Abstract
Ependymomas are rare primary tumors of the central nervous system in children and adults that comprise histologically similar but genetically distinct subgroups. The tumor biology is typically more associated with the site of origin rather than being age-specific. Genetically distinct subgroups have been identified by genomic studies based on locations in classic grade II and III ependymomas. They are supratentorial ependymomas with C11orf95-RELA fusion or YAP1 fusion, infratentorial ependymomas with or without a hypermethylated phenotype (CIMP), and spinal cord ependymomas. Myxopapillary ependymomas and subependymomas have different biology than ependymomas with typical WHO grade II or III histology. Surgery and radiotherapy are the mainstays of treatment, while the role of chemotherapy has not yet been established. An in-depth understanding of tumor biology, developing reliable animal models that accurately reflect tumor molecule features, and high throughput drug screening are essential for developing new therapies. Collaborative efforts between scientists, physicians, and advocacy groups will enhance the translation of laboratory findings into clinical trials. Improvements in disease control underscore the need to incorporate assessment and management of patients' symptoms to ensure that treatment advances translate into improvement in quality of life.
Keywords: Collaborative Ependymoma Research Network, ependymoma, management, molecular classification, rare disease
Ependymomas are primary tumors in the central nervous system (CNS). They are known as neoplasms of children and young adults and were thought to originate from the lining of cerebral ventricles or the spinal cord central canal. However, recent studies suggest that radial glial stem cells are the cell of origin.1 These tumors may occur at any site along the ventricular system and in the spinal cord but vary in different age groups and histological subgroups.
Ependymomas develop in all age groups but occur more frequently in children than in adults. According to the 2014 report published by the Central Brain Tumor Registry of the United States (CBTRUS), ependymomas account for 5.2% of all brain and CNS tumors in children and adolescents ages 0–19 years compared with 1.9% of adult patients. There also appears to be a racial disparity, with an incidence rate per 100,000 of 0.40 in whites versus 0.27 in African Americans.2
Ependymomas have traditionally been classified by the World Health Organization (WHO) Classification of Tumors of the Nervous System as grades I, II, and III based on their grade of anaplasia. The goal of WHO classification of the CNS tumors is to predict the different clinical prognoses for the different histological grades.3 However, there is an increasing body of research challenging the concept that histological grades predict prognosis, especially in different age groups and at different locations in the CNS.4,5 This suggests that genetic heterogeneity within the same histological grade dictates the biological behavior of ependymomas and is more predictive of outcomes.
The management of patients with different grades of ependymomas has not been standardized. Surgical resection and radiation therapies have been the mainstay therapies for this group of disease. Chemotherapy is of limited efficacy for ependymomas. For those with ependymomas, the overall 10-year survival rate is 79.2% according to the 2014 CBTRUS report.2 In general, the survival rate is the highest for those aged 20–44 years and decreases with increasing age at diagnosis. In fact, the 10-year survival rate is only 28.1% in those aged >75 years. In children and adolescents aged 0–19 years, the 10-year survival rate is 66%. European population-based studies on survival of childhood cancers and primary malignant brain tumors in adults have demonstrated the disparities between countries, which might be caused by limited drug supply, lack of specialized care, and poor management of the disease.6,7
Pathology and Current Histological Classifications
The WHO classification of the CNS tumors is currently accepted as the standard classification system, where ependymomas are classified from grades I to III. The classical histological findings for all grades of ependymoma are demonstrated and summarized in Fig. 1. As is true with most primary CNS tumors, the commonly used “TNM” (tumor, lymph nodes, and metastasis) cancer staging system does not apply to ependymomas because these tumors rarely spread outside the CNS or involve adjacent lymph nodes.
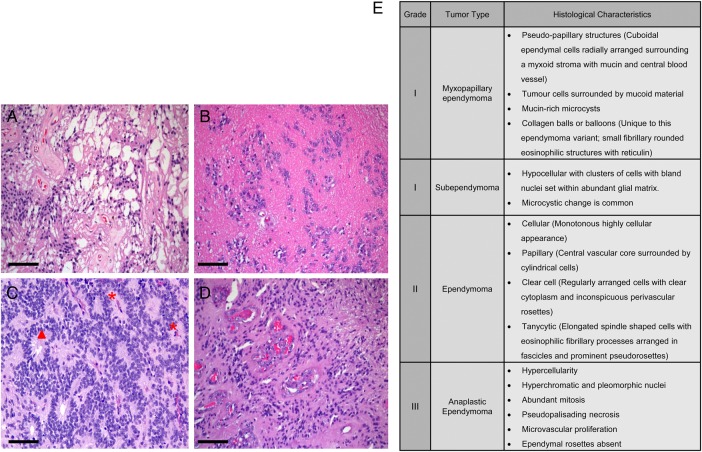
Fig. 1.
Histological features of (A) myxopapillary ependymoma, (B) subependymoma, (C) ependymoma, and (D) anaplastic ependymoma. (E) Description of histological characteristics of ependymomas of all grades. Red * indicates perivascular pseudorosettes, and red solid triangle indicates ependymal rosettes. Scale bar = 50 microns.
Grade I ependymomas include subependymomas and myxopapillary ependymomas, both of which are slow-growing and are often considered to be benign neoplasms. They have distinct localizations, imaging findings, and clinical features. Although their inclusion within the category of ependymoma is somewhat controversial, many reports continue to include subependymomas within the rubric of ependymoma. Clinically, subependymomas typically attach to a ventricle wall, most commonly the fourth ventricle, followed in frequency by the lateral ventricles. Subependymomas often appear to be sharply demarcated nodular masses that are nonenhancing on MRI scans. Patients with subependymomas usually present with symptoms of increased intracranial pressure as a consequence of ventricular obstruction. Subependymomas can be asymptomatic and discovered incidentally on MRI or even at autopsy.8 In contrast, myxopapillary ependymomas are almost exclusively located in the region of the conus medullaris, cauda equina, and filum terminale of the spinal cord, although uncommon locations such as the cervical thoracic spinal cord, lateral ventricle, or brain parenchyma have been reported.9–12 On MRI, myxopapillary ependymomas appear to be sharply circumscribed, sausage-shaped, and enhanced by the gadolinium contrast (Fig. 2). Chronic back pain is typically the most common presenting symptom for this group of patients.
