Abstract
Background
DOTATATE-based radionuclides have added new options in the diagnosis and treatment of meningiomas; however, a reliable predictor of tumor growth has still not been established.
Methods
We analyzed 64 meningiomas imaged with 68Ga-DOTATATE PET. Tumor growth rates were calculated by volumetric analysis of sequential MRI scans. Maximums of standardized uptake values (SUVmax) were correlated with tumor growth and covariates.
Results
World Health Organization (WHO) grades I and II meningiomas showed a correlation of SUVmax and tumor growth rate (meningiomas limited to the intracranial compartment: r = 0.757, P < .001, and transosseous growing meningiomas: r = 0.819, P = .024). SUVmax was significantly higher and the slope of the linear regression significantly steeper in transosseous compared with intracranial meningiomas (both P < .001). The association remained significant in multivariate analysis, and the prediction of tumor growth rate was independent of WHO grade. Anaplastic meningiomas showed no significant correlation of SUVmax and tumor growth.
Conclusions
68Ga-DOTATATE PET is a reliable predictor of tumor growth in WHO grades I and II meningiomas and provides additional information to conventional cross-sectional imaging modalities. Hence, 68Ga-DOTATATE PET can assist in selecting the time point for treatment initiation. Furthermore, meningiomas with fast tumor growth and transosseous expansion elicit the highest DOTATATE binding; therefore, they might be especially suited for DOTATATE-based therapy.
Keywords: DOTATATE-PET, DOTATOC-PET, meningioma, SSTR2
Meningiomas, with an annual incidence of 2.3 per 100 000, are the most common primary CNS tumors and comprise nearly 34% of all CNS tumors.1–3 According to the World Health Organization (WHO), ∼94% of meningiomas are classified as benign (grade I), 5%–7% as atypical (grade II), and 1%–3% as anaplastic (grade III).4–7 WHO grade I tumors have a good prognosis, with a recurrence rate of ∼5% after 5 years following gross total resection. WHO grades II and III meningiomas, however, remain challenging to treat and show 5-year overall recurrence rates of ∼40% and 80%, respectively.1,2 Multiple simultaneous meningiomas are recognized, especially in the context of neurofibromatosis II, and usually display a uniform histology.8,9
The cross-sectional imaging modalities CT and MRI are commonly applied for the identification and assessment of meningiomas.10 In meningioma management, the assessment of tumor growth rate (TGR) is critical to select an appropriate time point for therapy initiation and type of treatment, with surgery as the mainstay of primary treatment. However, surgery is challenging in meningiomas located in the skull base region, due to frequent transosseous growth and the close relationship to cranial nerves and other delicate anatomical structures.11
Positron emission tomography with specific radiotracers is an imaging modality that adds molecular information. As meningiomas generally express somatostatin receptor subtype 2 (SSTR2),12 octreotide-based radiotracers with high affinity to these receptors are suitable tumor-imaging tracers.13 Primarily, gallium-68 (68Ga)–labeled dodecanetetraacetic acid–tyrosine-3-octreotate (DOTATATE) and DOTA-(Tyr3)-octreotide (DOTATOC) tracers are used to determine the extent of SSTR2-positive meningiomas.14–18 Using 68Ga-DOTATOC, Afshar-Oromieh and colleagues17,19 showed that PET has superior sensitivity in meningioma detection. Rachinger et al18 demonstrated better tumor-to-nontumor discrimination of PET/CT over contrast-enhanced MRI. Furthermore, 68Ga-DOTATOC PET/CT information may strongly complement MRI and CT for intensity-modulated radiation therapy planning in patients with complex meningiomas of the skull base region.20
In recent years, initial studies have described use of yttrium-90 or lutetium-177 radiolabeled somatostatin analogs to treat SSTR2-positive meningiomas. Bartolomei and colleagues21 demonstrated disease stabilization in 66% of patients with meningiomas after peptide receptor radionuclide therapy (PRRT), which was confirmed in a recent study that reported a high percentage of patients with disease stabilization.22 Accordingly, binding of the therapeutic radionuclide correlates with the pretherapeutic standardized uptake value (SUV) in PET, and ultimately with SSTR2 expression of meningiomas.18,23 However, these results are conflicting, since SSTR2 expression itself is supposed to lead to growth inhibition in neoplastic and non-neoplastic tissue.24,25 Alternatively, binding may reflect only a more benign tumor behavior rather than beneficial response to therapy.
