 27.Crossey PA, Richards FM, Foster K et al. Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype. Hum Mol Genet. 1994;3(8):1303–1308. [DOI] [PubMed] [Google Scholar]
- 28.Ong KR, Woodward ER, Killick P et al. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2007;28(2):143–149. [DOI] [PubMed] [Google Scholar]
- 29.Pavesi G, Feletti A, Berlucchi S et al. Neurosurgical treatment of von Hippel-Lindau-associated hemangioblastomas: benefits, risks and outcome. J Neurosurg Sci. 2008;52(2):29–36. [PubMed] [Google Scholar]
- 30.ISTAT Rapporto annuale 2008. http://www3.istat.it/dati/catalogo/20090526_00/rapporto_annuale_2008.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.