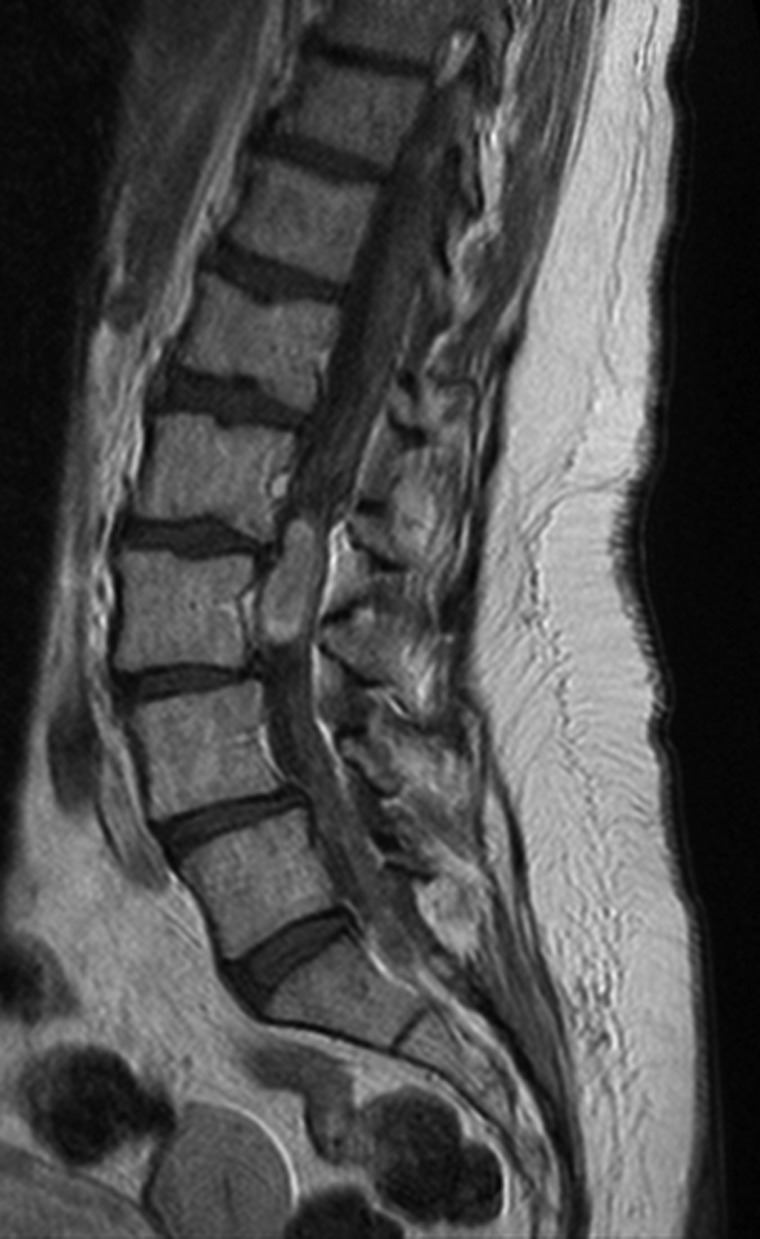
Fig. 2.
MRI with gadolinium showing a myxopapillary ependymoma of the filum terminale presenting as a contrast-enhanced spinal mass.
Grade II tumors are designated as ependymoma , whereas grade III tumors are called anaplastic ependymomas. The pathognomonic histological features are perivascular pseudorosettes, which originate from tumor cells arranged radially around the blood vessels with a perivascular anuclear zone (Fig. 1C) and true ependymal rosettes or tubules, which are ependymal cells arranged around a central lumen (Fig. 1C). Multiple histological variants of ependymoma have been reported including cellular, papillary, clear cell, and tanycytic ependymomas (Fig. 1E). Tumor geographic necrosis is not a diagnostic feature of the malignancy by itself without associated vascular proliferation and frequent mitotic activity or high proliferative index.13–15 In the grade II tumors, nonpalisading geographic foci of necrosis can be observed, while pseudopalisading necrosis and microvascular proliferation are common in anaplastic ependymomas. Increased cellularity and brisk mitotic activity are also frequently observed in anaplastic ependymomas. Clinical manifestations of both grades are largely location-dependent.
Prognostic Factors Are Age- and Site-Specific
Identification of prognostic factors in ependymomas remains an important but controversial topic that relies on correct diagnosis (ideally from a central review), formal study with an adequate number of subjects who are well stratified by their age, and tumor location. Rodriguez et al identified and studied 2408 ependymoma cases, including 2132 grade II and 276 grade III tumors, from the Surveillance, Epidemiology and End Results (SEER) database 1997–2005.16 Factors including younger age, male sex, higher tumor grade, intracranial location, and failure to undergo extensive surgical resection were found to be associated with poor clinical outcome. Although these are important findings, the use of the central registry does raise concerns regarding accuracy of the diagnosis. In fact, analysis of ependymoma cases in a single institution found that nearly 20% of them had been misdiagnosed as another histological type of neoplasm prior to the expert review,17 suggesting that accurate diagnosis for clinical studies remains an important consideration.
Recognizing that prognostic factors for pediatric patients may be different from those of adults, Amirian et al identified the ependymoma cases from the SEER database; the prognostic factors were analyzed separately for pediatric and adult groups.18 Anaplastic histology and infratentorial location of tumors were associated with an increased mortality rate in pediatric cases, while a supratentorial location was associated with higher mortality rate in adult patients. Completed surgical resection conferred a survival benefit for both pediatric and adult patients. The unfavorable prognostic impact of a supratentorial location was again demonstrated by univariate analysis from a study involving 70 patients older than aged 17 years. However, only older age, and not supratentorial location, was found to be an unfavorable prognostic factor by multivariate analysis from this study.19 A single institution study of 123 adult ependymoma patients was conducted at the University of Texas MD Anderson Cancer Center. Forty patients had tumors in the brain, 80 in the spinal cord, and 3 at both locations. Although the majority of tumors were grade I or II, the study was able to demonstrate that a brain location (versus spinal cord) and tumor anaplasia were associated with a worse outcome in adults measured by both overall survival (OS) and progression-free survival (PFS).