We aimed to study the SUV reflecting SSTR2 expression as measured by 68Ga-DOTATATE PET and its correlation to tumor growth in subsequent MR scanning. We hypothesized that SSTR2 uptake in 68Ga-DOTATATE PET not only accurately delineates meningiomas as previously reported but also provides critical information on tumor behavior for further treatment planning.
Materials and Methods
Patient Population
All patients who received a 68Ga-DOTATATE PET scan for meningioma imaging from January 2011 to December 2014 (n = 45) were included in this study. Twenty-two patients were excluded because of insufficient MRI follow-up data, cerebral radiation therapy within the last 2 years, or incomplete histological workup. In patients with multiple meningiomas, we assessed up to a maximum of 5 separate lesions and analyzed the tumors with the highest volumes, resulting in 64 meningiomas from 23 patients. The study was approved by the local ethics committee (KEK-ZH 2014-0605).
PET and MR Data Acquisition
PET imaging was performed on a full-ring PET/CT system (Discovery VCT, GE Healthcare). For attenuation correction purposes, a low-dose CT scan of the head was initially performed (10 mA, 120 kV, pitch = 0.984:1, collimation = 64 × 0.625 mm). The PET scan was initiated directly after the injection of 150 MBq 68Ga-DOTATATE, and emission data were acquired over 60 min (axial field of view = 153 mm covering the entire brain with one bed position). Emission data were corrected for randoms, dead time, scatter, and attenuation and iteratively reconstructed (3 iterations, 18 subsets) using CT.
We selected one brain MRI examination before 68Ga-DOTATATE PET and another afterwards, resulting in at least 4 months of temporal difference between both images (median 11, minimum 4, maximum 20 mo). All MRI examinations included axial T1- and T2-weighted images and axial, coronal, and sagittal T1-weighted images after the injection of gadolinium contrast media (T1Gd).
PET and MR Image Analysis
A board-certified nuclear medicine physician analyzed the PET images using PMOD v3.4 and measured the maximal SUV (SUVmax) in all meningiomas. For the quantitative evaluation of the PET data, frames acquired between 40 and 60 min were averaged. In the PET dataset, volumes of interest were adjusted in 3 planes so that the entire meningioma was included.
A second independent reader, blinded to the SUV, determined TGR of meningiomas in each patient. Meningiomas “en plaque” were not assessed, as they are prone to measurement inaccuracies. As all other intracranial meningiomas corresponded approximately to a rotational ellipsoid, volume was calculated by multiplying the product of the 3 diameters by 0.523 (according to the formula 4/3*π*a*b*c, where a, b, c are the lengths of each radius). The volumes of all transosseous meningiomas were determined by 3D volumetric measurements (contour outline on all axial slices) on T1Gd images. Finally, the monthly growth rate was calculated by dividing the percentage change in size by the numbers of months between the 2 examinations.
Grading and Histological Assessment
Histological grading according to WHO classification was performed in at least one of the meningiomas by a board-certified neuropathologist blinded to clinical and radiographic details.4 Median time interval between histology and PET imaging was 5.9 months. Since the majority of simultaneously occurring multiple meningiomas show a uniform histology,8,9 all meningiomas from a given patient, even those that were not surgically sampled, were assigned the same WHO grade. To assess for potential bias when analyzing multiple meningiomas per patient, an additional evaluation with only one biopsied meningioma per patient was performed. For this additional assessment, we selected the largest meningioma when histology of multiple meningiomas was available.
Concomitant Therapy
We assessed concomitant therapy during MR follow-up; 5 patients received focal therapy (all WHO grade I or II), including stereotactic radiation (protons or photons) and embolization (total of 6/57 meningiomas), and 1 patient with WHO grade III meningioma received systemic therapy with interferon alpha 2a. As all treatments aimed at tumor volume reduction or growth inhibition, we pooled treatments in statistical analysis.
Statistical Analysis
Statistical analyses were performed using SPSS v21. We calculated Pearson's r and linear regression analysis, with values of R2 below 0.4 considered weak, between 0.4 and 0.6 moderate, and above 0.6 strong. We calculated significance of differences in regression slopes using the Chow test. In multivariate linear regression, SUVmax, age, sex, WHO grade, time between histology and PET, duration of follow-up, initial volume, and concomitant therapy during follow-up were set as independent variables, and TGR as the dependent variable. Differences in mean values were tested with Student's t-test. Significance was defined as P < .05.
Results
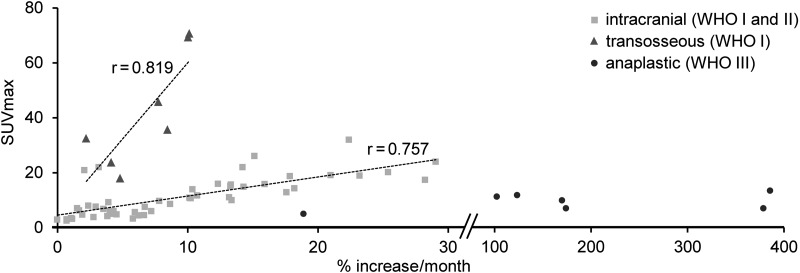
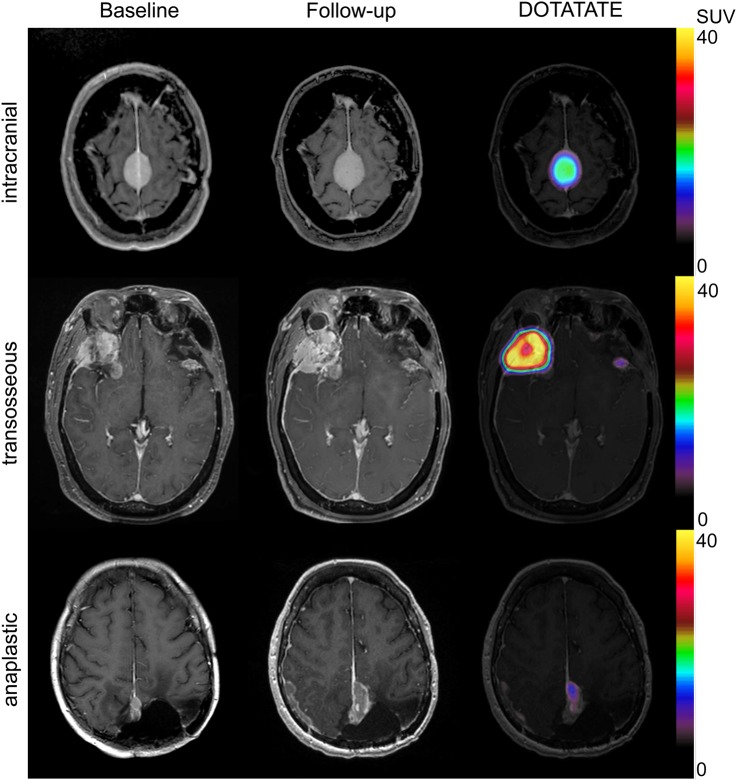
Sixty-four meningiomas in 23 consecutive patients were analyzed (29 WHO grade I, 28 grade II, and 7 grade III). TGR in relation to SUVmax of 68Ga-DOTATATE PET is summarized in Fig. 1. Meningiomas were separated into 3 different clusters: transosseous growth, intracranial meningiomas of WHO grades I and II, and meningiomas of WHO grade III, which were analyzed separately. Examples of PET and MR imaging of meningiomas are shown in Fig. 2.
Fig. 1.
Correlation between TGR and SUVmax. Meningiomas separated into 3 different clusters (intracranial growth, transosseous growth, and anaplastic).
Fig. 2.
Examples of intracranial, transosseous, and anaplastic meningiomas. From left to right: baseline MRI, follow-up MRI, and 68Ga-DOTATATE PET.