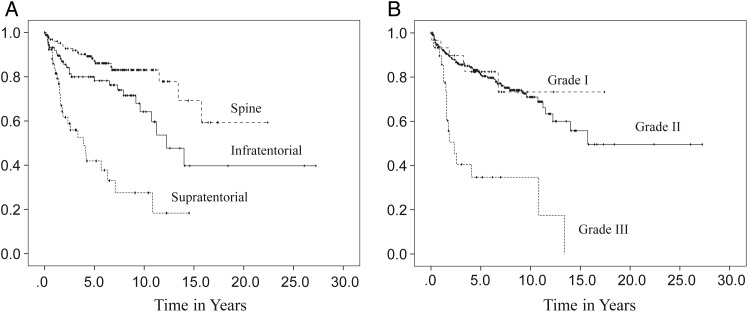
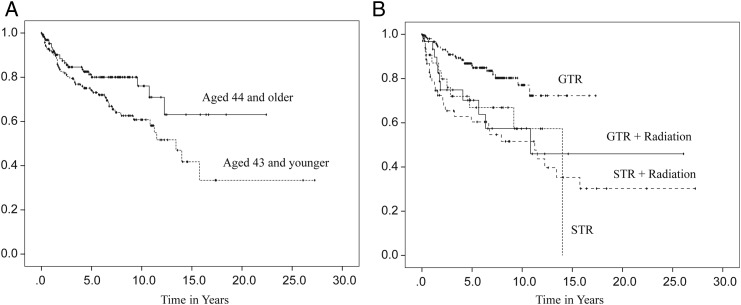
In order to address the limitations— such as quality of the study data, extensiveness of information regarding clinical features, and small numbers of samples from single institutions—the Collaborative Ependymoma Research Network (CERN) developed a multi-institutional, clinically annotated tissue repository in 2009. Tissue samples that were collected represent all locations and histological grades and were confirmed by experienced neuropathologists. Extensive clinical information, including tumor characteristics, diagnostic details, treatment history, and all demographical information were collected. Using this sophisticated data repository, the CERN investigators analyzed data from 282 cases with the goal of further defining the clinical and demographic factors associated with PFS in adult patients with ependymomas involving the brain and spinal cord.20 Participants were equally male and female with a medium age at diagnosis of 43 years. All 3 grades of WHO classification were represented, with grade II tumors being the most common histological type (78%). The spinal cord (46%) was the most common tumor location. Most participants underwent gross total resection (GTR) followed by observation. Radiation was given after both subtotal resection (STR) and GTR. Fewer than 5% of the patients received chemotherapy agents. Approximately one-third of patients had a recurrence at the time of the analysis. Although the median time to recurrence was 14 years for all patients, there were significant differences in PFS by the tumor location. The median time to progression was 3.9 years for the supratentorial brain region, 12.3 years for the infratentorial brain region, and was not reached by patients with a spinal cord location (Fig. 3A). When reported by tumor grade, grade III had the shortest PFS with the median PFS being 2.3 years, whereas PFS was not significantly different between grade I and grade II tumors (Fig. 3B). The lack of correlation of grade I and II tumors with clinical outcomes suggests that molecular features, rather than histological grades, may have greater impact in tumor biology. Differences in PFS following initial treatment were also found to be significant (Fig. 4B). Those who underwent STR had a shorter PFS compared with those who underwent GTR, regardless of the subsequent radiation treatment. Patients younger than aged 44 years had a shorter PFS than those older than aged 44 years at the time of diagnosis (Fig. 4A). The multivariate analysis of PFS demonstrated that tumor location, tumor grade, age, and initial treatment all contributed significantly to PFS (Table 1). When a separate multivariate analysis was done for each location, only the factor of initial treatment significantly contributed to PFS in the supratentorial and spinal tumors, whereas both tumor grade and initial treatment significantly contributed to PFS in infratentorial tumors. Extent of initial surgery impacted PFS, with less than GTR increasing risk for early progression in ependymomas at all 3 locations.
Fig. 3.
Progression-free survival distributions according to tumor location (A) and tumor grade (B). (Adapted from Vera-Bolanos et al, 2015, and used with permission of Oxford University Press).20
Fig. 4.
Progression-free survival distributions by age at diagnosis (A) and initial treatment (B). (Adapted from Vera-Bolanos et al, 2015, and used with permission of Oxford University Press).20
Table 1.
Multivariate analysis of progression-free survival
| Predictor | Variable | HR | 95% CI | P Value | Median PFS (y) |
|---|---|---|---|---|---|
| Age at diagnosis (y)a | 44 and older | 1 | Not reached | ||
| 43 and younger | 1.22 | 0.74–2.02 | .428 | 13.4 | |
| Location* | Supratentorial | 1 | 3.9 | ||
| Infratentorial | 0.33 | 0.18–0.63 | .1 | 12.3 | |
| Spine | 0.15 | 0.07–0.31 | <.001 | Not reached | |
| Tumor grade* | Grade II | 1 | 15.8 | ||
| Grade I | 1.90 | 0.73–4.96 | .189 | Not reached | |
| Grade III | 3.00 | 1.45–6.07 | .003 | 2.3 | |
| Initial treatment*,b | GTR | 1 | |||
| STR | 2.61 | 1.27–5.36 | .009 | 14 | |
| STR + radiation | 2.44 | 1.36–4.36 | .003 | 11.3 | |
| GTR + radiation | 0.62 | 0.27–1.45 | .271 | 10.8 |
Abbreviations: CI, confidence Interval; GTR + radiation, gross total resection followed by radiation; HR, hazard ratio; STR, subtotal resection followed by observation; STR + radiation, subtotal resection followed by radiation; y, years.
(Adapted from Vera-Bolanos et al, 2015, and used with permission of Oxford University Press).20
aBased on median age.
bInitial treatment variable is categorized as gross total followed by observation.
*Significant at P < .05.
Histological features have been traditionally used to grade ependymomas.21 However, the prognostic importance of some of the histological findings has been challenged for pediatric ependymoma when the correlation of WHO grade (II versus III) with prognosis has been questioned.22 Attempts have been made to find particular histological parameters that may be more indicative of specific ependymoma tumor biology. For example, the CERN tissue repository was used to evaluate 238 ependymoma cases to correlate individual histological features with clinical outcomes.23 Among posterior fossa ependymomas, the presence of hypercellular areas, necrosis, microvascular proliferation, and elevated mitotic rate, but not extensive ependymal canal formation, was significantly associated with worse clinical outcomes. Similar to posterior fossa tumors, microvascular proliferation and elevated mitotic rates were associated with a worse PFS in supratentorial tumors. However, in contrast to posterior fossa tumors, extensive ependymal canal formation, but not hypercellularity and necrosis, was found to be associated with a worse PFS significantly. In contrast to both supratentorial and infratentorial ependymomas, none of these histological features were found to be associated with PFS in ependymomas located within the spinal cord. These findings highly suggest that the clinical relevance of specific histological features in ependymomas appears to be related to the anatomical sites of their origins.
Advanced Genomic Studies Decode Ependymoma Biology
Despite extensive efforts to create histological criteria for segregating ependymoma into risk categories, tremendous variability has been seen in outcomes despite similarities in microscopic characteristics. These observations highly suggest that ependymomas are a group of diseases with discrete tumor biology that cannot be fully elucidated by a traditional histological classification. Therefore, molecular analyses have been undertaken to try to elucidate the pathogenesis of these tumors and provide better prognostic groupings and potentially novel therapeutic targets.
In order to understand the biological basis for the regional heterogeneity in ependymomas, many investigators have performed genomic sequencing and demonstrated that molecular changes are site-specific.23,24 Gilbertson et al performed a cross-species study, which demonstrated that ependymomas from different CNS locations share the same gene expression profiles with radial glial stem cells in the corresponding region of the developing brain or spinal cord.25 They observed that human ependymomas could be segregated into similar subgroups by using messenger RNA, microRNA, and DNA copy number alteration profiles, suggesting that the subgroups are truly distinct biological entities when defined by anatomic location. To further support this hypothesis, the investigators demonstrated that EPHB2, an oncogene identified from human supratentorial ependymomas, can transform the radial glial stem cells from forebrain to give rise to ependymomas. In contrast, the transformation by this oncogene does not occur in radial glial stem cells isolated from hindbrain and spinal cords, suggesting a unique site-specific genetic event.
Previous studies have not only demonstrated that there are location-specific molecular profiles but also a high degree of intertumoral heterogeneity within each CNS location.25–27 In order to understand intertumoral heterogeneity within each anatomical compartment (supratentorial, infratentorial, and spinal cord), researchers performed whole genome and whole exome sequencing of ependymomas in both supratentorial28 and posterior fossa27,29 ependymomas.