Eighteen patients with 50 meningiomas of WHO grades I and II had tumor growth limited to the intracranial compartment. TGR of these meningiomas strongly correlated with SUVmax of 68Ga-DOTATATE PET (r = 0.757, P < .001) and SUVmax correlated weakly with initial tumor volume in intracranial meningiomas (r = 0.316, P = .025) (Fig. 1). In the patient analysis of biopsied meningiomas, correlation did not change (r = 0.907, P < .001). Both TGR and SUVmax did not differ in meningiomas with concomitant therapy during follow-up (n = 4) versus nontreated meningiomas (n = 46) (TGR: 9.8 ± 6.4 vs 9.8 ± 8.1, P = .998 and SUVmax: 10.5 ± 3.4 vs 11.2 ± 7.4, P = .728). Correlation coefficient of TGR and SUVmax was stable when calculated for medicated meningiomas only but, because of low patient numbers, not to a significant degree (r = 0.770, P = .23). In multivariate linear regression, SUVmax remained a strong predictor for TGR (β = 0.863, P < .001; only covariate: initial tumor volume β = –0.335, P < .001, R2= 0.7). Patients with intracranial meningiomas are summarized in Table 1.
Table 1.
Intracranial meningiomas
| ID | Age (y) | Sex | WHO Grade | Lesion with Histology | Time between MRI Scans (mo) | Initial Volume (mm3) | Growth Rate (%) | SUVmax (g/mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 42.0 | Male | I | x | 16 | 5726 | 14.58 | 22 |
| 3107 | 15.51 | 26.1 | ||||||
| 460 | 8.92 | 8.6 | ||||||
| 431 | 22.88 | 32 | ||||||
| 2a | 29.6 | Female | I/II | 10 | 2561 | 1.87 | 6.3 | |
| I/II | 2312 | 3.77 | 6.8 | |||||
| II | x | 1689 | 21.51 | 19.1 | ||||
| I/II | 952 | 13.79 | 10 | |||||
| I/II | 795 | 7.51 | 6 | |||||
| 3b | 44.3 | Female | I | 11 | 759 | 10.47 | 10.9 | |
| 544 | 13.67 | 15.1 | ||||||
| 483 | 11.09 | 11.7 | ||||||
| 4 | 68.2 | Male | I | 11 | 4142 | 14.71 | 14.9 | |
| 435 | 13.58 | 11.1 | ||||||
| x | 345 | 10.58 | 10.7 | |||||
| x | 251 | 4.26 | 6 | |||||
| 5 | 49.7 | Female | I | 8 | 1841 | 12.76 | 16 | |
| x | 1054 | 18.38 | 18.8 | |||||
| 748 | 26.05 | 20.2 | ||||||
| 483 | 16.40 | 15.9 | ||||||
| 460 | 29.76 | 24 | ||||||
| 6 | 45.2 | Female | I | x | 5 | 8186 | 4.33 | 9.3 |
| 7 | 42.5 | Female | II | x | 6 | 483 | 4.91 | 4.8 |
| 408 | 18.81 | 14.3 | ||||||
| 8 | 73.4 | Female | II | 12 | 259 | 6.05 | 3.3 | |
| 157 | 6.92 | 4.6 | ||||||
| x | 146 | 6.20 | 5.6 | |||||
| 94 | 6.50 | 4.5 | ||||||
| 9 | 62.0 | Female | II | 13 | 314 | 8.09 | 9.7 | |
| x | 167 | 4.53 | 6 | |||||
| 10 | 37.2 | Male | II | 20 | 1849 | 0.82 | 3 | |
| 1657 | 28.81 | 17.4 | ||||||
| x | 598 | 1.24 | 3.2 | |||||
| 366 | 0.81 | 2.6 | ||||||
| 362 | 1.16 | 3.5 | ||||||
| 11 | 28.4 | Female | II | x | 13 | 226 | 2.96 | 3.8 |
| x | 176 | 4.04 | 4.2 | |||||
| 75 | 4.48 | 5 | ||||||
| 12 | 7.0 | Male | II | x | 10 | 925 | 2.12 | 4.7 |
| 13 | 63.1 | Female | II | x | 7 | 1255 | 7.08 | 7.5 |
| 14 | 68.1 | Female | II | x | 6 | 316 | 1.83 | 7.1 |
| 774 | 18.18 | 12.9 | ||||||
| 15 | 52.2 | Female | II | 12 | 10 632 | 3.38 | 22 | |
| 1245 | 2.24 | 20.9 | ||||||
| x | 322 | 2.57 | 8 | |||||
| 16 | 75.1 | Male | II | 10 | 2457 | 10.72 | 14 | |
| x | 1809 | 3.18 | 7.6 | |||||
| 17 | 64.5 | Female | II | x | 11 | 63 | 0.16 | 2.9 |
| 18c | 68.7 | Male | II | 5 | 264 | 23.96 | 18.9 | |
| 188 | 13.91 | 15.7 |
aPatient with ID #2 had meningiomas with WHO grades I and II in histology (intracranial and transosseous); therefore, meningiomas without biopsy were not allocated to a specific WHO grade (as indicated by “I/II”).