In a study of supratentorial ependymomas, Parker et al discovered that there are genomic structures clustered within a highly focal region on chromosome 11q12.1-q13.3.28 This region contains gross interchromosomal and intrachromosomal rearrangement, consistent with chromothripsis. The chromosome fragments from this area fused a poorly characterized gene, C11orf95, to RELA, a known principal effector of canonical NF-κB signaling pathways. This fusion occurs in about 70% of supratentorial tumors and is strictly specific for this location. This study also identified YAP1-MAMLD1 fusions in the supratentorial ependymomas without RELA fusion. Both RELA-C11orf95 and YAP1-MAMLD1 fusions were further confirmed by the DNA methylation study,30 which demonstrated that YAP1 fusion-positive cases characterize a subgroup of supratentorial ependymomas that are negative for RELA fusions. In contrast to C11orf95-RELA fusion, no chromothripsis was identified to underlie YAP1 fusions. Both fusion proteins are oncogenic.
A transcriptional profiling study of 2 independent sets of ependymomas, including both grade II and III, revealed that there are 2 demographically, genetically, and clinically distinct subgroups of ependymomas in the posterior fossa (PF) that have been designated as group A (PFA) and group B (PFB).27 PFA occurs predominantly in infants, is located laterally in the PF with a balanced genome, and is associated with a poor clinical prognosis. Conversely, PFB usually occurs in older children and adults and is associated with a better prognosis. In analyzing the signal pathways differentiating PFA and PFB, upregulation of many cancer-related molecular pathways (eg, angiogenesis, PDGFR, EGFR, and TGFβ signaling) were observed. Laminin alpha-2 (LAMA-2) and neural epidermal growth factor like-2 (NELL-2) were found and confirmed as biomarkers for PFA and PFB, respectively.
To better understand the biological basis of PF ependymomas, Mack et al proposed that PFA ependymomas might be driven by epigenetic mechanisms after they detected absence of a recurrent and significant single nucleotide variation and DNA copy number alterations in PF ependymomas.29 In a study of DNA methylation pattern in a cohort of 79 ependymomas, the finding of unsupervised consensuses clustering of CpG methylation profiles demonstrated 3 distinct subgroups including supratentorial, PF, and mixed spinal/PF tumors in a pattern similar to that yielded by unsupervised clustering of gene expression profile. Very distinct methylomes were identified in PFA and PFB ependymomas, with a higher extent of CpG island methylation found in PFA compared with PFB ependymomas. Therefore, the authors suggested that PFA ependymomas can be referred to as PFA CpG island methylator phenotype (CIMP)-positive ependymomas and PFB ependymomas as PFB CIMP-negative ependymomas. Interestingly, the genes CpG methylated in PFA ependymomas are similar to the genes that are silenced by polycomb repressive complex 2(PRC2) in embryonic stem cells. PRC2 controls all forms of methylation of lysine 27on histone H3 (H3K27) and is responsible for silencing the genes involved in cell differentiation and cancers.31,32 Both H3K27 trimethylation (H3K27Me3) and H3K27Me3 target genes were found in a separate cohort of PFA-CIMP+, but not in the PFB-CIMP- ependymomas, suggesting that tumor suppressor gene silencing by CpG hypermethylation secondary to an enhanced activity of PRC2 contributes to the pathogenesis of PFA and potentially explains the poor prognosis of patients with this subgroup of ependymomas.
Spinal ependymomas can be a major disease component of neurofibromatosis type 2 (NF2), indicating the potential role of the NF2 gene in ependymomas with spinal cord location. There have been studies showing increased frequency of NF2 gene mutation.33,34 Spinal ependymomas in the setting of NF2 have more indolent clinical courses. Deletion of chromosome 22 and increased expression of the homeobox (HOX) family genes encoding transcription factors involved in embryonic anteroposterior tissue pattern development were found in spinal ependymomas.34–36
Myxopapillary ependymoma and subependymoma have been classified under the great category of ependymoma for their unique histological appearance. However, their clinical features and tumor biology are quite distinct from grade II and III ependymomas. An analysis of a series of 35 spinal ependymomas revealed that, in addition to distinct morphologic differences between grade II and myxopapillary ependymomas, there are profound genomic and biologic differences. This study revealed that myxopapillary ependymomas demonstrated alterations in metabolic pathways such as upregulation of HIF1α and other proteins consistent with the aerobic glycolysis seen in cancer and commonly referred to as the Warburg effect.37 Subependymoma was found to be in all CNS locations by DNA-methylation profiling study, while myxopapillary ependymoma is only found at a spinal cord location.
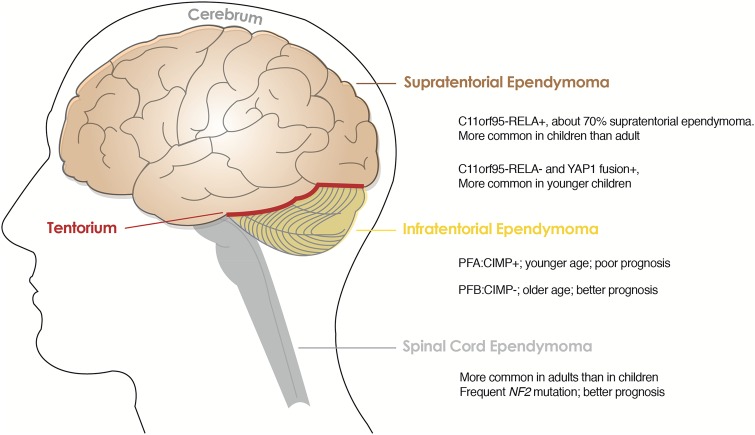
These genomic studies make it clear that ependymomas have different biology when they arise from different CNS locations and that tumors from the same location possess distinct molecular features that may be utilized to classify their biologically unique subgroups. More recently, DNA methylation profiling of a series of 500 ependymal tumors of all histological grades and anatomical locations allowed more detailed molecular classification.30 As summarized in Fig. 5, there are genetically distinct subgroups identified in the classical ependymomas, grade II and III, at each supratentorial, infratentorial, and spinal cord location.: C11orf95-RELA+ and C11orf95-RELA-, but YAP1+ supratentorial ependymomas; CIMP + and CIMP- infratentorial ependymomas and spinal cord ependymomas.27–30
Fig. 5.
Genetically distinct subgroups in supratentorial, infratentorial and spinal cord ependymomas of WHO grades II and III. Abbreviations: PFA: CIMP+, posterior fossa group A: CpG island methylator phenotype positive; PFB: CIMP-, posterior fossa group B: CpG island methylator phenotype negative.
Management of Ependymomas Is Challenging
Surgical Resection and Radiation Remain the Mainstays of Treatment for Ependymomas
Surgical resection remains a critical component of ependymoma management for all histological grades. The surgical procedure is essential to establish a pathological diagnosis and collect tissues for a genomic analysis to determine each ependymoma's unique molecular profile. If the tumor cannot be resected safely due to its location, a biopsy of the lesion is still warranted prior to the initiation of treatment. When tumor resection is feasible, the most extensive resection is always desirable as many clinical studies have reported that extensive resection is associated with both PFS and OS.38–40 In the largest case series investigating the clinical course and PFS, data from 282 patients with ependymomas suggested that GTR is the only prognostic factor significantly associated with increased PFS in supratentorial ependymomas.20 Patients who underwent subtotal resection (STR), even with the addition of radiation, have a higher chance for ultimately developing progressive disease. The same effects of GTR were also confirmed in ependymomas in the spinal cord region. Myxopapillary ependymomas are quite different from the rest of intramedullary ependymomas in the spinal cord. They are WHO grade I and almost always arise in conus medullaris but can extend to cauda equina. Several studies have demonstrated that a GTR without violating the tumor capsule may lead to cure. For this reason, it is important to maintain the integrity of the capsule to avoid tumor dissemination and to prevent a worse clinical outcome.41–43
An immediate postoperative brain MRI scan, ideally within 24–48 hours after surgery, is recommended to determine the extent of surgery. If there is extensive tumor burden appearing on the postoperative MRI and the residual disease can be readily resected, several groups have proposed a second-look surgery to maximize the extent of surgical resection.44,45 In addition to providing tissue diagnosis and improving prognosis, the tumor resection also relieves hydrocephalus, which is common with some supratentorial ependymomas and more frequently with posterior fossa ependymomas. In most patients, resection of the primary tumor is sufficient to manage the obstructive hydrocephalus without having external ventricular drainage or ventriculoperitoneal shunt.
Staging of ependymomas is highly recommended with imaging of the entire neuraxis and cerebrospinal fluid (CSF) analysis. The CSF analysis should be delayed for a minimum of 2 weeks after surgery to avoid confusing findings in the CSF. Dissemination through the CSF is not common and is estimated to occur in 15% of patients,42 but it will impact treatment planning markedly.
Postoperative radiation has been established as an effective adjuvant therapy for patients with grade III ependymomas or those with low-grade ependymomas who cannot undergo a complete resection. There is less clarity for grade II ependymomas with GTR. The optimal radiation field and dosage are still matters of debate. However, most experts in the field of neuro-oncology recommend focal radiation therapy, rather than whole brain or craniospinal radiation, unless there is a sign of tumor dissemination.46,47 Dosages of 5400 cGy in 30 fractions for low-grade ependymomas and 5940 cGy in 33 fractions for high-grade tumors have been tested in the treatment protocols.48,49 In nondisseminated diseases, a total dosage up to 6000 cGy brings the same benefit.50 Results from multivariate analysis of PFS by tumor location, conducted by CERN investigators, demonstrated that radiation following GTR may significantly increase PFS for infratentorial ependymomas but not supratentorial and spinal ependymomas.20
As described above, complete resection of a grade I myxopapillary ependymoma can be curative if the integrity of the capsule is maintained. However, some studies have suggested that routine radiotherapy should be included for young patients with spinal myxopapillary ependymomas, especially in their first 2 decades of life.51 There have been no prospective clinical studies to test the survival benefit of radiation therapy after a complete resection in grade II tumors. Therefore, it would be a reasonable approach to follow these patients closely in the setting of GTR. Due to a poor prognosis in (WHO grade III) anaplastic ependymomas, there is a consensus in the field that tumor resection should be followed by radiotherapy.
Proton therapy is increasingly being considered in the management of ependymomas due to its unique property of sparing normal tissue, suggesting that it may be superior to photon therapy for the management of pediatric ependymomas. Proton therapy may be most beneficial for younger patients with tumors that require radiotherapy near critical structures in the CNS.52 In a retrospective analysis of children with ependymomas receiving different modalities of radiotherapy, similar tumor volume coverage was achieved with intensity-modulated proton therapy (IMPT), proton therapy, and intensity-modulated radiation therapy (IMRT). However, substantial sparing of normal tissue was observed in ependymomas treated with proton therapy when compared with IMRT. Use of IMPT will allow for additional sparing of some critical structures.53 Some studies showed less acute toxicity after proton therapy in children compared with those who received photon therapy.54 Interestingly, a retrospective study that compared imaging findings after postoperative IMRT and IMPT in 72 patients with nonmetastatic intracranial ependymomas has demonstrated that postradiation MRI changes are more common after IMPT in patients younger than aged 3 years.55 Prospective studies with extended follow-up are warranted to evaluate the role of the 2 radiation modalities in both pediatric and adult ependymomas.
The Role of Chemotherapy Is Less Well Established
The role of chemotherapy in the management of ependymomas has not been well defined. Chemotherapy has been used in younger patients in an attempt to defer radiation to the developing nervous system.56–58 In a prospective clinical trial conducted by the French Society of Pediatric Oncology, 73 children younger than age 5 years with ependymomas were treated with 16 months of multiagent chemotherapy, including alternating procarbazine and carboplatin, etoposide and cisplatin, and vincristine and cyclophosphamide after surgery. The 2- and 4-year survival rates were 40% and 23%, respectively. Although treatment was well tolerated during the 5 year follow-up time, no tumors had shown more than 50% reduction as measured by imaging.59 The benefit of chemotherapy for adults with newly diagnosed ependymomas has not been studied prospectively. In patients with recurrent disease, retrospective analyses suggested that platinum-based chemotherapy regimens appear to result in higher response rates with lower rates of progression than nitrosourea-based regimens;60 however, cisplatin-based chemotherapy did not prolong PFS or OS, despite achieving a higher objective response rate. These findings are based on the results from a multicenter retrospective analysis in adults with recurrent intracranial ependymomas.61
Temozolomide (TMZ), an oral alkylating agent with a good overall toxicity profile, has become the standard treatment for several primary CNS cancers. Although there is a case report of one patient with recurrent intracranial ependymomas who stayed in remission 10 years after being treated with TMZ,62 other small series of case studies have demonstrated little efficacy in a cohort of adults with recurrent intracranial ependymomas that are platinum-refractory.63 In contrast, a recent retrospective study of response to TMZ in18 patients with recurrent ependymomas suggested that TMZ has a role in recurrent chemo-naïve adult patients with intracranial ependymoma and should be considered as the possible first-line treatment in the recurrent setting.64 The better response rate to TMZ noted in this study compared with the prior report,63 may be the result of treating chemotherapy-naïve patients. Findings from Buccoliero et al demonstrated that O6-methylguanine-DNA-methyltransferase is increased in recurrent anaplastic ependymomas, suggesting a possibility that a dose-dense dosing schedule of TMZ may have better efficacy to deplete MGMT and subsequently lead to better clinical outcomes.65 The CERN investigators have incorporated this preclinical finding (described in detail below) into their clinical trial rationale to test dose-dense TMZ in combination with lapatinib in adults with recurrent ependymomas in all CNS locations. There was evidence of treatment efficacy manifested as disease control and some objective responses; however, the contribution of each of the treatment components cannot be determined as a combination regimen.66
Development of Targeted Therapy Requires In- depth Understanding of Tumor Biology
Identifying unique molecular features and genomic alterations has provided an insight for developing clinical trial concepts in targeted treatments for ependymomas. Gilbertson et al discovered that elevated co-expression of ERBB2 and ERBB4 in > 75% of ependymomas and a high level of expression of ERBB receptors is associated with aggressive behavior such as increased cell proliferation. Pharmacological inhibition of ERBB2 receptor tyrosine kinase activity can downregulate the downstream pathway signals, such as AKT phosphorylation, suggesting a potential therapeutic role for targeting the ERBB2 receptor.67 CERN investigators developed a novel clinical trial for adults with recurrent ependymoma, testing a combination treatment of lapatinib, a dual tyrosine kinase receptor inhibitor of EGFR/ERBB1 and ERBB2, and dose-dense TMZ with the intention of depleting MGMT (CERN 08-02, NCT00826241). Treatment was well-tolerated with only modest myelotoxicity, and the results using PFS as a metric demonstrated activity in the spectrum of ependymoma defined by location and tumor grade, which was most efficacious in spinal cord tumors. Preliminary gene expression analysis of a tumor subset showed a statistically significant correlation of better treatment response with a higher ERBB2 expression at the mRNA level. Ongoing molecular profiling will determine whether ERBB2 is a predictive marker for response to this treatment regimen.66
Elevated VEGF expression has been observed in most of the ependymomas and found to be associated with short survival and poor prognosis, thereby providing the rationale for using antiangiogenic therapy to treat ependymomas, especially recurrent ependymomas with their more aggressive behavior.68,69 Lapatinib, combined with bevacizumab has been tested on children in a clinical trial by pediatric neuro-oncologists at the CERN Foundation (CERN 08-01, NCT00883688). However, the study accrual was stopped because of low response rate.70 Given that platinum-based chemotherapy has been associated with a higher response rate in intracranial ependymomas,61 the CERN investigators are accruing adult patients with recurrent ependymomas to test a combination of carboplatin with bevacizumab, a humanized VEGF monoclonal antibody (CERN 09-01, NCT01295944).