bPatient with ID #3 had intracranial and transosseous meningiomas; the latter was operated on and histology was obtained.
cPatient with ID #18 received total resection of another meningioma from which histology was obtained.
Seven meningiomas from 5 patients showed transosseous growth into the skull base or temporal squama (all WHO grade I; 3 patients had meningiomas with transosseous growth as well as tumors limited to the intracranial compartment) (Table 2). SUVmax of these meningiomas were remarkably higher, with a mean SUVmax of 43.3 g/mL (range 18–70.7 g/mL) compared with a mean SUVmax of 11.2 g/mL (range 2.6–32.0 g/mL) for meningiomas without transosseous growth (P < .001). Additionally, the slope of the linear regression coefficient was steeper in meningiomas with transosseous growth compared with meningiomas restricted to the intracranial compartment (a = 5.5 and a = 0.70, respectively; P < .001). Again, TGR of transosseous meningiomas correlated significantly with SUVmax of 68Ga-DOTATATE PET (r = 0.819, P = .024) (Fig. 1). The correlation coefficient remained stable at a high level in per patient analysis of biopsied meningiomas, but because of low patient numbers, not to a significant degree (r = 0.789, P = .113). TGR and SUVmax were concordantly lower in transosseous meningiomas (n = 2) with treatment during follow-up versus nonmedicated meningiomas (n = 5) (TGR: 5.8 ± 4.4 vs 7.6 ± 2.9, P = .667 and SUVmax: 34.1 ± 2.7 vs 45.5 ± 24.6, P = .361). SUVmax did not correlate with initial tumor volume in these meningiomas (P = .251) and it was the only predictor of TGR in multivariate linear regression analysis (β = 0.818, P = .024, R2= 0.7).
Table 2.
Transosseous meningiomas
| ID | Age (y) | Sex | WHO Grade | Lesion with Histology | Time between MRI Scans (mo) | Initial Volume (mm3) | Growth Rate (%) | SUVmax (g/mL) |
|---|---|---|---|---|---|---|---|---|
| 1a | 42.0 | Male | I | x | 16 | 23 995 | 10.32 | 69.3 |
| 1271 | 7.98 | 45.8 | ||||||
| 1391 | 4.31 | 23.8 | ||||||
| 2a | 29.6 | Female | I | x | 10 | 941 | 10.42 | 70.7 |
| 3a | 44.3 | Female | I | x | 11 | 1640 | 5.06 | 18 |
| 19 | 46.5 | Female | I | x | 5 | 565 | 8.93 | 35.7 |
| 20 | 52.2 | Female | I | x | 4 | 7362 | 2.67 | 32.5 |
aThese patients elicited intracranial and transosseous meningiomas.
WHO grade III meningiomas showed no significant correlation between TGR and SUVmax (P = .518) (Fig. 1). The monthly growth was remarkably high, with relatively low SUVmax (Table 3).
Table 3.