The molecular subtyping described above provides insights into tumor biology for potential therapeutic targets. However, this may dictate that specific treatment regimens are developed for the subgroups of ependymomas. For example, drugs that target DNA CpG methylation, EZH2 or histone demethylase inhibitors may be considered as potential therapeutic agents for treating PFA-CIMP+ ependymomas in young children since it may decrease H3K27 trimethylation and lessen silencing on its downstream gene targets. Conversely, the RELA fusion found in most supratentorial ependymomas results in constitutive activation of the NFκB, a potential therapeutic target.
This subgrouping of patients, which leads to very small numbers of patients eligible for clinical trials, underscores the need for preclinical models. Atkinson et al combined multicell high throughput screening, kinome-wide binding assays, and in vivo efficacy studies to identify potential treatments for ependymomas by using a mouse model of a specific subtype of human ependymomas.71 This approach established a model for expediting the process of prioritizing therapies tested in human clinical trials for specific subtypes of ependymomas. Using this drug screening system, an ongoing effort was undertaken with the aim of repurposing FDA-approved drugs and compounds that have not been used in brain cancers. This effort will dramatically shorten the time it takes to bring those drugs through different phases of clinical trials. A collection of several drugs have already been completed through this entire screening process and are now being considered for clinical trials.
Symptom Management Is Essential for Improving Quality of Life
Recognizing that oncology care is not just about using different treatment modalities to let patients live longer, we need to ensure that patients have the best quality of life (QOL) possible at every step of their survivorship. Therefore, in addition to ependymoma treatments, management of symptoms from the disease and treatment-induced toxicities are equally important in caring for patients with ependymomas. Understanding the impact of the disease and its treatments is essential for strategizing the treatments to improve patients' QOL and provide insights into the design of future clinical trials. In a recent survey of ependymoma patients, there are varied treatment approaches as well as a variety of physicians caring for adults with ependymomas.72 Consequently, it may be difficult for the individual provider to understand the impact of the disease upon the individual patient.
Investigators from CERN have developed ependymoma outcomes projects in both adults and children, from which they are collecting precise information on ependymoma epidemiology, the social and psychological impact of this disease, and treatment effects on patients' QOL. Through the project, patient presentation, clinical course, and current health status are measured and reported. At diagnosis, patients often present with multiple symptoms (an average of 3 symptoms) together with reports of symptoms occurring for 6 months on average prior to a diagnostic surgical procedure.72 Overall, patients with ependymoma involving the spine reported more severe symptoms than those with ependymoma involving the brain, despite these tumors being primarily low-grade.73 During the follow-up period, the majority of patients described significant symptom burden, and nearly 50% of patients participating in the survey reported being unable to return to work because of ongoing symptoms related to ependymomas. The neurocognitive outcomes following various treatment modalities in children have been studied extensively due to the special concern about treatment toxicities to the developing CNS.74–76 The knowledge obtained from the project will be used as an evidence-based resource for patients and their caregivers to better manage the symptoms and thus improve QOL. More importantly, the patient outcome measurement will become a critical part of future clinical trials of treatment for ependymomas, incorporating these measures into the assessment of efficacy of therapeutic regimens.
Clinical Research Challenges in Management of Ependymomas
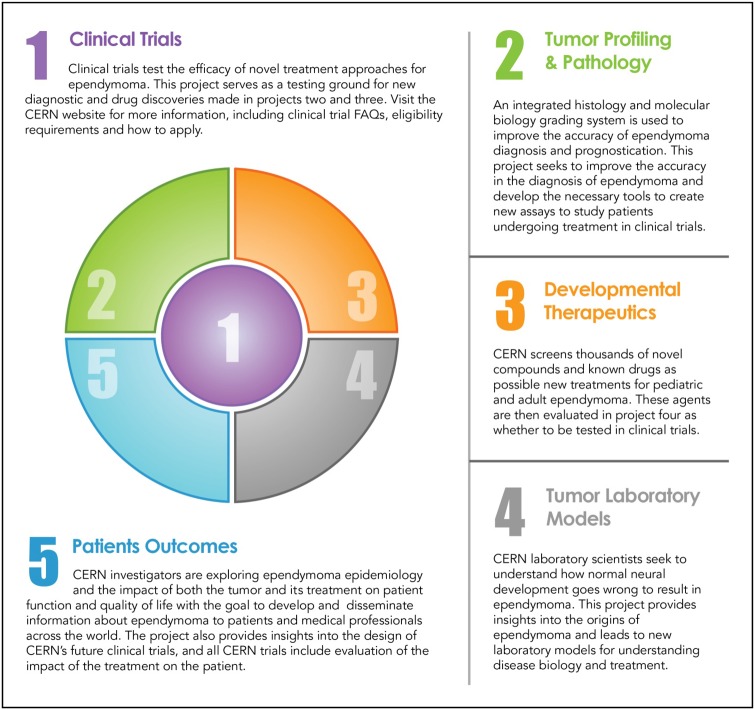
Despite better understanding of ependymoma biology and molecular classification, management of patients with ependymomas remains challenging. The rarity of the disease, especially in adults and children, makes it difficult to perform large scale or randomized clinical trials. This is compounded by the added complexity by the recent findings that although histologically similar, tumor biology may vary based on molecular subtype, thereby mandating either separate studies or stratification into subgroups based on their distinct genomic and epigenomic features. The recent molecular discoveries have clearly demonstrated that the ependymoma subgroups are biologically distinct and likely require different treatment approaches; these additional challenges are shared by many other rare cancers. Clinical trials testing agents against subgroup-specific targets have only a subset of an already uncommon disease. This makes accrual more challenging, and the decision to proceed with a particular therapeutic regimen is more critical since the time required to complete the study will likely be years. Therefore, robust preclinical models that recapitulate the disease subtypes are critical so that putative therapies can undergo laboratory testing prior to implementation of a clinical trial. As with many other rare diseases, lack of institutional support for rare diseases can make clinical research for ependymoma more complicated. In ependymoma, initiatives such as CERN foster collaborations between laboratory and clinical investigators and focus on the development of clinical trials, tumor profiling and pathology, developmental therapeutics, laboratory tumor models, and patient outcome (Fig. 6). This collaboration between scientists and clinicians specializing in adults and pediatric neuro-oncology should foster rapid translation of laboratory findings into clinical trials to maximize productivity of the research. The combination of philanthropic support with collaborative efforts between scientists and clinicians holds great promise for creating significant advances in management of patients with ependymomas—and may also serve as a model for studying other rare cancers.