Anaplastic meningiomas
| ID | Age (y) | Sex | WHO Grade | Lesion with Histology | Time between MRI Scans (mo) | Initial Volume (mm3) | Growth Rate (%) | SUVmax (g/mL) |
|---|---|---|---|---|---|---|---|---|
| 21 | 39.0 | Female | III | x | 7 | 31 777 | 19.48 | 5 |
| 22 | 78.2 | Female | III | x | 7 | 2921 | 104.42 | 10.7 |
| 23 | 61.8 | Female | III | 7 | 1486 | 126.02 | 11.2 | |
| x | 205 | 173.29 | 9.4 | |||||
| 377 | 392.60 | 12.8 | ||||||
| 78 | 177.53 | 6.6 | ||||||
| 59 | 385.33 | 6.6 |
Discussion
Our study provides convincing evidence that high expression of SSTR2 as measured by SUVmax in 68Ga-DOTATATE PET predicts faster growth in WHO grades I and II meningiomas, whereas WHO grade III meningiomas did not show an association of TGR with tracer binding. Meningiomas with transosseous growth elicited considerably higher 68Ga-DOTATATE binding.
These data are surprising, since SSTR2 expression itself is supposed to lead to growth inhibition in various neoplastic tissues.24,25 The (patho)physiological relevance of SSTR2 overexpression in meningiomas is not well understood,26 and growth stimulation in the presence of somatostatin and its analog octreotide has been reported.27 The inhibitory effect of somatostatin in meningiomas is further challenged by the disappointing results of current treatment trials using somatostatin analogs.28,29
The predictive value of TGR with 68Ga-DOTATATE PET may be especially informative in meningiomas adjacent to delicate structures such as cranial nerves or arteries. Specifically, TGR with 68Ga-DOTATATE PET may help in planning the optimal time point for surgery and for follow-up imaging. This is especially valuable for newly diagnosed meningiomas in such critical locations. SUVmax remains an independent predictor of TGR estimation in multivariate analysis, including tumor size and WHO grade, providing additional information to these estimates. Further studies have to assess the potential changes in management by the treating physician or the advisory tumor board in order to establish corresponding recommendations.
Our results also support further development of PRRT in meningiomas. As noted, Rachinger and colleagues showed a strong congruence of SUVmax in 68Ga-DOTATATE PET with SSTR2 expression in the histological preparations. In addition, 68Ga-DOTATATE PET SUVmax correlates with radionuclide uptake in PRRT in meningiomas.23 This information taken together with the physical properties of 177Lu or 90Y with a very limited maximal tissue penetration of the emitted β particles of 2 mm (maximum energy = 0.5 MeV) and 12 mm (max energy = 2.3 MeV), respectively, allows for radiation dose estimation and consequently high tumor radiation doses while minimizing the total radiation dose to normal brain parenchyma. As faster growing meningiomas have higher SUVmax, they will potentially also have higher radiation doses in PRRT. Accordingly, this treatment option might be particularly target oriented. Furthermore, transosseous meningiomas elicited considerably high DOTATATE binding; therefore, PRRT appears especially suited to complement surgery or external radiation therapy for skull base and spheno-orbito-maxillar meningiomas.
Finally, PET generates functional and morphological information that enhances treatment planning and dose calculation for intensity-modulated radiation therapy.20 Notably, PET morphometry facilitates more accurate delineation of skull base meningiomas compared with contrast-enhanced MRI alone.18
WHO grade III meningiomas showed no correlation between SUVmax and TGR. Furthermore, the monthly growth was remarkably high with relatively low SUVs. This observation implies advanced dedifferentiation of the meningioma cells and limits the accuracy of predicting the growth rate with 68Ga-DOTATATE PET. The clinical role of the other PET tracers, like the widely used 18F-fluorodeoxyglucose (FDG), in patients with dedifferentiated meningioma is still not clear. Arita and colleagues30 did not find any correlation between 18F-FDG uptake and WHO grading or tumor-doubling time. On the other hand, Lee et al31 reported that FDG uptake was a significant prognostic factor regarding tumor recurrence. Regarding 18F-fluoro-tyrosine and C11-methionine, markers of l-amino acid transport and protein synthesis, only small numbers of dedifferentiated meningiomas have been assessed in recently published studies,32,33 thus their value as biomarkers for TGR or prognosis has not yet been established. Importantly, WHO grade III meningiomas show morphological features on MRI which help to diagnose this entity and guide PET use.10 In meningiomas with an unclear tumor–brain interface, irregular tumor margins, heterogeneous contrast enhancement, and missing capsular enhancement, the probability of high-grade histology was 98%.34 However, the present study does not allow any conclusion on the effect of PRRT on grade III meningiomas, as dedifferentiated cells may react differently to this therapy.
The study design was retrospective, and therefore a selection bias cannot be ruled out. Another potential limitation is the relatively high percentage of patients with multiple meningiomas; at least one lesion from these patients was histologically evaluated, but not all lesions were subjected to histological assessment. Previous studies revealed that multiple meningiomas usually display a uniform histology, and anaplastic meningiomas (WHO grade III) were rare among these.9,35 Therefore, we analyzed meningiomas of WHO grades I and II together.
Standardized uptake value, reflecting the SSTR2 density measured by 68Ga-DOTATATE PET, is a reliable predictor of TGR in WHO grades I and II meningiomas. This finding underlines its clinical importance for surgical planning and radiation therapy, since meningiomas with high SUVmax are at risk for progression and require either local or systemic treatment. Our study suggests that patients with high SUVmax might benefit from PRRT treatment, not only regarding dosimetric considerations but also with respect to risk for progression. This holds especially true for transosseous meningiomas eliciting very high SSTR2 density. However, further studies are indicated to evaluate the therapeutic effect of PRRT in different histological types of meningiomas and according to their SSTR2 expression.
Funding
No funding was acquired for this work.
Conflict of interest statement. The authors declare no conflicts of interest.
References
- 1.Claus EB, Bondy ML, Schildkraut JM et al. . Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088–1095; discussion 1088–1095. [DOI] [PubMed] [Google Scholar]
- 2.Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99(3):393–405. [DOI] [PubMed] [Google Scholar]
- 3.CBTRUS. Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 (March 23, 2012 Revision). Hinsdale, IL: Central Brain Tumor Registry of the United States.
- 4.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5(12):1045–1054. [DOI] [PubMed] [Google Scholar]
- 5.Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23(4):E3. [DOI] [PubMed] [Google Scholar]
- 6.Perry AL, Scheithauer BW, Budka H et al. . Meningiomas. In: Louis DN, et al, ed. World Health Organization Classification of Tumors of the Central Nervous System. Lyon, France: IARC: IARC; 2007:164–172. [Google Scholar]
- 7.Kshettry VR, Ostrom QT, Kruchko C et al. . Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015;17(8):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocker K, Holland H, Ahnert P et al. . Multiple meningioma with different grades of malignancy: case report with genetic analysis applying single-nucleotide polymorphism array and classical cytogenetics. Pathol Res Pract. 2011;207(1):67–72. [DOI] [PubMed] [Google Scholar]
- 9.Butti G, Assietti R, Casalone R et al. . Multiple meningiomas: a clinical, surgical, and cytogenetic analysis. Surg Neurol. 1989;31(4):255–260. [DOI] [PubMed] [Google Scholar]
- 10.Saloner D, Uzelac A, Hetts S et al. . Modern meningioma imaging techniques. J Neurooncol. 2010;99(3):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkhardt JK, Zinn PO, Graenicher M et al. . Predicting postoperative hydrocephalus in 227 patients with skull base meningioma. Neurosurg Focus. 2011;30(5):E9. [DOI] [PubMed] [Google Scholar]
- 12.Reubi JC, Schaer JC, Waser B et al. . Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994;54(13):3455–3459. [PubMed] [Google Scholar]
- 13.Klutmann S, Bohuslavizki KH, Brenner W et al. . Somatostatin receptor scintigraphy in postsurgical follow-up examinations of meningioma. J Nucl Med. 1998;39(11):1913–1917. [PubMed] [Google Scholar]
- 14.Cornelius JF, Langen KJ, Stoffels G et al. . PET imaging of meningioma in clinical practice: review of literature and future directions. Neurosurgery. 2012;70(4):1033–1041. [DOI] [PubMed] [Google Scholar]