Fig. 6.
The core research projects are built around and support the development of new treatments through clinical trials.
Acknowledgments
The authors would like to thank Ms. Kristin Odom for her artwork in figure production for this article.
Conflict of interest statement. M.G. was on advisory boards for AbbVie, Genentech/Roche, Foundation Medicine and Wellcome Trust and received honoraria from AbbVie, Genentech/Roche, Foundation Medicine and Wellcome Trust.
References
- 1.Taylor MD, Poppleton H, Fuller C et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl 4):iv1–i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfraind C. Classification and controversies in pathology of ependymomas. Childs Nerv Syst. 2009;25(10):1185–1193. [DOI] [PubMed] [Google Scholar]
- 5.Mack SC, Taylor MD. The genetic and epigenetic basis of ependymoma. Childs Nerv Syst . 2009;25(10):1195–1201. [DOI] [PubMed] [Google Scholar]
- 6.Gatta G, Botta L, Rossi S et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5--a population-based study. Lancet Oncol . 2014;15(1):35–47. [DOI] [PubMed] [Google Scholar]
- 7.Visser O, Ardanaz E, Botta L et al. Survival of adults with primary malignant brain tumours in Europe; Results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2231–2241. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura A, Ahyai A, Hori A, Schaake T. Intracerebral subependymomas. Clinical and neuropathological analyses with special reference to the possible existence of a less benign variant. Acta Neurochir. 1989;96(1-2):15–25. [DOI] [PubMed] [Google Scholar]
- 9.Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56(4):883–893. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Ohmura K, Mizushima M, Ito J, Kuyama H. Myxopapillary ependymoma of the lateral ventricle. A study on the mechanism of its stromal myxoid change. Acta Pathol Jpn. 1983;33(5):1017–1025. [PubMed] [Google Scholar]
- 11.Lim SC, Jang SJ. Myxopapillary ependymoma of the fourth ventricle. Clin Neurol Neurosurg. 2006;108(2):211–214. [DOI] [PubMed] [Google Scholar]
- 12.Warnick RE, Raisanen J, Adornato BT et al. Intracranial myxopapillary ependymoma: case report. J Neurooncol. 1993;15(3):251–256. [DOI] [PubMed] [Google Scholar]
- 13.Korshunov A, Golanov A, Timirgaz V. Immunohistochemical markers for intracranial ependymoma recurrence. An analysis of 88 cases. J Neurol Sci. 2000;177(1):72–82. [DOI] [PubMed] [Google Scholar]
- 14.Kurt E, Zheng PP, Hop WC et al. Identification of relevant prognostic histopathologic features in 69 intracranial ependymomas, excluding myxopapillary ependymomas and subependymomas. Cancer. 2006;106(2):388–395. [DOI] [PubMed] [Google Scholar]
- 15.Rawlings CE III, Giangaspero F, Burger PC, Bullard DE. Ependymomas: a clinicopathologic study. Surg Neurol. 1988;29(4):271–281. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005). J Surg Res. 2009;156(2):340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR. Adult ependymal tumors: prognosis and the M. D. Anderson Cancer Center experience. Neuro Oncol. 2010;12(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amirian ES, Armstrong TS, Aldape KD, Gilbert MR, Scheurer ME. Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology . 2012;39(2):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reni M, Brandes AA, Vavassori V et al. A multicenter study of the prognosis and treatment of adult brain ependymal tumors. Cancer . 2004;100(6):1221–1229. [DOI] [PubMed] [Google Scholar]
- 20.Vera-Bolanos E, Aldape K, Yuan Y et al. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015;17(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis DN, International Agency for Research on Cancer., World Health Organization. WHO Classification of Tumours of the Central Nervous System 4th ed Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 22.Ellison DW, Kocak M, Figarella-Branger D et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghunathan A, Wani K, Armstrong TS et al. Histological predictors of outcome in ependymoma are dependent on anatomic site within the central nervous system. Brain Pathol. 2013;23(5):584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modena P, Lualdi E, Facchinetti F et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24(33):5223–5233. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RA, Wright KD, Poppleton H et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466(7306):632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wani K, Armstrong TS, Vera-Bolanos E et al. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012;123(5):727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witt H, Mack SC, Ryzhova M et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20(2):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker M, Mohankumar KM, Punchihewa C et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506(7489):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mack SC, Witt H, Piro RM et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506(7489):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajtler KW, Witt H, Sill M et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari KJ, Scelfo A, Jammula S et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol Cell. 2014;53(1):49–62. [DOI] [PubMed] [Google Scholar]
- 32.Ohm JE, McGarvey KM, Yu X et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39(2):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birch BD, Johnson JP, Parsa A et al. Frequent type 2 neurofibromatosis gene transcript mutations in sporadic intramedullary spinal cord ependymomas. Neurosurgery. 1996;39(1):135–140. [DOI] [PubMed] [Google Scholar]
- 34.Ebert C, von Haken M, Meyer-Puttlitz B et al. Molecular genetic analysis of ependymal tumors. NF2 mutations and chromosome 22q loss occur preferentially in intramedullary spinal ependymomas. Am J Pathol. 1999;155(2):627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korshunov A, Neben K, Wrobel G et al. Gene expression patterns in ependymomas correlate with tumor location, grade, and patient age. Am J Pathol. 2003;163(5):1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425(6961):926–933. [DOI] [PubMed] [Google Scholar]
- 37.Mack SC, Agnihotri S, Bertrand KC et al. Spinal Myxopapillary Ependymomas Demonstrate a Warburg Phenotype. Clin Cancer Res. 2015;21(16):3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metellus P, Barrie M, Figarella-Branger D et al. Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain. 2007;130(Pt 5):1338–1349. [DOI] [PubMed] [Google Scholar]
- 39.Paulino AC, Wen BC, Buatti JM et al. Intracranial ependymomas: an analysis of prognostic factors and patterns of failure. Am J Clin Oncol. 2002;25(2):117–122. [DOI] [PubMed] [Google Scholar]
- 40.Healey EA, Barnes PD, Kupsky WJ et al. The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery. 1991;28(5):666–671; discussion 671–662. [DOI] [PubMed] [Google Scholar]