- 15.Thorwarth D, Henke G, Muller AC et al. . Simultaneous 68Ga-DOTATOC-PET/MRI for IMRT treatment planning for meningioma: first experience. Int J Radiat Oncol Biol Phys. 2011;81(1):277–283. [DOI] [PubMed] [Google Scholar]
- 16.Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S et al. . Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J Nucl Med. 2005;46(5):763–769. [PubMed] [Google Scholar]
- 17.Afshar-Oromieh A, Giesel FL, Linhart HG et al. . Detection of cranial meningiomas: comparison of (68)Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–1415. [DOI] [PubMed] [Google Scholar]
- 18.Rachinger W, Stoecklein VM, Terpolilli NA et al. . Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 19.Afshar-Oromieh A, Wolf MB, Kratochwil C et al. . Comparison of 68Ga-DOTATOC-PET/CT and PET/MRI hybrid systems in patients with cranial meningioma: initial results. Neuro Oncol. 2015;17(2):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehler B, Paulsen F, Oksuz MO et al. . [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartolomei M, Bodei L, De Cicco C et al. . Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging. 2009;36(9):1407–1416. [DOI] [PubMed] [Google Scholar]
- 22.Marincek N, Radojewski P, Dumont RA et al. . Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med. 2015;56(2):171–176. [DOI] [PubMed] [Google Scholar]
- 23.Hanscheid H, Sweeney RA, Flentje M et al. . PET SUV correlates with radionuclide uptake in peptide receptor therapy in meningioma. Eur J Nucl Med Mol Imaging. 2012;39(8):1284–1288. [DOI] [PubMed] [Google Scholar]
- 24.Zhou T, Xiao X, Xu B et al. . Overexpression of SSTR2 inhibited the growth of SSTR2-positive tumors via multiple signaling pathways. Acta Oncol. 2009;48(3):401–410. [DOI] [PubMed] [Google Scholar]
- 25.Arena S, Barbieri F, Thellung S et al. . Expression of somatostatin receptor mRNA in human meningiomas and their implication in in vitro antiproliferative activity. J Neurooncol. 2004;66(1-2):155–166. [DOI] [PubMed] [Google Scholar]
- 26.Schulz S, Pauli SU, Schulz S et al. . Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype SST2A. Clin Cancer Res. 2000;6(5):1865–1874. [PubMed] [Google Scholar]
- 27.Koper JW, Markstein R, Kohler C et al. . Somatostatin inhibits the activity of adenylate cyclase in cultured human meningioma cells and stimulates their growth. J Clin Endocrinol Metab. 1992;74(3):543–547. [DOI] [PubMed] [Google Scholar]
- 28.Norden AD, Ligon KL, Hammond SN et al. . Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology. 2015;84(3):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson DR, Kimmel DW, Burch PA et al. . Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011;13(5):530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arita H, Kinoshita M, Okita Y et al. . Clinical characteristics of meningiomas assessed by 11C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neurooncol. 2012;107(2):379–386. [DOI] [PubMed] [Google Scholar]
- 31.Lee JW, Kang KW, Park SH et al. . 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36(10):1574–1582. [DOI] [PubMed] [Google Scholar]
- 32.Cornelius JF, Langen KJ, Stoffels G et al. . Positron emission tomography imaging of meningioma in clinical practice: review of literature and future directions. Neurosurgery. 2012;70(4):1033–1041; discussion 1042. [DOI] [PubMed] [Google Scholar]
- 33.Cornelius JF, Stoffels G, Filss C et al. . Uptake and tracer kinetics of O-(2-(18)F-fluoroethyl)-L-tyrosine in meningiomas: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42(3):459–467. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara Y, Nakada M, Hayashi Y et al. . Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol. 2012;108(1):147–152. [DOI] [PubMed] [Google Scholar]
- 35.Tomita T, Kurimoto M, Yamatani K et al. . Multiple meningiomas consisting of fibrous meningioma and anaplastic meningioma. J Clin Neurosci. 2003;10(5):622–624. [DOI] [PubMed] [Google Scholar]