- 41.Bagley CA, Wilson S, Kothbauer KF, Bookland MJ, Epstein F, Jallo GI. Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurg Rev. 2009;32(3):321–334; discussion 334. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010;10(3):240–247. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura M, Ishii K, Watanabe K et al. Long-term surgical outcomes for myxopapillary ependymomas of the cauda equina. Spine (Phila Pa 1976) . 2009;34(21):E756–E760. [DOI] [PubMed] [Google Scholar]
- 44.Massimino M, Solero CL, Garre ML et al. Second-look surgery for ependymoma: the Italian experience. J Neurosurg Pediatr . 2011;8(3):246–250. [DOI] [PubMed] [Google Scholar]
- 45.Foreman NK, Love S, Gill SS, Coakham HB. Second-look surgery for incompletely resected fourth ventricle ependymomas: technical case report. Neurosurgery. 1997;40(4):856–860; discussion 860. [DOI] [PubMed] [Google Scholar]
- 46.Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neurooncol. 2005;75(3):287–299. [DOI] [PubMed] [Google Scholar]
- 47.Taylor RE. Review of radiotherapy dose and volume for intracranial ependymoma. Pediatr Blood Cancer. 2004;42(5):457–460. [DOI] [PubMed] [Google Scholar]
- 48.Paulino AC. The local field in infratentorial ependymoma: does the entire posterior fossa need to be treated? Int J Radiat Oncol Biol Phys. 2001;49(3):757–761. [DOI] [PubMed] [Google Scholar]
- 49.Paulino AC, Wen BC. The significance of radiotherapy treatment duration in intracranial ependymoma. Int J Radiat Oncol Biol Phys . 2000;47(3):585–589. [DOI] [PubMed] [Google Scholar]
- 50.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kukreja S, Ambekar S, Sin AH, Nanda A. Cumulative survival analysis of patients with spinal myxopapillary ependymomas in the first 2 decades of life. J Neurosurg Pediatr. 2014;13(4):400–407. [DOI] [PubMed] [Google Scholar]
- 52.Wright KD, Gajjar A. Current treatment options for pediatric and adult patients with ependymoma. Curr Treat Options Oncol. 2012;13(4):465–477. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald SM, Safai S, Trofimov A et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979–986. [DOI] [PubMed] [Google Scholar]
- 54.Amsbaugh MJ, Grosshans DR, McAleer MF et al. Proton therapy for spinal ependymomas: planning, acute toxicities, and preliminary outcomes. Int J Radiat Oncol Biol Phys. 2012;83(5):1419–1424. [DOI] [PubMed] [Google Scholar]
- 55.Gunther JR, Sato M, Chintagumpala M et al. Imaging Changes in Pediatric Intracranial Ependymoma Patients Treated With Proton Beam Radiation Therapy Compared to Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;93(1):54–63. [DOI] [PubMed] [Google Scholar]
- 56.Duffner PK, Horowitz ME, Krischer JP et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24):1725–1731. [DOI] [PubMed] [Google Scholar]
- 57.Siffert J, Allen JC. Chemotherapy in recurrent ependymoma. Pediatr Neurosurg. 1998;28(6):314–319. [DOI] [PubMed] [Google Scholar]
- 58.Souweidane MM, Bouffet E, Finlay J. The role of chemotherapy in newly diagnosed ependymoma of childhood. Pediatr Neurosurg. 1998;28(5):273–278. [DOI] [PubMed] [Google Scholar]
- 59.Grill J, Le Deley MC, Gambarelli D et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19(5):1288–1296. [DOI] [PubMed] [Google Scholar]
- 60.Gornet MK, Buckner JC, Marks RS, Scheithauer BW, Erickson BJ. Chemotherapy for advanced CNS ependymoma. J Neurooncol. 1999;45(1):61–67. [DOI] [PubMed] [Google Scholar]
- 61.Brandes AA, Cavallo G, Reni M et al. A multicenter retrospective study of chemotherapy for recurrent intracranial ependymal tumors in adults by the Gruppo Italiano Cooperativo di Neuro-Oncologia. Cancer. 2005;104(1):143–148. [DOI] [PubMed] [Google Scholar]
- 62.Rehman S, Brock C, Newlands ES. A case report of a recurrent intracranial ependymoma treated with temozolomide in remission 10 years after completing chemotherapy. Am J Clin Oncol. 2006;29(1):106–107. [DOI] [PubMed] [Google Scholar]
- 63.Chamberlain MC, Johnston SK. Temozolomide for recurrent intracranial supratentorial platinum-refractory ependymoma. Cancer. 2009;115(20):4775–4782. [DOI] [PubMed] [Google Scholar]
- 64.Ruda R, Bosa C, Magistrello M et al. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: a retrospective study. Neuro Oncol. 2016;18(2):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buccoliero AM, Castiglione F, Rossi Degl'Innocenti D et al. O6-Methylguanine-DNA-methyltransferase in recurring anaplastic ependymomas: PCR and immunohistochemistry. J Chemother. 2008;20(2):263–268. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert MR, Yuan Y, Wu JM et al. A phase II study of lapatinib and dose dense temozolomide(TMZ) for adults with recurrent ependymoma: a CERN clinical trial. Neuro Oncol. 2014;16(v13). [Google Scholar]
- 67.Gilbertson RJ, Bentley L, Hernan R et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8(10):3054–3064. [PubMed] [Google Scholar]
- 68.Korshunov A, Golanov A, Timirgaz V. Immunohistochemical markers for prognosis of ependymal neoplasms. J Neurooncol. 2002;58(3):255–270. [DOI] [PubMed] [Google Scholar]
- 69.Preusser M, Wolfsberger S, Haberler C et al. Vascularization and expression of hypoxia-related tissue factors in intracranial ependymoma and their impact on patient survival. Acta Neuropathol. 2005;109(2):211–216. [DOI] [PubMed] [Google Scholar]
- 70.DeWire M, Fouladi M, Turner DC et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: a collaborative ependymoma research network study (CERN). J Neurooncol. 2015;123(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atkinson JM, Shelat AA, Carcaboso AM et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20(3):384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Armstrong TS, Vera-Bolanos E, Gilbert MR. Clinical course of adult patients with ependymoma: results of the Adult Ependymoma Outcomes Project. Cancer. 2011;117(22):5133–5141. [DOI] [PubMed] [Google Scholar]
- 73.Walbert T, Mendoza TR, Vera-Bolanos E, Acquaye A, Gilbert MR, Armstrong TS. Symptoms and socio-economic impact of ependymoma on adult patients: results of the Adult Ependymoma Outcomes Project 2 . J Neurooncol. 2015;121(2):341–348. [DOI] [PubMed] [Google Scholar]
- 74.Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early Cognitive Outcomes Following Proton Radiation in Pediatric Patients With Brain and Central Nervous System Tumors. Int J Radiat Oncol Biol Phys. 2015;93(2):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sung KW, Lim do H, Lee SH et al. Tandem high-dose chemotherapy and autologous stem cell transplantation for anaplastic ependymoma in children younger than 3 years of age. J Neurooncol. 2012;107(2):335–342. [DOI] [PubMed] [Google Scholar]
- 76.Willard VW, Conklin HM, Boop FA, Wu S, Merchant TE. Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. Int J Radiat Oncol Biol Phys. 2014;88(4):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]